Summary
We screened for the impact of hyperthermal regimes varying in the cumulative equivalent minutes at 43°C (CEM43°C) and media composition on tumour development using an original teratoma in vitro model. Rat embryos (three germ layers) were microsurgically isolated and cultivated at the air‐liquid interface. During a two week period, ectodermal, mesodermal and endodermal derivatives developed within trilaminar teratomas. Controls were grown at 37°C. Overall growth was measured, and teratoma survival and differentiation were histologically assessed. Cell proliferation was stereologically quantified by the volume density of Proliferating Cell Nuclear Antigen. Hyperthermia of 42°C, applied for 15 minutes after plating (CEM43°C 3.75 minutes), diminished cell proliferation (P ˂ .0001) and enhanced differentiation of both myotubes (P ˂ .01) and cylindrical epithelium (P ˂ .05). Hyperthermia of 43°C applied each day for 30 minutes during the first week (CEM43°C 210 minutes) impaired overall growth (P ˂ .01) and diminished cell proliferation (P ˂ .0001). Long‐term hyperthermia of 40.5°C applied for two weeks (CEM43°C 630 minutes) significantly impaired survival (P ˂ .005). Long‐term hyperthermia of 40.5°C applied from the second day when differentiation of tissues begins (CEM43°C 585 minutes) impaired survival (P ˂ .0001), overall growth (P ˂ .01) and cartilage differentiation (P ˂ .05). No teratomas survived extreme regimes: 43°C for 24 hours (CEM43°C 1440 minutes), hyperthermia in the scant serum‐free medium (CEM43°C 630 minutes) or treatment with an anti‐HSP70 antibody before long‐term hyperthermia 40.5°C from the second day (CEM43°C 585 minutes). This in vitro research provided novel insights into the impact of hyperthermia on the development of experimental teratomas from their undifferentiated sources and are thus of potential interest for future therapeutic strategies in corresponding in vivo models.
Keywords: anti‐HSP, differentiation, embryo, growth, hyperthermia, teratoma
1. INTRODUCTION
Contemporary thermal therapy of cancer aims at the targeted destruction of the tumour and is usually used as an adjuvant therapy.1, 2, 3 It is also important to avoid normal tissue damage that can or cannot be caused by different thermal exposures.4 To calculate a thermal isoeffect dose, the cumulative equivalent minutes at 43°C (CEM43°C) was introduced and used in studying the thermal damage in various systems.4, 5, 6 For in vitro systems, CEM43°C can be easily calculated, but for a number of biological, physiological and technical reasons, it is still difficult to apply CEM43°C for mild hyperthermia treatment monitoring in vivo at temperatures between 40 and 44°C (as reviewed by van Rhoon).7
Recently, hyperthermia was proposed to specifically target cancer stem cells.8 As cancer stem cells seem to be the most resistant to conventional therapy, it is important to develop new strategies to combat them.9, 10 Indeed, malignancies may develop not only as the consequence of gene mutations but also as a result of epigenetically deregulated development of stem cells of which germ cell tumours are the best example.11, 12, 13 Therefore, at this crossroad between normal and tumour development, it is important to be aware of the influence of hyperthermia on different developmental processes in order to design adequate and appropriate hyperthermal regimes for the therapy of cancer.
Hyperthermia has been shown to induce malformations in numerous animal models and has been associated with human abnormalities, but the precise molecular mechanism(s) for translation into specific cellular functions remains largely unknown.14, 15
In vivo experimental studies on animals provided data about the type of malformations caused by exposure to the heat, the most sensitive developmental stages and the critical doses for the teratogenic effect of heat.16, 17, 18, 19
In vivo studies on animals were not so accurate because of the variability in the developmental stages found in a female and the quantity of heat affecting an embryo through the heating of the mother. By the in vitro approach, such problems and the influence of the mother's metabolic response could be omitted.20 In the whole rat in vitro culture (WEC) used as an alternative to in vivo experiments in animals,21 malformations of the brain, face and eyes were found and the temperature thresholds were defined during the short‐term (2‐day) culture in vitro.22, 23, 24, 25
In an original experimental teratoma model in vitro, it was possible to screen for embryotoxic or teratogenic substances during rat development,11, 26, 27 and also reduce the need for screening experiments in living animals accordingly to the 3Rs of Russel and Burch.21 In this model, the development starts at the most critical period of development (gastrulation) when the embryo consists of the 3 germ layers containing only stem cells for the 3 basic cell lineages (ectodermal, mesodermal, endodermal). Development is followed up to 2 weeks in vitro, which is longer than in WEC, and simultaneous assessment of several endpoints such as the survival, overall growth, cell proliferation and differentiation is possible. This model also has an advantage over various cell cultures because it is a natural 3D system where tissue interactions are not as disrupted as in cell cultures.
Our aim was now to assess the impact of hyperthermal regimes varying in the cumulative equivalent minutes at 43°C (CEM43°C) and media composition upon the development of the experimental teratoma during 2 weeks in vitro to discover the most pronounced effects that could be proposed for the therapy of malignancies in corresponding models in vivo.11
2. MATERIAL AND METHODS
2.1. Ethical statement
All procedures with animals were conducted according to the Directive 2010/63/EU and Croatian Law on protection of experimental animals. They were approved by the Ethical Committee of the School of Medicine, University of Zagreb, Croatia.
2.2. Animals
Fisher inbred strain rats were kept in conventional cages, with standard diet and water ad libitum. Three months old females were mated with males overnight, and noon of the following day was assigned as the 0.5 day of pregnancy if sperm was found in the vaginal smear. At least four females were chosen for each experimental group.
At 9.5 dpc, pregnant rat females were anesthetized with 0.8 mL/kg of ketamine (Narketan®; Vétoquinol, Bern, Switzerland) and 0.6 mL/kg of xylazine (Xylapan®; Vétoquinol). Uteruses were removed, and animals were sacrificed by this procedure. All deciduas were removed from the uteruses. Under the dissecting microscope, egg cylinders were isolated from deciduas with watchmaker's forceps, and Reichert's membranes and ectoplacental cones were removed. Then, the extraembryonic part was cut away at the level of the amnion leaving just the embryo proper consisting of the three germ layers.
2.3. In vitro culture
All isolated embryos were randomly divided into respective experimental or control groups and plated in vitro. In each culture dish, three explanted embryos were placed on a lens paper supported by a stainless steel grid placed in a 60 × 15 mm centre‐well of an organ culture dish (BD Falcon™, Oxford, UK) and culture medium was added to the well to wet the lens paper. Explants were grown in Eagle's minimum essential medium (MEM) with 50% of rat serum at the air‐liquid interface. The serum was collected from the blood of anesthetized male rats (0.8 mL/kg of ketamine (Narketan®; Vétoquinol) and 0.6 mL/kg of xylazine (Xylapan®; Vétoquinol) of the same strain and heat inactivated.28 For serum‐free culture conditions, MEM was supplemented with 50 μg/mL of human holo‐transferrin (Sigma, St. Louis, MO, USA). Control explants were grown in an incubator at 37°C for 2 weeks. Cultures were grown in 5% CO2 and 95% humidified air. The medium was changed on alternate days for two weeks.
2.4. Hyperthermal regimes
Explanted embryos were cultivated in hyperthermal conditions in a separate incubator precalibrated one day before to the elevated temperature. After exposure to hyperthermia, explants were returned to another incubator at 37°C where control explants were cultivated from the beginning of culture.
If the sample was heated in n time intervals i = 1…n, and each time interval is at the temperature T i, then the cumulative equivalent minutes at 43°C (CEM43°C) was calculated using where the contribution of the i‐th interval of length Δt i at constant temperature T i is given by the relation where .6 Alternatively, we write , where .
Explanted embryos were subjected to short‐term hyperthermia at 42°C for 15 minutes (CEM43°C 3.75 minutes). The second group of explants was subjected either to 43°C (5°C higher than the normal rat body temperature) for 30 minutes every day during the first 7 days of culture (CEM43°C 210 minutes) or continuously during the first 24 hours of culture (CEM43°C 1440 minutes).
In the long‐term hyperthermia experiments, explants were exposed to the milder temperature at 40.5°C. The first group was exposed to 40.5°C during whole 2 weeks of culture (CEM43°C 630 minutes) in Eagle's minimum essential medium supplemented with 50% rat serum or in serum‐free conditions with 50 μg/mL of holo‐transferrin (T0665; Sigma) added to MEM.29 The second group was treated with 40.5°C from the second day of culture until the end of the culture period (CEM43°C 585 minutes) in MEM supplemented with 50% rat serum. The third group was treated with one dose of 100 μL/mL of antibody to heat shock protein 70 (HSP70; AM289‐5M; Biogenex, San Ramon, USA) before exposure to mild temperature from the second day (CEM43°C 585 minutes).
2.5. Overall growth
The overall growth of all isolated explants was followed from the moment of plating explanted embryos throughout the two week culture period during which teratomas are developing.30 Measures were included in the formula for ellipse area (A = π × major diameter × minor diameter/4). All values were normalized by the division to values of the initial measurement and used as the measure of overall growth (A/A 0) as described before.31 Therefore, A/A 0 was 1 for the day 0 when the explants were plated at the air‐liquid surface.
2.6. Histology and immunohistochemistry
After two weeks of culture, experimental teratomas were placed for 24 hours into mild St. Marie fixative solution (1% glacial acetic acid in 96% EtOH), dehydrated and embedded in paraffin. Uninterrupted or serial step sections (5 μm) were prepared and processed for routine histology or immunohistochemistry. For histological analysis of survival and differentiation, haematoxylin‐eosin‐stained sections were used as previously described.32
For immunohistochemistry, several teratomas were chosen at random from the control and experimental regimes. For single immunohistochemical labelling, Animal Research Peroxidase Kit (Dako, Glostrup, Denmark) was used according to the manufacturer's instructions. Briefly, microscopic slides with serial sections were deparaffinized, cleared in xylene and hydrated to PBS in gradient alcohols. Antigen retrieval was performed in Dako Target Retrieval Buffer pH = 6.0 in a microwave oven on 700 W. After boiling, slides were cooled for one minute, which was repeated three times. Slides were then cooled for 20 minutes, blocked with Peroxidase Blocking reagent (0.03% H2O2) for five minutes and rinsed three times in PBS. Primary antibodies were diluted in 1% BSA/PBS and incubated for 15 minutes with Biotinylation Reagent and five minutes with Blocking Reagent. Primary antibodies used in this study were mouse anti‐human Proliferating Cell Nuclear Antigen (PCNA) monoclonal antibody (Clone PC‐10; M0879; 1:100; Dako), mouse anti‐rat nestin monoclonal antibody (Rat 401; Developmental Studies Hybridoma Bank, Iowa, IA; 1:5) or mouse anti‐Neurofilament 160 kDa (anti‐NF160) monoclonal antibody (N‐5264;1:20; Sigma). Slides were incubated with primary antibody for one hour, washed three times for five minutes with PBS and incubated for 15 minutes with Dako streptavidin‐horseradish peroxidase complex. Visualization of the signal was performed with DAB (3,3′‐diaminobenzidine) and chromogen‐substrate complex for one minute and stopped in dH2O. Slides were contrasted with haematoxylin, washed with tap water for 20 minutes and covered with glycerol/PBS solution (1:1).
For double immunolabelling, En Vision Doublestain kit (Dako) was used. Staining with anti‐rat nestin monoclonal antibody was performed as described above, but after being treated with DAB, slides were washed for five minutes in H2O and blocked for 10 minutes with En Vision Doublestain Block Reagent. Primary antibody to PCNA (1:100; Dako) and monoclonal mouse anti‐human glial fibrillary acidic protein (GFAP) antibody (N 1506; Dako Ready‐To‐Use) or anti‐nestin antibody were diluted in 1% BSA/PBS and applied on slides for one hour. Slides were washed three times for five minutes with PBS and incubated with alkaline phosphatase‐labelled polymer for 15 minutes. After washing for three times in PBS, Fast Red and chromogen‐substrate for alkaline phosphatase were applied for five minutes. The reaction was stopped after one minute in dH2O, after which the slides were contrasted with haematoxylin, washed in tap water, dehydrated and covered with glycerol/PBS solution (1:1).
2.7. Stereology
Step serial sections of complete explants that were immunohistochemically stained with an antibody to PCNA were quantified by estimating the volume density of PCNA‐positive cells in the sections of control and experimental explants. The analysis was performed under the Nikon Alphaphot binocular light microscope (Nikon, Vienna, Austria) using Weibel's M42 test system, made of 42 short test lines each with two ends as test points.33 Volume density of PCNA‐positive cells was estimated by counting points of the test system, which hit PCNA‐stained nuclei, and points which hit the reference space at the magnification of 400 × (reference space being defined as hits on any part of an explant). Volume density (V v) of PCNA is the ratio between the hits falling in all PCNA‐stained nuclei (P i) and hits falling in the reference space (P t; V v = P i/P t), and is expressed in mm0 (mm3/mm3).33, 34 Before analysis, for each experimental group, the stereological orientation measurement was carried out to define the number of fields to be tested.34 At least 90 fields were stereologically assessed.
2.8. Statistical analysis
Statistics were performed by an SPSS Statistics 10.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA, USA).
Descriptive analysis was made for all data, and the normal distribution was tested by Kolmogorov‐Smirnov test or Shapiro‐Wilk test after which parametric or nonparametric tests were chosen to compare variances.
The overall growth of explants assessed as described above was tested by parametric t test or one‐way analysis of variance (ANOVA) or nonparametric Kruskal‐Wallis or Mann‐Whitney U test. For survival and differentiation analysis, proportions of survived explants or differentiated tissues in experimental teratomas were compared by either chi‐square test or Fisher's exact test.
Differences in the volume density of PCNA‐positive cells were tested using Student's t test or one‐way ANOVA with the post hoc Tukey's multiple comparison test.
The statistical significance level was P < .05.
3. RESULTS
3.1. Survival
3.1.1. Short‐term hyperthermia
In the first part of the screening, experiments were carried out with hyperthermia of duration shorter than the two week culture period and at higher temperatures (Table 1).
Table 1.
Experimental teratoma survival after 14 d of in vitro culture in short hyperthermal regimes. After the two week culture, survival was assessed by classical histology. All teratomas survived in controls and in the hyperthermal regime at 43°C for 30 min during 7 d. Note that at 43°C applied for 24 h no teratomas survived, which was significantly different in comparison with controls
| Regime | 37°C | 42°C 15′ | 43°C 30′/7 d | 43°C 24 h | ||||
|---|---|---|---|---|---|---|---|---|
| CEM43°C (min) | Control | 3.75 | 210 | 1440 | ||||
| N | % | N | % | N | % | N | % | |
| Explanted embryos | 122 | 100 | 109 | 100 | 19 | 100 | 12 | 100 |
| Teratomas developed in vitro | 122a | 100 | 107 | 98.2 | 19 | 100 | 0a | 0 |
Fisher's exact test: a P < .0001.
Almost all explanted embryos survived for two weeks when the temperature of 42°C was applied for the short period of 15 minutes at the beginning of culture (CEM43°C 3.75 minutes).
To test the ability for survival in more severe hyperthermal conditions, two experiments on a smaller number of explants were conducted. First, 43°C was applied for 30 minutes each day during 7 days (CEM43°C 210 minutes). Surprisingly, the survival was the same as in controls. However, when explants were treated with 43°C for 24 hours (CEM43°C 1440 minutes), survival was impaired to the extent that no teratomas for the histological analysis were obtained.
3.1.2. Long‐term hyperthermia
In the second part of the screening, experiments were carried out with milder hyperthermia of 40.5°C but applied for a longer time (long‐term hyperthermia; Table 2). To test the response to hyperthermia with the stage of teratoma development, hyperthermia was applied either from the first day (CEM43°C 630 minutes) or the second day of culture (CEM43°C 585 minutes). The survival was significantly lower in both groups treated with 40.5°C in comparison with controls. Previously applied, the antibody to HSP70 completely disabled survival of explants treated with hyperthermia from the second day (CEM43°C 585 minutes).
Table 2.
Experimental teratoma survival after 14 d of in vitro culture in long hyperthermal regimes at 40.5°C. After the two week culture, survival was assessed by classical histology. Almost all teratomas survived in controls while in the hyperthermal regime at 40.5°C from both the first, and the second day, survival was significantly impaired in comparison with controls. Note that at 40.5°C from the second day with the addition of antibody against HSP70 no teratomas survived which was significantly different in comparison with controls
| Regime | 37°C | 40.5°C from second day | 40.5°C from second day + anti‐HSP70 | 40.5°C | ||||
|---|---|---|---|---|---|---|---|---|
| CEM43°C (min) | Control | 585 | 585 | 630 | ||||
| N | % | N | % | N | % | N | % | |
| Explanted embryos | 59 | 100 | 29 | 100 | 8 | 100 | 27 | 100 |
| Teratomas developed in vitro | 58a , b , c | 98.31 | 24a | 82.76 | 0b | 0 | 20c | 74.07 |
Fisher's exact test: a P < .05; b P < .0001, c P < .005.
Long‐term hyperthermia (40.5°C) applied from the start of culture (CEM43°C 630 minutes) caused complete necrosis of the serum‐free cultivated explants (Table 3).
Table 3.
Experimental teratoma survival after 14 d of in vitro culture in the serum‐free medium (MEM supplemented with 50 μg/mL human holo‐transferrin) in a long hyperthermal regime at 40.5°C. After the two week culture, survival was assessed by classical histology. Note that only a portion of teratomas cultivated in the chemically defined medium with transferrin (controls) survived, while in the hyperthermal regime at 40.5°C no teratomas survived which was significantly different in comparison with controls
| Regime | 37°C in MEM + transferrin | 40.5°C in MEM + transferrin | ||
|---|---|---|---|---|
| CEM43°C (min) | Control | 630 | ||
| N | % | N | % | |
| Explanted embryos | 34 | 100 | 37 | 100 |
| Teratomas developed in vitro | 22a | 64.71 | 0a | 0 |
Fisher's exact test: a P < .0001.
3.2. Overall growth
3.2.1. Short‐term hyperthermia
The explants treated once with 42°C for a short period of 15 minutes (CEM43°C 3.75 minutes) were surprisingly larger than controls on days 5 and 9 (Figure 1A).
Figure 1.
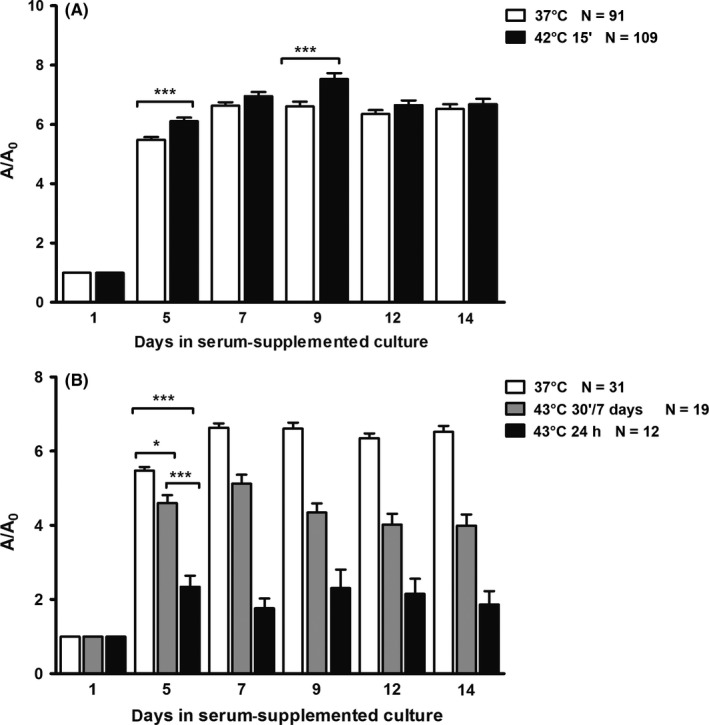
Overall growth of teratomas in short‐term hyperthermal regimes (mean ± SEM). A/A 0 ‐ explant area/explant area at the beginning of culture. A, Growth of teratomas in short‐term hyperthermal regime at 42°C (CEM43°C 3.75 min) was significantly higher during the first part of the culture in comparison with controls cultivated at 37°C. Student's t test: ***P < .0001; B, growth of explants in short‐term hyperthermal regimes at 43°C. Note that at 43°C for 30 min during 7 d (CEM43°C 210 min), growth was significantly lower than in controls cultivated at 37°C, while at 43°C applied for 24 h (CEM43°C 1440 min) growth was even lower. ANOVA: *P < .01; ***P < .0001
Hyperthermia of 43°C severely affected overall growth of experimental teratomas, which were smaller than controls during the whole culture period (Figure 1B). Treatment with 43°C for 24 hours leads to a significant decrease in size on the fifth day of culture (CEM43°C 1440 minutes). The group treated at 43°C for 30 minutes during the first seven days (CEM43°C 210 minutes) showed a smaller, although still significant decrease, starting at the same day, till the end of the culture period. Therefore, comparison between the groups treated with 43°C showed that the group treated periodically during seven days for 30 minutes was significantly larger than the group treated once for 24 hours.
3.2.2. Long‐term hyperthermia
When explants were treated with 40.5°C from the start of the culture (CEM43°C 630 minutes), no difference in the overall growth during the whole culture period was detected in comparison with controls. However, when treated with 40.5°C from the second day (CEM43°C 585 minutes), they were significantly smaller than controls during the whole culture period but also smaller than the group treated on 40.5°C from the start (CEM43°C 630 minutes; Figure 2A).
Figure 2.
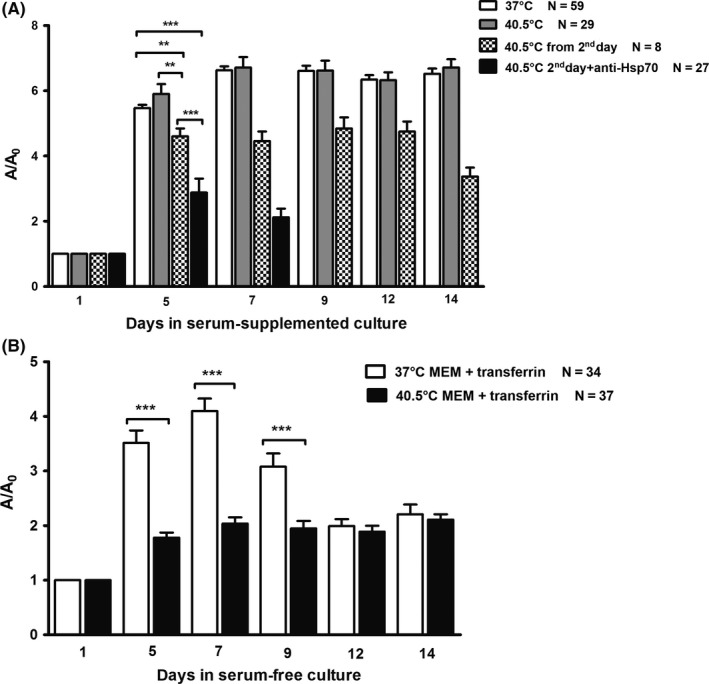
Overall growth of teratomas in long‐term hyperthermal regimes (mean ± SEM). A/A 0 ‐ explant area/explant area at the beginning of culture. A, Growth of explants in the serum‐supplemented medium in the hyperthermal regime at 40.5°C from the first day (CEM43°C 630 min) was as in controls and that was significantly higher than in the hyperthermal regime at 40.5°C from the second day (CEM43°C 585 min). Note that growth of teratomas at 40.5°C from the second day with the addition of antibody against HSP70 (CEM43°C 585 min) was the lowest and significantly different in comparison with controls and the two other regimes at 40.5°C. ANOVA: **P < .001; ***P < .0001; B, growth of teratomas in the chemically defined medium with transferrin as the only protein was significantly lower during the first week at 40.5°C (CEM43°C = 630 min) in comparison with controls cultivated at 37°C. Student's t test: ***P < .0001
When the antibody to heat shock protein HSP70 was added to the medium on the second day before long‐term hyperthermia, explants have shown a decrease in size already in the first week of culture (CEM43°C 585 minutes). They were significantly smaller compared to control and also to those treated with hyperthermia without the antibody (Figure 2A).
As expected, in the chemically defined serum‐free medium explants were always smaller than those cultivated with serum.29 Exposure to long‐term hyperthermia (40.5°C; CEM43°C 630 minutes) significantly decreased explant growth in comparison with controls, especially in the first week (Figure 2B).
3.3. Differentiation and cell proliferation
In the control and experimental hyperthermal regimes, all surviving embryonic explants developed into teratoma‐like structures. By light microscopy, differentiated tissues among which ectodermal derivatives such as epidermis and neural tissue (Figure 3), mesodermal derivatives such as myotubes and cartilage (Figure 4) and endodermal derivative, the columnar epithelium (Figure 5) were easily detected after the two week culture period. Stratified squamous epithelium (epidermis) contained basal and suprabasal keratinocytes with exfoliated keratin at the top (Figure 3A,B). Cells of the stratum germinativum, for example basal cells situated next to basement membrane and cells of the stratum spinosum, the second layer of epidermal cells, were positive for the proliferating cell nuclear antigen (PCNA; Figure 3B), as expected. Neural tissue contained typical big light nuclei scattered within the neuropil (Figure 3A). Within the neural tissue, some cells expressed cell proliferation marker PCNA (Figure 3C,E). Nestin, a neural stem cell marker but also a marker of specific neurons in the adult brain,35 was also expressed, surrounding only a few cells within the neural tissue (Figure 3D). Markers of neural tissue differentiation, glial fibrillary acidic protein (GFAP; Figure 3E) and neurofilaments (NF; Figure 3F) were also detected. Myotubes, precursors of the skeletal muscle, typically contained several nuclei in a row (Figure 4A) and were expressing nestin (Figure 4B). Cartilage contained typical chondroblasts with elongated cell shape in the zone of appositional growth, while chondrocytes were typically situated in lacunae embedded in the extracellular matrix. Chondrocytes, in the zone of interstitial growth, were characterized by the formation of isogenic groups typically containing at least two cells within a single lacuna (Figure 4C). PCNA was abundantly expressed in nuclei of cartilage cells (Figure 4D). Simple columnar epithelium typically expressed PCNA in the majority of cells (Figure 5A). Nestin expression was abundant in the vicinity of the columnar gut‐like epithelium, sometimes connecting several small cells which were reminiscent of the enteric ganglia (Figure 5B).
Figure 3.
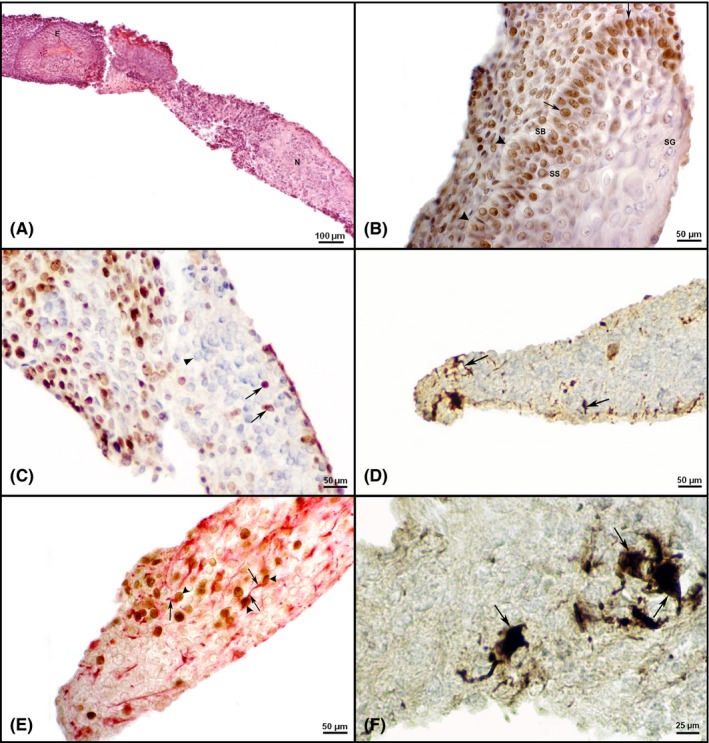
Differentiation of ectodermal derivatives and ability for cell proliferation in teratomas after two weeks of in vitro culture. A, Keratinized epidermis (E) and neural tissue (N) in a teratoma in vitro developed after 14 d of culture on 40.5°C (CEM43°C 630 min; HE). B, PCNA expression in the nuclei of epidermal cells (arrow) after a two week hyperthermia on 40.5°C starting from the 2nd day (CEM43°C 585 min). Note the basement membrane (arrowhead) and well‐developed epidermal cell layers: stratum basale (SB), stratum spinosum (SS) and stratum granulosum (SG). C, PCNA expression (arrow) in nuclei of neural tissue cells found after two weeks of hyperthermia on 40.5°C (CEM43°C 630 min) starting from the second day. Note that majority of neuroblasts are negative for proliferation marker (arrowhead). IHC, DAB, counterstained by haematoxylin. D, Expression of nestin (arrow) in neural tissue developed after treatment with 43°C for 30 min daily for seven days. (CEM43°C = 210 min). IHC, DAB, counterstained by haematoxylin. E, Expression of GFAP (arrow) and PCNA (arrowhead) in neural tissue after a two week hyperthermia at 40.5°C (CEM 43°C = 630 min). IHC, double immunolabelling, DAB, Fast Red, counterstained by haematoxylin. F) Neurons containing neurofilaments (arrow) in the neural tissue of a control cultivated at 37°C. IHC, DAB, counterstained by haematoxylin [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
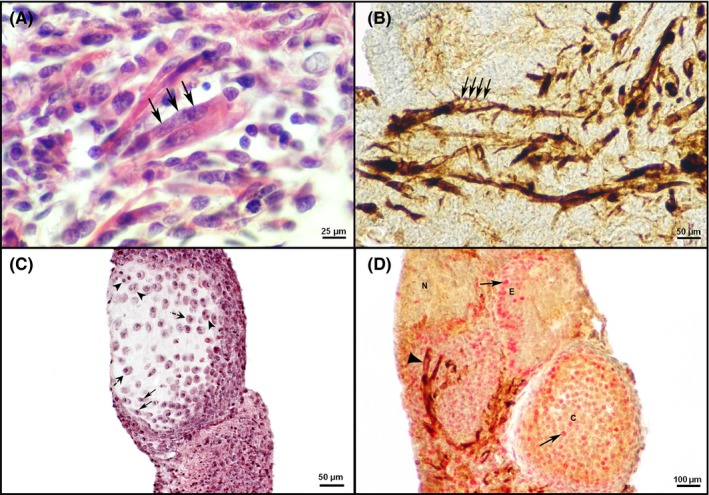
Differentiation of mesodermal derivatives (myotubes and cartilage) and ability for cell proliferation in teratomas after 2 wk of in vitro culture. A, Myotubes with several nuclei (arrow) found in a teratoma in vitro after hyperthermia of 42°C applied for 15 min (CEM43°C 3.75 min; HE). B, Perinuclear nestin expression in differentiated myotubes (arrow) after hyperthermia of 43°C for 30 min (CEM43°C 210 min) during 7 d. IHC, DAB, counterstained by haematoxylin. C, Cartilage with chondroblasts (arrow), chondrocytes situated in lacunae (dashed arrow) and isogenic groups of chondrocytes (arrowhead) after a two week hyperthermia on 40.5°C starting from the second day (CEM43°C 585 min; HE). D, Expression of PCNA (arrow) in the cartilage (C), in some cells of the neural tissue (N) and epidermis (E) and expression of nestin (arrowhead) in teratoma treated with 42°C for 15 min (CEM43°C 3.75 min). IHC, double immunolabelling, Fast Red, DAB, counterstained by haematoxylin [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
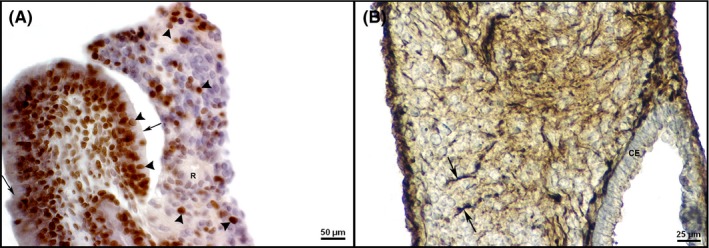
Differentiation of endodermal derivative, the columnar epithelium and ability for cell proliferation in teratomas after two weeks of in vitro culture. A, PCNA expression (arrowhead) in nuclei of the columnar epithelium cells (arrow). Note also the PCNA expression in adjacent neural tissue forming rosettes (R) in an explant treated with hyperthermia of 43°C for 30 min during 7 d (CEM43°C 210 min). IHC, DAB, counterstained by haematoxylin. B, Columnar epithelium (CE) and nestin expression (arrow) in enteric ganglia‐like structures of a control cultivated at 37°C. IHC, DAB, counterstained by haematoxylin [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3.1. Short‐term hyperthermia
In explanted embryos treated with 42°C for 15 minutes (CEM43°C 3.75 minutes), myotubes (Figure 4A) and columnar epithelium differentiated with significantly higher incidence than in controls (Table 4). PCNA expression was present, but the volume density was the lowest and significantly different in comparison with controls (Figure 6).
Table 4.
Experimental teratoma differentiation in a short hyperthermal regime at 42°C for 15 min at the beginning of culture. After the two week culture, differentiation in teratomas was assessed by classical histology. Note that in the hyperthermal regime at 42°C applied for 15 min the incidence of myotubes and columnar epithelium was significantly higher than in controls at 37°C
| Regime | 37°C | 42°C 15′ | ||
|---|---|---|---|---|
| CEM43°C (min) | Control | 3.75 | ||
| N | % | N | % | |
| Teratomas developed in vitro | 77 | 100 | 93 | 100 |
| Epidermis | 71 | 92.21 | 86 | 92.47 |
| Neural tissue | 65 | 84.42 | 77 | 82.80 |
| Cartilage | 66 | 85.71 | 74 | 79.57 |
| Myotubes | 48a | 62.34 | 75a | 80.65 |
| Columnar epithelium | 70b | 90.91 | 92b | 98.92 |
Chi‐square test: a P < .01; Fisher's exact test: b P < .05.
Figure 6.
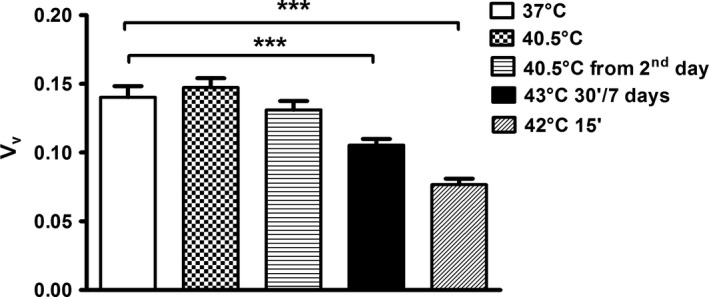
Volume density (V v) of PCNA‐positive cells in hyperthermal regimes. No difference in cell proliferation was discovered in hyperthermal regimes at 40.5°C (CEM43°C 630 min, from 1st day and CEM43°C 585 min, from the second day) in comparison with controls. Note significantly lower cell proliferation ability in hyperthermal regimes at 43°C for 30 min (CEM43°C 210 min) during seven d and 42°C for 15 min (CEM43°C 3.75 min) in comparison with controls. Mann‐Whitney U test: ***P < .0001
In experimental teratomas resulting from the periodical treatment with 43°C for 30 minutes during first 7 days of culture (CEM43°C 210 minutes; Table 5), PCNA (Figure 5A) and nestin (Figures 3D and 4B) expression was distributed as has been described above. However, the volume density of PCNA‐labelled cells at the end of culture was significantly reduced in comparison with controls (Figure 6).
Table 5.
Experimental teratoma differentiation in a short hyperthermal regime at 43°C applied for 30 min during the first week of culture. After the 2‐wk culture, differentiation in teratomas was assessed by classical histology. No differences in the tissue incidence were observed between controls cultivated at 37°C and those cultivated at 43°C for 30 min during 7 d
| Regime | 37°C | 43°C 30′ 7 d | ||
|---|---|---|---|---|
| CEM43°C (min) | Control | 210 | ||
| N | % | N | % | |
| Teratomas developed in vitro | 11 | 100 | 6 | 100 |
| Epidermis | 11 | 100 | 6 | 100 |
| Neural tissue | 10 | 90.91 | 6 | 100 |
| Cartilage | 9 | 81.82 | 3 | 50.00 |
| Myotubes | 10 | 90.91 | 6 | 100 |
| Columnar epithelium | 11 | 100 | 6 | 100 |
3.3.2. Long‐term hyperthermia
In both long‐term hyperthermia regimes at 40.5°C, differentiated tissues were detected. In the group of embryos treated with 40.5°C after the first 24 hours of culture (CEM43°C 585 minutes), differentiation of cartilage was significantly impaired (Table 6), while in the group treated from the start of the culture (CEM43°C 630 minutes), this impairment was not significant (Table 7).
Table 6.
Experimental teratoma differentiation in a long hyperthermal regime at 40.5°C from the second day of culture. After the two week culture, differentiation in teratomas was assessed by classical histology. Note that only the incidence of cartilage was significantly lower in teratomas cultivated at 40.5°C from the second day in comparison with controls cultivated at 37°C
| Regime | 37°C | 40.5°C from second day | ||
|---|---|---|---|---|
| CEM43°C (min) | Control | 585 | ||
| N | % | N | % | |
| Teratomas developed in vitro | 11 | 100 | 11 | 100 |
| Epidermis | 11 | 100 | 11 | 100 |
| Neural tissue | 9 | 81.82 | 10 | 90.91 |
| Cartilage | 9a | 81.82 | 4a | 36.36 |
| Myotubes | 9 | 81.82 | 6 | 54.55 |
| Columnar epithelium | 11 | 100 | 10 | 90.91 |
Fisher's exact test: a P < .05.
Table 7.
Experimental teratoma differentiation in a long hyperthermal regime at 40.5°C for two weeks. After the two week culture, differentiation in teratomas was assessed by classical histology. Note that the incidence of tissues in teratomas cultivated at 40.5°C was similar to the incidence of tissues in controls cultivated at 37°C
| Regime | 37°C | 40.5°C | ||
|---|---|---|---|---|
| CEM43°C (min) | Control | 630 | ||
| N | % | N | % | |
| Teratomas developed in vitro | 9 | 100 | 10 | 100 |
| Epidermis | 9 | 100 | 10 | 100 |
| Neural tissue | 7 | 77.78 | 8 | 80 |
| Cartilage | 8 | 88.89 | 7 | 70 |
| Myotubes | 7 | 77.78 | 7 | 70 |
| Columnar epithelium | 8 | 88.89 | 9 | 90 |
PCNA expression was still observed after long‐term hyperthermia (Figure 3B,C,E). In both long‐term hyperthermia experiments, PCNA expression (V v) was not significantly changed in comparison with controls and no difference in PCNA expression between the two treatments on 40.5°C (from the first or the second day of culture) could be found (Figure 6).
4. DISCUSSION
Results of screening for the impact of hyperthermia on the experimental teratomas developed from gastrulating embryos that contained only the stem cells for three basic lineages (ectodermal, mesodermal and endodermal) were positive for every hyperthermal regime that we applied (40.5‐43°C) where CEM43°C was in the range from 3.75 to 1440 minutes. Therefore, this in vitro model seems to be as sensitive to this externally applied physical factor as it was to various biologically active substances, among which a DNA‐demethylating agent (5‐azacytidine) used today in the therapy of myelodysplastic syndrome.26, 27 We may suppose that the similar effects may be obtained at least in the experimental teratoma model in vivo that originates from transplanted embryos to an ectopic environment.11 In 2011, Yarmolenko et al reviewed CEM43°C and thermal threshold damage data (≥20 ‐ 1.5 × 104 minutes) mostly in rodent models and found that they are different for various tissues and species. In 2016, van Rhoon states that clinical trials aspire to use 43°C but probably achieve 39.5‐41°C for 69‐90 minutes. As reviewed by Seifert et al,36 in 2016, adjuvant hyperthermia was used in paediatric patients for treatment of refractive or recurrent sarcomas and germ cell tumours. In a study on 35 children with germ cell tumours, 86% had an objective response to treatment with combination of one hour regional deep hyperthermia (41‐43°C) on days 1 and 4 and chemotherapy.37 Indeed, hyperthermia, either localized, regional or whole‐body hyperthermia, has been used in clinical trials to destroy various tumours or impede their growth. Usually, it is regarded as an adjuvant therapy for radiotherapy and chemotherapy because it causes secondary changes that increase susceptibility of tumour tissue to destruction, but more studies are needed to identify hidden effects of hyperthermia as reviewed by Behrouzkia et al.38
Although parameters of development were almost always negatively affected in our in vitro study with experimental teratomas, the experiment at the 42°C for 15 minutes did not affect survival and at the beginning even increased the growth of explants. These results differ from in vivo studies where the temperature of 42°C applied for 10 minutes caused resorptions.16 In WEC, after 2 days, this dose caused only mild retardation of development but severe craniofacial defects if the duration was prolonged to 40 minutes. A slightly higher temperature, 42.5°C for 15 minutes caused craniofacial defects in 100% embryos.25 It may be that during our two week cultivation period, explants were protected by overexpression of heat shock proteins followed by induction of growth factor expression, similarly to the findings reported in mouse tumour development.39 Moreover, a positive effect on growth was suggested to arise from the inhibition of protein synthesis of negative growth regulators.40 However, at the end of culture, the ability for cell proliferation was lower than in controls, which can be explained by the fact that terminal differentiation that we find occurs after the exit from the cell cycle.
Hyperthermia of 42°C for 15 minutes also enhanced differentiation of the columnar epithelium and myotubes which is in concordance with studies on erythroleukaemia cells41 where hyperthermia acted as an inducer of differentiation while in brain malignant cells it potentiated differentiation therapy.42 Constitutive heat shock proteins such as HSP70 are present during the mouse myoblast differentiation period,43 while HSP90 inhibition affected protein kinases regulating pathways required for myoblast differentiation.44 Multiple transduction proteins, which are essential for differentiation, are client proteins of HSP90.45 In porcine satellite cells, a mild heat shock increased transcription and translation of HSP90, HSP70, HSP25/27, while in fused satellite cells, protein synthesis rates were significantly increased, and degradation rates decreased.46 A treatment of 43°C for 5 minutes causes upregulation of myogenin and MyHC genes in mouse embryos.47 The higher incidence of the columnar epithelium in our experiment may also be related to the expression of thermoprotective heat shock proteins because of their proposed role in differentiation and development.48 A stronger induction of HSP72 was detected in gut tissue after 42°C 15‐minutes hyperthermia, and the colon seems to require an enhanced HSP72 response.49 Enhancement of differentiation that we discovered might also be of interest for regenerative medicine strategies.50
We have to explain that our results on growth and differentiation imply that such dysregulation of developmental processes may lead to teratogenic effects in utero. Although it was quite difficult to generalize data on teratogenicity induced by hyperthermia, because of the variety of species and procedures, it is important to stress that our lowest CEM43°C 3.75 is slightly above CEM43°C 1‐2 minutes reported to be teratogenic for mammals as reviewed by van Rhoon et al in 2013.
Although CEM43°C 210 minutes in the regime of 43°C for 30 minutes each day during the first week is in the range of CEM43°C 180‐240 minutes where ablation of the prostate cancer was reported, it might be that our CEM43°C was too low to affect survival because for some other solid tumours, CEM43°C ˃ 240 was necessary.51 Anyway, the rather unexpected result that the survival was not at all affected may be explained by the thermotolerance. The short exposure of embryos to the temperature of 42°C could prevent subsequent treatment with severe hyperthermia of 43°C for 7.5 minutes from causing severe malformations.25, 52 A higher dose, 43°C for 30‐60 minutes, is more teratogenic because it decreases cell proliferation.53 Indeed, 43°C, periodically applied, significantly reduced the size of explants in our study, similarly as in other in vitro and in vivo studies at this temperature25, 54 and decreased the ability for cell proliferation (V v for PCNA). Obviously, cell cycle dynamics were affected, similarly as in other in vitro studies on rat embryos.55
Based on the previous research with the metabolically poor protein‐free medium and an embryotoxic substance,26, 27, 29, 32 our results with the 3 most extreme regimes discussed below corroborate the idea that in our model a simple simultaneous assessment of survival and overall growth endpoints can efficiently screen for the most deleterious conditions of the microenvironment.
Although 3D systems were less sensitive to temperatures from 37 to 57°C,56 our 3D system treated by the whole‐day regime at 43°C, a 5°C higher temperature than the normal rat body temperature16 was extremely sensitive. Melanoma cells at 45°C for 30 minutes (approximate CEM43°C 120 minutes) produced HSPs and some cells survived for two weeks,57 while our exposure time of 24 hours at 43°C with the highest CEM43°C 1440 minutes was probably too severe to elicit HSP production and thermotolerance.
The heat was defined as a physical agent that acts directly on the embryo and seems not to be influenced by metabolic factors.19, 58 Chinese hamster ovary HA‐1 cells have shown that heat sensitivity was not dependent on serum addition.59 In contrast to that, in chemically defined medium that we used, with transferrin as the only protein supplement, deficiency of nutrients seems to have altogether blocked the competitive ability of cells to adapt to CEM43°C 630 minutes and no survival was detected while in serum‐supplemented medium CEM43°C 630 minutes several embryos survived.
The last regime with CEM43°C 585 minutes and an antibody to HSP70 has rendered experimental teratomas totally unprotected from the heat probably because this antibody recognizes both the constitutive (HSC73) and inducible (HSP72) forms of HSP70 that have a protective function helping the cell to cope with lethal conditions.60 HSPs may also determine the cell fate by orchestrating the decision of apoptosis vs differentiation.45 Indeed, in our two week model apoptosis has been elicited by a chemical agent already at the beginning of culture when differentiation just begins (our unpublished observation). Therefore to understand the impact of apoptosis on the overall growth, we shall do a study in another model of a shorter duration. In four to eight cell embryos, the addition of anti‐HSP70 was found to reduce development by induction of apoptosis and degradation of mammalian embryos.61, 62 HSP90 inhibitor caused antiproliferative and apoptotic effect in adult and paediatric glioblastoma cells in vitro and in vivo.63 Heat Shock Factor 1 (HSF1), the master regulator of the response to the heat, in cancer cells has a specific transcriptional signature that enhances malignancy.64 Inhibition of the HSF1 by an RNA aptamer in cancer cells induced apoptosis and abolished their colony‐forming capability.65 The above results speak in favour of therapeutic inhibition of the HSPs. However, extracellularly localized and membrane‐bound HSPs elicit an immunological response against cancer and are the basis of anticancer vaccines.66 Whether antibodies against HSPs, sometimes found in the organism of cancer or other patients, are “a friend or a foe” is questionable.67 Regardless of this dilemma, our result suggests the benefit of anti‐HSP therapy with the therapeutic hyperthermia of cancer.
The dose of 40.5°C over 2 days (CEM43°C 90 minutes) was defined as a threshold dose for the development of malformations in 9.5 days old rat embryos in vivo and in vitro16, 68 but was effective even after 40 minutes (approximate CEM43°C 1.25 minutes) when applied to the uterus ex vivo.20 In our study, CEM43°C were much higher in long‐term regimes, but the long‐term hyperthermia of 40.5°C caused a more pronounced stage‐specific overall growth inhibition in embryos treated from the second day. In this organ culture model, differentiation starts from the second day30; 69, so it is possible that differentiating cells are more susceptible to the heat shock than undifferentiated, especially because cell lines vary in response to small temperature elevations.70 Moreover, stem cells express HSPs that protect them “against external and internal stressors, thereby maintaining their stemness”71 and that may be the reason that just explanted embryos were more resistant to the hyperthermia in our system. Survival was also affected in both groups, but the ability for cell proliferation at the end of culture remained the same as in controls. Studies on CHO or Hela cells report a chronic tolerance and a progression through cell cycle during chronic heating at an even higher temperature, 41.5°C.72 The negative effect on cartilage development could be related to changes in the Hox gene expression 19 and skeletal development.55, 73 It was suggested that a single acute exposure to 42°C be a threshold dose in skeletal development.17 However, our results support assumption that increasing the duration of temperature elevation may lower threshold for malformations to 40‐41°C19 as has been recently published for the chick embryo.74 Other in vitro studies on mammalian skeletal muscle cells have shown that hyperthermia at 41°C for 72 hours inhibited myotube formation.75
5. CONCLUSION
Based on the results in our experimental teratoma model, we may conclude that hyperthermia can directly target the exact source of stem cells for ectodermal, mesodermal and endodermal lineages, in a natural 3‐dimensional system reminiscent of a solid tumour. Alongside negative effects upon survival, growth and cell proliferation in various regimes, our in vitro screening result with the local short‐term hyperthermia at 42°C applied for 15 minutes (CEM43°C 3.75) is in line with a rare previous result on hyperthermal differentiation therapy.41 It should be noted that the concept of differentiation therapy has been used for a long time for the treatment of acute promyelocytic leukaemia.76 Therefore, hyperthermal differentiation therapy may represent a challenging task for future research.
Finally, we may single out our result where the application of an antibody against HSP70 before long‐term (13 days) hyperthermia of 40.5°C (CEM43°C 585 minutes) leads to the total destruction of experimental teratomas that was not achieved at the same CEM43°C without the antibody. A recent study in vivo reduced off‐target systemic tumorigenic effects and distant tumour growth caused by hyperthermia by an adjuvant HSP inhibition.77 However, thermal ablation at 60°C for 10 minutes and 90°C for two minutes far exceeded much milder CEM43°C at a much milder temperature used in our system that should be harmless, for example for local application to the skin. Therefore, our second proposal for the local hyperthermal therapy strategy in vivo includes the application of an anti‐HSP therapy to avoid thermotolerance elicited by HSPs. It is also possible that anti‐HSPs will destroy tumours in a shorter time interval because CEM43°C depends linearly on the heating time interval and exponentially on heating temperature. This remains to be studied in future in vitro and in vivo experiments.
DECLARATION OF INTEREST
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the study.
ACKNOWLEDGEMENT
This work was supported by the Croatian Ministry of Science, Education and Sport under Grant (No. 108‐1080399‐0335) Experimental Embryonic Tumours and Development of Mammalian Embryo in vitro and in vivo; University of Zagreb under Supportive Grants (No. 1.2.1.17, No. 1101310 BM1.22) and by the European Union through the European Regional Development Fund, Operational Programme Competitiveness and Cohesion, under grant agreement No. KK.01.1.1.01.0008, Reproductive and Regenerative Medicine—Exploring New Platforms and Potentials.
The authors wish to thank Dr. Robert Beuc and MD Marta Himerleich Peric for their valuable suggestions and Milan Kopač, and Mariana Druga for their technical assistance.
Katusic Bojanac A, Rogosic S, Sincic N, et al. Influence of hyperthermal regimes on development of the experimental teratoma in vitro. Int J Exp Path. 2018;99:131–144. 10.1111/iep.12273
REFERENCES
- 1. Cho M, Cervadoro A, Ramirez MR, et al. Assembly of iron oxide nanocubes for enhanced cancer hyperthermia and magnetic resonance imaging. Nanomaterials. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 2007;19:418‐426. [DOI] [PubMed] [Google Scholar]
- 3. Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia. 2015;31:272‐279. [DOI] [PubMed] [Google Scholar]
- 4. Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27:320‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re‐setting the biologic rationale for thermal therapy. Rev Int J Hyperthermia. 2005;21:779‐790. [DOI] [PubMed] [Google Scholar]
- 6. van Rhoon GC, Samaras T, Yarmolenko PS, Dewhirst MW, Neufeld E, Kuster N. CEM43°C thermal dose thresholds: a potential guide for magnetic radiofrequency exposure level? Eur Radiol. 2013;23:2215‐2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Rhoon GC. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia. 2016;32:50‐62. [DOI] [PubMed] [Google Scholar]
- 8. Oei AL, Vriend LE, Krawczyk PM, Horsman MR, Franken NA, Crezee J. Targeting therapy‐resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;2:1‐12. [DOI] [PubMed] [Google Scholar]
- 9. Ben‐David U, Biran A, Scaffidi P, et al. Elimination of undifferentiated cancer cells by pluripotent stem cell inhibitors. J Mol Cell Biol. 2014;6:267‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107:5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bulic‐Jakus F, Katusic Bojanac A, Juric‐Lekic G, Vlahovic M, Sincic N. Teratoma: from spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip Rev Dev Biol. 2016;5:186‐209. [DOI] [PubMed] [Google Scholar]
- 12. Hernandez‐Vargas H, Sincic N, Ouzounova M, Herceg Z. Epigenetic signatures in stem cells and cancer stem cells. Epigenomics. 2009;1:261‐280. [DOI] [PubMed] [Google Scholar]
- 13. Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7:319‐327. [DOI] [PubMed] [Google Scholar]
- 14. Bennett GD. Hyperthermia: malformations to chaperones. Birth Defects Res B Dev Reprod Toxicol. 2010;89:279‐288. [DOI] [PubMed] [Google Scholar]
- 15. Czeizel AE, Puho EH, Acs N, Bánhidy F. Delineation of a multiple congenital abnormality syndrome in the offspring of pregnant women affected with high fever‐related disorders: a population‐based study. Congenit Anom. 2008;48:158‐166. [DOI] [PubMed] [Google Scholar]
- 16. Germain MA, Webster WS, Edwards MJ. Hyperthermia as a teratogen ‐ parameters determining hyperthermia‐induced head defects in the rat. Teratology. 1985;31:265‐272. [DOI] [PubMed] [Google Scholar]
- 17. Kimmel CA, Cuff JM, Kimmel GL, et al. Skeletal development following heat exposure in the rat. Teratology. 1993a;47:229‐242. [DOI] [PubMed] [Google Scholar]
- 18. Skreb N, Frank Z. Developmental abnormalities in rat induced by heat shock. J Embryol Exp Morphol. 1963;11:445‐457. [PubMed] [Google Scholar]
- 19. Kimmel GL, Williams PL, Claggett TW, Kimmel CA. Response‐surface analysis of exposure‐duration relationships: the effects of hyperthermia on embryonic development of the rat in vitro. Toxicol Sci. 2002;69:391‐399. [DOI] [PubMed] [Google Scholar]
- 20. Mirkes PE, Fantel AG. Maternal influences on development In: Copp AJ, Cockroft DL, eds. Postimplantation Mammalian Embryos: A Practical Approach. Oxford, UK; New York: IRL Press; 1990:235‐248. [Google Scholar]
- 21. Tonk ECM, Robinson JF, Verhoef A, Theunissen PT, Pennings JL, Piersma AH. Valproic acid‐induced gene expression responses in rat whole embryo culture and comparison across in vitro developmental and non‐developmental models. Reprod Toxicol. 2013;41:57‐66. [DOI] [PubMed] [Google Scholar]
- 22. Kimmel GL, Cuff JM, Kimmel CA, Heredia DJ, Tudor N, Silverman PM. Embryonic‐development in vitro following short‐duration exposure to heat. Teratology. 1993b;47:243‐251. [DOI] [PubMed] [Google Scholar]
- 23. Mirkes PE, Doggett B. Accumulation of heat‐shock protein‐72 (HSP‐72) in postimplantation rat embryos after exposure to various periods of hyperthermia (40‐degrees‐43‐degrees‐c) in vitro‐evidence that heat‐shock protein‐72 is a biomarker of heat‐induced embryotoxicity. Teratology. 1992;46:301‐309. [DOI] [PubMed] [Google Scholar]
- 24. Mirkes PE. Heat‐shock response and hyperthermia‐induced teratogenesis in rat embryos. Teratology. 1985;31:A30. [Google Scholar]
- 25. Walsh DA, Klein NW, Hightower LE, Edwards MJ. Heat‐shock and thermotolerance during early rat embryo development. Teratology. 1987;36:181‐191. [DOI] [PubMed] [Google Scholar]
- 26. Bulic‐Jakus F, Vlahovic M, Juric‐Lekic G, Crnek‐Kunstelj V, Šerman D. Gastrulating rat embryo in a serum‐free culture model: changes of development caused by teratogen 5‐azacytidine. Altern Lab Anim. 1999;27:25‐933. [DOI] [PubMed] [Google Scholar]
- 27. Skreb N, Bulic‐Jakus F, Crnek V, Stepic J, Vlahovic M. Differentiation and growth of rat egg‐cylinders cultured in vitro in a serum‐free and protein‐free medium. Int J Dev Biol. 1993;37:151‐154. [PubMed] [Google Scholar]
- 28. Steele CE, New DAT. Serum variants causing formation of double hearts and other abnormalities in explanted rat embryos. J Embryol Exp Morphol. 1974;31:707‐719. [PubMed] [Google Scholar]
- 29. Bulic‐Jakus F, Skreb N, Jurić‐Lekić G, Svajger A. Transferrin enhances lentoid differentiation in rat egg cylinders cultivated in a chemically defined medium. Int J Dev Biol. 1990;34:275‐279. [PubMed] [Google Scholar]
- 30. Skreb N, Crnek V. Development of embryo‐derived teratomas in vitro. Results Probl Cell Differ. 1980;11:283‐289. [DOI] [PubMed] [Google Scholar]
- 31. Muzic V, Bojanac AK, Juric‐Lekic G, et al. Epigenetic drug 5‐azacytidine impairs proliferation of rat limb buds in an organotypic model‐system in vitro. Croat Med J. 2013;54:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skreb N, Bulic F. Partial differentiation of rat egg cylinders in serum‐free and protein‐ free medium. Dev Biol. 1987;120:584‐586. [DOI] [PubMed] [Google Scholar]
- 33. Zorc M, Vraspir‐Porenta O, Zorc‐Pleskovic R, Radovanović N, Petrovic D. Apoptosis of myocytes and proliferation markers as prognostic factors in end‐stage dilated cardiomyopathy. Cardiovasc Pathol. 2003;12:36‐39. [DOI] [PubMed] [Google Scholar]
- 34. Serman L, Vlahovic M, Sijan M, et al. The impact of 5‐azacytidine on placental weight, glycoprotein pattern and proliferating cell nuclear antigen expression in rat placenta. Placenta. 2007;28:803‐811. [DOI] [PubMed] [Google Scholar]
- 35. Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE. Expression of nestin by neural cells in the adult rat and human brain. PLoS ONE. 2011;6:e18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seifert G, Budach V, Keilholz U, Wust P, Eggert A, Ghadjar P. Regional hyperthermia combined with chemotherapy in paediatric, adolescent and young adult patients: current and future perspectives. Radiat Oncol. 2016;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wessalowski R, Schneider DT, Mils O, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non‐testicular malignant germ‐cell tumours: an open‐label, non‐randomised, single‐institution, phase 2 study. Lancet Oncol. 2013;14:843‐852. [DOI] [PubMed] [Google Scholar]
- 38. Behrouzkia Z, Joveini Z, Keshavarzi B, et al. Hyperthermia: how can it be used? Oman Med J. 2016;31:89‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanamori S, Nishimura Y, Okuno Y, Horii N, Saga T, Hiraoka M. Induction of vascular endothelial growth factor (VEGF) by hyperthermia and/or an angiogenesis inhibitor. Int J Hyperthermia. 1999;15:267‐278. [DOI] [PubMed] [Google Scholar]
- 40. Kuhl NM, Rensing L. Heat shock effects on cell cycle progression. Cell Mol Life Sci. 2000;57:450‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharif‐Khatibi L, Kariminia A, Khoei S, Goliaei B. Hyperthermia induces differentiation without apoptosis in permissive temperatures in human erythroleukaemia cells. Int J Hyperthermia. 2007;23:645‐655. [DOI] [PubMed] [Google Scholar]
- 42. Ebert PS, Salcman M. Differentiation therapy is potentiated by chemotherapy and hyperthermia in human and canine brain‐tumor cells in‐vitro. Neurosurgery. 1994;34:657‐664. [DOI] [PubMed] [Google Scholar]
- 43. Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol. 2003;548:837‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yun BG, Matts RL. Differential effects of HSP90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp Cell Res. 2005;307:212‐223. [DOI] [PubMed] [Google Scholar]
- 45. Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamanga‐Sollo E, Pampusch MS, White ME, Hathaway MR, Dayton WR. Effects of heat stress on proliferation, protein turnover, and abundance of heat shock protein messenger ribonucleic acid in cultured porcine muscle satellite cells. J Anim Sci. 2011;89:3473‐3480. [DOI] [PubMed] [Google Scholar]
- 47. Lee J, Mirkes PE, Paik DJ, Kim WK. Effects of maternal hyperthermia on myogenesis‐related factors in developing upper limb. Birth Defects Res A Clin Mol Teratol. 2009;85:184‐192. [DOI] [PubMed] [Google Scholar]
- 48. Davidson SM, Loones MT, Duverger O, Morange M. The developmental expression of small HSP. Prog Mol Subcell Biol. 2002;28:103‐128. [DOI] [PubMed] [Google Scholar]
- 49. Ruell PA, Hoffman KM, Chow CM, Thompson MW. Effect of temperature and duration of hyperthermia on HSP72 induction in rat tissues. Mol Cell Biochem. 2004;267:187‐194. [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Jiang M, Duan D, et al. Hyperthermia‐conditioned OECs serum‐free‐conditioned medium induce NSC differentiation into neuron more efficiently by the upregulation of HIF‐1 alpha and binding activity. Transplantation. 2014;97:1225‐1232. [DOI] [PubMed] [Google Scholar]
- 51. Dewey Retired WC, Diederich CJ. Hyperthermia classic commentary: “Arrhenius relationships from the molecule and cell to the clinic”. Int J Hyperth. 1994;10:457‐483. [DOI] [PubMed] [Google Scholar]
- 52. Walsh D, Li K, Wass J, et al. Heat‐shock gene‐expression and cell‐cycle changes during mammalian embryonic‐development. Dev Genet. 1993;14:127‐136. [DOI] [PubMed] [Google Scholar]
- 53. Hunt CR, Pandita RK, Laszlo A, et al. Hyperthermia activates a subset of ataxia‐telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010‐3017. [DOI] [PubMed] [Google Scholar]
- 54. Padmanabhan R, Al‐Menhali NM, Ahmed I, Kataya HH, Ayoub MA. Histological, histochemical and electron microscopic changes of the placenta induced by maternal exposure to hyperthermia in the rat. Int J Hyperthermia. 2005;21:29‐44. [DOI] [PubMed] [Google Scholar]
- 55. Breen JG, Claggett TW, Kimmel GL, Kimmel CA. Heat shock during rat embryo development in vitro results in decreased mitosis and abundant cell death. Reprod Toxicol. 1999;13:31‐39. [DOI] [PubMed] [Google Scholar]
- 56. Song AS, Najjar AM, Diller KR. Thermally induced apoptosis, necrosis, and heat shock protein expression in three‐dimensional culture. J Biomech Eng. 2014;136:071006‐1‐10 10.1115/1.4027272. [DOI] [PubMed] [Google Scholar]
- 57. Garcia MP, Cavalheiro JRT, Fernandes MH. Acute and long‐term effects of hyperthermia in B16‐F10 melanoma cells. PLoS ONE. 2012;7:e35489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperthermia. 2003;19:295‐324. [DOI] [PubMed] [Google Scholar]
- 59. Lin PP, Hahn GM. Growth‐factors and hyperthermia. II. Viability of Chinese‐hamster ovary HA‐1 cells during serum starvation and hyperthermia. Radiat Res. 1988;113:513‐525. [PubMed] [Google Scholar]
- 60. Velazquez JM, Lindquist S. HSP70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984;36:655‐662. [DOI] [PubMed] [Google Scholar]
- 61. Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829‐837. [DOI] [PubMed] [Google Scholar]
- 62. Olexikova L, Makarevich AV, Pivko J, Chrenek P. Antibody to HSP70 alters response of rabbit preimplantation embryos to hyperthermia in vitro. Anim Reprod Sci. 2010;119:130‐136. [DOI] [PubMed] [Google Scholar]
- 63. Gaspar N, Sharp SY, Eccles SA, et al. Mechanistic evaluation of the novel HSP90 inhibitor NVP‐AUY922 in adult and pediatric glioblastoma. Mol Cancer Ther. 2010;9:1219‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mendillo ML, Santagata S, Koeva M, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS ONE. 2014;9:e96330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shevtsov M, Multhoff G. Heat shock protein‐peptide and HSP‐based immunotherapies for the treatment of cancer. Front Immunol. 2016;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu T, Tanguay RM. Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones. 2006;11:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cockroft DL, New DAT. Abnormalities induced in cultured rat embryos by hyperthermia. Teratology. 1978;17:277‐283. [DOI] [PubMed] [Google Scholar]
- 69. Crnek‐Kunstelj V, Stipié J, Stipié‐Marković A. Differentiation in embryo‐derived teratomas in vitro can be mediated by retinoic acid and dibutyryl‐Camp. Libri Oncologici. 2001;29:133‐141. [Google Scholar]
- 70. Morrissey JJ, Higashikubo R, Goswami PC, Dixon P. Mild hyperthermia as a potential mechanism to locally enhance cell growth kinetics. J Drug Target. 2009;17:719‐723. [DOI] [PubMed] [Google Scholar]
- 71. Fan GC. Role of Heat Shock Proteins in Stem Cell Behavior. Prog. Mol. Biol. Trans. Sci. 2012;111:305‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mackey MA, Morgan WF, Dewey WC. Nuclear fragmentation and premature chromosome condensation induced by heat‐shock in S‐phase Chinese‐hamster ovary cells. Cancer Res. 1988;48:6478‐6483. [PubMed] [Google Scholar]
- 73. Cuff JM, Kimmel GL, Kimmel CA, Heredia DJ, Tudor N, Chen J. Relationship between abnormal somite development and axial skeletal defects in rats following heat exposure. Teratology. 1993;48:259‐266. [DOI] [PubMed] [Google Scholar]
- 74. Hutson MR, Keyte AL, Hernández‐Morales M, et al. Temperature‐activated ion channels in neural crest cells confer maternal fever–associated birth defects. Sci Signal. 2017;10:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yamaguchi T, Suzuki T, Arai H, Tanabe S, Atomi Y. Continuous mild heat stress induces differentiation of mammalian myoblasts, shifting fiber type from fast to slow. Am J Physiol Cell Physiol. 2010;298:C140‐C148. [DOI] [PubMed] [Google Scholar]
- 76. Ades L, Guerci A, Raffoux E, et al. Very long‐term outcome of acute promyelocytic leukemia after treatment with all‐trans retinoic acid and chemotherapy: the EuropeanAPL Group experience. Blood. 2010;115:1690‐1696. [DOI] [PubMed] [Google Scholar]
- 77. Muneeb A, Kumar G, Gourevitch S. Radiofrequency ablation (RFA) induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int J Hyperthermia. 2018;9:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]


