Abstract
Ameloblastoma is a histologically benign but locally aggressive tumor of the jaws. Accurate preoperative imaging facilitates complete surgical resection with clear margins for long term cure. Adjuvant radiotherapy is effective for microscopic positive margins. The aim of this study was to review clinical, radiological and pathological features of the patients with ameloblastoma of the mandible and maxilla and report outcomes of treatment. A retrospective cohort study was performed on a consecutive series of 30 patients who had their primary treatment of ameloblastoma of the mandible or maxilla, at Memorial Sloan Kettering Cancer Center between 1987 and 2013. Data was collected on age, sex, clinical and imaging findings, management, histologic patterns, surgical margins, length of follow-up, time to recurrence, and treatment of recurrence. Factors impacting upon recurrence, and the role of radiotherapy were also studied. The gender and site distribution (mandible / maxilla) were equal. All but two patients, with negative margins were cured. Adjuvant post operative radiotherapy offered local control in all patients at high risk of recurrence, or with positive margins. Complete excision with negative margins remains the mainstay of curative treatment. Post operative radiotherapy is effective in preventing recurrence in patients with microscopic positive margins
Introduction
Ameloblastoma is the most common benign tumor of the jaws, comprising 1% of all cysts and tumors of the jaws and approximately 10% of odontogenic tumors (1). Malassez in 1885 first described ameloblastoma, suggesting that it arises from epithelial remnants of the developing root sheath (2, 3). However, the exact genesis of ameloblastoma largely remains unknown. The question of whether ameloblastoma originates in dental cysts or whether ameloblastoma becomes cystic would appear to be not resolvable without delineation of precise diagnostic criteria concerning ameloblastoma and dental cysts. Ameloblastoma is classically described as a locally aggressive benign tumor, but it has the potential to rarely metastasize to lymph nodes and even distant locations (4, 5). It is more common in the mandible (nearly 80%) and is often asymptomatic (2). The disease occurs at all ages with a peak incidence in the third and fourth decades of life (6). Various authors have shown an equal gender distribution. In Caucasians, over 50% of cases occur in the molar-ramus region of the mandible (7). Most mandibular lesions arise in the third molar region. However, a predilection for mandibular symphysis has been reported in Nigerians and Asian Indians (8). There are three classic varieties of ameloblastoma: solid/multicystic, unicystic and peripheral. In a review of 3677 cases, the solid/multicystic type comprised 92% of ameloblastomas, 6% were unicystic type, and 2% peripheral type (9).
The most common presenting symptom is a painless swelling. Other symptoms include, loosening of teeth, malocclusion and altered sensation in the teeth and occasionally pain. Local progression of the tumor, leads to progressive bone destruction, initially within the bone, but eventually the tumor breaks through the cortical bone and extends in to adjacent soft tissues. Although the tumor has characteristic radiological features, a biopsy is essential for accurate diagnosis (10). Surgical resection is the most definitive treatment; however the extent of surgery is often debated in the literature (11). In a previous report from our institution by Sehdev et al, complete resection by a segmental or marginal mandibulectomy was recommended, since local excision or curettage lead to a prohibitively high local recurrence rate (12). This view has been challenged in the literature, where a more conservative approach has been recommended by some for early stage, localized ameloblastomas (13).
The objective of this study was to review a contemporary series of patients with ameloblastomas of the mandible and maxilla treated at a tertiary care cancer center over a 26 year period, and report the clinical and radiological features, as well as pathological variants reflecting their biological behavior, treatments employed and long term outcomes.
Materials and Methods:
After obtaining Institutional Review Board approval, we performed a retrospective review of the records of 42 patients with the diagnosis of Ameloblastoma of the upper and lower jaw at Memorial Sloan Kettering Cancer Center between the years 1987 to 2013. Of these, 10 patients who received initial treatment elsewhere were excluded and an additional 2 patients were excluded, because on review of their pathology slides, their tumors were not true ameloblastomas. This left 30 patients eligible for study. Histological slides were available for review on 25 patients. All available slides were reviewed by a senior pathologist (RG), to confirm the diagnosis and classify into various histological variants. The remaining 5 patients were included in the study based on their final pathology report in medical record.
Details on clinical characteristics, pathology characteristics, treatment and outcomes were collected by retrospective chart review. We collected details on clinical presentation, symptoms, radiological features, type of surgery performed, status of the margins, adjuvant treatments employed, and follow up in the long term.
Statistical considerations:
This study is primarily descriptive, reporting a summary of our experience with ameloblastoma of the head and neck. Using the statistical software SPSS (Version 24.0), survival curves were generated by way of Kaplan Meier methods. Recurrence free survival was calculated as the time from date of surgery to date of recurrence or last disease assessment. Recurrences consisted of local, regional and distant events.
Results
The median age of patients in the study group was 61.5 years (range 19-81) and 15 were males. Majority of the patients (80%) were Caucasians, 2 were African Americans, and one each were Chinese, Indian and Filipino. The distribution of tumors was equal, with 15 patients with tumors in the mandible, and 15 in maxilla.
All patients underwent definitive surgery at our institution. The goal of the treatment was to achieve a complete resection. In spite of this, 5 patients had positive margins on final pathological analysis. Twenty-three had clear margins, and margin status was not reported in two patients. The details of treatment and outcomes are reported separately for mandible and maxilla.
Mandible
Details of patients with ameloblastoma of the mandible are shown in Table 1.
Table 1.
Clinical, pathology, treatment and outcomes details on mandibular ameloblastoma
| Gender | Location | Radiology | Pathology | Surgerical | Procedure in the neck | Reconstruction | Margins | Local recurrence | Follow ups (months) | Status at last follow up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Retromolar trigone | Solid | Acanthomatous | Segmental | Parotidectomy, infratemporal fossa dissection | Rectus abdominis | Positive | Yes (12 months) | 99 | NED |
| 2 | F | Posterior lower gum | Multicystic | Follicular | Segmental | Supraomohyoid | FFF | Negative | Yes(127 month after op) | 127 | AWD |
| 3 | M | Posterior lower gum | Unicystic/cortical | Follicular | Marginal | No | None | Negative | No | 0 | NED |
| 4 | F | Posterior lower gum | Multicystic | Follicular | Segmental | Supraomohyoid | FFF | Negative | No | 141 | NED |
| 5 | F | Posterior lower gum | Multicystic | Follicular | Segmental | Neck exploration | FFF | Negative | No | 196 | NED |
| 6 | M | Posterior lower gum | Multicystic | Follicular | Segmental | No | FFF | Negative | No | 61 | NED |
| 7 | F | Posterior lower gum | Multicystic | Follicular | Segmental | Supraomohyoid | FFF | Negative | No | 24 | NED |
| 8 | M | Posterior lower gum | Unicystic | Follicular | Marginal | No | None | Negative | No | 4 | NED |
| 9 | M | Posterior lower gum | Unicystic | Follicular | Segmental | Modified radical neck dissection | FFF | Negative | No | 66 | NED |
| 10 | F | Buccal mucosa | Peripheral | Acanthomatous | Excision | No | Skin graft from thigh | Positive | No | 5 | NED |
| 11 | M | Posterior lower gum | Multicystic | Plexiform | Segmental | Supraomohyoid | FFF | Negative | No | 16 | NED |
| 12 | F | Posterior lower gum | Unicystic | Follicular | Curettage | No | None | Negative(frag)* | No | 45 | NED |
| 13 | F | Posterior lower gum | Multicystic | Granular | Marginal | No | None | Negative | No | 24 | NED |
| 14 | M | Posterior lower gum | Multicystic | Follicular | Segmental | Neck exploration | FFF | Negative | No | 1 | NED |
| 15 | M | Posterior lower gum | Multicystic | Follicular | Segmental | No | FFF | Negative | No | 3 | NED |
Clinical presentation
All tumors were located in the molar region. Symptoms consisted of swelling, tenderness, or pain in 12 patients. The tumors in the remaining three were discovered incidentally. Previous unrelated interventions included, tooth extraction or root canal in 5.One patient had a history of odontogenic keratocyst, and one patient had two previous biopsies. Eight patients had no previous intervention.
Radiological findings
Computer tomography was performed preoperatively in all patients. However, the scans were not available for review in one patient. The most common diagnostic radiological feature was that of a “soap bubble” appearance in an expansile lesion (Figure 1).
Figure 1.
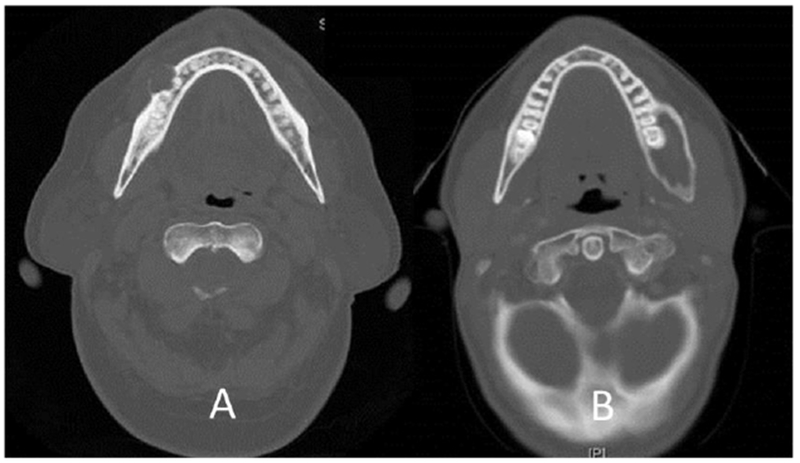
Unicystic ameloblastoma with cortical erosion (A) and Unicystic ameloblastoma with medullary expansion(B)
Surgical treatment
Surgical treatment consisted of segmental mandibulectomy in 10, marginal mandibulectomy in 3, peroral excision of a peripheral ameloblastoma in 1 and curettage in 1 patient. This patient was referred after a biopsy proven cystic ameloblastoma, in a cystic lesion measuring 6.7 × 4.6 cm, which appeared to arise from the coronoid process of the mandible and extending into the ascending ramus. Review of the pathology slides at our institution indicated it to be a benign dentigerous cyst. Therefore, curettage, of the cystic lesion was performed. However, the final pathology report showed it to be a unicystic ameloblastoma-follicular subtype. After discussion with the patient, it was decided to keep her under surveillance. The surgery was performed in 2007, and at last follow up in 2011, patient remains free of any evidence of recurrence. 4 patients had supraomohyoid neck dissection done at the same time, 1 had modified radical neck dissection, and one had parotidectomy and infratemporal fossa dissection. The surgical resection in 5 patients was performed intra orally.
All the patients undergoing segmental mandibulectomy had some type of immediate reconstruction of the mandible. Fibula free flap was used in 9 patients, one patient with extensive tumor extending to the infratemporal fossa had reconstruction with rectus abdominis free flap, and the remaining patient with a peripheral ameloblastoma in the cheek had only split thickness skin graft. Four patients, who had either per oral resection or marginal mandibulectomy, did not require any specific reconstructive procedure. No major post operative complications were observed.
Pathological findings
Pathology report showed eleven patients with (73% 11/15) follicular ameloblastoma, 2 had (13%) acanthomatous, 1 plexiform (7%) and 1 granular (7%) variety.12 patients (80%, 12/15) had negative surgical margins. Ten of the 12 had follicular subtype, and one each had granular and plexiform subtype. 2 patients (13%) had positive surgical margins; and both of them had acanthomatous subtype. Margin status was not reported in 1 patient. One patient with positive margin had advanced disease located in retromolar trigone involving both mandible and maxilla, with a 3.3X6.3 cm mass involving the medial pterygoid muscle with destruction of the posterior wall of the maxillary sinus, requiring resection in the infra temporal fossa. The other was a peripheral ameloblastoma in buccal mucosa. No immediate treatment was instituted for positive margins in this patient. One of these two patients developed local recurrence, and received post operative radiotherapy after re resection of local recurrence.
Outcomes
The Kaplan Meier plot for recurrence free survival is shown in Figure 3.
Figure 3.
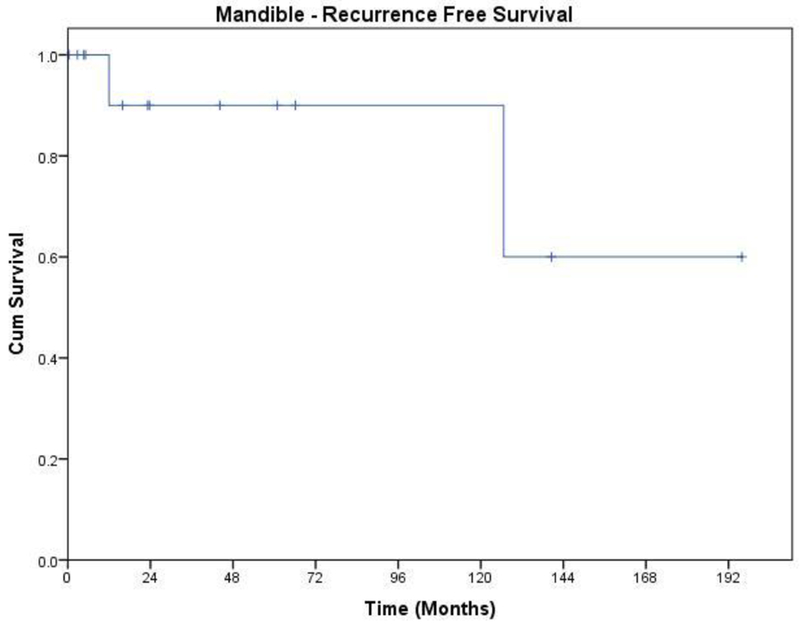
Recurrence free survival of patients with Ameloblastoma of the mandible
Two patients developed local recurrence and one of them also developed regional recurrence. The patient with loco regional recurrence developed it after a year from primary surgery. He had acanthomatous ameloblastoma and positive soft tissue margin. The other patient developed local recurrence ten years after initial treatment. She had follicular subtype and negative margins at initial operation. Her slides were not available for review. She received further treatment elsewhere, and is lost to follow up. Salvage treatment for the patient with loco regional recurrence consisted of per oral excision of local recurrence in conjunction with modified neck dissection, followed by post operative RT (6300cGy). He remained free of further recurrence for 7 years, and died of other causes.
Maxilla
Details of patients with ameloblastoma of the maxilla are shown in Table 2.
Table 2.
Clinical, pathology, treatment and outcomes details on ameloblastoma of maxilla
| Gender | Location | Radiology | Pathology | Surgery | Procedure in the neck | Reconstruction | Margins | Local recurrence | Follow ups (months) | Status at last follow up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Maxillary sinus and ethmoid | Solid | Basal | Partial maxillectomy | No | Obturator | Positive | No | 109 | NED |
| 2 | M | Posterior Upper gum | Unicystic | Basal | Partial maxillectomy | No | Obturator | Negative | No | 173 | NED |
| 3 | F | Hard palate, alveolus | Unicystic | Follicular | Maxillectomy | No | Obturator | Negative | No | 5 | NED |
| 4 | M | Maxillary antrum | Solid | Malignant | Maxillectomy | No | Obturator | Negative | No | 125 | NED |
| 5 | M | Upper alveolus | Multicystic | Malignant | Maxillectomy | No | Obturator | Negative | Distal | 209 | NED |
| 6 | F | Posterior upper gum | Unicystic | Desmoplastic | Partial maxillectomy | No | Obturator | Negative | No | 57 | NED |
| 7 | F | Maxillary sinus, nasal cavity | Solid | Follicular | Maxillectomy | No | Obturator | Positive | Local (4 times) | 229 | NED |
| 8 | F | Anterior hard palate, nasal cavity | Multicystic | Desmoplastic | Partial maxillectomy | No | Obturator | Positive | No | 34 | NED |
| 9 | M | Maxillary sinus | Solid/Multicystic | Plexiform | Partial maxillectomy | No | Obturator | Negative | No | 107 | NED |
| 10 | M | Maxillary sinus, retromolar trigone | Solid | Biphasic (plexiform and granular) | Partial maxillectomy | No | Obturator | Negative | No | 88 | NED |
| 11 | F | Posterior upper gum | Multicystic | Follicular | Partial maxillectomy | No | Obturator | Negative | No | 24 | NED |
| 12 | F | Maxillary sinus | Solid/Multicystic | Follicular | Partial maxillectomy | No | Obturator | Negative | No | 63 | NED |
| 13 | M | Posterior upper gum | Multicystic | Follicular | Partial maxillectomy | No | Obturator | NA | No | 17 | NED |
| 14 | M | Posterior upper gum | Multicystic | Follicular | Partial maxillectomy | No | Obturator | Negative | No | 17 | NED |
| 15 | F | Maxillary sinus | Solid | Folicular | Maxillectomy | No | Obturator | Negative | Local | 120 | NED |
Clinical presentation
There were 15 patients with ameloblastoma in the maxilla. Seven patients had their tumors located in the molar region, 6 presented with the bulk of the tumor in the maxillary sinus, and one each involved the hard palate and pre maxilla. 13 patients (87%) had symptoms consisting of loose teeth, swelling or nasal obstruction. The tumor was diagnosed incidentally in one patient. Six patients had previous interventions, which included, tooth extractions in 4 and one had endoscopic sinus surgery and the other had a Caldwell Luc procedure.
Radiological imaging
Nearly all patients with tumors in the maxilla had CT or MRI scans performed. The radiological features of these lesions were similar to those seen in the mandible, with a unicystic, multicystic / solid presentation (Figure 2).
Figure 2.
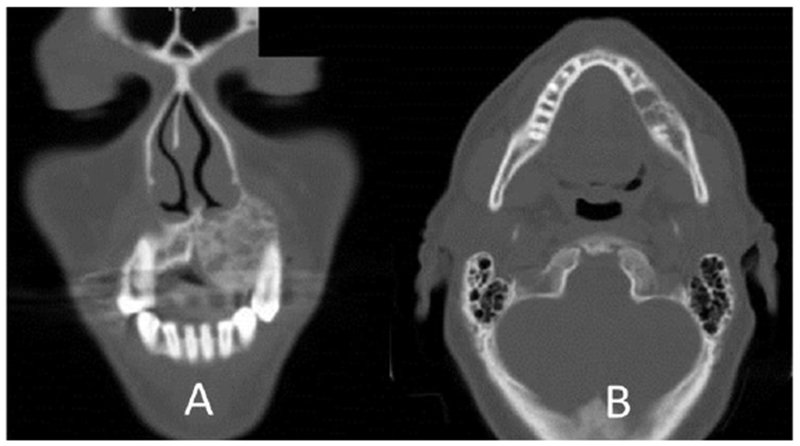
Multicystic / Solid ameloblastoma in maxilla (A) and mandible (B)
Surgical treatment
Surgical resections included partial maxillectomy in 10 patients (67%), and total maxillectomy in the remaining five. Rehabilitation of the maxillectomy defect required a prosthetic obturator in all.
Pathological findings
Review of the slides showed follicular ameloblastoma in 7 patients (47%), basaloid in two, desmoplastic in two (13%), and one each were plexiform (7%) and biphasic (plexiform and granular). Two patients showed ameloblastoma with malignant features. Surgical margins were negative in 11 patients (73% 11/15); five with follicular subtype, one each of the basal, biphasic, desmoplastic and plexiform variety. Both patients with ameloblastoma with malignant features had negative margins. Surgical margins were reported to be positive in 3 patients (one each were basaloid, follicular, and desmoplastic subtype). Information on surgical margins was not available in 1 patient.
Outcomes
The Kaplan Meier plot for recurrence free survival is shown in Figure 4. There were 3 recurrences (20% 3/15), 2 local and 1 distant. Both patients with local recurrence had a follicular variety requiring total maxillectomy with orbital preservation. One of these two had a positive surgical margin. The one with positive margin developed recurrence two years after primary surgery, and the one with negative margin 7 years after initial surgery. Patient with distant recurrence developed late solitary pulmonary metastasis, 13 years after maxillectomy. His primary location was left upper alveolus, and the primary tumor was a malignant ameloblastoma.
Figure 4.
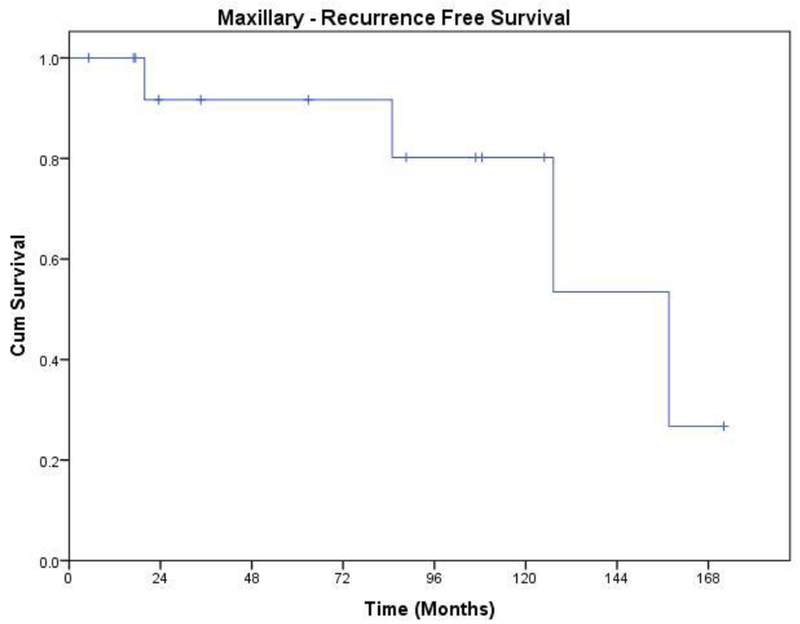
Recurrence free survival of patients with Ameloblastoma of the maxilla.
Salvage treatment in the patient with initial positive margin, consisted of excision of the local recurrence followed by post operative radiation therapy (5580 cGy). The other patient who developed recurrence after 7 years had only re-resection, and remains free of recurrence ten years following salvage surgery.
The other patient who developed solitary pulmonary metastasis had a lobectomy performed, and is alive with no evidence of disease at 20 years after primary surgery and seven years after lung resection.
Discussion
Ameloblastoma is a slow growing benign odontogenic tumor. Not much is known about the cause of ameloblastomas in either human beings or animals. Experimental induction of ameloblastomas has not been very successful (14) and has not helped clarify the pathogenesis. Nor has study of the only relatively common spontaneous animal model of ameloblastoma, the canine acanthomatous ameloblastoma (15). This lesion is known as the acanthomatous epulis in veterinary medicine but is basically an intraosseous lesion and not an epulis. The onset of this tumor in the human jaws is very insidious, and it may take years before development of symptoms. Often the tumor is diagnosed incidentally during routine dental examination.
Often patients present with the history of a painless swelling of the jaw, and occasionally with loose teeth. Clinical examination shows a submucosal mass arising from the underlying mandible or maxilla. The clinical differential diagnosis is usually between odontogenic cysts, and primary neoplasms of the mandible such as chondro or osteo sarcoma. Radiological evaluation is absolutely mandatory, for assessment of the features of the tumor and extent of bone involvement (10). Radiologically ameloblastomas can be unicystic, solid and/or multicystic, with cortical expansion or peripheral.
Occasionally they may be extra osseous; arising in the soft tissues around the jaws, from epithelial rests of the odontoma (10). Radiological studies, may involve a simple orthopantomogram, occlusal films, a CT scan or an MRI. A denta scan (CT scan) gives a very thorough assessment of the mandible, but CT scan with soft tissue and bone windows is best for assessment of the maxilla (16). Radiologically there are three classic varieties of ameloblastomas: solid/multicystic, unicystic and peripheral (extraosseous). The solid/multicystic and unicystic types have several histologic variants that can have different biological behavior. Reichart (9) conducted the biggest review of 3677 cases where solid/multicystic ameloblastoma comprised of 92% of cases, unicystic 6% and peripheral accounted for 2% In our series, 14 (47%) had multicystic lesions,7 were unicystic, 8 were solid/multicystic, and one patient had peripheral ameloblastoma. Sixteen patients, (53%), had extension of tumor outside of the bone into soft tissues.
Tissue diagnosis is crucial before embarking upon definitive treatment. Temptation to extract teeth and curettage should be avoided. An incisional biopsy harvesting adequate tissue from an area, which will be encompassed in definitive surgical resection, is desirable. Consultations with bone pathologists, and or oral pathologists with expertise in odontogenic lesions are advisable. Nearly all ameloblastomas are histologically benign, but may manifest locally aggressive behavior, with progressive bone destruction, and even extra osseous extension to adjacent soft tissues.
Histologically, ameloblastomas are classified into, a) follicular, the most common subtype of the solid/multicystic ameloblastoma, b) acanthomatous, characterized by extensive squamous metaplasia and keratin production; c) plexiform, d) granular cell, commonly seen in young patients and have an aggressive behavior; e) basal cell, and f) desmoplastic. Majority of patients in our study had follicular histologic pattern (60%), which was a predominant pattern in both mandible and maxilla. Acanthomatous, basal, desmoplastic, plexiform, and ameloblastoma with malignant features were present in two patients in each category. There was only one patient each with biphasic (plexiform and granular pattern) and granular variety. Locally aggressive behavior is often seen in follicular, granular and acanthomatous variants of ameloblastomas (17). However, in our series, from the 18 follicular ameloblastomas, two patients with the tumor in maxilla recurred after initial maxillectomy, requiring further surgery in one and surgery with radiotherapy in the other, and both remain alive without further recurrence. In addition there was one patient with follicular pattern in the mandible that developed local recurrence 10 years after segmental mandibulectomy. He received further treatment elsewhere, and is lost to follow up since. One of the two patients with advanced acanthomatous ameloblastoma of the mandible required extensive initial surgery, with parotidectomy and resection in the infratemporal fossa, with free flap reconstruction. He had positive margins of resection, and received post operative RT, and remains alive with disease at 8 years follow up. On rare occasions malignant transformation may take place in a benign ameloblastoma, presenting as an ameloblastic carcinoma. With malignant transformation, the risk of nodal metastases as well as distant metastases exists, and therefore appropriate pre operative evaluation in such cases is crucial for proper treatment planning (18). Occasionally, distant metastases have been reported from histologically benign ameloblastoma (19).Only two patients in our study had ameloblastoma with malignant features in their final pathology reports. One of them did develop pulmonary metastasis, thirteen years after initial surgery, underwent lobectomy, and remains free of disease for seven years since that surgery. However, these are extremely rare cases.
The term malignant ameloblastoma refers to a lesion with histologic similarity to conventional ameloblastoma, but it has metastasized. The ameloblastic carcinoma shows some features of ameloblastoma, but it has histologic features of malignancy. The ameloblastic carcinoma is considered a more aggressive lesion than conventional ameloblastoma (4). Metastases are likely to be to the lung or regional lymph nodes generally, along with reports of metastases to liver, brain, bone, kidneys, and the gastrointestinal system (20). The reported rate of metastases from ameloblastoma is approximately 2%. The likelihood of metastases is increased with large initial tumors, delay in treatment, recurrence, and primary mandibular tumors (21).
The definitive treatment of ameloblastoma is surgical resection. Curettage is not recommended as definitive treatment. Sehdev (12) reported a 90% recurrence rate of the solid/multicystic ameloblastoma of the mandible when treated with curettage alone. Complete excision with negative margins is the hallmark of a curative resection. The goals of the surgical procedure should be to achieve a curative resection, while minimizing functional and esthetic consequences of ablative surgery. A radical approach, either marginal or segmental resection with adequate margin ensures complete removal of solid/multicystic ameloblastoma, minimizing the recurrence rate (0-10%) and the risk of metastasis (22, 23, 24). Some literature suggests that ameloblastoma can extend into cancellous bone histologically at a mean of 4.5 mm beyond the radiographic boundary (24) and currently, the literature recommends the use of 1-1.5 cm resection margins (25). Marx et al showed that microscopic tumor cells can extend up to 8 mm beyond the radiographic extent of the lesion (24). Therefore, the standard treatment of solid/multicystic ameloblastoma is surgical resection extending a minimum of 1 cm beyond the clinical/radiological extent of the tumor. All but two patients with negative surgical margins in our series were cured, without any evidence of recurrence at the time of last follow up. The treatment of malignant ameloblastoma is primarily surgical, because ameloblastoma is relatively resistant to irradiation (26). Currently, no chemotherapeutic agent has shown efficacy in ameloblastoma.
Approximately 80% of ameloblastomas occur in the mandible, compared with ~ 20% in the maxilla (2). Of those that occur in the maxilla, most are in the posterior maxilla. Due to the thin cortical bone of maxilla, compared to the mandible, these tumors have the ability to penetrate the cortical bone with earlier soft tissue extension. The maxillary ameloblastoma is more difficult to visualize clinically, and, on plain radiographs, which may prohibit early detection (27). When planning treatment of ameloblastoma, imaging with a CT scan with bone windows is imperative in order to view the tumor extension in three dimensions and to evaluate for cortical perforation and extraosseous extension of tumor.
Marginal resection for small, solid, multicystic ameloblastoma of the mandible is advantageous since it preserves the inferior margin of the mandible and avoids the need for a complex bone reconstruction (20). However, if marginal resection is likely to result in jaw instability and an increased risk of pathological fracture, segmental resection with bone reconstruction is the preferred option (28). Furthermore, the unexcised inferior margin of mandible may present a source of tumor recurrence (20).
Most ameloblastomas of the mandible with invasion of the cancellous part of the mandible will require segmental resection with adequate margins, (at least 1 cm, beyond the radiological extent of the tumor), and mandible reconstruction.
In maxillary ameloblastoma, radical resection is especially necessary due to the frequency of extraosseous extension of tumor into soft tissues. Although less common and histologically similar to mandibular ameloblastoma, maxillary ameloblastoma manifest clinically more aggressive behavior, probably due to the thin bony walls of the maxilla, allowing the tumor to break thru the bone early in the course of the disease. Furthermore, maxillary ameloblastoma can potentially extend to the skull base and invade the brain. (12, 29, 30) and in that setting the rate of surgical cure decreases significantly (31). Either partial, total or extended maxillectomy may be necessary, depending on the extent of the tumor, to achieve a complete resection with negative margins.
Peripheral ameloblastomas typically occur in soft tissues adjacent to the alveolar process of the mandible or maxilla. Adequate resection of the soft tissue surrounding the tumor should be undertaken to get clear margins. If cupping or saucerization of alveolar bone is seen, the associated periosteum and/or bone should be excised. Recurrence rates of 16% to 19% are reported, and long-term follow-up is still imperative (32).
Inadequate initial surgical treatment of ameloblastoma yields a high chance of local recurrence. Positive margins clearly increase the risk of local recurrence. Only 80% of recurrent ameloblastoma are cured with re resection (12).
Due to the slow-growing nature of ameloblastoma, many recurrences occur after more than 5 years, and as long as 30 years after the initial treatment. Recurrence of ameloblastoma typically occurs within a decade from the initial presentation; however, there have been reports of tumor recurrence 30 years after initial treatment (33). Tumor surveillance in asymptomatic patients should consist of clinical exams and orthopantomograms every 6 months for 1 year, then once per year for minimum of 10 years. Routine use of computer tomography (CT) scans for monitoring of maxillary ameloblastomas is reasonable, due to anatomic overlap of structures in this region. Due to the potential for late recurrence with all types of ameloblastoma and the importance of long-term and vigorous follow-up, patients unable or unwilling to follow such recommendations may be candidates for initial radical resection, regardless of histologic variant of ameloblastoma, to minimize the risk of recurrence.
The role of radiotherapy (RT) either as initial definitive treatment, or as an adjunct after surgery has been reported in the literature with conflicting results. Kennedy et al reported on six cases treated with radiotherapy, and recommended radiation as an adjunct to reduce the risk of recurrence (34). Koukourakis et al, in their literature review regarding the role of radiotherapy, concluded that modern methods of delivering radiation including IMRT and proton beam therapy may have a role in an adjuvant setting after surgery to improve local control (35). In our series, two patients with positive margins after maxillary resection had post operative RT, and remain free of recurrence at 9 and 10 years after treatment. In addition two patients received post op RT after re resection of their local recurrence, and remained free of recurrence at last follow up. One of them died seven years after re resection and post op RT, from extensive melanoma, without evidence of recurrent ameloblastoma. The other patient developed multiple local recurrences, four times. The first three recurrences were treated by additional surgery. The fourth recurrence was resected, followed by post op RT. She remained free from further recurrence for 14 years, and has been lost to follow up since.
Based on this limited experience in these four patients, we can state that post operative radiotherapy in an adjuvant setting has a role in local control. The indications for post op RT are microscopic positive margins, when re resection is not feasible or advisable, high risk for local failure in tumors with extensive invasion of soft tissues, and after salvage surgery for recurrent tumors.
Summary
Ameloblastoma of the mandible and maxilla is generally a benign tumor of the odontoma with locally aggressive behavior. Occasionally the tumor breaks through cortical bone and extends into adjacent soft tissues. Complete surgical resection with negative margins is the hallmark for curative resection. Local recurrences are rare, and usually amenable to re- resection. Post operative radiotherapy for patients at increased risk of local recurrence improves local tumor control.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Bibliography
- 1.Haluk Vayvada, Fahri Mola, Adnan Menderes, Mustafa Yilmaz. Surgical management of ameloblastoma in the mandible: segmental mandibulectomy and immediate reconstruction with free fibula or deep circumflex iliac artery flap (evaluation of the long-term esthetic and functional results) J Oral Maxillofac Surg. 2006; 64:1532–1539. [DOI] [PubMed] [Google Scholar]
- 2.Vickers RA, Gorlin RJ. Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer. 1970; 26:699–710. [DOI] [PubMed] [Google Scholar]
- 3.Zemann W, Feichtinger M, Kowatsch E, Kärcher H Extensive ameloblastoma of the jaws: surgical management and immediate reconstruction using microvascular flaps. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2007; 103(2):190–196. [DOI] [PubMed] [Google Scholar]
- 4.Hall JM, Weathers DR, Krishnan K. Ameloblastic carcinoma: an analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007; 103(6):799–807. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin EH. Metastasizing ameloblastoma. Cancer. 1989. August 1;64(3):776–80 [DOI] [PubMed] [Google Scholar]
- 6.Olaitan AA, Adeola DS, Adekeye EO. Ameloblastoma: Clinical features and management of 315 cases from Kaduna, Nigeria. J Craniomaxillofac Surg. 1993; 21:351–5. [DOI] [PubMed] [Google Scholar]
- 7.Huffman GG, Thatcher JW (1974) Ameloblastoma—the conservative surgical approach to treatment: report of four cases. J Oral Surg 32: 850–854 [PubMed] [Google Scholar]
- 8.Akinosi JO, Williams AO. Ameloblastoma in Ibadan, Nigeria. Oral Surg Oral Med Oral Pathol. 1969;27(2):257–265. [DOI] [PubMed] [Google Scholar]
- 9.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995; 31B:86–99. [DOI] [PubMed] [Google Scholar]
- 10.Eversole LR, Leider AS, Strub D. Radiographic characteristics of cystogenic ameloblastoma. Oral Surg Oral Med Oral Pathol. 1984; 57:572–7. [DOI] [PubMed] [Google Scholar]
- 11.D’Agostino A, Fior A, Pacino GA, Bedogni A, Santis D, Nocini PF Retrospective evaluation on the surgical treatment of jaw bones ameloblastic lesions. Experience with 20 clinical cases. Minerva Stomatol. 2001. Jan-Feb;50(1–2):1–7 [PubMed] [Google Scholar]
- 12.Sehdev MK, Huvos AG, Strong EW, Gerold FP, Willis GW (1974) Proceedings: Ameloblastoma of maxilla and mandible. Cancer 33: 324–333 [DOI] [PubMed] [Google Scholar]
- 13.Ghandhi D, Ayoub AF, Pogrel MA, MacDonald G, Brocklebank LM, Moos KF. Ameloblastoma: A surgeon’s dilemma. J Oral Maxillofac Surg. 2006; 64:1010–4.). [DOI] [PubMed] [Google Scholar]
- 14.Gardner DG. Experimentally induced ameloblastomas: a critical review. J Oral Pathol Med 1992;21:337–9 [DOI] [PubMed] [Google Scholar]
- 15.Gardner DG. Canine acanthomatous epulis: the only common spontaneous ameloblastoma in animals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;79:612–5 [DOI] [PubMed] [Google Scholar]
- 16.Apajalahti S, Kelppe J, Kontio R, Hagstrom J, Imaging characteristics of ameloblastomas and diagnostic value of computed tomography and magnetic resonance imaging in a series of 26 patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015. August; 120(2):e118–30. [DOI] [PubMed] [Google Scholar]
- 17.Hong J, Yun PY, Chung IH, Myoung H, Suh JD, Seo BM, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007; 36:283–8. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z,Chen F, Wang T, Hu Q., Sun G, The variability and complexity of ameloblastoma: carcinoma ex ameloblastoma or primary ameloblastic carcinoma J Craniomaxillofac Surg. 2013. April;41(3):190–3. [DOI] [PubMed] [Google Scholar]
- 19.Berger AJ, Son J, Desai NK. Malignant ameloblastoma: concurrent presentation of primary and distant disease and review of the literature. J Oral Maxillofac Surg. 2012. October;70(10):2316–26. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg SE, Steinberg B. Surgical management of ameloblastoma: current status of the literature. Oral Surg Oral Med Oral Pathol. 1996;81:383. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso A, Lazow SK, Solomon MP, et al. Metastatic ameloblastoma to the cervical lymph nodes: a case report and review of literature. J Oral Maxillofac Surg. 2009; 67:1163–1166. [DOI] [PubMed] [Google Scholar]
- 22.Muller H, Slootweg PJ. The ameloblastoma, the controversial approach to therapy. J Maxillofac Surg. 1985; 13:79. [DOI] [PubMed] [Google Scholar]
- 23.Simon EN, Merkx MA, Kalyanyama BM, Shubi FM, Stoelinga PJ (2013) Immediate reconstruction of the mandible after resection for aggressive odontogenic tumours: a cohort study. Int J Oral Maxillofac Surg 42: 106–112. [DOI] [PubMed] [Google Scholar]
- 24.Marx RE, Smith BH, Smith BR, Fridrich KL (1993) Swelling of the retromolar region and cheek associated with limited opening. J Oral Maxillofac Surg 51: 304–309. [DOI] [PubMed] [Google Scholar]
- 25.Pogrel MA, Montes DM (2009) Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg 38: 807–812. [DOI] [PubMed] [Google Scholar]
- 26.Goldwyn R, Constable J, Murray JE, Ameloblastoma of the jaw. A clinical study. N Engl J Med . 1963. July 18; 269:126–9. [DOI] [PubMed] [Google Scholar]
- 27.Williams TP. Management of ameloblastoma: A changing perspective. J Oral Maxillofac Surg. 1993; 51:1064. [DOI] [PubMed] [Google Scholar]
- 28.Carlson ER, Marx RE (2006) The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg 64: 484–494 [DOI] [PubMed] [Google Scholar]
- 29.Bredenkamp JK, Zimmerman MC, Mickel RA (1989) Maxillary ameloblastoma. A potentially lethal neoplasm. Arch Otolaryngol Head Neck Surg 115: 99–104 [DOI] [PubMed] [Google Scholar]
- 30.Nastri AL, Wiesenfeld D, Radden BG, Eveson J, Scully C (1995) Maxillary ameloblastoma: a retrospective study of 13 cases. Br J Oral Maxillofac Surg 33: 28–32. [DOI] [PubMed] [Google Scholar]
- 31.Komisar A (1984) Plexiform ameloblastoma of the maxilla with extension to the skull base. Head Neck Surg 7: 172–175. [DOI] [PubMed] [Google Scholar]
- 32.Philipsen HP, Reichart PA, Nikai H, Takata T, Kudo Y. Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol. 2001; 37(1):17–27. [DOI] [PubMed] [Google Scholar]
- 33.Hayward JR (1973) Recurrent ameloblastoma 30 years after surgical treatment. J Oral Surg 31: 368–370. [PubMed] [Google Scholar]
- 34.Kennedy WR, Werning J W, Kaye F J and Mendenhall W M. Treatment of Ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur Arch Otorhinolaryngol. January 2016. [DOI] [PubMed] [Google Scholar]
- 35.Georgios VK, Anthoula M, Anastasia SL. Ameloblastoma, a rare benign odontogenic tumour: an interesting tumour review targeting the role of radiation therapy. Clin Transl Oncol. 2011;13:793–797 [DOI] [PubMed] [Google Scholar]


