Abstract
Aim of the study:
To investigate the roles of endoplasmic reticulum (ER) transmembrane sensor inositol-requiring enzyme-1 (IRE1)α signaling in ER stress-induced chondrocyte apoptosis, and to determine the molecular mechanisms underlying chondroprotective activity of 5,7,3′,4′-tetramethoxyflavone (TMF) from Murraya exotica.
Materials and methods:
IRE1α was knocked down by siRNA transfection in chondrocytes, which were harvested from rats’ knee cartilages. Chondrocytes with IRE1α deficiency were administrated with tunicamycin (TM) and TMF. Chondrocyte apoptosis was quantified by flow cytometry and DAPI/TUNEL staining. Expression of mRNA and proteins was quantified by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western-blot, respectively.
Results:
IRE1α deficiency significantly increased the rate of TM-induced chondrocyte apoptosis, down-regulated the expression of pro-survival factors XBP1S and Bcl-2, and up-regulated pro-apoptotic factors CHOP, p-JNK, and caspase-3. TMF suppressed TM-induced chondrocyte apoptosis by activating the expression of IRE1α, which reversed the expression patterns of downstream pro-survival and pro-apoptotic factors due to IRE1α deficiency.
Conclusion:
The mechanism of TMF in protecting chondrocytes against ER stress-induced apoptosis might be associated with regulating the activity of ER sensor IRE1α and its downstream pathway.
Keywords: CHOP, ER stress, IRE1α, TMF, XBP1
Introduction
5,7,3′,4′-tetramethoxyflavone (TMF), one of the major polymethoxyflavones isolated from Murraya exotica, has been reported to be chondroprotective by inhibiting ER stress-induced chondrocyte apoptosis (1). TMF has been shown to have anti-fungal, anti-inflammatory, anti-myco-bacterial, and anti-malarial activities (2), decrease the concentration of inflammatory cytokines TNFα, IL-1β, and PGE2 in the knee synovial fluid, and protect cartilage through down regulation of EP/cAMP/PKA signaling in a rat model of osteoarthritis (OA) (1,3). Additionally, TMF inhibits Wnt/β-catenin signaling pathway in OA chondrocytes (3). However, the mechanism underlying the TMF protective activity against chondrocyte apoptosis is unknown.
Recently, it has been demonstrated that the cytokine PGE2 may induce endoplasmic reticulum (ER) stress through up regulation of inositol-requiring enzyme-1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor-6 (ATF6) signaling pathways in chondrocytes (1). ER is the membrane organelle dealing with the folding and maturation of protein, the biosynthesis of lipid, and the balance of calcium. In order to maintain cellular homeostasis, the unfolded protein response (UPR), a unique self-protecting mechanism, has been developed to adapt to new micro-environments produced by detrimental stimuli. Three ER transmembrane sensors including IRE1, PERK, and ATF6 are linked to UPR (4). The persistence of homeostatic imbalance leads to ER stress, characterized by accumulation of unfolded or mis-folded proteins in ER. Thus, UPR initiates a protective role through up regulating ER chaperones expression to help protein folding, decreasing the load of proteins entering ER, and attenuating the genes translation, so as to restore cell functions in balance. However, once these stimuli are overwhelming and ER stress persists, cells are programmed to switch from pro-survival to proapoptosis (5), through activating the downstream factors such as C/EBP-homologous protein (CHOP) and c-Jun N-terminal kinase (JNK) (6).
ER stress has been involved in many diseases including cancers, metabolism diseases, and degenerative diseases, such as OA. Under ER stress, IRE1, a Ser/Thr protein kinase and endoribonuclease, undergoes transautophosphorylation and oligomeric assembly (7). Active IRE1α can splice the mRNA of XBP1 into XPB1S, which exhibits transcriptional regulating activity and promotes cell survival. In addition, XBP1S can also inhibit the pro-apoptotic activities of CHOP and JNK (8). Recently, IRE1 RNase activity is reported to exhibit regulated IRE1-dependent decay (RIDD) activity to degenerate ER-localized mRNAs preferentially. RIDD can either promote cell survival or induce apoptosis, depending on the time course and intensity of ER stress (9). On the other hand, IRE1α activation also leads to a cascade of phosphorylation events, including activation of JNK and MAP3K apoptosis signal-regulated kinase 1 (ASK1) (10).
In this study, we tested the role of ER transmembrane sensor IRE1α in regulating ER stress-induced chondrocyte apoptosis. We focused on testing whether the chondroprotective effects of TMF are achieved by its regulation of IRE1α.
Materials and methods
Materials
TMF was prepared as described previously (1). Tunicamycin (TM) was obtained from Sigma Chemicals (St. Louis, MO). Dulbecco’s modified Eagle’s minimum essential medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Gibco (Life Technologies, Grand Island, NY). IRE1α, XBP1S, CHOP, JNK, p-JNK, Bcl-2, caspase-3, and GAPDH monoclonal antibodies and the peroxidase-conjugated secondary antibody were purchased from Abcam (Cambridge, UK).
Primary cell isolation and culture
Under sterile conditions, four-week-old rats were sacrificed, and cartilages were isolated from the knee joints. The cartilages were cut into small pieces, and 0.25% pancreatic enzymes were employed to digest for 30 min to remove other tissues and cells, following with employment of 0.2% collagenase II to digest at 37°C for 4 h. The chondrocytes (about 5 × 106 chondrocytes in total) were isolated from cartilages, which were obtained from several individual rats. They were then cultured under the standard conditions, such as DMEM (low glucose) supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C with 5% CO2. The cultured chondrocytes have been identified as previously mentioned by toluene-blue staining and immunohistochemical staining (11). The passage 2 of chondrocytes was used. The study was approved by the Institutional Animal Care and Use Committee of Gannan Medical University.
Small interference RNA (siRNA) analysis
IRE1α siRNA (the sequence is UUGAUGGGCAGAGACUAUCdTdT) was purchased from Gene Pharma (Shanghai). Scrambled siRNA (the sequence is UCACAACCUCCUAGAAAGAGUAGA) was constructed. Chondrocytes were transfected using optimized concentration of IRE1α siRNA. The transfection procedures were carried out according to the manufacturer’s instructions. Specifically, the density of chondrocytes in the cultured plate was less than 50%. The mixture of siRNA-lipo2000 was prepared by combining siRNA with the diluted lipo2000 solvent. After 20 min of stationary standing, the siRNA-lipo2000 mixture exposed to chondrocytes for 6 h. Then, the cultured medium containing siRNA-lipo2000 mixture was replaced with standard medium. The different dosages of TMF (5 and 20 μg/mL, respectively) were administrated to chondrocytes. 48 h after transfection, the chondrocytes were lysed. Quantitative reverse transcription polymerase chain reaction and western-blotting were applied to determine mRNA and protein expression to confirm siRNA-mediated knockdown.
Quantitative analysis of apoptosis
Annexin V-FITC apoptosis assay was employed to quantify the changes of chondrocytes apoptosis by flow cytometry according to the apoptosis detection kit (Nanjing KeyGEN Biological Technology Development Co., Ltd., Nanjing, China) procedures. Chondrocytes were incubated for 48 h, and then treated with 1 μM TM and TMF. Chondrocytes (1 × 106 /mL) were collected and incubated in the buffer containing FITC-annexin V and PI. The apoptosis ratio of chondrocytes was determined by a flow cytometer (FACSCalibur BD, San Jose, CA).
TUNEL and DAPI staining assays were processed using a TUNEL and DAPI staining kit (Abcam, Cambridge, MA). Chondrocytes were cultured and administrated with 1 μM TM and TMF. After 48 h treatment, chondrocytes were harvested and fixed with 4% paraformaldehyde. Chondrocytes apoptosis analysis was performed after staining according to the instructions recommended by the kit. All fluorescent images were examined using a Leica DM3000 microscope and photos graphed using a DFC 420 camera (Leica, Wetzlar, Germany).
Gene expression analysis
The Easy-spin total RNA extraction kit (iNtRON Biotechnology, Seoul, South Korea) was employed to extract the total RNA from chondrocytes. According to the standard protocols, the first-strand of cDNA was synthesized by reverse-transcribing 2 μg of total RNA for each sample using M-MLV (Promega, USA). qRTPCR was employed to determine the expression levels of IRE1α, CHOP, XBP1, Bcl-2, and caspase-3 genes using EzOmics SYBR qPCR kits (Mastercycler, Eppendorf, Germany). Their individual primer sequences are listed in Table 1. The procedure for amplification was as follows: 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, 56°C for 45 s, 72°C for 45 s, and finally at 72°C for 10 min. The PCR reactions were performed by using the iCycler iQ real time PCR system (Bio-Rad).
Table 1.
Primer sequences for different genes.
| Gene symbol | Primers | Sequences | References |
|---|---|---|---|
| IRE1α | forward | 5′- AGAGAGGCGGGAGAGCCGTG -3′ | (12) |
| reverse | 5′- CGAGGAGGTGGGGGA AGCGA -3′ | ||
| CHOP | forward | 5′- ATATCTCATCCCCAGGAAACG -3′ | (13) |
| reverse | 5′- TCTTCCTTGCTCTTCCTCCTC -3′ | ||
| XBP1 | forward | 5′- AGTTAAGAACACGCTTGGGAAT -3′ | (13) |
| reverse | 5′- AAGATGTTCTGGGGAGGTGAC -3′ | ||
| Bcl-2 | forward | 5′- CCGGGAGATCGTGATGAAGT -3′ | (14) |
| reverse | 5′- ATCCCAGCCTCCGTTATCCT -3′ | ||
| caspase-3 | forward | 5′- TGTCATCTCGCTCTGGTACG -3′ | (15) |
| reverse | 5′- AAATGACCCCTTCATCACCA -3′ | ||
| GAPDH | forward | 5′-CAGTGGCAAAGTGGAGATTG-3′ | (16) |
| reverse | 5′-AATTTGCCG-TGAGTGGAGTC-3′ |
Four duplicates were performed for all the PCR reactions. GAPDH was used as an internal standard control. For each target, the experimental protocols were designed and optimized for efficiency near one per assumption underlying the 2−ΔΔCT method (16).
Western blot analysis
The lysis buffer (2% SDS, 10% glycerol, 10 mmol/L Tris, pH6.8, 100 mmol/L DTT) was used to lyse chondrocytes to prepare for immunoblotting. A BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA) was employed to determine the protein concentrations with bovine serum albumin as a standard. The samples were then combined with gel loading buffer (50 mmol/L Tris-HCl, pH6.8, 2% SDS, 10% glycerol, and 0.1% bromphenol blue) and denatured for 5 min, following with electrophoresing on 10% SDS-PAGE gel for IRE1α, XBP1s, CHOP, JNK, p-JNK, Bcl-2, and caspase-3. Proteins were western-blotted onto polyvinylidene difluoride (PVDF) transfer membranes. Tris-buffered saline (TBS) containing 5% non-fat milk was used to block blots for 1h, which were incubated with IRE1α, XBP1s, CHOP, JNK, p-JNK, Bcl-2, and caspase-3 at 4°C overnight, respectively. After that, the blots were rinsed and incubated with HRP-conjugated goat anti-rat IgG for 1 h. The blots were washed and developed by use of a Super Enhanced chemiluminescence detection kit (Applygen Technologies Inc., Beijing, China). Then, the protein bands were visualized after exposure of the membranes to Kodak film (Rochester, NY). GAPDH was used as the internal control.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical analysis of gene expression data were analyzed by a paired t-test. Differences were considered significant at p < 0.05.
Results
IRE1α deficiency results in a significant increase of ER stress-induced chondrocyte apoptosis
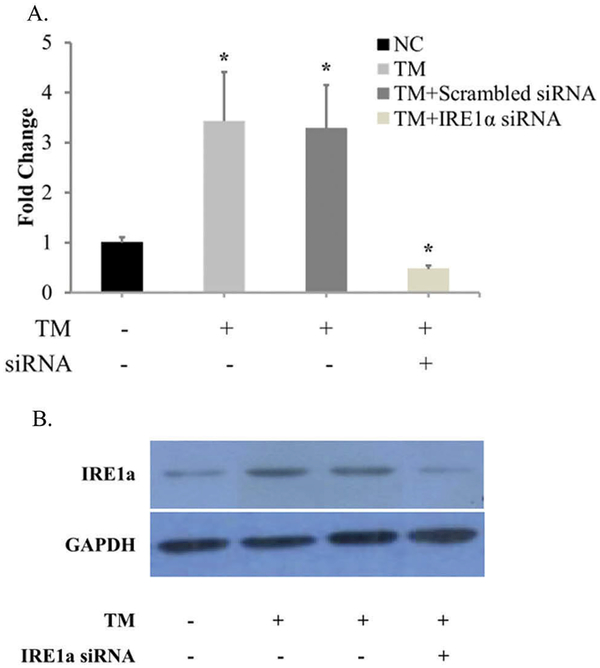
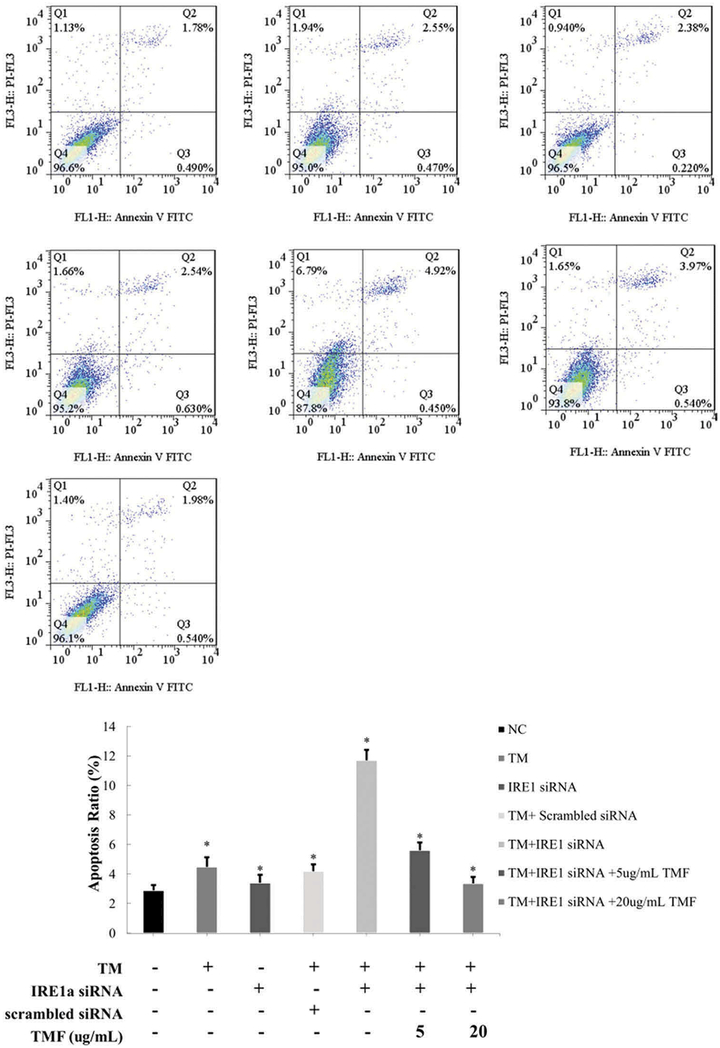
To determine the role of IRE1α in ER stress-induced chondrocyte apoptosis, the passage 2 of rat chondrocytes with IRE1α deficiency was prepared. While an ER stress-inducing agent TM (1 μM) induced IRE1α mRNA and protein levels, IRE1α siRNA transfection greatly inhibited IRE1α expression in chondrocytes (Figure 1). While TM treatment for 4 h or IRE1α deficiency alone induced chondrocyte apoptosis(4.49% ± 0.65% and 3.42% ± 0.54%, respectively), IRE1α deficiency significantly potentiates TM-induced ER stress promoted chondrocyte apoptosis(11.71% ± 0.72%) (Figure 2). Thus, IRE1α is required for ER stress response to suppress apoptosis in chondrocytes.
Figure 1.
IRE1α is expressed in chondrocytes transfected with scrambled siRNA or IRE1α siRNA. (A) Analysis of IRE1α mRNA expression with qRT-PCR. (B) Determination of IRE1α protein expression with western blot. GAPDH was used as internal control. Data were presented by mean ± standard deviation of four replicates. *p < 0.05 compared with control.
Figure 2.
TMF reduced IRE1α siRNA-induced apoptosis after treatment with 1 μM TM for 4 h in chondrocytes. The chondrocytes were incubated for 48 h. Chondrocytes in the negative control (NC) group was incubated without adding any medicines. Group (TM) was the chondrocytes incubated with 1 μM TM for 4 h. Group (IRE1 siRNA) was the chondrocytes transfected with IRE1α siRNA. Group (TM+scrambled siRNA) was the chondrocytes transfected with scrambled siRNA incubated with 1 μM TM for 4 h. Group (TM+ IRE1 siRNA) was the chondrocytes transfected with IRE1α siRNA incubated with 1 μM TM for 4 h. Group (TM+ IRE1 siRNA + TMF) were the groups that the chondrocytes transfected with IRE1α siRNA incubated with 1 μM TM and 5, 20 μg/mL TMF for 4 h, respectively. The summarized data in histogram were indicating the chondrocytes apoptosis ratio, as detected by the flow cytometry. Data were presented by mean ± standard deviation of 4 replicates. *p < 0.05 compared with control.
TMF protects chondrocytes from apoptosis induced by ER stress and IRE1α deficiency
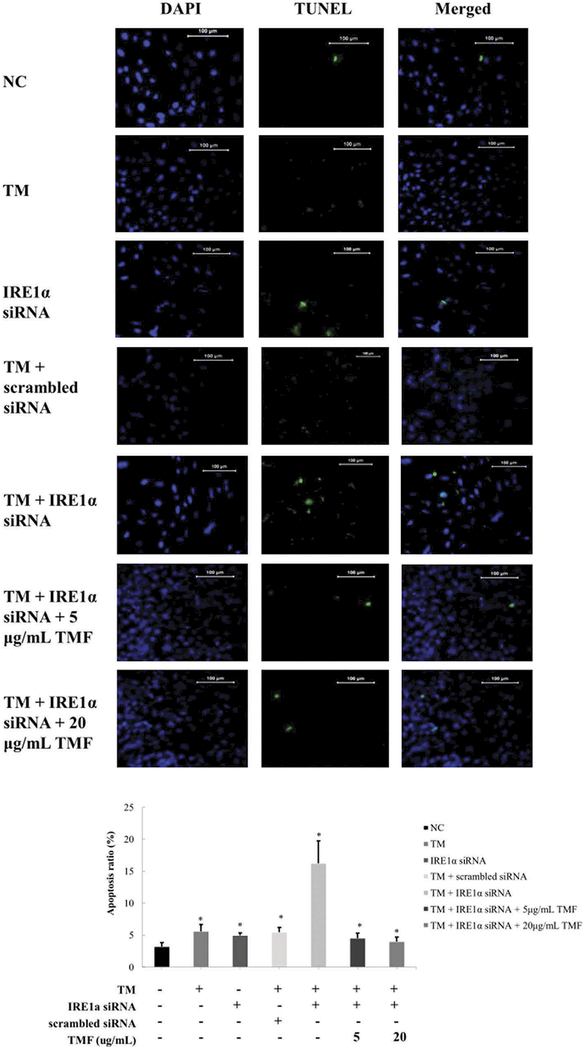
To determine whether TMF affects chondrocyte apoptosis induced by ER stress and IRE1 α deficiency, we co-administrated IRE1α deficiency chondrocytes with 5 or 20 μg/mL TMF and 1 μM TM for 4 h simultaneously. Flow cytometry analysis indicated that the apoptosis ratio of chondrocytes was significantly decreased (5.62% ± 0.53% and 3.38% ± 0.44%, respectively) in comparison to without TMF treatment (Figure 2). Similar trends were also found in chondrocyte apoptosis rates quantified by DAPI/TUNEL staining (Figure 3).
Figure 3.
TMF reduced IRE1α siRNA-induced apoptosis after treatment with 1 μM TM for 4 h in chondrocytes. The chondrocytes were incubated for 48 h. Chondrocytes in the negative control (NC) group was incubated without adding any medicines. Group (TM) was the chondrocytes incubated with 1 μM TM for 4 h. Group (IRE1 siRNA) was the chondrocytes transfected with IRE1α siRNA. Group (TM+scrambled siRNA) was the chondrocytes transfected with scrambled siRNA incubated with 1 μM TM for 4 h. Group (TM+ IRE1 siRNA) was the chondrocytes transfected with IRE1α siRNA incubated with 1 μM TM for 4 h. Group (TM+ IRE1 siRNA + TMF) were the groups that the chondrocytes transfected with IRE1α siRNA incubated with 1 μM TM and 5, 20 μg/mL TMF for 4 h, respectively. The summarized data in histogram were indicating the chondrocytes apoptosis ratio, as detected by the DAPI/TUNEL staining. Data were presented by mean ± standard deviation of four replicates. *p < 0.05 compared with control.
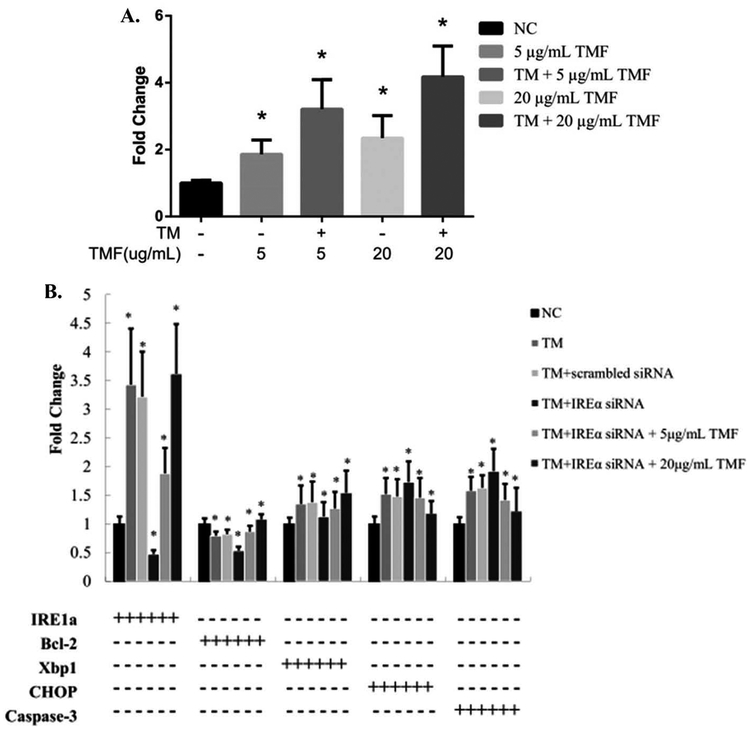
TMF activates IRE1α expression in the presence or absence of ER stress
To determine whether TMF protects chondrocytes from apoptosis through regulating IRE1α, we treated rat chondrocytes with TMF in the presence or absence of ER stress. In the absence of ER stress, TMF treatment stimulated IRE1α mRNA levels (Figure 4A). In the presence of TM-induced ER stress, TMF treatment further induced IRE1α mRNA levels (Figure 4A). While transfection of IRE1α siRNA significantly reduced IRE1α mRNA levels, TMF treatment significantly raised IRE1α mRNA levels by overcoming the knockdown effects of siRNA (Figure 4B).
Figure 4.
(A) The mRNA expression changes of IRE1α are induced by 5 and 20 μg/mL TMF or in combination of TM for 4 h. (B) The mRNA expression changes of IRE1α, CHOP, XBP1, Bcl-2 and caspase-3 in IRE1α siRNA transfected chondrocytes incubated with 1 μM TM and 5 and 20 μg/mL TMF for 4 h. Changes in mRNA expression of these genes were detected by qRT-PCR. GAPDH was used as internal control. Data were presented by mean ± standard deviation of four replicates. *p < 0.05 compared with control.
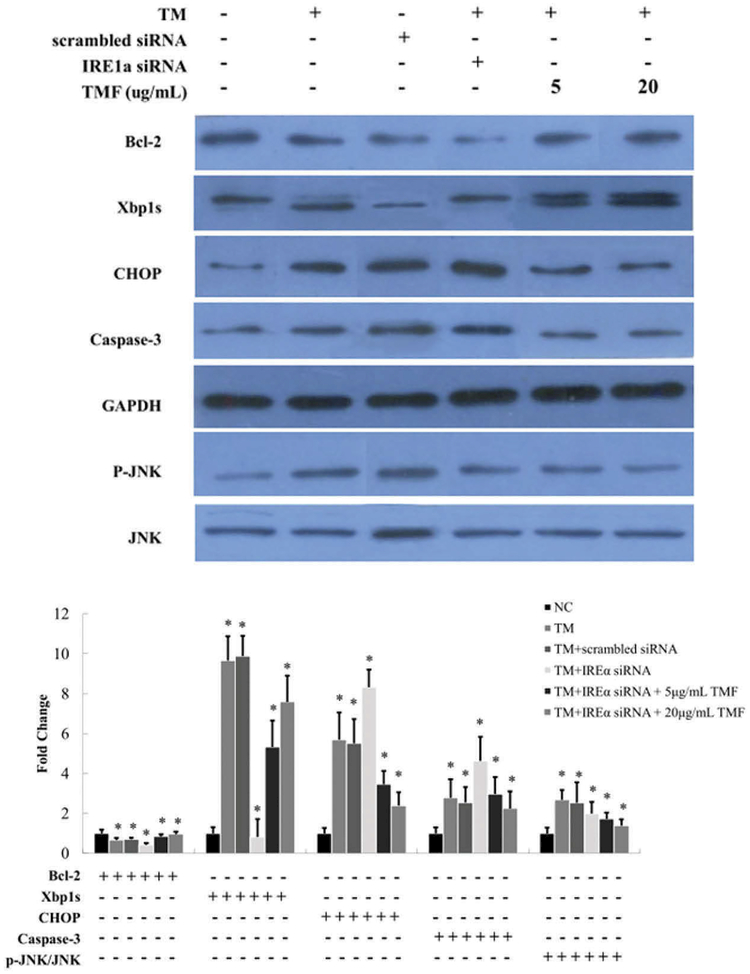
TMF reverses the expression pattern of ER stress genes due to IRE1α deficiency
To determine whether TMF treatment affects downstream gene expression in the ER stress pathway, we quantified mRNA and protein levels by real-time qRTPCR (Figure 4B) and western blot analysis (Figure 5), respectively. While IRE1α deficiency and ER stress significantly decreased Bcl-2 expression, TMF treatment reversed the decrease of Bcl-2 in chondrocytes (Figures 4B and 5). TMF treatment at 20 μg/mL also significantly increased the protein level of another pro-survival factor XBP1S (Figure 5). In contrast, while IRE1α deficiency and ER stress significantly increased the expression of pro-apoptotic factors CHOP and caspase-3, TMF treatment reversed those increases (Figures 4B and 5). In addition, the activity of the stress kinase JNK was induced by IRE1α deficiency and ER stress while TMF treatment inhibited such induction (Figure 5).
Figure 5.
Protein expression changes of Bcl-2, XBP1, CHOP, caspase-3, p-JNK, and JNK in IRE1α siRNA transfected chondrocytes incubated with 1 μM TM and 5 and 20 μg/mL TMF for 4 h. Changes in protein expression were detected by Western blot. GAPDH was used as internal control. The ratio of p-JNK/JNK was indicated in Figure 4B. Data were presented by mean ± standard deviation of four replicates. *p < 0.05 compared with control.
Discussion
Increasing evidence has suggested that ER stress is involved in chondrocyte apoptosis during OA (17). In response to ER stress, IRE1α, the most conserved branch of UPR, is activated and exhibits both endoribonuclease and protein kinase activities. IRE1α has been demonstrated to act as an important protective factor to regulate the homeostasis of intestinal epithelium (18). Our previous studies show that TMF could exhibit chondroprotective activity by inhibiting ER stress (1). To determine whether IRE1α is involved in maintaining homeostasis in response to ER stress in chondrocytes, we further investigated the pro-survival role of IRE1α in ER stress-induced chondrocytes apoptosis. Furthermore, we determined whether the protective role of TMF involves the activation of the IRE1α pathway.
Our results showed that TMF protected chondrocytes against apoptosis through regulation of IRE1α signaling. Specifically the activation of ER transmembrane sensor IRE1α is required for suppressing apoptosis in response to ER stress in chondrocytes (19). IRE1α signaling pathway has been shown to be critical in UPR and restores ER homeostasis (20). To study the role of IRE1α in the early phase of ER stress, we harvested chondrocytes after TM treatment for 4 h. The apoptosis rate in IRE1α deficient chondrocytes increased significantly, demonstrating the activation of IRE1α is necessary for protecting chondrocytes under ER stress.
We also showed that IRE1α deficiency and its reversal by TMF treatment involve downstream factors in the IRE1α pathway including XBP1, Bcl-2, Chop, caspase-3, and JNK. Unlike the physiological glucose, exogenous chemicals, such as thapsigargin (TG) or TM, can activate both IRE1α RNase activities (21). It has been demonstrated that both XBP1 mRNA splicing activity contributed to the maintenance of ER homeostasis, when cells are in the phase of adaptation (22). During the time course of ER stress response, XBP1 splicing activity comes to a peak within 1 h after treatment with TG, and lasts for 4 h before it decreases (23). Paradoxically, no significant changes in chondrocytes cells death but dysregulated proliferation have been found in Xbp1CartΔEx2 and wildtype mice. Ablation of XBP1 activity does not trigger the activation of a classic UPR in the growth plate. However, IRE1 is hyperactivated through a feedback loop in Xbp1CartΔEx2 mice (24). These underlie a complicated mechanism in IRE1/XBP1 signaling activation, which is distinct from induction by ER stress (24). Some reports show that apoptosis induced by ER stress is mediated independently of XBP1 (25). On the other hand, IRE1α can also serve as a scaffold to recruit pro-apoptotic protein factors (26), such as tumor necrosis factor receptor-associated factor 2 (TRAF2), Bax, and Bak, which trigger a cascade of phosphorylation events, including activation of ASK1 and JNK (10). Bcl-2 family proteins can regulate UPR and affect ER homeostasis. Overexpression of Bcl-2 enhances the endoribonuclease activity of IRE1α to promote the production of XBP1S. Although double knockout of Bax/Bak delays cell apoptosis, it also impairs the endoribonuclease activity of IRE1α, leading to decreased XBP1S (27).
We showed that IRE1α signaling and its modulation by TMF involves not only pro-survival factors but also pro-apoptotic factors. Recently, it has been demonstrated that IRE1α deficiency up-regulates pro-apoptotic PERK signaling, leading to phosphorylation of eIF2α and expression of CHOP (18). In rat mesangial cells, it has been found that high glucose could induce CHOP expression, resulting in cell apoptosis, which is accompanied by down regulation of XBP1S expression (28). Consistent with these, we found that IRE1α knockdown could up-regulate CHOP expression, while it down-regulates XBP1S, leading to chondrocytes apoptosis under ER stress. Thus, IRE1α acts as the switcher to turn from pro-survival to pro-apoptosis to determine cell fate.
ER stress-induced apoptosis regulated by CHOP are mainly through IRE1-TRAF2-JNK signaling or CHOP-mediated transcriptional regulation of Bcl-2 family (29). The pathway that CHOP induces cell apoptosis through suppressing the activity of Bcl-2 is a widely accepted mechanism. In response to ER stress, CHOP could induce apoptosis through down regulating the expression of Bcl-2, but not Bcl-X (30). Recently, it has been demonstrated that CHOP could up regulate the protein expression of Bax and caspase-3 (31). In this article, we suggested that IRE1α could up- regulate the expression of Bcl-2 at mRNA and protein levels, and down-regulate the expression of CHOP and caspase-3.
We found that TMF could protect chondrocytes against ER stress-induced apoptosis through up-regulating the expression of pro-survival factor IRE1α, XBP1S, and bcl-2 and down-regulating the expression of pro-apoptotic factors CHOP, caspase-3, and p-JNK. The natural flavonoids have been reported to exhibit many benefits and medicinal characters. It has been found that quercetin could inhibit Pb-induced ER stress through regulating the PI3K/Akt and IRE1α/JNK signaling pathways (32). In addition, quercetin is also reported to target ER stress pathways and inhibit ADMA-induced GEnC apoptosis (33). The chondroprotective property of TMF raises the possibility of developing TMF as an ER stress resistant and counter-apoptosis therapeutics for joint degenerative diseases.
Acknowledgments
Funding
This study was financially supported by the National Science Foundation of China (81360277 and 81660371), the National Science Foundation of Jiangxi Province (20161BAB215219), Scientific Research Fund of Jiangxi Provincial Education Department (GJJ150959), and NIH P20GM104937.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Yang J, Liu H, Li L, Liu H, Shi W, Wu L. The chondroprotective role of TMF in PGE2-induced apoptosis associating with endoplasmic reticulum stress. Evidence-Based Complem Altern Med 2015;2015:297423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu WC, Sheen JF, Hwang LS, Wei GJ. Identification of 5,7,3’,4’-tetramethoxyflavone metabolites in rat urine by the isotope-labeling method and ultra-high-performance liquid chromatography-electro-spray ionization-mass spectrometry. J Agric Food Chem 2012;60:8123–8128. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Liu H, Li L, Liu H, Yang K, Liu Z, Huang H. 5,7,3’,4’-Tetramethoxyflavone exhibits chondroprotective activity by targeting beta-catenin signaling in vivo and in vitro. Biochem Biophys Res Commun 2014;452:682–688. [DOI] [PubMed] [Google Scholar]
- 4.Boot-Handford RP, Briggs MD. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res 2010;339:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishitoh H CHOP is a multifunctional transcription factor in the ER stress response. J Biochem 2012;151:217–219. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Kuan CP, Lin JY, Lai JS, Ho TF. Tanshinone IIA facilitates TRAIL sensitization by up-regulating DR5 through the ROS-JNK-CHOP signaling axis in human ovarian carcinoma cell lines. Chem Res Toxicol 2015;28:1574–1583. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu N, Chiang WC, Kurt TD, Sigurdson CJ, Lin JH. Multiple mechanisms of unfolded protein response-induced cell death. Am J Pathol 2015;185:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol 2010;8: e1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci 2014;39:245–254. [DOI] [PubMed] [Google Scholar]
- 10.Jager R, Bertrand MJ, Gorman AM, Vandenabeele P, Samali A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell 2012;104:259–270. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Liu H, Zhang R, Li L, Li J, Hu H, Huang H. Chondroprotective activity of Murraya exotica through inhibiting beta -catenin signaling pathway. Evidence-Based Complementary Altern Med 2013;2013:752150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, Baird S, Pearce N, Grönberg S, Wilson B, Prakesh M, Aman A, Isaac M, Mamai A, Uehling D, Al-Awar R, Falls T, Alain T, Stojdl DF. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer Cell 2011;20:443–456. [DOI] [PubMed] [Google Scholar]
- 13.Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metabol 2009;9:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Song Q, Cai Y, Wang P, Wang M, Zhang D. RLIP76-dependent suppression of PI3K/AKT/Bcl-2 pathway by miR-101 induces apoptosis in prostate cancer. Biochem Biophys Res Commun 2015;463:900–906. [DOI] [PubMed] [Google Scholar]
- 15.Ganai AA, Jahan S, Ahad A, Abdin MZ, Farooqi H. Glycine propionyl l-carnitine attenuates d-Galactosamine induced fulminant hepatic failure in wistar rats. Chemico-Biol Interact 2014;214:33–40. [DOI] [PubMed] [Google Scholar]
- 16.Lu YC, Song J, Cho HY, Fan G, Yokoyama KK, Chiu R. Cyclophilin a protects Peg3 from hypermethylation and inactive histone modification. J Biol Chem 2006;281:39081–39087. [DOI] [PubMed] [Google Scholar]
- 17.Haywood J, Yammani RR. Free fatty acid palmitate activates unfolded protein response pathway and promotes apoptosis in meniscus cells. Osteoarthritis Cartilage 2016;24:942–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li XX, Yuan TL, He SQ, Yu Y, Shao ML, Liu Y, Bai CG, Ling ZQ, Li M, Liu Y, Fang J. The endoplasmic reticulum stress sensor IRE1alpha in intestinal epithelial cells is essential for protecting against colitis. J Biol Chem 2015;290:15327–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Zhou J, Zhang P, Song F, Jiang R, Li M, Xia F, Guo FJ. IRE1alpha dissociates with BiP and inhibits ER stress-mediated apoptosis in cartilage development. Cell Signall 2013;25:2136–2146. [DOI] [PubMed] [Google Scholar]
- 20.So JS, Cho S, Min SH, Kimball SR, Lee AH. IRE1alpha-dependent decay of CReP/Ppp1r15b mRNA increases eukaryotic initiation factor 2alpha phosphorylation and suppresses protein synthesis. Mol Cell Biol 2015;35:2761–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eletto D, Eletto D, Boyle S, Argon Y. PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1. FASEB J 2016;30:653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, Glimcher LH. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol 2015;16:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam AB, Koong AC, Niwa M. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep 2014;9:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron TL, Gresshoff IL, Bell KM, Pirog KA, Sampurno L, Hartley CL, Sanford EM, Wilson R, Ermann J, Boot-Handford RP, Glimcher LH, Briggs MD, Bateman JF. Cartilage-specific ablation of XBP1 signaling in mouse results in a chondrodysplasia characterized by reduced chondrocyte proliferation and delayed cartilage maturation and mineralization. Osteoarthritis Cartilage 2015;23:661–670. [DOI] [PubMed] [Google Scholar]
- 25.Cameron TL, Bell KM, Gresshoff IL, Sampurno L, Mullan L, Ermann J, Glimcher LH, Boot-Handford RP, Bateman JF. XBP1-Independent UPR pathways suppress C/EBP-beta mediated chondrocyte differentiation in ER-stress related skeletal disease. PLoS Genet 2015;11:e1005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol 2008;442:395–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chonghaile TN, Gupta S, John M, Szegezdi E, Logue SE, Samali A. BCL-2 modulates the unfolded protein response by enhancing splicing of X-box binding protein-1. Biochem Biophys Res Commun 2015;466:40–45. [DOI] [PubMed] [Google Scholar]
- 28.Lim JC, Lim SK, Park MJ, Kim GY, Han HJ, Park SH. Cannabinoid receptor 1 mediates high glucose-induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. Am J Physiol - Renal Physiol 2011;301:F179–F188. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Cheng M, Lao Y, Wang X, Wu J, Zhou L, Zhang Y, Xu H, Xu N. DNA damage and ER stress contribute to oblongifolin C-induced cell killing in Bax/Bak-deficient cells. Biochem Biophys Res Commun 2015;457:300–306. [DOI] [PubMed] [Google Scholar]
- 30.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J 1998;17:3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh MR, Kim JI, Han SJ, Lee TJ, Park KM. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim Biophys Acta 2015;1852:1895–1901. [DOI] [PubMed] [Google Scholar]
- 32.Liu CM, Zheng GH, Ming QL, Sun JM, Cheng C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radical Res 2013;47:192–201. [DOI] [PubMed] [Google Scholar]
- 33.Guo W, Ding J, Zhang A, Dai W, Liu S, Diao Z, Wang L, Han X, Liu W. The inhibitory effect of quercetin on asymmetric dimethylarginine-induced apoptosis is mediated by the endoplasmic reticulum stress pathway in glomerular endothelial cells. Int J Mol Sci 2014;15:484–503. [DOI] [PMC free article] [PubMed] [Google Scholar]