Abstract
Background
We aimed to study the continence between intermediate and high-risk cancer patients and the influential factors to recover continence.
Materials and methods
In total, 655 patients underwent surgery by robot-assisted radical prostatectomy between 2010 and 2015. Of 655 patients, 294 were classified according to D'Amico risk groups as intermediate risk or high risk and completed the micturition protocol. Patients with intermediate risk were matched in a 1:1 ratio to patients with high risk for age and body mass index. Urine loss ratio (ULR) was defined as urine loss divided by micturition volumes. Immediate continence was defined with the best cut-off value of ULR.
Results
In total, 117 patients with intermediate risk were matched to those with high risk. The comparison did not show any statistically significant difference in the ULR value (P = 0.359) or continence rate (P = 0.449). Predictive analysis was performed for the 294 patients (intermediate and high risk), of which 9.5% were classified as incontinent (>1 pad/d). Immediate continence was defined as ULR < 0.049 in 232 (78.9%) patients. Age, preoperative hemoglobin, and duration of catheterization were found influent by univariate analysis. Only age [odds ratio (OR) = 1.072; 95% confidence interval (CI) = 1.020–1.127; P = 0.006] and duration of catheterization (OR = 1.060; 95% CI = 1.003–1.120; P = 0.040) were independent influential factors to predict immediate continence.
Conclusion
D'Amico intermediate- and high-risk groups do not differ in continence terms. The ULR value of < 0.049 identifies those patients who recover continence earlier. Age and duration of catheterization were influential factors in predicting immediate continence.
Keywords: Predictive value of tests, Prostatectomy, Prostatic neoplasm, Urinary incontinence
1. Introduction
Prostate cancer is one of the most prevalent solid cancer in men, which is treated by surgery or radiation therapy.1 Nowadays, D'Amico low-risk patients can also start a program of active surveillance, receive treatment by focal therapy, brachytherapy, or minimally-invasive radical prostatectomy (RP).1, 2 The benefit of radical treatment is in doubt.3 In this scenario, surgery has taken advantage for D'Amico intermediate- or high-risk patients. RP has undergone an evolution over time from retropubic to laparoscopic and finally to robot assisted RP (RARP). All techniques have changed with the aim to improve outcomes; however, urinary incontinence (UI) is still a secondary effect.4, 5 UI may appear in 4–31% cases after surgery and reduce the quality of life.6 Identifying those patients who will have difficulties in recovering continence would be useful for the physician, since patients frequently ask for incontinence outcome. In 2006, a new parameter was introduced to address this question: the urine loss (UL) volume.7 In 2007, it was reported that UL ratio (ULR) parameter predicts time to continence.8 Continuing with the micturition protocol of this study, we aimed to study the continence between intermediate- and high-risk cancer patients and the influential factors to recover continence.
2. Materials and methods
2.1. Study design
The study was a non-randomized and non-placebo study with retrospective view of prospective recorded data.
Between 2010 and 2015, a total of 655 patients underwent surgery by RARP, performed by one surgeon. Patients were diagnosed after transrectal prostate biopsy if elevated levels of prostate-specific antigen and/or suspicious digital rectal examination results were found, and then classified according to D'Amico risk groups as low risk, intermediate risk, or high risk. Surgical treatment was performed using Heilbronn technique9 with or without unilateral or bilateral nerve sparing technique (NST), bladder neck–sparing procedure, and Van Velthoven anastomosis technique.10 Surgical techniques did not differ between risk groups, except in selected cases.
A cystogram was performed 5 days after the surgery, in case of no leakage, the urethral catheter was removed. If leakage, the cystogram was repeated at 7 days or 10 days after surgery. If there was urine debit through the drainage during immediate surgical recovery, the cystogram was performed in the absence of leakage. Difficult cases with blood loss, increased anastomosis time or difficulty, evidence of slight leakage during surgery, or urine debit in drainage were the main reasons to maintain the urethral catheter.
A total of 294 patients completed the micturition protocol and were suitable for inclusion. These 294 patients were classified as intermediate risk and 224 patients as high risk. Furthermore, 117 patients with intermediate risk were matched in a 1:1 ratio to patients with high risk for age and body mass index (BMI).
2.2. UI
Continence status was evaluated after 12 months by a self-administered modified International Continence Society (ICS) questionnaire by mail. Incontinence status was defined as the need for more than one pad/day after 12 months of recovery.
2.3. Micturition protocol
The protocol was performed 24 hours after removing the catheter. A 24-hour modified pad test was performed to measure UL. The micturition volumes were collected, and ULR was calculated on the last day of the patient's hospital stay. ULR was defined as UL divided by micturition volumes. Immediate continence was defined with the best cut-off value of ULR.
2.4. Data analysis
Clinical characteristics of patients were collected and results stated in absolute value or percentage.
Matched-pair analysis was performed manually choosing controls depending on the match criteria (age and BMI). To test the normality of the distribution, Shapiro–Wilk test was performed. A comparison between patient characteristics of matched groups was performed by Student t test or Mann–Whitney U test for mean comparison, or by Pearson's Chi-square test or Fisher exact test. The best cut-off value of ULR was obtained by the minimum description length principle method and confirmed by a sensitivity/1-specificity chart.11
Univariate and multivariate logistic regression analyses were performed to identify the influential variables in predicting immediate continence.
All data were collected prospectively in a specific database (Microsoft Excel Version 14. 2010. Microsoft, Washington, USA). All statistical analyses were performed using IBM SPSS Statistics for MAC version 21.0 (IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered to be statistically significant.
3. Results
Patients characteristics of all patients included in analysis are shown in Table 1. The matched population is divided into intermediate- and high-risk groups according to age and BMI. All continuous variables obtained a P value < 0.05 by Shapiro–Wilk test, implying a non-normal distribution.
Table 1.
Patient characteristics of the study
| Matched population |
P | |||
|---|---|---|---|---|
| Intermediate risk | High risk | |||
| No. of patients (%) | 294 (100%) | 117 (39.7%) | 117 (39.7%) | – |
| Age (y) | 0.851 | |||
| Mean | 65 | 65.37 | 65.53 | – |
| Range | 44–80 | 45–77 | 46–76 | – |
| Body mass index (kg/m2) | 0.820 | |||
| Mean | 26.97 | 26. 7 | 26.8 | – |
| Range | 16.59–37.74 | 16.59–37.74 | 18.7–37.74 | – |
| TURS volume (cc) | 0.286 | |||
| Mean | 40.57 | 39.78 | 43.09 | – |
| Range | 10–150 | 10–135 | 10–150 | – |
| Prior TURP | 20 (6.8%) | 8 (6.8%) | 7 (6.8%) | – |
| DRE abnormal | 175 (59.5%) | 49 (41.9%) | 93 (79.5%) | 0.79 |
| PSA (ng/mL) | ||||
| Median | 13.53 | 8 | 19.5 | – |
| Range | 1–425 | 1.2–19.2 | 1.8–425 | – |
| D'Amico risk categories | ||||
| Intermediate risk | 146 (49.7%) | – | – | |
| High risk | 148 (50.3%) | – | – | |
| NST | ||||
| Bilateral | 256 (87.1%) | 115 (98.3%) | 106 (90.6%) | 0.019 |
| Surgical time (min) | ||||
| Mean | 217 | 217 | 218.5 | 0.644 |
| Range | 104–500 | 104–500 | 50–2000 | – |
| Estimated blood loss (mL) | ||||
| Mean | 485 | 485 | 485 | 0.446 |
| Range | 50–2000 | 50–2000 | 50–2000 | – |
| Pathological stage | ||||
| T2 | 153 (52%) | 72 (61%) | 53 (46.1%) | – |
| T3a | 70 (23.8%) | 27 (23.1%) | 24 (20.9%) | – |
| T3b | 64 (21.8%) | 14 (12%) | 38 (33%) | – |
| Pathological Gleason | ||||
| 2–6 | 29 (10.3%) | 11 (9.4%) | 12 (10.5%) | – |
| 7 | 201 (71.3%) | 92 (78.6%) | 73 (64%) | – |
| 8–10 | 52 (18.4%) | 7(6%) | 29 (25.4%) | – |
| Catheterization time (d) | ||||
| Mean | 9.21 | 9.48 | 9.18 | 0.668 |
| Median | 7 | 7 | 7 | |
| Range | 5–35 | 5–29 | 5–35 | |
| ULR | ||||
| Mean | 0.04 | 0.0291 | 0.049 | |
| Median | 0.01 | 0.01 | 0.01 | 0.359 |
| Range | 0–0.49 | 0–0.25 | 0–0.49 | |
| Incontinence: | ||||
| >1 pad/d | 28 (9.5%) | 12 (8.2%) | 16 (10.8%) | 0.449 |
DRE, digital rectal examination; NST, nerve sparing technique; PSA, prostatic specific antigen; TURP, transurethral resection of the prostate; TURS, transrectal ultrasound; ULR, urine loss ratio.
No differences were found in prostate volume, transurethral resection of the prostate, time of surgery, and catheterization time between the groups. Only NST differed between the groups. After matching intermediate-risk patients with high risk patients, according to age and BMI, we did not find differences in terms of continence prevalence or ULR.
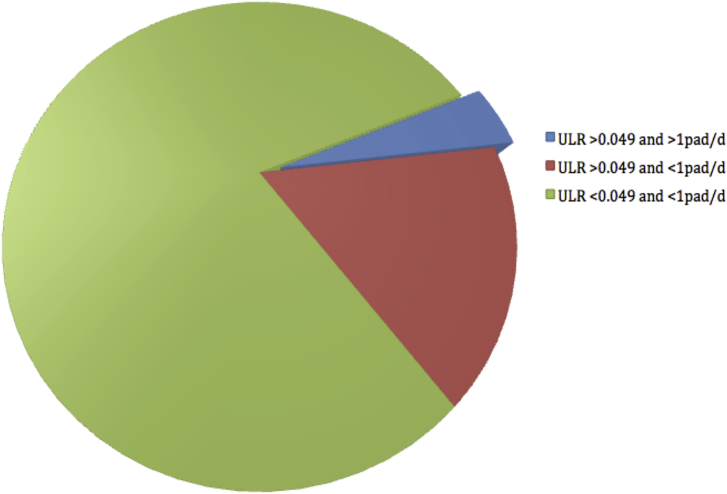
We continued the analysis with the whole population that completed the micturition protocol (n = 294). The best cut-off value of ULR was searched and matched with the previously reported value of 0.049 (P = 0.001).8 In total, 232 (78.9%) patients had ULR < 0.049 and were defined as immediate continent. Thus, 217 patients (93.5%) of immediate continent patients, 73.8% of the total, will use no pad or just one safety pad per day (Fig. 1, green color). Furthermore, 49 (79%) patients with ULR > 0.049, 16.6% of the total, will recover continence (Fig. 1, red color). Of those with ULR > 0.049, 20.9% will not recover continence, (positive predictive value) concerning to 13 patients, 4.4% of the total (Fig. 1, blue color). Finally 28 patients, 9.5% of the total, are classified as incontinent (> 1 pad/d), after 1 year, with a mean score of Incontinence Questionnaire-Short Form of 9.36 (range, 4–17).
Fig. 1.
Continence status according to urine loss ratio and definition (> 1pad/d). ULR, urine loss ratio.
The micturition protocol and ULR showed a negative predictive value of 93.5%. Clinical, surgical, and post-surgical characteristics were analyzed to predict immediate continence (ULR < 0.049). Table 2 shows a comparative analysis between immediate continence and the remainder.
Table 2.
Comparative analysis between groups
| ULR < 0.049 | ULR > 0.049 | P | |
|---|---|---|---|
| No. of patients (%) | 232 (79%) | 62 (21%) | – |
| Age (y) | 64.34 (± 7.08) | 67.6 (± 6.05) | 0.001 |
| Body mass index (kg/m2) | 27.16 (± 3.6) | 26.29 (± 3.5) | 0.097 |
| TURS volume(cc) | 40.75 (± 21.6) | 39.9 (± 24.3) | 0.792 |
| Prior TURP | 12 (6%) | 6 (9.7%) | 0.312 |
| DRE abnormal | 137 (60.6%) | 38 (62.3%) | 0.812 |
| PSA (ng/mL) | 12.43 (± 14.2) | 17.7 (± 54.6) | 0.453 |
| Hemoglobin (mg/mL) | 14.89 (± 1.03) | 14.4 (± 1.5) | 0.019 |
| D'Amico risk categories | |||
| Intermediate risk | 121 (82.8%) | 25 (17.2%) | – |
| High risk | 111 (75%) | 37 (25%) | – |
| NST | 219 (94%) | 57 (92%) | 0.47 |
| Surgical time (min) | 220 (± 53) | 207 (± 38) | 0.034 |
| Estimated blood loss (mL) | 492 (± 254) | 458 (± 277) | 0.410 |
| Pathological stage | 0.5 | ||
| T2 | 124 (55%) | 29 (47%) | – |
| T3a | 53 (23%) | 17 (27%) | – |
| T3b | 48 (21%) | 16 (26%) | – |
| Pathological Gleason | 0.72 | ||
| 2–6 | 22 (10%) | 7 (11.5%) | – |
| 7 | 160 (72%) | 41 (67.2%) | – |
| 8–10 | 39 (17.6%) | 13 (21.3%) | – |
| Catheterization time | 8.8 (± 4.7) | 10.5 (± 6.3) | 0.026 |
| Incontinence, >1 pad/d | 15 | 13 | 0.449 |
DRE, digital rectal examination; NST, nerve sparing technique; PSA, prostatic specific antigen; TURP, transurethral resection of the prostate; TURS, transrectal ultrasound; ULR, urine loss ratio.
With the factors that differ by groups, a univariate analysis was performed as shown in Table 3. We collected anastomosis time, with a mean of 17 minutes (range, 8–50 minutes) finding a weak but significant correlation between anastomosis time and catheterization days (r = 0.124; P = 0.04). Additionally, we found a correlation between age and hemoglobin (r = –0.199; P = 0.001).
Table 3.
Logistic regression in group
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.081 | 1.031–1.133 | 0.001 | 1.072 | 1.020–1.127 | 0.006 |
| Hemoglobin | 1.081 | 1.031–1.133 | 0.001 | – | – | 0.186 |
| Surgical time | 0.995 | 0.989–1.001 | 0.079 | – | – | – |
| Catheterization time | 1.056 | 1.005–1.109 | 0.030 | 1.060 | 1.003–1.120 | 0.040 |
CI, confidence interval; OR, odds ratio.
A multivariate analysis was performed with variables such as age, preoperative hemoglobin, and duration of catheterization. Only age and duration of catheterization were independently influential to predict immediate continence.
4. Discussion
UI has been one of the biggest fears since prostate cancer started to be treated by surgery.12 Since the appearance of active surveillance and focal therapies, surgical treatment for low-risk patients is discussed.1, 2 Moreover, there is a current debate regarding the use of surgical or radiation therapy treatment for high-risk patients, and one reason is the risk of secondary effects.13, 14 Our first aim was to compare the probability of incontinence between D'Amico intermediate- and high-risk patients. We decided to design a matched-pair study, and according to the last review, adjusted by age, one of the most influential factors, and BMI.6
The definition of UI we used was “the use of more than one safety pad per day”. This definition has been previously used by Wille et al15, Greco et al16, Murphy et al17, and Samadi et al18. However, ICS made a report of standardization of terms including a more strict definition of UI, “the complaint of any involuntary loss of urine”, which implies a higher prevalence.19
Although the incidence rate could vary a lot, the rates of incontinent patients matched with other series.6, 20 As reported, we did not find differences between D'Amico intermediate- and high-risk patients. This is an important concern because patients with high-risk prostate cancer could benefit from surgery without compromising continence. We only adjusted by age and BMI, but the analysis shows that adjusting by prostate volume would estimate the same result (P = 0.792).
The ULR value obtained by the micturition protocol is very useful to confirm the continence status due to a negative predictive value of 93.5%; remarkably, only 20.9% of those patients with a value of ULR > 0.049 will use more than one pad per day.
Second, we aimed to identify the factors that may predict immediate incontinence in D'Amico intermediate- or high-risk patients. We obtained a cut-off value of ULR of 0.049 (P = 0.001). This value matched with the first study performed. This study was performed with patients who underwent laparoscopic RP and studied the ULR value at the 1st day after the removal of catheter, obtaining the best value of 15% (0.15), and at the last day obtaining a value of 0.049, reporting an incontinence rate of 10.7%.8
Age, preoperative hemoglobin, surgical time, and catheterization time varied between immediate continence patients and the remainder. Age is a well-known factor.12, 21, 22, 23 Increasing age plays an important role in the recovery of continence but also in previous urinary status. We found differences in preoperative hemoglobin; initially, it was surprising, but hemoglobin levels reportedly decreased by increasing age.24, 25 We also found a significant, although weak, correlation in our study (r = –0.199; P = 0.001). The surgical time is not easy to understand and could be influential because of the difficulty of dissection. Instead, we did not find any influence of the blood loss over UI.26
Finally, some studies have reported catheterization as an influential factor. In 2003, Koch et al27 reported that an earlier removal of the catheter proved favorable for continence rates without complications; in 2011, Gacci et al28 found that the catheterization time significantly influenced in multivariate analysis.
On the other hand, we did not found influence of BMI or prostate volume. NST was neither influence over continence, but we have to notice that there were no differences between continent and incontinent in rate of NST (94% vs 92%, P = 0.47). Other series have found it in them analysis as a very influential factor.29, 30
In 2006, Saito et al7 analyzed the UL volume, thereby finding a relationship with UI. Similarly, in 2009, Kampen et al31 reported that the most important predictive factor of UI was UL after catheter withdrawal on Day 1.
The present work has some limitations. We excluded all low-risk patients due to the study design. The applicability of the results could be limited because some centers could treat low-risk patients by surgery. The definition of incontinence should be taken into account because changes on the definition could make the results not comparable. The number of patients was limited. Furthermore, this is a prospective study that started in 2010, and it is the first study that deals with ULR using robot-assisted approach.
To conclude, D'Amico-classified intermediate- and high-risk patients do not differ in continence terms. A ULR value of < 0.049 identified those patients who recover continence earlier with a negative predictive value of 93.5%. Age and duration of catheterization were found influential in predicting immediate continence.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
The present work has been possible with the help of the EAU and EUSP (European Urological Scholarship program).
References
- 1.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L., Emberton M. Management of low risk prostate cancer-active surveillance and focal therapy. Nat Rev Clin Oncol. 2014;11:324–334. doi: 10.1038/nrclinonc.2014.73. [DOI] [PubMed] [Google Scholar]
- 3.Wilt T.J., Jones K.M., Barry M.J., Andriole G.L., Culkin D., Wheeler T. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;13(377):132–142. doi: 10.1056/NEJMoa1615869. [DOI] [PubMed] [Google Scholar]
- 4.Son S.J., Lee S.C., Jeong C.W., Jeong S.J., Byun S.S., Lee S.E. Comparison of continence recovery between robot-assisted laparoscopic prostatectomy and open radical retropubic prostatectomy: a single surgeon experience. Korean J Urol. 2013;54:598–602. doi: 10.4111/kju.2013.54.9.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ficarra V., Novara G., Artibani W., Cestari A., Galfano A., Graefen M. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Ficarra V., Novara G., Rosen R.C., Artibani W., Carroll P.R., Costello A. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–417. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Saito S., Namiki S., Numahata K., Satoh M., Ishidoya S., Ito A. Relevance of postcatheter removal incontinence to postoperative urinary function after radical prostatectomy. Int J Urol. 2006;13:1191–1196. doi: 10.1111/j.1442-2042.2006.01529.x. [DOI] [PubMed] [Google Scholar]
- 8.Ates M., Teber D., Gozen A.S., Tefekli A., Hruza M., Sugiono M. A new postoperative predictor of time to urinary continence after laparoscopic radical prostatectomy: the urine loss ratio. Eur Urol. 2007;52:178–185. doi: 10.1016/j.eururo.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Rassweiler J., Marrero R., Hammady A., Erdogru T., Teber D., Frede T. Transperitoneal laparoscopic radical prostatectomy: ascending technique. J Endourol. 2004;18:593–599. doi: 10.1089/end.2004.18.593. –600. [DOI] [PubMed] [Google Scholar]
- 10.Van Velthoven R.F., Ahlering T.E., Peltier A., Skarecky D.W., Clayman R.V. Technique for laparoscopic running urethrovesical anastomosis:the single knot method. Urology. 2003;61:699–702. doi: 10.1016/s0090-4295(02)02543-8. [DOI] [PubMed] [Google Scholar]
- 11.Fayyad U.M., Irani K. Proceedings of the 13th International Joint Conference on Artificial Intellingence. Nagoya; 1993. Multi-interval discretization of continuous-valued attributes for classification learning; pp. 1022–1027. [Google Scholar]
- 12.Bauer R.M., Bastian P.J., Gozzi C., Stief C.G. Postprostatectomy incontinence: all about diagnosis and management. Eur Urol. 2009;55:322–333. doi: 10.1016/j.eururo.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Petrelli F., Vavassori I., Coinu A., Borgonovo K., Sarti E., Barni S. Radical prostatectomy or radiotherapy in high-risk prostate cancer: a systematic review and metaanalysis. Clin Genitourin Cancer. 2014;12:215–224. doi: 10.1016/j.clgc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Abern M.R., Terris M.K., Aronson W.J., Kane C.J., Amling C.L., Cooperberg M.R. The impact of pathologic staging on the long-term oncologic outcomes of patients with clinically high-risk prostate cancer. Cancer. 2014;1(120):1656–1662. doi: 10.1002/cncr.28647. [DOI] [PubMed] [Google Scholar]
- 15.Wille S., Heidenreich A., von Knobloch R., Hofmann R., Engelmann U. Impact of comorbidities on post-prostatectomy incontinence. Urol Int. 2006;76:223–226. doi: 10.1159/000091623. [DOI] [PubMed] [Google Scholar]
- 16.Greco K.A., Meeks J.J., Wu S., Nadler R.B. Robot-assisted radical prostatectomy in men aged > or =70 years. BJU Int. 2009;104:1492–1495. doi: 10.1111/j.1464-410X.2009.08718.x. [DOI] [PubMed] [Google Scholar]
- 17.Murphy D.G., Kerger M., Crowe H., Peters J.S., Costello A.J. Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol. 2009;55:1358–1366. doi: 10.1016/j.eururo.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Samadi D.B., Muntner P., Nabizada-Pace F., Brajtbord J.S., Carlucci J., Lavery H.J. Improvements in robot-assisted prostatectomy: the effect of surgeon experience and technical changes on oncologic and functional outcomes. J Endourol. 2010;24:1105–1110. doi: 10.1089/end.2010.0136. [DOI] [PubMed] [Google Scholar]
- 19.Abrams P., Cardozo L., Fall M., Griffiths D., Rosier P., Ulmsten U. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 20.Wei J.T., Dunn R.L., Marcovich R., Montie J.E., Sanda M.G. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164:744–748. doi: 10.1097/00005392-200009010-00029. [DOI] [PubMed] [Google Scholar]
- 21.Campodonico F., Manuputty E.E., Campora S., Puntoni M., Maffezzini M. Age is predictive of immediate postoperative urinary continence after radical retropubic prostatectomy. Urol Int. 2014;92:276–281. doi: 10.1159/000353414. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson A.E., Schumacher M.C., Johansson E., Carlsson S., Stranne J., Nyberg T. Age at surgery, educational level and long-term urinary incontinence after radical prostatectomy. BJU Int. 2011;108:1572–1577. doi: 10.1111/j.1464-410X.2011.10231.x. [DOI] [PubMed] [Google Scholar]
- 23.Barry M.J., Gallagher P.M., Skinner J.S., Fowler F.J. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol. 2012;10(30):513–518. doi: 10.1200/JCO.2011.36.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson-Ehle H., Jagenburg R., Landahl S., Svanborg A. Blood haemoglobin declines in the elderly: implications for reference intervals from age 70 to 88. Eur J Haematol. 2000;65:297–305. doi: 10.1034/j.1600-0609.2000.065005297.x. [DOI] [PubMed] [Google Scholar]
- 25.Zakai N.A., French B., Arnold A.M., Newman A.B., Fried L.F., Robbins J. Hemoglobin decline, function, and mortality in the elderly: the cardiovascular health study. Am J Hematol. 2013;88:5–9. doi: 10.1002/ajh.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djavan B., Agalliu I., Laze J., Sadri H., Kazzazi A., Lepor H. Blood loss during radical prostatectomy: impact on clinical, oncological and functional outcomes and complication rates. BJU Int. 2012;110:69–75. doi: 10.1111/j.1464-410X.2011.10812.x. [DOI] [PubMed] [Google Scholar]
- 27.Koch M.O., Nayee A.H., Sloan J., Gardner T., Wahle G.R., Bihrle R. Early catheter removal after radical retropubic prostatectomy: long-term followup. J Urol. 2003;169:2170–2172. doi: 10.1097/01.ju.0000065860.16392.19. [DOI] [PubMed] [Google Scholar]
- 28.Gacci M., Carini M., Simonato A., Imbimbo C., Gontero P., Briganti A. Factors predicting continence recovery 1 month after radical prostatectomy: results of a multicenter survey. Int J Urol. 2011;18:700–708. doi: 10.1111/j.1442-2042.2011.02826.x. [DOI] [PubMed] [Google Scholar]
- 29.Burkhard F.C., Kessler T.M., Fleischmann A., Thalmann G.N., Schumacher M., Studer U.E. Nerve sparing open radical retropubic prostatectomy–does it have an impact on urinary continence? J Urol. 2006;176:189–195. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 30.Suardi N., Moschini M., Gallina A., Gandaglia G., Abdollah F., Capitanio U. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2013;111:717–722. doi: 10.1111/j.1464-410X.2012.11315.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Kampen M., Geraerts I., De Weerdt W., Van Poppel H. An easy prediction of urinary incontinence duration after retropubic radical prostatectomy based on urine loss the first day after catheter withdrawal. J Urol. 2009;181:2641–2646. doi: 10.1016/j.juro.2009.02.025. [DOI] [PubMed] [Google Scholar]