Abstract
Periodontal disease (PD), a severe form of gum disease, is among the most prevalent chronic infection in humans and is associated with complex microbial synergistic dysbiosis in the subgingival cavity. The immune system of the body interacts with the microbes as the plaque extends and propagates below the gingival sulcus. Once bacteria reach the gingival sulcus, it can enter the blood stream and affect various areas of the human body. The polymicrobial nature of periodontal disease, if left untreated, promotes chronic inflammation, not only within the oral cavity, but also throughout the human body. Alterations seen in the concentrations of healthy gut microbiota may lead to systemic alterations, such as gut motility disorders, high blood pressure, and atherosclerosis. Although gut microbiome has been shown to play a vital role in intestinal motility functions, the role of oral bacteria in this setting remains to be investigated. It is unclear whether oral microbial DNA is present in the large intestine and, if so, whether it alters the gut microbiome. In addition, polybacterial infection induced PD reduced nitric oxide (NO) synthesis and antioxidant enzymes in rodent colon. In this review, we will discuss the interactions between oral and gut microbiome, specifics of how the oral microbiome may modulate the activities of the gut microbiome, and possible ramifications of these alterations.
Keywords: microbiome, periodontal disease, gut motility, nitric oxide, antioxidants, colitis
2. Introduction
A microbiome is used to define the full collection of genes of all the microbes that reside in a community. Every human body has a unique microbiome that is capable of sustaining health, as well as eliciting disease. Specifically, multiple organs and organ systems in the human body have variable microbiomes. The bacteria in these microbiome communities communicate and function to protect the human body symbiotically. However, if the customary bacterial concentrations become dysregulated, detrimental occurrences may be observed. The oral cavity is the primary entry site of the human body, and the oral microflora provides crucial defenses. Studies have shown the constituents of oral bacteria directly relate to the constituents of the microbiota in the stool (1). The most heavily colonized portion of the human body is the gastrointestinal tract, and gut microbiota has recently been touted as an important asset to metabolism, immune function, nutrition, and gastrointestinal issues (1). Recent studies have related the function of gut microbiota in various diseases, such as obesity (2), malnutrition (3), diabetes (4), and chronic inflammatory diseases, such as irritable bowel syndrome (IBS) and Crohn’s disease (5). Intestinal bacteria represent a complex microbiome. Some bacteria of the gut are believed to play a role in gut motility and the start of inflammatory diseases of the gut, while other bacteria have a protective function (6). Understanding the importance of the oral-systemic connection can help prevent, treat, and manage disorders of the gastrointestinal tract.
3. Gut Microbiome
The intestinal microbiome plays a major role in health and disease. It is estimated that 25 million adults have functional gastrointestinal disorders, and 80% of those individuals do not consult their physician about their issues (7). Unfortunately, many common gastrointestinal disorders are of unknown etiology. The intestinal microbiota contains a plethora of bacteria, with most of them being obligate anaerobes from the phyla Bacteroides, Firmicutes, and Lactobacilli (8). This microbiota helps the body uptake certain vitamins and minerals, helps degrade starches and carbohydrates, and helps stimulate the immune system. Studies have shown that alterations in oral flora can cause an imbalance in the gut flora, which can contribute to the pathogenesis of gut disorders (1). Investigating the role of the oral microbiome on gut motility (1) and questions regarding the interrelationship of the oral and gut microbiome on gastrointestinal disorders have not been elucidated.
Early Development of the Gut Microbiome
The gut of a healthy individual contains mostly fastidious anaerobes and more than 50 phyla, with the most predominant being Bacteroides and Firmicutes. However, studies have shown that most individuals harbor over 1,000 microbial species-level phylotypes (9–11). Most microbial colonization of the gut occurs at birth. As the life of the infant progresses, the gut microbiome continuously evolves as a result of changes in diet, feeding habits, environmental conditions, and physical stresses, as well as many internal factors, such as intestinal pH (12). These same studies have shown that the microbiome of the infant gut is an indicator of the composition of the gut microbiome as an adult.
The Healthy Gut Microbiome
A healthy microbiome is described as one that is ecologically stable and can resist changes under stress or can return to homeostasis after a stress-related modification. In a healthy individual, the gut microbiome can prevent the attachment of exogenous pathogenic bacteria to the wall of the gastrointestinal tract and has direct bactericidal effects (12). Commensal bacteria foster a positive environment, modulate adaptive and innate immune responses, and prevent the adherence of pathogenic bacteria to the gastrointestinal tract. The microbiota of the lower intestinal tract is widely recognized as playing a symbiotic role in maintaining a healthy host physiology by participating in nutrient acquisition and bile acid recycling, among other activities (12). However, a specific population cannot define a healthy microbiome, as many studies have observed great inter-individual variation in health states (14–15).
The Pathogenic Gut Microbiome and Gastrointestinal Disorders
The presence of certain microbes may make an individual more susceptible to infection or disease. Pathogenic gut microbes have been known to cause disease, syndromes, and functional issues relating to the colon. Gastrointestinal disorders are of major public health concern. Crohn’s disease, Ulcerative Colitis, and other major inflammatory bowel syndromes (IBSs) that limit gut motility are chronic, relapsing enteropathies of unknown etiology (16). These diseases may exhibit symptoms such as abdominal pain, diarrhea, and changes in bowl habits (17). The most recent theories suggest that a dysregulated mucosal immune response to unknown constituents of a normal intestinal microbiome in a hereditarily predisposed host is at the core of these diseases (18). Therefore, involvement of intestinal bacteria is considered an important factor in the onset of irritable bowel syndrome. Reports indicate that antibiotics enhance the symptoms of IBD in patients, further implying the idea that bacteria have an imperative role in dysregulation of the colon (3,8,12).
Previous studies have shown that irritable bowel syndrome (IBS) is characterized by an increase in certain bacterial genus, such as Firmicutes, Ruminococcus, Clostridium, and Dorea, as well as a reduction in the amounts of Bifidobacterium and Faecalibacterium (19). In another study of pediatric patients with active IBS, an alteration in members of Firmicutes and Proteobacteria was noted, as well as a greater abundance of Dorea, Ruminococcus and Haemophilus (19). Furthermore, the genus Bacteroides was markedly lower in pediatric patients with IBS compared to those who were healthy (20). There are a variety of bacteria in the large intestine that promote infection and disease (Table 1). These pathogenic bacteria may potentiate or exacerbate the manifestation of IBS, such as Crohn’s disease. For example, increased numbers of pathogenic bacteria with mucolytic activity, such as Escherichia coli, are found in the intestinal tract of those with Crohn’s disease. Thus, the microbiota have closer contact with the mucosa during active Crohn’s Disease. The intestinal barrier plays an important role in how the immune system functions, as it provides a barrier to prevent the infiltration of bacteria through the mucosal surface.
Table 1.
Examples of Pathogenic Microbiota of the Large Intestine. The pathogenic oral bacteria in the large intestine may have negative ramifications in respect to gut health and motility disorders. Some of the more common pathogenic microbiota of the large intestine may promote motility disorders, such as Crohn’s disease and gastroenteritis. Information was adapted from the NIH Microbiome Database (Published 2008).
| Microbe | Function | NIH Microbiome Database Link |
|---|---|---|
| Helobacterium pylori | Causes gastric ulcers | A |
| Pseudomonas aeruginosa | Opportunistic pathogen | B |
| Clostridium perfringens | Causative agent of gas gangrene | C |
| Yersinia enterocolitica | Causes gastroenteritis | D |
| Salmonella enterica | Causes enteric infections | E |
| Vibrio parahaemolyticus | Causes gastroenteritis | F |
| Listeria monocytogenes | Causes listeriosis | G |
| Shigella flexneri | Causes enteric disease | H |
| Clostridium botulinum | Produces botulinum | I |
| Bacillus cerus | Can cause food poisoning | J |
| Campylobacter concisus | Causes gastroenteritis | K |
| Serratia marcescens | Opportunistic human pathogen | L |
| Escherichia coli O83:H1 Strain NRG 857C | Implicated in Crohn’s disease | M |
| Adult Diarrheal Rotavirus Strain J19 | Induces diarrhea | N |
(The URL in the hyperlinks cited in the above table will provide the detailed information of each microbe).
4. Oral Microbiome
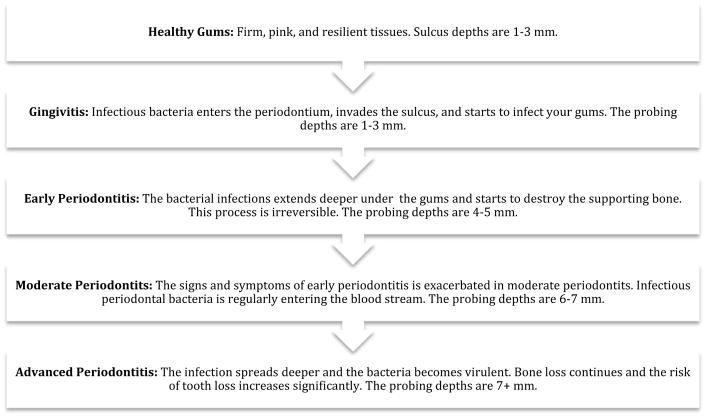
The oral microbiome is particularly important because it can instigate both oral and systemic diseases. However, as with the gastrointestinal tract, microbial shifts in the oral ecology can allow pathogenesis to manifest. The microbiome of the oral cavity differs from the microbiome of any other organ system because there are two types of surfaces on which bacteria may colonize: the sloughing surfaces (mucosa) and the hard tissues (teeth). This characteristic makes the oral cavity the ideal location for a wide variety of bacteria. Within the oral cavity, commensal bacteria turned opportunistic bacteria are known to cause many diseases or conditions, such as caries, dental cavities, gingivitis, periodontitis, peri-implantitis, endodontic infections, and tonsillitis. Figure 1 illustrates the progression of healthy gum tissue to one that is diseased and periodontally compromised. It is known that oral microflora can disseminate throughout the body and can incite several systemic diseases, including atherosclerotic vascular disease (ASVD), cardiovascular disease (CVD), adverse pregnancy outcomes (APO), Alzheimer’s disease (AD), rheumatoid arthritis (RA), colorectal cancer and diabetes.
Fig. 1.
Stages of Gingivitis and Periodontal Disease. Gingivitis typically precedes the various stages of periodontal disease. However, gingivitis does not always progress to periodontal disease. Periodontal disease is irreversible, and the vascular destruction in the oral cavity by pathogenic bacteria correlates with the amount of systemic vascular damage throughout the body (33).
The Healthy Oral Microbiome
The microbiome of a healthy oral cavity is markedly different than a diseased one. A study by Palmer et al. showed significant amounts of inter-individual differences exist, which means there are both core and variable microbiome within an environment (21). The major genera with the greatest representation in a healthy oral cavity include: Streptococcus, Veillonella, Granulicatella, Gemella, Actinomyces, Corynebacterium, Rothia, Fusobacterium, Porphyromonas, Prevotella, Capnocytophaga, Neisseria, Haemophilus, Treponema, Lactobacterium, Eikenella, Leptotrichia, Peptostreptococcus, Staphylococcus, Eubacteria, and Propionibacterium (21–23). The microbiome in a healthy oral cavity compared to one with periodontal disease (PD) is noticeably dissimilar, implying there may be a profile for central bacteria as an indicator of health (22). Moreover, sets of Gram-positive bacteria exist that are significantly enriched in healthy samples, such as Streptococcus, Actinomyces, and Granulicatella (15). These bacteria are also implicated in nitric oxide ([NO], an inhibitory neurotransmitter, a smooth muscle relaxant and a potent vasodilator) production. The same study also noted that neither Fusobacterium nor Porphyromonas are significantly more abundant in PD samples, even though they are implicated in PD (15). Creating an in-depth description of health, and understanding molecular differences between health and disease, can offer clinicians the capability to detect and diagnose disease before it reaches a non-reversible stage.
The Unhealthy Oral Microbiome and Periodontal Disease
Periodontal disease (PD) is a polymicrobial (e.g., bacteria, viruses, and fungi), chronic immune-inflammatory, dysbiotic disease (16). PD occurs succeeding a dramatic shift in the oral microbial community from a healthy state to a diseased state. PD promotes chronic inflammation due to its polybacterial nature and leads to a complex disease cascade, resulting in the destruction of the soft and hard tissues of the periodontium (16). Nitric Oxide (NO) producing bacteria, an indicator of health, are diminished in those with PD. Oral bacteria exhibit highly specific adherence mechanisms, and as a result, they colonize and cause disease, primarily in the gingiva. However, several studies have shown that PD is a significant risk factor and contributor to many systemic diseases, including ASVD, stroke, diabetes, and RA (16). Specifically, oral microbiota in Crohn’s disease patients, but not ulcerative colitis patients, show reduced diversity with reduced Firmicutes and Fusobacteria and an increase in Bacteroides (19).
The prominent pathogens that are normally found in PD are Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Fusobacterium nucleatum, Treponema denticola, and Aggregatibacter actinomycetemcomitans (24). However, certain studies have shown that certain Gram-negative bacteria (Selenomonas [25], Prevotella [26], Treponema [27], Tannerella [28], Haemophilus [29], and Catonella [30]) are particularly enriched in PD. These bacteria release proteolytic enzymes, which in turn release inflammatory signals that result in inflammation of the gingiva, apical migration of junctional epithelium, periodontal pocket formation, and alveolar bone resorption.
PD is incredibly difficult to treat because of its destructive nature. Once deep pockets form and alveolar bone is destroyed, PD is irreversible. Table 2 illustrates that many pathogenic bacteria in the oral cavity can induce oral infection and periodontal disease. Consequently, much controversy surrounds antimicrobial therapy for PD. Antibiotics for PD, such as Arestin, have been shown to work situationally, but such treatment should not be the first line of action. Using antibiotics to treat PD may destroy helpful bacteria, which can create an unhealthy environment in the oral cavity. Additionally, some bacteria have protective abilities, such as encapsulation and antibiotic-resistance, that make treating PD even more difficult (31). Even after the bacterial plaque or biofilm is removed in subgingival lesions with severe PD, there is a high chance of re-colonization of those bacteria because of the proliferation of Gram-negative bacteria in other sites of the oral cavity. The bacteria in the oral cavity should have a greater proportion of Gram-positive bacteria (i.e., Veillonella and Actinomyces) and a lesser proportion of Gram-negative bacteria; common pathogenic bacteria in the oral cavity are gram-negative (Table 2). These proportions will ensure that dental and periodontal damage remains minimal. The most adequate treatment for PD is reducing the amount of plaque and necrotic cementum from the tooth surface. Furthermore, antibiotic therapy (i.e., Arestin and tetracyclines) is used to control the propagation and proliferation of the bacteria (32).
Table 2.
Example of Pathogenic Microbiota of the Oral Cavity. Some of these bacteria have been implicated in diseases such as periodontal disease and root canal infections. Also, it is known that oral polybacteria can disseminate throughout the body and can colonize in organs such as the distal colon. Information was adapted from the NIH Microbiome Database (Published 2008).
| Microbe | Function | NIH Microbiome Database Link |
|---|---|---|
| Tannerella forsythia | Causes periodontal disease | O |
| Treponema denticola | Causes periodontal disease | P |
| Prevotella intermedia | Causes periodontal disease | Q |
| Porphyromonas gingivalis | Associated with severe and chronic periodontal disease | R |
| Cryptobacterium curtum | Causes periodontal disease | S |
| Selenomonas artemidis | Causes periodontal disease | T |
| Campylobacter rectus | Causes periodontal disease | U |
| Corynebacterium matruchotii | Associated with dental disease | V |
| Prevotella dentalis | Associated with root canal infections | X |
| Peptostreptococcus stomatis | Associated with periodontal disease, gingivitis, and root canal infections | Y |
| Wolinella succinogenes | Associated with dental root canal infections | Z |
(The URL in the hyperlinks cited in the above table will provide the detailed information of each microbe).
Biofilms and Saliva
The oral microbiome is particularly resistant to changing environments due to protective biofilms. Biofilms are defined as complex colonies of microorganisms that reside on the mucosal part of the oral cavity, as well as on the hard parts of the oral cavity. These biofilms can create an environment of health and homeostasis, or they can be a significant factor in oral diseases. The most common biofilm in the oral cavity is dental plaque (34). Dental plaque normally occurs within 48 hours of not practicing adequate oral hygiene. However, dental plaque can have a protective function in the oral cavity—it can capture oral pathogens and prevent them from proliferating. Moreover, it can prevent the pathogens from disseminating through saliva and spreading throughout the human body (34). However, bacteria can proliferate within this dental plaque and wreak havoc on the tooth and surrounding tissues.
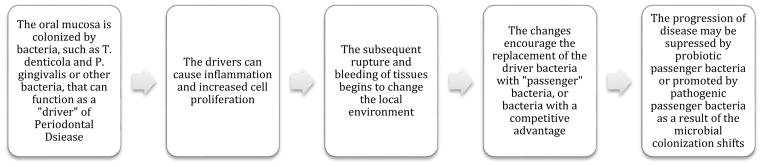
Dental biofilms occur in three stages: pellicle formation, bacterial colonization, and biofilm maturation. A pellicle is a protein film that selectively binds glycoproteins; it is formed as soon as the tooth is cleaned. In the second stage, early colonizers of the dental biofilm, Streptococcus and Actinomyces, bind to teeth. Intermediate colonizers, such as F. nucleatum, act as bridging organisms for binding early and late colonizers (35). Intermediate colonizers can adhere to and aggregate with both early and late colonizers. A “driver-passenger” model has been found in the final stages of biofilm formation and maturation and has been proposed to describe the ending stages of periodontal biofilm development (35). The “driver” pathogenic organisms, such as P. gingivalis or T. denticola, can produce virulence factors that alter the biofilm structure after the healthy bacteria are disrupted, ultimately affecting bacterial load and gene expression of “passenger” species and further challenging the immune response (Figure 2) (35). Flynn et al. also describes a polymicrobial synergy and dysbiosis (PSD) model, indicating potential for intermediate species to express multiple adhesins, proteolytic enzymes, and pro-inflammatory surface ligands, which can enter epithelial cells in the oral cavity and create an environment that can sustain the late colonizers in the biofilm (35). Ultimately, this model illustrates the possibility that the community can support itself nutritionally and can increase the prevalence of pro-inflammatory tissue destruction. Detachment of the biofilms in the oral cavity is extremely important for dental health.
Fig. 2.
A Description of the Driver-Passenger Model of the Oral Cavity. The gingiva of those who have periodontal disease are intrinsically colonized by pathogenic bacteria of the red complex or “drivers” of the disease process. These drivers can cause inflammation and increased cell proliferation. This leads to bleeding and rupturing of the tissue, which alters the microenvironment and the selective pressure on the local microbiota. These changes facilitate the gradual replacement of driver bacteria by “passengers,” consisting of opportunistic pathogens or other bacteria with a competitive advantage in the disease process. Progression of the disease may be either suppressed (by healthy passenger bacteria) or promoted (by pathogenic passenger bacteria) because of these microbial colonization changes.
Saliva is another important component of a healthy oral cavity. Saliva helps provide numerous antimicrobial enzymes, proteins, and minerals that help influence biofilm growth (36). Specifically, saliva provides minerals that help in re-mineralizing the dentition, and the antimicrobial enzymes and various proteins help defend the oral cavity and rest of the human body from exogenous bacteria. Studies have shown that salivary flow is unique and dependent on the individual, and various states of oral health are related to salivary flow, regardless of oral hygiene (36). Moreover, a difference in plaque biomass, individualistic immune responses, and pH may be a consequence of a predecessor of health and disease; this may explain why some individuals are prone to oral healthcare issues, regardless of differences in oral healthcare habits (36).
5. Nitric Oxide
The tiny molecule, nitric oxide (NO), is involved in a vast array of physiological functions, such as neurotransmission, nerve transmission, and vasodilation (37). It also plays a significant role in gastrointestinal motility. Risk factors for diseases, such as atherosclerosis, include alterations of serum NO levels (37). Furthermore, studies have assessed NO and its end-metabolites in PD and tissue health (38). Inflammatory mediators that are known to diagnose PD include interleukins, lactate dehydrogenase (LDH), C-reactive protein, and NO (38). NO is produced in the body in two different ways: dependently and independently (38). The independent mechanism of NO production includes consuming nitrate (NO3−)- and nitrite (NO2−)-containing foods and the conversion and fermentation by the bacteria of the oral cavity and stomach acid. In the dependent method, three isoenzymes are involved with the production of NO (38). Endothelial NO synthase (eNOS) is expressed on the surface of endothelial cells, specifically in distinct compartments such as the endoplasmic reticulum. Neuronal NOS (nNOS) is expressed in brain neurons. Inducible NOS (iNOS) is produced by macrophages that are stimulated by inflammatory cytokines, such as TNF-α, IL-1, and IFN-γ; these cytokines are able to produce NO (38). Salivary NO can be produced from free nerve endings, secretory cells in the salivary gland, salivary gland endothelial cells, and bacteria in the oral cavity (39). Salivary glands and oral microbiota play an essential role in the conversion of NO3− and NO2− to NO. Spiegelhalder et al. have shown that up to 25% of NO3− in circulation is actively taken up by the salivary glands (40).
Thus, the oral cavity plays an important role in NO production, and specifically, the nitrate-nitrite-NO pathway is taken by commensal bacteria in the oral cavity. Our studies in mice infected with P. gingivalis and T. denticola presented with significantly reduced (P<0.05) levels of circulatory NO compared to age-matched controls (41). This study demonstrated that reduced circulatory levels of NO, along with increased atherosclerotic plaque areas in mice orally challenged with P. gingivalis and T. denticola, suggest that either local infection with or dissemination of bacteria causes an impairment of NO bioavailability, leading to endothelial-dependent vascular dysfunction (41). Examples of oral bacteria which have been shown to produce NO3− to NO2− are: Streptococcus salivarius, S. mitis, S. bovis, Veillonella spp., Staphylococcus aureus and S. epidermidis, Norcordia spp., and Corynebacterium sp (42–43). In this pathway, nitrate is obtained by the salivary gland and is then concentrated in the saliva. Various facultative anaerobic bacteria on the dorsum of the tongue effectively reduces NO3− to NO2− (43). The bacteria then use the NO3− and NO2− as electron acceptors in their respiration process, helping the host in the first steps of converting NO3− to NO. The salivary NO3− then reaches the systemic circulation, and various enzymatic reactions occur so that reduction of NO and other reactive nitrogen intermediates (44).
6. The Link between Oral and Gut Microbiome
The microbiome of the oral cavity changes following birth and progresses through adult life (i.e., microbial shift). The emergence of primary and permanent teeth, caries, root canals, and dental appliances all change the microbiome of the oral cavity. In addition, antibiotics and proper nutrition also play a role in the microbiome of the oral cavity. The microbes in the oral cavity and the oropharynx inevitably travel to the stomach, and if the bacteria can withstand the harsh pH of the stomach, it can ultimately reside and proliferate in the gastrointestinal tract. For example, Helicobacter pylori, an infectious bacterium that leads to gastric cancer, also has a presence in dental plaque (45). In subjects with gastric H. pylori infection, high levels of H. pylori were found in dental plaque. Moreover, it was shown that the quantification of the bacteria in the dental plaque was not caused by pre-existing conditions or bad hygiene. Other studies show that H. pylori patients treated for dental plaque had a lower H. pylori gastric reinfection rate (19.4%) in comparison to those who received no treatment for plaque (84.3%) (45). The intestinal microbiome has been of great interest to researchers in the past decade because gut microbiome controls much of our health and quality of life. Roughly 45% of the bacteria of the large intestine and the oral cavity overlap (31). Furthermore, humans are constantly seeding the gastrointestinal tract with bacteria by doing everyday activities, such as eating and swallowing.
The oral cavity is the primary entryway into the human body. Thus, many microbes that inhabit this area have the ability to disseminate throughout the body. Oral bacteria enter the blood stream during mastication, chewing, tooth brushing, and flossing teeth and during dental procedures in patients with severe periodontitis (48–51). Oral microbes may colonize in the colon and alter standard immune responses. Numerous studies examine the role of oral bacteria, such as F. nucleatum, and their role in gastrointestinal carcinoma. A study by Mima et al. reported that F. nucleatum was detected in 76 of 598 (13%) colorectal carcinomas (stages I–IV) within United States cohort studies. In adjacent non-tumor tissues, the amount of F. nucleatum was higher in colorectal carcinoma tissue than in paired adjacent non-tumor tissue (52). Figure 3 illustrates the hypothetical model of periodontal disease in individuals with colitis. If periodontal disease is left untreated, members of the red complex have the potential to migrate to the colon and alter its function.
Fig. 3.
Hypothetical Diagram of Action of Periodontal Infection in the Induction of Colitis. As a first step, infection with periodontal pathogens led to increased pro-inflammatory cytokines and periodontal infection. When PD is left untreated, the oral pathogens migrate to various parts of the body and colonize locally. Alternatively, either directly or indirectly by modulating gut microbiome, these pathogens can cause reduced tetrahydrobiopterin (BH4, a cofactor for nitric oxide synthesis) in the large intestine. Reduced BH4 biosynthesis will lead to uncoupling of nitric oxide synthase decreased NO synthesis, suppression of NRF2-Phase II expression, colon dysmotility and colitis.
7. Link between Nitric Oxide and Microbiome in Gut Motility
Many studies have shown that oral bacteria can cause systemic diseases. However, the link between PD and alteration of gut motility is not well established. Recent studies from our group demonstrated that oral bacteria that induced periodontitis in mice led to impairment of the NO synthesis and tetrahydrobiopterin (BH4), a cofactor in NOS in both the vasculature and colon (16). Furthermore, these studies have shown that antioxidant protein expression, nuclear factor erythroid 2-related factor (Nrf2-Phase II), is reduced with polybacterial infection in both the vasculature and colon. Nrf2 is another vital element (an antioxidant) and is involved in defense mechanisms. Additionally, Nrf2 is a transcription factor that protects the cells from oxidative stress by activating the phase II antioxidant enzymes, such as GCSm, GCSc and HO-1 (53–54). Several lines of evidence suggest that impairment of BH4 and NO synthesis are associated with altered antioxidant mechanism and gastrointestinal motility disorders (56–57). Reduced NO bioavailability, as well as an impaired NO-mediated vasodilation, has been known to play a detrimental role in cardiovascular pathologies as a result of endothelial nitric oxide synthase (eNOS) uncoupling and increased reactive oxygen species (ROS) (58). Higashi et al studies demonstrated that periodontitis is associated with NO dependent endothelial dysfunction in the patients with coronary artery disease (59). Collectively, the above studies revealed it is possible that periodontal pathogens that travel hematogenously cannot only damage peripheral vasculature, but can also affect the large intestine and alter motility of the gut, leading to impaired gut motility. We speculate that oral bacteria possibly modulate the gut microbiome environment either directly or indirectly through other mechanism(s), such as increased free radicals, inflammatory cytokines that are known to inhibit NOS activity, and production of NO (Figure. 3). Preliminary data from our laboratory indicate that specific oral pathogens known to cause colitis are colonized in the large intestine in polybacterial-infected mice. Currently, studies are underway to investigate the relationship between the oral and gut microbiome in this setting.
8. Conclusion
The oral microbiome is extremely important and dynamic. With our oral cavity, we breathe, eat, talk, drink, and communicate. Thus, the oral microbiome has functions that affect the prevalence of bacterial activity. The oral microbiome has great ramifications on the health of other organ systems, such as the gastrointestinal system. Practicing good oral hygiene, maintaining a stable oral biofilm, and having an ample amount of NO-producing bacteria in the oral cavity may help ameliorate gastrointestinal disorders. The study of microbiomics and metagenomics will continue to demystify the relationship between the oral and gut microbiome and will ultimately allow for more personalized primary care and dental medicine.
Table 3.
Pathogenic Oral Bacteria Found in Colitis Rodent and Human Models. Polybacterial infection was performed in mice as described in our publication (16). We analyzed the BLAST results – pathogenic oral bacteria were characterized and classified using published literature. Our preliminary data show that polybacterial infection alters the colon microbiome, and modulates the amount of healthy oral and healthy gut bacteria in the distal colon.
| Bacteria Genus | Function Related to Colitis |
|---|---|
| Eubacterium | Certain species, such as Eubacterium rectale is found predominately in self-limiting colitis (60) |
| Prevotella | Invades terminal ileum and colon of patients with ulcerative colitis (61) |
| Bacteriodes | Commensal Bacteriodes induce colitis in host (62) |
| Fusobacterium | The species Fusobacterium nucleatum may correlate with IBS (63) |
| Mesorhizobium | Prevalent in those with Crohn’s disease (64) |
Acknowledgments
We thank the UNC Microbiome Core Facility at UNC, Chapel Hill, NC, and Microarray Bioinformatics Core Facility at Meharry Medical College, Nashville, TN, for microbiome analysis. Authors thank the Meharry Medical College Center of Excellence (COE) at the School of Dentistry, Nashville, TN, and Meharry Translational Research Center (MeTRC; 5U54MD007593—08) pilot grant funded to Dr. Gangula. We thank the Meharry Office for Scientific Editing and Publications for scientific editing support (S21MD000104).
Footnotes
Disclosure statement: Authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Singhal S, Dian D, Keshavarzian A, et al. The role of oral hygiene in inflammatory bowel disease. Digestive diseases and sciences. 2011;56(1):170–175. doi: 10.1007/s10620-010-1263-9. [DOI] [PubMed] [Google Scholar]
- 2.Ley R, Turnbaugh P, Klein S, Gordon J. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 3.Kau A, Ahern P, Griffin N, Goodman A, Gordon J. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin J, Li Y, Cai Z, Li S, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 5.Frank D, St Amand A, Feldman R, Boedeker E, Harpaz N, Pace N. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infection and immunity. 2008;76(8):3360–3373. doi: 10.1128/IAI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton C, PA Question: What is a Functional GI disorder? n.d Retrieved August 20, 2016, from https://www.med.unc.edu/ibs/files/educational-gi-handouts/WhatIsFunctionalGI.pdf.
- 8.Papa Eliseo, Docktor Michael, Smillie Christopher, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PloS one. 2012;7(6):e39242. doi: 10.1371/journal.pone.0039242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 10.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 11.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 12.Bull MJ, Plummer NT. Part 1: the human gut microbiome in health and disease. Integrative Medicine: A Clinician's Journal. 2014;13(6):17. [PMC free article] [PubMed] [Google Scholar]
- 13.Dewhirst Floyd E, Chen Tuste, Izard Jacques, et al. The human oral microbiome. Journal of bacteriology. 2010;192(19):5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy” core microbiome” of oral microbial communities. BMC microbiology. 2009;9(1):1. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Faller LL, Klitgord N, et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PloS one. 2012;7(6):e37919. doi: 10.1371/journal.pone.0037919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangula Pandu, Ravella Kalpana, Chukkapalli Sasanka, et al. Polybacterial Periodontal Pathogens Alter Vascular and Gut BH 4/nNOS/NRF2-Phase II Enzyme Expression. PloS one. 2015;10(6):e0129885. doi: 10.1371/journal.pone.0129885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014;20(12):3255–64. doi: 10.3748/wjg.v20.i12.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PA. The Tantalizing Links Between Gut Microbes and the Brain. 2015 Oct 16; doi: 10.1038/526312a. Retrieved August 14, 2016, from 11. http://www.nature.com/news/the-tantalizing-links-between-gut-microbes-and-the-brain-1.18557. [DOI] [PubMed]
- 19.Guinane Caitriona M, Cotter Paul D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therapeutic advances in gastroenterology. 2013;6(4):295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socransky Sigmund S, Haffajee Anne D. Dental biofilms: difficult therapeutic targets. Periodontology. 2000;28(1):12–55. doi: 10.1034/j.1600-0757.2002.280102.x. 2002. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, Robert J. Composition and development of oral bacterial communities. Periodontology 2000. 2014;64(1):20–39. doi: 10.1111/j.1600-0757.2012.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aas Jørn A, et al. Defining the Normal Bacterial Flora of the Oral Cavity. Journal of Clinical Microbiology. 2005;43(11):5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. PMC. Web. 5 Jan. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaura Egija, Keijser Bart JF, Huse Susan M, Crielaard Wim. Defining the healthy. BMC microbiology. 2009;9(1):259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eick S, Habil PD, Schaumann R, Habil PDM. Microbial profile of patients with periodontitis compared with healthy subjects. 2012 [PubMed] [Google Scholar]
- 25.Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, et al. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol. 2008;23:112–118. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darby I, Curtis M. Microbiology of periodontal disease in children and young adults. Periodontol. 2001;26:33–53. doi: 10.1034/j.1600-0757.2001.2260103.x. [DOI] [PubMed] [Google Scholar]
- 28.Mineoka T, Awano S, Rikimaru T, Kurata H, Yoshida A, et al. Site-specific development of periodontal disease is associated with increased levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in subgingival plaque. J Periodontol. 2008;79:670–676. doi: 10.1902/jop.2008.070398. [DOI] [PubMed] [Google Scholar]
- 29.Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979;6:351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 30.Siqueira JF, Jr, Rocas IN. Catonella morbi and Granulicatella adiacens: new species in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:259–264. doi: 10.1016/j.tripleo.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Segata N, Haake SK, Mannon P, et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13(6):R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horz HP, Conrads G. Diagnosis and anti-infective therapy of periodontitis. Expert review of anti-infective therapy. 2007;5(4):703–715. doi: 10.1586/14787210.5.4.703. [DOI] [PubMed] [Google Scholar]
- 33.Carranza FA, Newman MG, Takei HH, Klokkevold PR. Carranza's clinical periodontology. St. Louis, Mo: Saunders Elsevier; 2012. [Google Scholar]
- 34.Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2(5):435–444. doi: 10.4161/viru.2.5.16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn KJ, Baxter NT, Schloss PD. Metabolic and Community Synergy of Oral Bacteria in Colorectal Cancer. mSphere. 2016;1(3):e00102–16. doi: 10.1128/mSphere.00102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CS, King CP, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. The Journal of the American Dental Association. 2006;137(3):322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 37.Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, Kesavalu L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoE null Mice. PloS one. 2015;10(11):e0143291. doi: 10.1371/journal.pone.0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poorsattar Bejeh-Mir Arash, et al. Diagnostic Role of Salivary and GCF Nitrite, Nitrate and Nitric Oxide to Distinguish Healthy Periodontium from Gingivitis and Periodontitis. International Journal of Molecular and Cellular Medicine. 2014;3(3):138–145. [PMC free article] [PubMed] [Google Scholar]
- 39.Bayindir YZ, Polat MF, Seven N. Nitric oxide concentrations in saliva and dental plaque in relation to caries experience and oral hygiene. Caries Res. 2005;39:130–3. doi: 10.1159/000083158. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14(6):545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 41.Velsko IM, Chukkapalli SS, Rivera, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9(5):e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Duncan C, Townend J, et al. Nitrate-reducing bacteria on rat tongues. Appl Environ Microbiol. 1997;63:924–930. doi: 10.1128/aem.63.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmerini CA, Palombari R, Perito S, Arienti G. NO synthesis in human saliva. Free Radical Res. 2003;37:29–31. doi: 10.1080/1071576021000028398. [DOI] [PubMed] [Google Scholar]
- 44.Hyde Embriette R, et al. Metagenomic Analysis of Nitrate-Reducing Bacteria in the Oral Cavity: Implications for Nitric Oxide Homeostasis. In: Jourd’heuil David., editor. PLoS ONE. 3. Vol. 9. 2014. p. e88645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral diseases. 2015;21(1):7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 46.Yang Jing, et al. Association Between Helicobacter Pylori Infection and Risk of Periodontal Diseases in Han Chinese: A Case-Control Study. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2016;22:121–126. doi: 10.12659/MSM.894583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World journal of gastroenterology. 2016;22(2):557. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomas I, Diz P, Tobias A, Scully C, Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39:213–28. doi: 10.1111/j.1600-051X.2011.01784.x. [DOI] [PubMed] [Google Scholar]
- 49.Daly CG, Mitchell DH, Highfield JE, Grossberg DE, Stewart D. Bacteremia due to periodontal probing: a clinical and microbiological investigation. J Periodontol. 2001;72:210–4. doi: 10.1902/jop.2001.72.2.210. [DOI] [PubMed] [Google Scholar]
- 50.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–13. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 51.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–7. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 52.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA oncology. 2015;1(5):653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMahon M, Itoh K, Yamamoto M, Chanas S, et al. The cap ‘n’ collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 54.Hirayama A, Yoh K, Nagase S, Ueda A, et al. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic Biol Med. 2003;34:1236–1242. doi: 10.1016/s0891-5849(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 55.Gangula PR, Sekhar RK, Mukhopadhyay S. Gender bias in gastroparesis: Is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–7. doi: 10.1007/s10620-011-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhopadhyay S, Sekhar R, Konjeti, Hale A, Channon KM, Williams K, Farrugia G, Freeman M, Gangula PRR. Loss of Nrf2 impairs gastric nitirergic stimulation and function. Free Radical Biology and Medicine. 2011;51:619–25. doi: 10.1016/j.freeradbiomed.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gangula PRR, Chinnathambi V, Hale A, Mukhopadhyay S, Channon KM, Ravella P. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor (LDLR) deficient female mice. Neurogastroenterology & Motility. 2011;23:773–e335. doi: 10.1111/j.1365-2982.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali ZA, Rinze R, Douglas G, Hu Y, Xiao Q, Qi W, et al. Tetrahydrobiopterin determines vascular remodeling through enhanced endothelial cell survival and regeneration. Circulation. 2013;128:s50–58. doi: 10.1161/CIRCULATIONAHA.112.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higashi Y, Goto C, Hidaka T, Soga J, Nakamura S, Fujii Y, et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis. 2009;206:604–610. doi: 10.1016/j.atherosclerosis.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 60.Swidsinski A, Loening-Baucke V, Herber A. Mucosal flora in Crohn’s disease and ulcerative colitis-an overview. J Physiol Pharmacol. 2009;60(Suppl 6):61–71. [PubMed] [Google Scholar]
- 61.Sasaki Maiko, Klapproth Jan-Michael A. The role of bacteria in the pathogenesis of ulcerative colitis. Journal of signal transduction. 2012;2012 doi: 10.1155/2012/704953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bloom Seth M, Bijanki Vinieth N, Nava Gerardo M, Sun Lulu, Malvin Nicole P, Donermeyer David L, Michael Dunne W, Allen Paul M, Stappenbeck Thaddeus S. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell host & microbe. 2011;9(5):390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strauss Jaclyn, Kaplan Gilaad G, Beck Paul L, Rioux Kevin, Panaccione Remo, DeVinney Rebekah, Lynch Tarah, Allen-Vercoe Emma. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflammatory bowel diseases. 2011;17(9):1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 64.Andoh Akira, Kuzuoka Hiroyuki, Tsujikawa Tomoyuki, Nakamura Shiro, Hirai Fumihito, Suzuki Yasuo, Matsui Toshiyuki, Fujiyama Yoshihide, Matsumoto Takayuki. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. Journal of gastroenterology. 2012;47(12):1298–1307. doi: 10.1007/s00535-012-0605-0. [DOI] [PubMed] [Google Scholar]