Abstract
Intrafamilial relationships among clubtail dragonflies (Gomphidae) have been the subject of many morphological studies, but have not yet been systematically evaluated using molecular data. Here we present the first molecular phylogeny of Gomphidae. We include six of the eight subfamilies previously suggested to be valid, and evaluate generic relationships within them. We have included examples of all genera reported from the Nearctic except Phyllocycla. This sample includes all North American species of Ophiogomphus, which has allowed us to explore intrageneric relationships in that genus. Our particular focus is on the closest relatives of the genus Gomphus, especially those North American species groups that have been commonly treated as subgenera of Gomphus. The Gomphus complex is split into additional genera, supported by molecular and morphological evidence: Phanogomphus, Stenogomphurus, Gomphurus and Hylogomphus are here considered to be valid genera. The genus Gomphus, in our restricted sense, does not occur in the western hemisphere; in addition, G. flavipes is transferred to Stylurus.
Introduction
Few insects inspire both public and academic interest; among these are dragonflies (Anisoptera), colourful, ubiquitous insects whose likeness is incorporated into many aspects of human culture (Sarot, 1958). Of the close to 3000 species of dragonflies, the majority of known species belong to either the family Libellulidae (∼1000) or the family Gomphidae (∼960); given the cryptic habits and apparently relatively low vagility of gomphids compared with most other Anisoptera, they might ultimately be found to exceed Libellulidae in species number. Gomphidae are medium to large dragonflies, males of which usually have expanded apical abdominal segments that give the appearance of a club (the common name for Gomphidae is clubtails). Female clubtails have a vestigial ovipositor and oviposit exophytically (i.e. not using plant material). Nymphs tend to be burrowers or hiders, concealing themselves with mud and debris in lentic and lotic habitats (Corbet, 1999). Previous work has suggested that the relatively high diversities of both Gomphidae and Libelluloidea were brought about by parallel shifts in behaviour and niche space: in both groups the nymphs acquired the habit of hiding within or sprawling on aquatic substrates (F.L. Carle, personal communication); this in turn made them less dependent on concealment in vegetation, which allowed for exophytic oviposition and, consequently, the probable convergent reduction in the gonapophyses.
No extensive modern phylogeny of Gomphidae has yet been published. Carle (1986) provided the most recent and comprehensive classification, which included descriptions and lists of purported morphological synapomorphies for all subfamilies and tribes, and some genera and subgenera, but did not provide a phylogenetic analysis. Briefly, Carle recognized four ‘Divisions’, each with one to three subfamilies: Hagenius (Hageniinae), Gomphus (Octogomphinae, Gomphinae), Epigomphus (Epigomphinae, Austrogomphinae) and Lindenia (Phyllogomphinae, Onychogomphinae, Lindeniinae). Under this scheme, most North American species are placed in Gomphinae [Arigomphus Needham 1897, Dromogomphus Selys 1854, Gomphurus Needham 1901, Gomphus Leach 1815 (including Hylogomphus Needham, Westfall & May 2000), Phanogomphus Carle, 1986, Stenogomphurus Carle, 1986, Stylurus Needham 1897] or Onychogomphinae (Erpetogomphus Hagen in Selys 1858, Ophiogomphus Selys 1854), with a few Octogomphinae (Lanthus Needham 1897, Octogomphus Selys 1873, Stylogomphus Fraser 1897) and Lindeniinae (Aphylla Selys, 1854, Phyllocycla Calvert 1948, Phyllogomphoides Belle 1978, Progomphus Selys, 1854). Molecular analyses have often included Gomphidae in larger ordinal level studies (e.g. Bybee et al., 2008, which included fossil and extant taxa in a morphological and molecular analysis; Carle et al., 2008, which focused taxon sampling on Coenagrionidae; Dumont et al., 2010, an ordinal phylogeny which used nuclear ribosomal genes; Ware et al., 2014, which focused taxon sampling on Petaluridae; Letsch et al., 2016, for which diversification rate analyses were done; and Carle et al., 2015, which included mitochondrial and nuclear data), but no comprehensive Gomphidae-focused, molecular-based phylogeny has been reconstructed until now.
In particular, the classification of North American gomphids, especially within or very close to the genus Gomphus Leach 1815, has long been in dispute. Needham (1948) reviewed the North American taxa and recognized five subgenera: Arigomphus, Gomphurus, Gomphus (including the Eurasian generotype, G. (Gomphus) vulgatissimus (L. 1758), Hylogomphus and Stylurus Needham, 1897. Over time, Arigomphus and Stylurus have been recognized as genera in their own right (Needham et al., 2014; Garrison et al., 2006). Walker (1958) concluded that a satisfactory classification within Gomphus would have to wait until all related Old World species are compared with the North American fauna; this still has not been attempted. Carle (1986) considered that Needham’s Hylogomphus was actually synonymous with the ‘true’ Gomphus of the Palaearctic. He proposed the name Phanogomphus for the species that Needham had placed in his subgenus Gomphus, and also erected Stenogomphurus for two somewhat aberrant species formerly placed in Gomphurus. Both the latter names have heretofore received only lukewarm acceptance (e.g. Garrison et al., 2006).
Certainly, a revision of Gomphidae is much needed, as its phylogenetic relationships have not been examined in detail, and its placement within Anisoptera has varied widely with gene selection or morphological character set (e.g. Bybee et al., 2008; Carle et al., 2015 recover it as sister to Petaluridae using molecular data; Carle, 1982; Blanke et al., 2013 recover it as sister to all other Anisoptera using morphology). While past studies suggest that Gomphidae may comprise a separate superfamily, Gomphoidea, which molecular data suggest is probably related to the Petaluroidea (e.g. Carle et al., 2015), the lack of a comprehensive Gomphidae-rich phylogeny prevents such hypotheses from being tested. Here we use a multi-locus approach with broad taxon sampling across the Gomphidae, with emphasis on North American taxa, to determine intergeneric and subfamilial relationships. We also discuss possible taxonomic revisions within the highly speciose genus Gomphus, using morphological characters and molecular data.
Materials and methods
Taxon sample
We focused on North American taxon sampling, but included a number of taxa from the Old World (Table S1), especially from Gomphus and its putative close relatives. Only Zonophora Selys, 1854 is strictly Neotropical; Aphylla, Phyllogomphoides and Progomphus are very likely of Neotropical origin and Erpetogomphus may be, although we used Nearctic examples in all cases. In all, we included 33 genera (out of 94 total), and 136 species (out of 961 total), from six of the eight subfamilies recognized by Carle (1986) (see Dijkstra et al., 2013). Aeshnidae, Petaluridae and selected members of the Cavilabiata (Bechly, 1996) were used as outgroup taxa.
Molecular data collection
We used nuclear and mitochondrial protein coding [histone 3 (H3), and cytochrome c oxidase subunit I (COI)] and ribosomal (12S, 16S, and 28S) primers for amplification. The selected regions were amplified by polymerase chain reaction (PCR) in 20 𝜇L reactions with 2 𝜇L of 10X Qiagen (Germantown, MD, U.S.A.) PCR buffer with MgCl2, 0.6 𝜇L of 25 mm MgCl2, 0.4 𝜇L of 10 mm dNTPs, 0.5 𝜇L of each 10 mm primer (Table 1), 4.0 𝜇L of 1X bovine serum albumin, and 0.1 𝜇L of Qiagen Taq polymerase, 9.9 𝜇L sterile water, and 2 𝜇L of template DNA (∼10 ng/𝜇L). The thermal cycler programme was 94∘C for 150 s, then 35 cycles of 94∘C for 30 s, 46–56∘C for 60 s, and 72∘C for 60 s, and concluded with 10 min at 72∘C. PCR products were visualized in 1.5% agarose gels stained with ethidium bromide and successful amplifications were cleaned with the QIAquick (Qiagen, Germantown, MD, U.S.A.) PCR kit. Sequencing PCR in both directions was done with the ABI Big Dye Terminator Cycle Sequencing Ready Reaction Kit 3.1 (Carlsbad, CA, U.S.A.), and sequences were then purified with DyeEx 96 Kit from Qiagen, dried and re-eluted with formamide, and run on an ABI Prism 3730xl DNA Analyzer. All sequenced have been deposited into GenBank (see Table S1 for accession numbers).
Table 1.
Primer sequences
| Locus | Primer name |
Primer sequence | Lengths (bp) |
Annealing temperature |
References |
|---|---|---|---|---|---|
| 12S | 12Sai | AAACTAGGATTAGATACCCTATTAT | 350 – 358 | 50°C | Simon et al. (1994) |
| 12Sbi | AAGAGCGACGGGCGATGTGT | ||||
| 16S | 16S-F | TTACGCTGTTATCCCTAA | 384–388 | 46°C | Simon et al. (1994) |
| 16S-R | CGCCTGTTTATCAAAAACAT | ||||
| 28S | D2up4 | GAGTTCAAGAGTACGTGAAACCG | 479–504 | 50°C | Kjer et al. (2001) |
| D2dnB | CCTTGGTCCGTGTTTCAAGAC | ||||
| Histone 3 | H3F | ATGGCTCGTACCAAGCAGACGGC | 328 | 50°C | Ogden and Whiting (2003) |
| H3R | ATATCCTTGGGCATGATGGTGAC | ||||
| COI | LCO1490 | GGTCAACAAATCATAAAGATATTGG | 655–658 | 46°C | Simon et al. (1994) |
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | ||||
| GomphLCO | CAACAAATCATAAAGATATTGGAA |
Alignment
We aligned ribosomal fragments with reference to secondary structure, as in Kjer (1995, 2004) and Kjer et al. (2006). All other fragments were aligned using clustal x 2.0 (Larkin et al., 2007), followed by manual alignment in mesquite (version 2.75, 2011). The resulting alignment contained 2182 nucleotides: 1360 constant characters, 767 parsimony-informative characters and 55 autapomorphic characters.
Phylogenetic analyses
Bayesian inference (mrbayes v3.2.2) (Huelsenbeck & Ronquist, 2002; Ronquist & Huelsenbeck, 2003) was used to infer a posterior probability (PP) distribution of topologies and branches, applying uniform priors to tree topologies and an exponential prior (10) to branch lengths. Based on the results of jmodeltest (Posada, 2008), GTR+ Gamma was used as the evolutionary model for each of the fragments. We applied two different Metropolis coupled Markov runs (four chains, 20 000 000 generations and every 1000th generation sampled). After discarding the first 10% of generations as ‘burn-in’, posterior probabilities were calculated, using a 50% majority-rule consensus tree, from the concatenated set of trees, generated in all Markov chain Monte Carlo runs. Convergence and mixing of parameters were assessed by inspection of the trace plots and the effective sample sizes using tracer 1.7.0 (Rambaut & Drummond, 2007). Additional maximum likelihood (ML) analyses were conducted with garli 2.0 (Zwickl, 2006). We applied bootstrapping and best likelihood tree search in two steps. To reduce the risk of being trapped in a local optimum, inference of the best likelihood tree was conducted 100 times with different random starting trees, obtained via maximum parsimony, with a final optimization of the best tree. Subsequently, 1000 bootstrap replicates were conducted. All ML analyses were calculated with the GTR model. Model parameters were estimated from the data and among site rate variation modelled with gamma-distributed rates across sites with four discrete rate categories.
Morphological observations
Morphological observations of Gomphini were completed using a Wild M5A stereomicroscope (Gais, Switzerland) with calibrated ocular micrometer. Observations were made using Gomphini specimens in the private collection of Ken Tennessen; photographs of each genus can be found in Needham et al. (2014). Where possible, multiple individuals were examined. All Gomphini were examined, but for Gastrogomphus, for which we could not obtain specimens.
Postfrons length
Metafemur length
Abdominal segment length
Male cerci curvature, presence/absence of spines
Anterior hamule shape
Penis vesicle shape
Head capsule with/without tubercules
Female subgenital plate shape
Nymphal tibia with or without hooks/spines.
Results
Maximum likelihood and Bayesian analyses
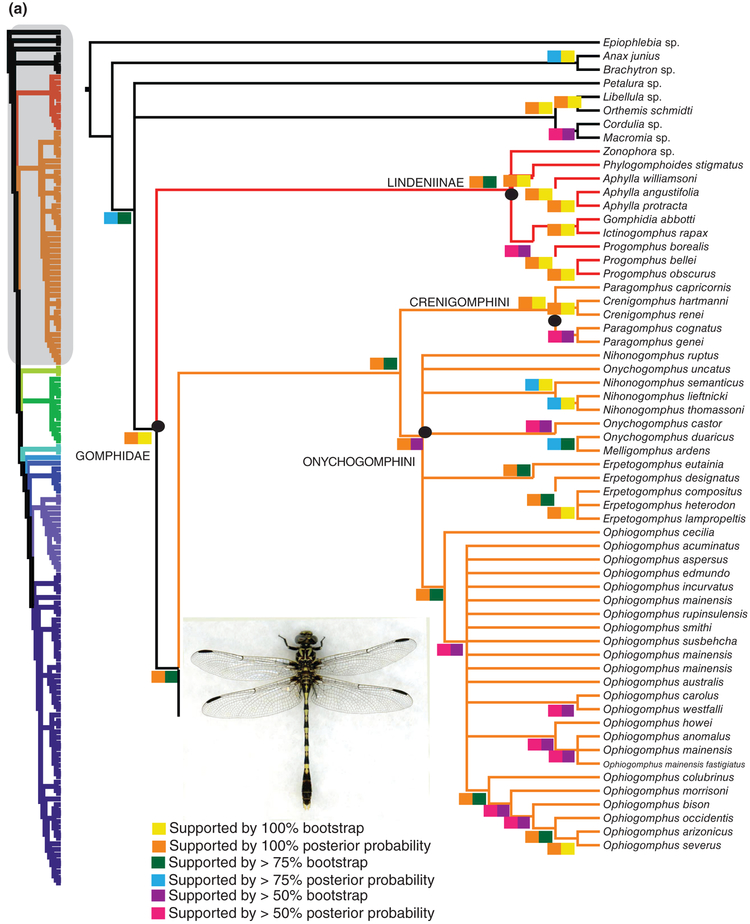
Both analyses reconstructed a monophyletic Gomphidae [100% bootstrap support (BS), PP], comprising several well-supported clades (Fig. 1a, b). The two analyses are largely in agreement, but are incongruent in some places. In both trees, the deepest split is between Lindeniinae and the remainder of the family, with 97% BS/100% PP and 83% BS/100% PP, respectively. Within the remainder of the family, Onychogomphinae and one of its constituent tribes, Crenigomphini, have high support, as was suggested also by Dijkstra & Kalkman (2012), but support for species of Onychogomphini is weaker, and Onychogomphus Selys 1854, Nihonogomphus Oguma 1926 and Melligomphus Chao 1990 are intermixed, making it unclear whether these taxa are valid; more specimens would be needed to evaluate the monophyly of these genera, and resolve the polytomy in which they are recovered. The mostly North American genus Erpetogomphus and the Holarctic Ophiogomphus Selys 1854 are each recovered as monophyletic (94% BS/100% PP, 80% BS/100% PP, respectively).
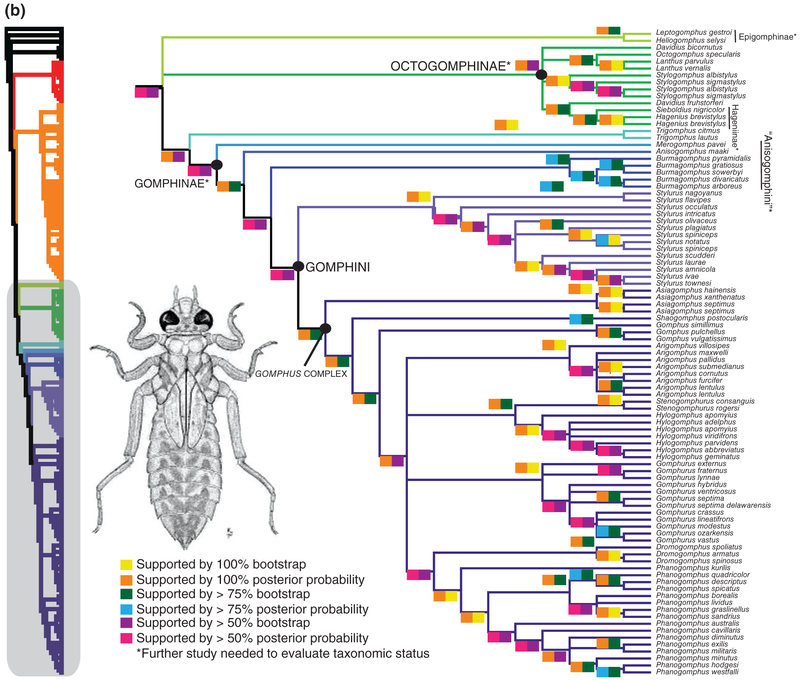
Fig. 1.
(a, b) Majority rule consensus tree garli 1000 pseudoreplicate bootstrap analysis. Coloured boxes above and below branches indicate node support from (1) bootstrap analysis and (2) from 20 million generation Bayesian analyses, respectively. Coloured boxes below nodes indicate branch support from Bayesian and maximum likelihood analyses.
Carle & Cook (1984) established the tribe Octogomphini (raised by Carle, 1986, to subfamily status) to accommodate a number of small gomphids characterized by a relatively distal second costal brace and with the interspace between veins MP and CuA of the hindwing rather sharply divergent distally. We failed to recover this subfamily, as the purported constituent genera constitute a paraphyletic assemblage with Hagenius+Sieboldius (Hageniinae, Hageniini), albeit with ambiguous support (51% BS, 99% PP), and with Trigomphus as sister to the gomphines.
The remaining taxa, which include the North American Gomphus species, form a monophyletic group that is consistent with Carle’s Gomphinae. This clade includes four subordinate clades arranged in pectinate fashion. The two basalmost nodes give rise to Merogomphus Martin 1904 and Anisogomphus Selys 1857 (both in Carle’s Anisogomphini; Fig. 1b), the third to Burmagomphus Williamson 1907 (Cyclogomphini), and the last corresponds approximately to Carle’s (1986) Gomphini. The latter comprises Stylurus plus a series of clades that are distinct from Stylurus with very high support (97% BS/100% PP). Stylurus includes the European ‘Gomphus’ flavipes (Charpentier), as well as the Asian S. nagoyanus (Asahina) and S. oculatus (Asahina) and presumably other Asian species usually placed in that genus.
The taxa in the clade that is sister to Stylurus had all (with the exception of Dromogomphus Selys 1854) usually been placed in Gomphus, until Chao (1984) and Asahina (1985) distinguished Shaogomphus Chao 1984 and Asiagomphus Asahina 1985 as valid genera. This series, again a largely pectinate array from Asiagomphus to Phanogomphus (Fig. 1b), is here called the Gomphus complex (comprising Arigomphus, Asiagomphus, Dromogomphus, Gomphus, Gomphurus, Hylogomphus, Phanogomphus, Shaogomphus and Stenogomphurus; Fig. 1b). We are here treating each of these taxa as genus-level taxa. Asiagomphus is sister to the remainder of the Gomphus complex with 97%BS/100%PP support, and Shaogomphus is similarly well-separated from the other taxa. The genus Gomphus s.l., including Gomphus s.s. (i.e. the clade containing G. vulgatissimus) plus the North American clades previously considered to be subgenera by Needham et al. (2014), are in a clade with Dromogomphus and Arigomphus; we consider each of the former subgenera to be genus-level taxa. The latter clade, Dromogomphus+ Arigomphus+Stenogomphurus+ Hylogomphus+ Gomphurus +Phanogomphus, has quite substantial support (71% BS/100% PP). Each of the nested clades within the Gomphus complex is strongly supported (86–100% BS/00% PP).
Discussion
Phylogeny
Monophyly of the family Gomphidae has not been disputed, and it is confirmed with molecular evidence here. We recover a group of genera usually placed in Lindeniinae (Yakobson & Bianchi, 1905) as sister to all other Gomphidae. A similar arrangement was reported by Carle et al. (2015) except that they also included in Lindeniinae (their Ictinogomphinae) three genera, not sampled here, that were placed in Octogomphinae by Carle (1986).
Within the non-ictinogomphine clade, the Onychogomphinae (Chao, 1984; Carle, 1986) are clearly separated from other taxa, and fall into two groups corresponding to Crenigomphini (including Crenigomphus Selys 1892 and Paragomphus Cowley 1934; Carle, 1986) and Onychogomphini, including, in our sample, Melligomphus ardens, Nihonogomphus, Onychogomphus (Palaearctic and Indo-Malayan), Erpetogomphus (Nearctic, Neotropical) and Ophiogomphus (Holarctic). Although Erpetogomphus and Ophiogomphus are each well supported as monophyletic, Nihonogomphus and Onychogomphus are not. In the latter case, however, only O. uncatus (Charpentier) is undoubtedly close to the generotype, O. forcipatus (L.), both morphologically and geographically; Bridges (1994) placed O. duaricus Fraser in Nychogomphus Carle, 1986 and Fraser moved (1934) O. risi (Fraser) to Lamelligomphus Fraser 1922, although it has since usually been listed in Onychogomphus. Clearly these and related genera (Davidioides Fraser 1922, Nepogomphus Fraser, 1934, Nihonogomphus and others) require more extensive sampling and analysis and possibly revision of generic assignments (Wilson & Xu, 2009).
Garrison (1994) divided Erpetogomphus into three monophyletic groups, of which our sample includes two; we did not recover either, but the small taxon sample precludes any firm conclusion about this genus. Ophiogomphus, which reaches its greatest diversity in North America, is well represented in our analysis. It is recovered as monophyletic, and, in fact, it is striking how little genetic difference exists among many species (garli best tree, supplementary material); perhaps as a result, five specimens of O. mainensis are in unresolved positions on the tree. Future studies with faster evolving loci and more specimens of Ophiogomphus species may be needed to determine the status of these species and subspecies. Carle (1986) recognized three subgenera within Ophiogomphus: Ophionurus, including O. australis to O. mainensis in our phylogeny; Ophionuroides, including only O. howei and O. anomalus; and Ophiogomphus, including the Palaearctic species and six northern and western North American species included here (O. colubrinus to O. severus; Fig. 1a). The latter group appears to form a rather well-defined clade, but this does not include the generotype, O. cecilia, so, if this topology is confirmed, the name will have to be changed. Ophionuroides could also be monophyletic, as the presence of O. mainensis may be spurious (given the anomalous distribution of multiple individuals of that species in the tree) and needs to be evaluated with different loci to determine its phylogenetic position.
Carle & Cook (1984) suggested that Octogomphinae may be one of the most ancient lineages among Gomphidae, and the recent results of Carle et al. (2015) support that conclusion, despite not finding the subfamily to be monophyletic. Our analysis fails to confirm monophyly (Fig. 1b). Rather we recover Octogomphinae as a paraphyletic assemblage that also includes Hagenius and Sieboldius (Hageniinae) and several other taxa. Trigomphus, placed by Carle (1986) in Octogomphinae, Trigomphini, appears to be more closely related to Gomphinae, although Stylogomphus Fraser 1922, also placed in Trigomphini by Carle, is well within Octogomphinae in our analysis. The status of the octogomphines requires further study.
Likewise, the status of Hageniinae and Epigomphinae is left unresolved in our analysis. The pairs of genera representing these subfamilies are each recovered as monophyletic, but the rank at which they should be recognized and their relation to Octogomphinae, Gomphinae and other putative Epigomphinae are unclear.
Our principal focus here is on the classification of the North American Gomphus complex and its close Palaearctic relatives. Important earlier attempts, on which ours is based, include those of Needham (1948), using a variety of morphological characters, and Walker (1957), emphasizing principally the genitalia. Both of these efforts successfully identified most of the taxa that we recognize within Gomphus s.l., except Stenogomphurus and Dromogomphus, although neither explicitly used the concepts of cladistics or, apparently, of the usual assumption of binary taxon splitting. Nevertheless, these works have provided the essential underpinnings of subsequent understanding of our focal taxa.
Our data largely support the classification of Carle (1986) within that group, in particular in validating his (sub)genera Phanogomphus and Stenogomphurus. Our results, like those of Carle et al. (2015) fail to support Carle’s (1986) synonymy of the North American taxon Hylogomphus with Gomphus s.s.; the latter appears to be largely confined to the Palaearctic, with a few species reaching the Indo-Malayan region. Likewise, Shaogomphus (eastern Palaearctic) appears to be unequivocally distinct from Gomphus. Another unexpected result, although perhaps in retrospect not surprising, is the placement of Dromogomphus within the Gomphus complex. This genus, regarded as distinct since it was first described by Selys (1854), is unique in having several very long, stout spines on the metafemur, although the species are otherwise very like large Gomphus s.l. (Needham et al., 2014). Nymph morphology supports the inclusion of Dromogomphus within the Gomphus complex.
Although now usually recognized as a genus separate from Gomphus, Stylurusis still commonly seen as very closely related to the latter. Carle et al. (2015), using a chimeric sequence from three species of Stylurus, suggested that the genus should be shifted from Gomphini to Cylogomphini (including only Burmagomphus in our sample). Our data suggest that it might preferably remain in Gomphini, but they confirm that Stylurus is abundantly distinct from others in that tribe (of which our sample includes all genera proposed by Carle, 1986, except Gastrogomphus Needham 1941; Bridges, 1994, also placed Scalmogomphus Chao, 1990 and Melligomphus here, but these are very obviously onychogomphines; Chao, 1990). Fig. 1 also indicates that Palearctic and Indo-Malayan Stylurus are closely grouped with those from North America, although the latter do comprise a clade within the genus, except that S. intricatus (Hagen) is weakly associated with S. oculatus in the Bayesian analysis. Furthermore, the suggestion of Needham & Westfall (1955) that the North American group comprises two main subgroups – the plagiatus group and the amnicola group – is largely valid, although S. olivaceus (Selys) is paraphyletic relative to these two groups rather than being included in the first; S. intricatusis, indeed, the most distinctive North American species, genetically as well as morphologically, as Needham & Westfall (1955) also proposed.
Within the Gomphus complex itself, all of the recognized taxa investigated here are validated as monophyletic. It is notable, however, that no Nearctic species are included in the clade with the generotype, G. vulgatissimus, so we place none of the former in Gomphus s.s. Our sample of Palaearctic species is small, but given that our Nearctic species lacked only three species [Gomphurus dilatatus (Rambur), Gomphurus gonzalezi (Dunkle) and Phanogomphus oklahomensis (Pritchard)] of the entire New World Gomphus complex, it is clear that none of the Palaearctic clades occurs in North America. Thus we conclude that all the genus group names currently in use for divisions of the Gomphus complex are valid and that all species have been properly grouped (e.g. Needham et al., 2014) in genera or subgenera [we did not have access to specimens of Anatogomphurus Carle, 1986, but the type species, Gomphus personatus (Selys), seems to fit well in Asiagomphus, as proposed and illustrated by Asahina, 1985]. However, the species placed by Needham et al. (2014) in Gomphus s.s. should be transferred to Phanogomphus, and Gomphus s.s. should be restricted to the clade including G. vulgatissimus and its Palaearctic relatives.
Another somewhat unexpected result of our analysis is the placement of Stenogomphurus as sister to Hylogomphus rather than to Gomphurus, with which it has often been regarded as synonymous. Nevertheless, the genetic relationship is quite well supported, and, in fact, the genetic distance from the basal node of Stenogomphurus to the basal node of Hylogomphus is barely greater than, for example, that from Phanogomphus kurilis (Hagen) to the basal node of the remaining Phanogomphus. On that basis alone, one might suggest that the two taxa be united. Marked morphological differences exist, however, including shape of posterior hamuli (see our diagnosis, later, and Needham et al., 2014; figs 251, 266); ventroapical teeth on male cerci present and usually prominent in Hylogomphus, absent in Stenogomphurus; shape of subgenital plate not smoothly convex laterally and with tips usually directed posterolaterally in Hylogomphus, more or less smoothly convex and with tips directed posteriorly in Stenogomphurus; abdomen stocky with lateral margins of S8–S9 usually markedly expanded in Hylogomphus, relatively slender and with lateral margins of S8–S9 only slightly expanded in Stenogomphurus; nymphal prementum without a median tooth on the distal margin in Hylogomphus, with a small median tooth in Stenogomphurus.
The remaining taxa in the complex have all been recognized previously, although some have usually been accorded generic, and others only subgeneric, rank. Relationships among them are not entirely clear, although both analyses find Dromogomphus as sister to Phanogomphus – this is interesting in light of their morphology; in adults, the large size and elongate metafemur of the former seem to suggest an alliance with Gomphurus, but the nymphs of Dromogomphus are more similar to Phanogomphus than they are to Gomphurus in the dimensions of the abdominal segment 9 and the development of its dorsal hook. Within Phanogomphus, species appear to fall into two fairly well-supported species groups that seem separated largely along geographic lines: one [P. australis (Needham) to P. westfalli (Carle & May)] mainly confined to the southeastern and south-central United States [although P. exilis (Selys) extends northwards], the other [P. quadricolor (Walsh) to P. sandrius (Tennessen)] mostly northern and Midwestern, with P. kurilis of the Pacific Coast standing apart from both. There is incongruence between morphological and molecular data regarding the relationships of P. minutus, P. diminutus and P. westfalli; future studies should sequence several conspecifics of each to explore the relationships among these species.
The internal relationships among Gomphurus are partly consistent with Needham & Westfall’s division into a dilatatus group and a fraternus group, but several species do not fall into their predicted place, and support for most nodes is weak.
Classification of North American Gomphini
As Needham & Westfall (1955) pointed out, the genus Gomphus at one time encompassed all Gomphidae. As with many other early insect genera, the number of described species has steadily proliferated, and knowledge of their morphological, behavioural and geographic diversity has increased. In recent decades a revised understanding of the criteria defining taxa at all levels has resulted in an ongoing re-evaluation of how taxon names should be applied, both conceptually (taxa must be monophyletic) and practically (through use of new molecular and analytical techniques). In general, successive restrictions on the range of species included in Gomphus have been implemented.
Here we suggest continuing that trend. Within the Gomphus complex, Asiagomphus and Shaogomphus arise from nodes basal to Gomphus and are widely accepted as valid genera (Bridges, 1991; Schorr & Paulson, 2015), and we accept them as such. Two other generally accepted genera, Arigomphus and Dromogomphus, however, would leave Gomphus paraphyletic if removed. The only options are either to reduce Arigomphus and Dromogomphus to subgenera of Gomphus, or to elevate all the subgenera to generic status. We prefer the latter solution for three reasons. First, in most of these taxa the constituent species are very closely related to one another and separated from the other proposed taxa by relatively long genetic distances that are comparable to distances between many genera throughout Gomphidae (garli best tree, supplementary material). Second, if Gomphus is left intact, it remains a genus of unwieldy size (>60 spp.), much larger than other genera in Gomphinae sensu Carle (Burmagomphus is probably next in size with ∼25 spp.). Finally, most of the species in each taxon have a characteristic appearance that makes them recognizable as a member of their group, even in the field. Thus we recognize Gomphurus, Hylogomphus, Phanogomphus and Stenogomphurus, along with Gomphus s.s., as genera.
Taxonomy of Gomphini Rambur, 1842
Gomphus Leach, 1815. (type genus). Type species Gomphus vulgatissimus Linnaeus, 1758.
Includes also: pulchellus Selys, 1854, simillimus Selys, 1854, and probably others not included in this study (e.g. Carle et al., 2015 recovered G. graslini as sister to G. vulgatissimus; we hesitate, however, to include other supposed Palaearctic or Indo-Malayan species attributed to Gomphus, as in the past this genus has been a catch-all for gomphines that did not clearly belong elsewhere).
Arigomphus Needham, 1897. Type species Arigomphus pallidus (Rambur, 1842).
Includes also: cornutus (Tough, 1900), furcifer (Hagen, 1878), lentulus (Needham, 1902), maxwelli (Ferguson, 1950), subme- dianus (Williamson, 1914), villosipes (Selys, 1854).
Asiagomphus Asahina, 1985. Type species Asiagomphus melaenops (Selys, 1854).
Includes also: amamiensis (Asahina, 1962), auricolor (Fraser, 1926), coreanus (Doi & Okumura, 1937), corniger (Morton, 1928), cuneatus (Needham, 1930), giza Wilson, 2005, gongsha- nensis Yang, Mao & Zhang, 2006, hainanensis (Chao, 1953), hesperius (Chao, 1953), melanopsoides (Doi, 1943), motuoensis Liu & Chao in Chao, 1990, nilgiricus (Laidlaw, 1922), odoneli (Fraser, 1922), pacatus (Chao, 1953), pacificus (Chao, 1953), perlaetus (Chao, 1953), personatus (Selys, 1873), pryeri (Selys, 1883), reinhardti Kosterin & Yokoi, 2016, septimus (Needham, 1930), somnolens (Needham, 1930), xanthenatus (Williamson, 1907), yayeyamensis (Matsumura in Oguma, 1926).
Dromogomphus Selys 1854. Type species spinosus Selys, 1854.
Includes also: armatus Selys, 1854, spoliatus (Hagen, 1858).
Gastrogomphus Needham, 1944 (Not included in our analy- sis. Placed in Trigomphus by Davies & Tobin, 1985; Steinmann, 1997.). Type species abdominalis (McLachlan, 1884).
Gomphurus Needham, 1901, New status. Type species Gom- phurus vastus (Walsh, 1862).
Includes also: crassus (Hagen in Selys, 1878), dilatatus (Ram- bur, 1842), externus (Hagen in Selys, 1858), fraternus (Say, 1840), gonzalezi (Dunkle, 1992), hybridus (Williamson, 1902), lineatifrons (Calvert, 1921), lynnae (Paulson, 1983), modestus (Needham, 1942), ozarkensis (Westfall, 1975), septima (West- fall, 1956), ventricosus (Walsh, 1863).
Hylogomphus Needham, Westfall & May, 2000, New status.
Type species Hylogomphus adelphus (Selys, 1858).
Includes also: abbreviatus (Hagen in Selys, 1878), apomyius (Donnelly, 1966), geminatus (Carle, 1979), parvidens (Currie, 1917), viridifrons (Hine, 1901).
Phanogomphus Carle, 1986, New status. Type species
Phanogomphus minutus (Rambur, 1842).
Includes also: australis (Needham, 1897), borealis (Needham, 1901), cavillaris (Needham, 1902), descriptus (Banks, 1896), diminutus (Needham, 1950), exilis (Selys, 1854), graslinellus (Walsh, 1862), hodgesi (Needham, 1950), kurilis (Hagen in Selys, 1858), lividus (Selys, 1854), militaris (Hagen in Selys, 1858), oklahomensis (Pritchard, 1935), quadricolor (Walsh, 1863), sandrius (Tennessen, 1983), spicatus (Hagen in Selys, 1854), westfalli (Carle & May, 1987).
Shaogomphus Chao, 1986. Type species Shaogomphus lieftinki Chao, 1984.
Includes also: postocularis (Selys, 1869), schmidti (Asahina, 1956).
Stenogomphurus Carle, 1996, New status. Type species
Stenogomphurus consanguis (Selys, 1879).
Includes also: rogersi (Gloyd, 1936).
Stylurus Needham, 1897. Type species Stylurus plagiatus (Selys, 1854).
Includes also: amicus (Needham, 1930), amnicola (Walsh, 1862), annulatus (Djakonov, 1926), clathratus (Needham, 1930), endicotti (Needham, 1930), erectocornus Liu & Chao in Chao, 1990, falcatus Gloyd, 1944, flavicornis (Need- ham, 1931), flavipes (Charpentier, 1825), gaudens (Chao, 1953), gideon (Needham, 1941), intricatus (Selys, 1858), ivae Williamson, 1932, kreyenbergi (Ris, 1928), laurae Williamson, 1932, nagoyanus Asahina, nanningensis Liu, 1985, nobilis Liu & Chao in Chao, 1990, notatus (Rambur, 1842), occultus (Selys, 1878), oculatus (Asahina, 1949), olivaceus (Selys, 1873), placidus Liu & Chao in Chao, 1990, plagiatus (Selys, 1854), potulentus (Needham, 1942), scudderi (Selys, 1873), spiniceps (Walsh, 1862), takashii (Asahina, 1966), townesi Gloyd, 1936, tongrensis Liu, 1991.
Diagnoses of genera of Gomphini
As a result of our analysis and reclassification of Gomphini, Gomphus is now strictly a Eurasian genus and is no longer a part of the New World fauna. The following diagnoses include all the genera of Gomphini recognized by Carle (1986) and Bridges (1994) except Gastrogomphus, which we could not examine. Images of all the North American genera, showing the morphological features described in the following, can be found in Needham et al. (2014).
Gomphus – Postfrons about three times as wide as long; metafemur about 1.04 – 1.15 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.0 – 1.4 times that of segment 9; abdominal segments 7 – 9 expanded; male cerci slightly to moderately curved in lateral view, basolateral margin not carinate, usually with distinct subapical ventral angulation or tooth, sometimes also with lateral tooth at or just beyond midlength; anterior hamules with inner lobe not extending beyond outer edge, the latter with very small rounded denticles; first segment of penis (penis vesicle) loaflike, midventral groove (penile receiver) not extending through posteroventral rim of hood, in lateral view posterior margin of vesicle convex posteroventrally, slightly inflated, anterior surface concave; female without prominent tubercles or pyramidal projection on posterior surface of head capsule; subgenital plate about 2/5 length of ninth sternum, convex or slightly sinuate laterally, tips variable; nymphs with burrowing hooks on protibiae.
Arigomphus – Postfrons about three times as wide as long; metafemur about 1.1 times as long as greatest width of head and lacking unusually long spines; mid-dorsal length of abdominal segment 8 about 1.0 times that of segment 9; segments 7 – 9 of male abdomen barely expanded; male cerci usually straight in lateral view (slightly curved in A. furcifer), basolateral margin not carinate, often each with distinct lateral angulation and/or ventral spine or protu- berance (may be lacking or poorly developed in A. lentu- lus, A. maxwelli and A. villosipes); anterior hamules not peg-like, each with small, backward pointing terminal hook; first segment of penis (penis vesicle) elongate-pyramidal, midventral groove (penile receiver) extending through pos- teroventral rim of hood, in lateral view posterior margin of vesicle concave, anterior margin with strong constriction at base of hood; female without prominent tubercles or pyrami- dal projection on posterior surface of head capsule; subgen- ital plate 1/4 – 2/5 length of ninth sternum, convex laterally, tips parallel, usually blunt and contiguous; nymphs with bur- rowing hooks on protibiae.
Asiagomphus – Postfrons about three times as wide as long; metafemur about 1.0 – 1.5 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.1 – 1.4 times that of segment 9; abdominal segments 7 – 9 moderately to broadly expanded; male cerci straight or slightly curved in lateral view, baso- lateral margin not carinate, each often with subapical ven- tral swelling or low tooth, rarely also with ventrolateral lat- eral teeth or angulations; anterior hamules not peg-like, each excavated posterolaterally, the outer edge often with a row of denticles in distal half; first segment of penis (penis vesi- cle) loaflike, slightly elongate, or low-pyramidal, midventral groove (penile receiver) not extending through posteroven- tral rim of hood, margins of vesicle in lateral view with posteroventral and anterior surfaces nearly straight or with posteroventral surface convex but not strongly inflated, ante- rior surface straight or slightly concave; female without prominent tubercles or pyramidal projection on posterior sur- face of head capsule; subgenital plate 1/5 – 1/2 of ninth ster- num, sometimes diverging downward from sternum, sides usually straight or concave, tips parallel; nymphs with bur- rowing hooks on protibiae.
Dromogomphus – Postfrons about three times as wide as long; metafemur 1.6 – 2.0 times as long as greatest width of head, with four to eight unusually long spines intermixed with numerous smaller ones; middorsal length of abdominal segment 8 about 1.1 – 1.2 times that of segment 9; abdominal segments 7 – 9 of male moderately to strongly [D. spoliatus (Hagen)] expanded; male cerci straight in lateral view, lateral carina sometimes extending onto basolateral margin, with ventral flange o r e xpansion n ear m idlength, without lateral angulations; anterior hamules with inner lobe not extending beyond outer edge, the latter with minute rounded denticles; first segment of penis (penis vesicle) loaflike, with paired anterolateral protrusions; female without prominent tubercles or pyramidal projection on posterior surface of head capsule, sometimes with small medial tubercle on occipital crest; subgenital plate about 1/3 length of ninth sternum, laterally slightly to strongly sinuate, tips barely to markedly divergent; nymphs with burrowing hooks on protibiae.
Gastrogomphus – No specimens were available to us for analysis. In erecting the genus, Needham (1944) diagnosed the single species as follows: ‘A very long, thick abdomen about a third longer than the hind wing; anal vein 3 arises generally after, and sometimes opposite the anal crossing; no basal subcostal cross vein and no cross vein in any of the triangles, first and fifth an tenodals th ickened; a single row of large paranals in the forewing; anal triangle of the male three celled, and four postanals in the hindwing; [caudal] appendages of the male of about equal length and divergence. … there is a very wide differentiation in size among the cells of three wing areas; very large before the level of the arculus, a little smaller out to a line drawn from the stigma to the hind angle of the wing, and much smaller thence outward to the margin. The nymph … differs from all known related forms in having neither dorsal hooks nor lateral spines; in having the front border of the median labial lobe doubly produced (bilobed) and fringed at the sides of a bare median notch; and in having the strongly incurving terminal third of the lateral lobe very feebly denticulate on its concave inner margin.’ The length of the abdomen relative to the wings would distinguish G. abdominalis adults from all other Gomphini except some Stylurus spiniceps (Walsh) and the nymphal bilobed median labial lobe and complete absence of abdominal dorsal hooks and lateral spines are likewise unique.
Gomphurus – Postfrons about three times as wide as long; metafemur 1.3–1.6 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.2–1.4 times that of segment 9; abdominal segments 7–9 of male very strongly expanded; male cerci straight to moderately curved in lateral view, basolateral margin not carinate, each with ventral tooth or triangular projection at 1/2–3/4 its length, lateral angulation usually weak or absent; anterior hamules with inner lobe not extending beyond outer edge, the latter with sharp, spine-like denticles; first segment of penis (penis vesicle) subtriangular to almost columnar in lateral view, posteroventral margin usually straight or convex, distal two-thirds enclosing deep anterior trough, midventral groove (penile receiver) not extending through posteroventral rim of hood, in lateral view posterior margin of vesicle straight or slightly concave; female without prominent tubercles or pyramidal projection on posterior surface of head capsule; subgenital plate 1/3–3/4 length of ninth sternum, usually with constriction near base, tips parallel or divergent; nymphs with burrowing hooks on protibiae.
Hylogomphus – Postfrons about three times as wide as long; metafemur about as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.4–1.8 times that of segment 9; abdominal segments 7–9 of male abdomen moderately expanded; male cerci straight or slightly curved in lateral view, basolateral margin not carinate, each usually with stout ventroapical spine (small in H. geminatus and H. parvidens), lateral angulation absent; anterior hamules with inner lobe not extending beyond outer edge, the latter with very small rounded denticles; first segment of penis (penis vesicle) roughly triangular in lateral view, midventral groove (penile receiver) not extending through posteroventral rim of hood, in lateral view posterior margin of vesicle usually straight or concave (convex in H. apomyius, but not markedly inflated), anterior surface with concavity at or just basal to midlength; female without prominent tubercles or pyramidal projection on posterior surface of head capsule; subgenital plate 2/5 to full length of ninth sternum, usually slightly concave laterally with tips diverging slightly; nymphs with burrowing hooks on protibiae.
Phanogomphus – Postfrons about three times as wide as long; metafemur about 1.0–1.2 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 0.7 (P. australis)–1.35 times that of segment 9; abdominal segments 7–9 of male slightly to moderately expanded; male cerci straight or slightly curved in lateral view, with midlateral carina almost always extending onto basolateral margin, usually with ventral tooth or flange arising from medial margin, often also with prominent lateral tooth or angulation; anterior hamules usually curved slightly to markedly anteriorly, ending in a recurved spine varying from small and thorn-like to long and sickle-like (row of denticles posterior to short spine in P. borealis); first segment of penis (penis vesicle) highly variable, in lateral view from loaflike with broadly convex and somewhat inflated posteroventral surface, to pyramidal with straight or concave posteroventral surface, to nearly columnar, often with paired anterolateral protrusions, midventral groove (penile receiver) not extending through posteroventral rim of hood; female without prominent tubercles or pyramidal projection on posterior surface of head capsule; female with subgenital plate 1/10–3/5 length of ninth sternum, roughly triangular or with sides slightly concave, tips often slightly divergent; nymphs with burrowing hooks on protibiae.
Shaogomphus – Postfrons about three times as wide as long; metafemur about 1.1 – 1.2 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.4 times that of segment 9; abdominal segments 7 – 9 moderately expanded; male cerci strongly down-curved through almost 90∘ in lateral view, basolateral margin not carinate, each without ventral or lateral angulations or teeth; anterior hamules peg-like, rounded distally; first segment of penis (penis vesicle) in lateral view markedly inflated and smoothly convex over entire posterior, ventral and anterior surface, midventral groove (penile receiver) not extending through posteroventral rim of hood; female with pair of prominent postocular tubercles (S. lieftincki, S. postocularis) or a single large pyramidal projection (S. schmidti) on posterior surface of occiput; subgenital plate about 1/4 length of ninth sternum, sides concave so tips markedly divergent; nymphs with burrowing hooks on protibiae.
Stenogomphurus – Postfrons about three times as wide as long; metafemur about 1 – 1.1 times as long as greatest width of head and lacking unusually long spines; middorsal length of abdominal segment 8 about 1.45 – 1.6 times that of segment 9; abdominal segments 7 – 9 of male abdomen only slightly expanded; male cerci moderately curved in lateral view, basolateral margin not carinate, each with two small ventrolateral teeth, lateral angulations absent; anterior hamules not peg-like, narrowed at about half length, apical 1/3 – 1/4 bent sharply caudad, somewhat resembling a bird’s head; first segment of penis (penis vesicle) moderately to sharply pyramidal, midventral groove (penile receiver) not extending through posteroventral rim of hood, in lateral view posterior margin of vesicle straight to sharply; female without prominent tubercles or pyramidal projection on posterior surface of head capsule; subgenital plate about 1/2 length of ninth sternum, smoothly convex laterally, tips parallel; nymphs with burrowing hooks on protibiae.
Stylurus – Postfrons about four times as wide as long; metafemur about as long as greatest width of head and lack- ing unusually long spines; middorsal length of abdominal segment 8 about 0.82 (S. spiniceps) – 1.4 times that of seg- ment 9; segments 7 – 9 of male abdomen usually moderately expanded [broadly expanded in S. scudderi and S.(?) gideon]; male cerci straight or slightly curved in lateral view, usually without lateral or ventral teeth or sharp angulations (ven- trolateral tooth present in S. clathratus), often with midlat- eral carina extending onto basolateral margin; first segment of penis (penis vesicle) usually loaflike, midventral groove (penile receiver) not extending through posteroventral rim of hood, in lateral view with posteroventral surface straight or convex, sometimes distinctly inflated, anterior surface flat, sometimes with wide, low, paired anterolateral protrusions; anterior hamules peg-like, short, without hooks or spines; female without prominent tubercles or pyramidal projec- tion on posterior surface of head capsule; subgenital plate less than 1/4 length of ninth sternum, tips either rounded or pointed, not divergent; nymphs without burrowing hooks on protibiae.
Supplementary Material
Acknowledgements
We would like to thank those who contributed specimens to this analysis: Jerrell Daigle, Rosser Garrison, Oleg Kosterin, William Kuhn, Melissa Sanchez-Herrera, Dominic Evangelista, Manpreet Kohli and Ian Biazzo. Thanks also to the work of anonymous reviewers and the editors of Systematic Entomology, whose comments helped to improve this manuscript. Thank you to Rosser Garrison for additional Gomphus metafemur/head width ratio measurements.
References
- Asahina S (1985) A revisional study of Japanese and east Asiatic Gomphus species with the description of Asiagomphus gen. nov. Gekkan Mushi, 169, 6–17. [Google Scholar]
- Bechly G (1996) Morphologische untersuchungen am flügelgeäder der rezenten libellen und deren stamm- gruppenvertreter (Insecta; Pterygota; Odonata), unter besonderer berücksichtigung der phylogenetischen systematik und des grundplanes der *Odonata. Petalura Special Volume, 2, 1–402. [Google Scholar]
- Blanke A, Greve C, Mokso R, Stampanoni M, Beckman F & Misof B (2013) An updated phylogeny of Anisoptera including formal convergence analysis of morphological characters. Systematic Entomology, 38, 474–490. [Google Scholar]
- Bridges CA (1991) Catalogue of the family-group, genus-group and species-group names of the Odonata of the world. Bridges, Urbana, Illinois. [Google Scholar]
- Bridges CA (1994) Catalogue of the Family-Group, Genus-Group and Species-Group Names of the Odonata of the World, 3rd edn C.A. Bridges, Urbana, Illinois. [Google Scholar]
- Bybee SM, Ogden TH, Branham MA & Whiting MF (2008) Molecules, morphology and fossils: a comprehensive approach to odonate phylogeny and the evolution of the odonate wing. Cladistics, 24, 477–514. DOI: 10.1111/j.1096-0031.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- Carle FL (1982) A contribution to the knowledge of the Odonata. PhD thesis, Virginia Tech University, Blacksburg, Virginia. [Google Scholar]
- Carle FC (1986) The classification, phylogeny and biogeography of the Gomphidae (Anisoptera). I. Classification. Odonatologica, 15, 275–326. [Google Scholar]
- Carle FL & Cook C (1984) A new Neogomphus from South America, with extended comments on the phylogeny and biogeography of the Octogomphini trib. nov. (Anisoptera: Gomphidae). Odonatologica, 13, 55–70. [Google Scholar]
- Carle FL, Kjer KM & May ML (2008) Evolution of Odonata, with special reference to Coenagrionoidea (Zygoptera). Arthropod Systematics & Phylogeny, 66, 37–44. [Google Scholar]
- Carle FL, Kjer KM & May ML (2015) A molecular phylogeny of Anisoptera (Odonata). Arthropod Systematics and Phylogeny, 73, 281–301. [Google Scholar]
- Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Cornell University Press, Ithaca, New York. [Google Scholar]
- Chao H-F (1984) Reclassification of Chinese gomphid dragonflies with the establishment of a new subfamily and the descriptions of a new genus and species (Anisoptera: Gomphidae). Contributions of the Odonatologica, 13, 71–80. [Google Scholar]
- Chao H-F (1990) The Gomphid Dragonflies of China (Odonata: Gom- phidae), Special Publication No. 1. Contributions of the Biological Control Research Institute of Fujian Agricultural College, Science and Technology Publishing House, Fuzhou, China. [Google Scholar]
- Davies DAL & Tobin P (1985) The dragonflies of the world: a sys- tematic list of the extant species of Odonata, Vol. II. Anisoptera. Societas Internationalis Odonatologica Rapid Communications (Sup- plement), 5, 1–151. [Google Scholar]
- Dijkstra K-DB & Kalkman VJ (2012) Phylogeny, classification and taxonomy of European dragonflies and damselflies (Odonata): a review. Organisms Diversity & Evolution, 12, 209–227. DOI: 10.1007/s13127-012-0080-8. [DOI] [Google Scholar]
- Dijkstra K-DB, Bechly G, Bybee SM et al. (2013) The classifica- tion and diversity of dragonflies and damselflies (Odonata). Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness (ed. by Zhang Z-Q). Zootaxa, 3730, 36–45. [DOI] [PubMed] [Google Scholar]
- Dumont HJ, Vierstraete A & Vanfleteren JR (2010) A molecular phylogeny of the Odonata (Insecta). Systematic Entomology, 35, 6–18. [Google Scholar]
- Fraser FC (1934) Odonata The Fauna of British India, including Ceylon and Burma, Vol. 2 (ed. by Sewell RBS), pp. 157–315. Taylor & Francis, London. [Google Scholar]
- Garrison RW (1994) A revision of the New World genus Erpetogom- phus Hagen in Selys (Odonata: Gomphidae). Tijdschrift voor Ento- mologie, 137, 173–269. [Google Scholar]
- Garrison RW, von Ellenrieder N & Louton JA (2006) Dragonfly Genera of the New World: An Illustrated and Annotated Key to the Anisoptera. Johns Hopkins University Press, Baltimore, Maryland. [Google Scholar]
- Huelsenbeck JP & Ronquist F (2002) MrBayes 3: Bayesian Analysis of Phylogeny. University of California Press, Berkeley, California. [Google Scholar]
- Kjer KM (1995) Use of rRNA secondary structure in phylogenetic studies to identify homologous positions: an example of alignment and data presentation from the frogs. Molecular Phylogenetics and Evolution, 4, 314–330. [DOI] [PubMed] [Google Scholar]
- Kjer KM (2004) Aligned 18S and insect phylogeny. Systematic Biology, 53, 506–514 (*With cover illustration). [DOI] [PubMed] [Google Scholar]
- Kjer KM, Gillespie JJ & Ober KA (2006) Structural homology in ribosomal RNA, and a deliberation on POY. Arthropod Systematics and Phylogeny, 64, 71–76. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948. [DOI] [PubMed] [Google Scholar]
- Letsch H, Gottsberger B & Ware J (2016) Not going with the flow: a comprehensive time-calibrated phylogeny of dragonflies (Anisoptera: Odonata: Insecta) provides evidence for the role of lentic habitats on diversification. Molecular Ecology, 25, 1340–1353. DOI: 10.1111/mec.13562. [DOI] [PubMed] [Google Scholar]
- Needham JG (1944) Observations on Chinese Gomphine dragonflies. Bulletin of the Museum of Comparative Zoology at Harvard College, 94, 145–163. [Google Scholar]
- Needham JG (1948) Studies on the North American species of the genus Gomphus (Odonata). Transactions of the American Entomo- logical Society, 73, 307–347. [Google Scholar]
- Needham JG & Westfall MJ (1955) A Manual of the Dragonflies of North America, 1st edn University of California Press, Berkeley, California. [Google Scholar]
- Needham JG, Westfall MJ & May ML (2014) Dragonflies of North America, 3rd edn Scientific Publishers, Gainesville, Florida. [Google Scholar]
- Posada D (2008) jModelTest: phylogenetic model averaging. Molecu- lar Biology and Evolution, 25, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Rambaut A & Drummond AJ (2007) Tracer v1.4. [WWW docu- ment]. URL http://beast.bio.ed.ac.uk/Tracer [accessed on 31 October 2016].
- Ronquist F & Huelsenbeck JP (2003) MrBayes 3: bayesian phyloge- netic inference under mixed models. Bioinformatics, 19, 1572–1574. [DOI] [PubMed] [Google Scholar]
- Sarot EE (1958) Folklore of the Dragonfly A Linguistic Approach. Storia e Letteratura, Rome. [Google Scholar]
- Schorr M & Paulson D (2015) World Odonata List. Slater Museum of Natural History, University of Puget Sound, Tacoma, Washington: [WWW document]. URL http://www.pugetsound.edu/academics/ academic-resources/slater-museum/biodiversity-resources/ dragonflies/world-odonata-list/ [accessed on 14 June 2015]. [Google Scholar]
- Selys L.E.de. (1854) Synopsis des Gomphines. Bulletin de l’Académie Royale des Sciences de Belgique, 21, 23–114. [Google Scholar]
- Steinmann H (1997) World Catalogue of Odonata, Vol. II Anisoptera. Walter de Gruyter, Berlin. [Google Scholar]
- Walker (1957) The affinities of the North American species of Gomphus as revealed by the genitalia (Odonata, Gomphidae). Contributions of the Royal Ontario Museum Division of Zoology and Palaeontology, 46, 3–24. [Google Scholar]
- Walker EM (1958) The Odonata of Canada and Alaska. Vol. II, Part III: The Anisoptera – Four Families University of Toronto Press, Canada. [Google Scholar]
- Ware J, Beatty C, Sanchez Herrera M et al. (2014) The petal- tail dragonflies (Odonata: Petaluridae): mesozoic habitat special- ists that survive to the modern day. Journal of Biogeography, 41, 1291–1300. [Google Scholar]
- Wilson KDP & Xu Z (2009) Gomphidae of Guangdong and Hong Kong, China (Odonata: Anisoptera). Zootaxa, 2177, 1–62. [Google Scholar]
- Yakobson GG & Bianchi VP (1905) Orthoptera and Pseudoneu- roptera of the Russian Empire and Adjacent Countries. AF Defriena, St. Petersburg. (in Russian). [Google Scholar]
- Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion PhD Dissertation, The University of Texas at Austin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.