Abstract
This review article summarizes the efficacy, feasibility and potential mechanisms of the application of essential oils as antibiotic alternatives in swine production. Although there are numerous studies demonstrating that essential oils have several properties, such as antimicrobial, antioxidative and anti-inflammatory effects, feed palatability enhancement and improvement in gut growth and health, there is still a need of further investigations to elucidate the mechanisms underlying their functions. In the past, the results has been inconsistent in both laboratory and field studies because of the varied product compositions, dosages, purities and growing stages and conditions of animals. The minimal inhibitory concentration (MIC) of essential oils needed for killing enteric pathogens may not ensure the optimal feed intake and the essential oils inclusion cost may be too high in swine production. With the lipophilic and volatile nature of essential oils, there is a challenge in effective delivery of essential oils within pig gut and this challenge can partially be resolved by microencapsulation and nanotechnology. The effects of essential oils on inflammation, oxidative stress, microbiome, gut chemosensing and bacterial quorum sensing (QS) have led to better production performance of animals fed essential oils in a number of studies. It has been demonstrated that essential oils have good potential as antibiotic alternatives in feeds for swine production. The combination of different essential oils and other compounds (synergistic effect) such as organic acids seems to be a promising approach to improve the efficacy and safety of essential oils in applications. High-throughput systems technologies have been developed recently, which will allow us to dissect the mechanisms underlying the functions of essential oils and facilitate the use of essential oils in swine production.
Keywords: Essential oils, Organic acids, Medium chain fatty acids, Inflammation, Oxidative stress, Pigs
1. Introduction
Young piglets have a high susceptibility to various stressors, including bacterial pathogens, oxidative stress and inflammation, leading to reduced growth performance, high mortality and morbidity rates and compromised animal welfare. Antibiotic growth promoters (AGP) have widely been used in pig diets, especially in nursery diets, to control incidences of post-weaning diarrhea and to improve growth performance. Total consumption of antimicrobials in food animal production worldwide was estimated at 63,151 t in 2010 with an increasing trend and the annual consumption of antimicrobials per kilogram body weight is 148 mg/kg for pigs (Van Boeckel et al., 2015). This practice may lead to the spread of antimicrobial-resistant bacterial pathogens in both pigs and humans, posing a significant public health threat (Yang et al., 2015). The use of AGP in food animal production has been banned in European Union since 2006 (Bengtsson and Wierup, 2006). The U.S. Food and Drug Administration placed restrictions on antibiotic use in animals in December 2016 and Health Canada will ban the use of antibiotics in animal diets in December 2017 with more countries expected to follow. Several challenges are associated with the withdrawal of antibiotics from feeds (Zhao et al., 2007). Therefore, it is critical to develop cost-effective antibiotic alternatives for ensuring the long-term sustainability of pig production (Yang et al., 2015, Valenzuela-Grijalva et al., 2017). Organic acids (Eckel et al., 1992, De Lange et al., 2010), enzymes (Bedford and Cowieson, 2012, Kiarie et al., 2013), probiotics (Heo et al., 2004, Heo et al., 2013, Musa and Seri, 2009), antimicrobial peptides (Choi et al., 2013), medium chain fatty acids (Boyen et al., 2008) and essential oils (Windisch et al., 2008, Randrianarivelo et al., 2010, Gong et al., 2013) have been widely recognized as promising alternatives to antibiotics in feeds.
Essential oils are natural bioactive compounds derived from plant and have positive effects on animal growth and health (Puvača et al., 2013). Because of the antimicrobial, anti-inflammatory and antioxidative properties, essential oils have been widely used as traditional medicines to improve health or cure diseases in humans (Brenes and Roura, 2010, Kim et al., 2008). The bioactive components in essential oils have been identified and some progress have been made to elucidate the mechanisms underlying the functions of these compounds in animals, leading to increased research efforts to use essential oils to replace antibiotics in animal feeds (Li et al., 2012). However, the application of essential oils in the feed has been mainly based on the antimicrobial effects. Moreover, the minimum inhibitory concentration (MIC) of most essential oils are much higher than the acceptable levels in animal industry in terms of cost-effectiveness and feed palatability (Yang et al., 2015). Other than the varied results and unclear mechanisms, there are still several other challenges in using essential oils in animal feeds, including toxic effects, regulatory concerns and high inclusion costs. Therefore, it is vital to investigate the specific effects and target sites (either animal host or its microbiome) of individual compounds in essential oils to facilitate the application of essential oils in swine production.
2. Essential oils
Essential oils are aromatic, volatile and oily liquids extracted from plant materials such as seeds, flowers, leaves, buds, twigs, herbs, bark, wood, fruits and roots (Brenes and Roura, 2010). The word “essential” is postulated by Paracelsus in the theory of ‘quinta essentia’, which means that the quintessence can be useful medically due to its effectiveness (Oyen and Dung, 1999). Essential oils are a mixture of complex compounds which may vary in their individual chemical compositions and concentrations. For instance, the predominant components thymol and carvacrol found in thyme, researchers have found that they can be as low as 3% to as high as 60% of the total essential oils in thyme (Lawrence and Reynolds, 1983). Also, it was discovered that a major component of the cinnamon essential oils, cinnamaldehyde ranges from 60% to 75% of the total oil (Duke, 1986). These constituents of essential oils such as carvacrol and thymol present in thyme provide broad spectrum antimicrobial activities against Gram-negative and gram-positive bacteria, fungi and yeast (Roller and Seedhar, 2002, Abbaszadeh et al., 2014). Essential oils have greater effect against gram-positive (G+) than gram-negative (G−) bacterial pathogens because the entrance of hydrophobic compounds through the lipopolysaccharide structures of G bacteria are limited due to their outer membrane coating the cell wall (Vaara, 1992). Various researchers have proven essential oils as alternatives to antibiotics because they have antimicrobial, anti-inflammatory, antioxidative, and coccidiostatic properties. They enhance digestibility (Chitprasert and Sutaphanit, 2014) and immunity (Brenes and Roura, 2010), promote gut health by minimizing the effect of the pathogenic bacteria (Chitprasert and Sutaphanit, 2014), and control odor and ammonia emission (Varel, 2002).
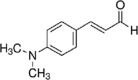
Essential oils have 2 major classes of compounds, terpenes (e.g., carvacrol and thymol) and phenylpropenes (e.g., cinnamaldehyde and eugenol). Terpenes are sub-divided in respect to the numbers of 5 – carbon building blocks known as isoprene units with mono (C10H6), sesqui (C15H24) and diterpenes (C20H32). There are some different sources of terpenes represented by the existence or non-existence of the ring structures, double bonds, and addition of oxygen or presence of stereochemistry (Lee et al., 2004). It is estimated that there are more than 1,000 monoterpenes and more than 3,000 sesquiterpenes based on various researchers (Cooke et al., 1998). There are just 50 phenylpropenes discovered (Lee et al., 2004). The commonly used essential oils in animals are carvacrol, thymol, citral, eugenol and cinnamaldehyde and their chemical properties and stability were described in Table 1.
Table 1.
Chemical properties of essential oils popularly used in pig feeds (Adapted from Michiels, 2009).
| Compound | Carvacrol | Thymol | Citral | Eugenol | Cinnamaldehyde |
|---|---|---|---|---|---|
| Chemical structure | 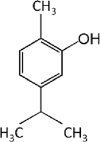 |
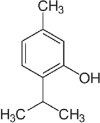 |
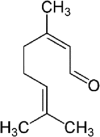 |
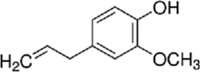 |
 |
| Formula | C10H14O | C10H14O | C10H16O | C10H12O2 | C9H8O |
| Molecular mass, g/mol | 150.2 | 150.2 | 152.2 | 164.2 | 132.2 |
| Density, kg/m3 | 976 | 969 | 893 | 1,067 | 1,050 |
| Melting point, °C | 0 to 2 | 49 to 52 | −10 | −12 to −10 | −7.5 |
| Boiling point, °C | 234 to 238 | 232 to 233 | 229 | 253 | 246 to 251 |
| Vapor pressure at 20 °C, Pa | 35 | 250 (50 °C) to 133 (64 °C) | 22 | 133 (78 °C) | 3.85 |
| Solubility in water, g/L | 0.83 to 1.10 | 0.85-1.01 to 1.4 (40 °C) | 0.59 | 0.80 to 2.41 | 1.42 to 1.45 |
| Solubility in ethanol, g/L | Good | 1,000 | Good | 500 (in 70%), good | 150 (in 60%), good |
| Octanol/water partition Coefficient, log Kow |
3.38 to 3.64 | 3.30 | 2.8 to 3.0 | 2.99 | 1.9 |
| pKa value | 10.4 | 10.4 | – | – | – |
| Physical appearance at room temperature | Colorless to pale yellow liquid | White crystalline powder or large colorless crystals | Pale yellow liquid | Colorless or pale yellow, thin liquid | Clear yellowish liquid |
3. Antimicrobial effects of essential oils
3.1. Mode of action of essential oils
Although carvacrol and thymol have several target sites in bacterial cells, the biosynthetic machinery of bacterial cell walls is their main target site (Faleiro, 2011, Yap et al., 2014). First, carvacrol and thymol can sensitize the cell walls (including membranes) and cause significant membrane damages, leading to integrity collapse of the bacterial cytoplasmic membrane, leakage of vital intracellular contents and eventually death of the bacterial cells. The leakage often happens through cell wall damage, cytoplasmic membrane damage, cytoplasm coagulation and membrane protein destruction (Conner and Beuchat, 1984, Cox et al., 1998, Helander et al., 1998, Ultee et al., 2002) as well as reduction of proton motive force (Nazzaro et al., 2013). Secondly, with their lipophilic structure, carvacrol and thymol can easily get into the bacterial membranes among the fatty acid chains and cause the membranes to expand and become more fluidity. With these properties, carvacrol and thymol are regarded as promising alternatives to antibiotics in swine production systems (Kim et al., 1995, Lambert et al., 2001, Delaquis et al., 2002). The position of functional groups (e.g., hydroxyl or alkyl) in essential oils plays very important roles in the antimicrobial activities of essential oils. Although thymol and carvacrol have similar antimicrobial effects, they have different effects on G+ or G− bacteria based on the positions of one or more functional groups in thymol and carvacrol. Their antimicrobial action highly depends on the hydroxyl group of the phenolic terpenoids and the presence of delocalized electrons (Lambert et al., 2001, Ultee et al., 2002), which often determine the level of their antimicrobial activity on different bacteria. Helander et al. reported that thymol and carvacrol were able to damage the outer membrane of Salmonella typhimurium and Escherichia coli O157:H7 because of their enhanced ability to release lipopolysaccharides and sensitize membrane (Helander et al., 1998). Both carvacrol and thymol have lipopolysaccharide releasing properties that make them have superior antimicrobial properties against some G− bacteria when compared to other essential oils. Another hypothesis is the proton exchanger model and carvacrol can act as a trans-membrane carrier by exchanging its hydroxyl proton for a potassium ion resulting in dissipation of the pH gradient and electrical potential over the membrane, reduced proton motive force and depletion of intracellular adenosine triphosphate (ATP) pools. Loss of potassium can also cause problems, since it plays very important roles in the activation of a number of cytoplasmatic enzymes, in maintaining osmotic pressure and in the regulation of intracellular pH. Generally speaking, bacteria can use ionic pumps to counter these effects and cell death does not always happen, but large amounts of energy are needed for this function and bacterial growth is compromised (Ultee et al., 1999, Ultee et al., 2002). Eugenol and cinnamaldehyde also have a phenolic functional group and their antimicrobial activities related to membrane effects and energy generation have been reported (Gill and Holley, 2004, Gill and Holley, 2006). A study has been reported that cinnamaldehyde and eugenol can effectively inhibit histidine decarboxylase activity of Enterococcus aerogenes at sublethal levels (Wendakoon and Sakaguchi, 1995). The hydroxyl group of eugenol and the carbonyl group of cinnamaldehyde are believed to bind to proteins, inhibiting the action of amino acid decarboxylases in E. aerogenes. Therefore, the primary mechanism of action for thymol, carvacrol, eugenol and cinnamaldehyde is related to their effects on the cytoplasmic membranes and energy metabolism.
3.2. Minimum inhibitory concentration of essential oils
The definition of MIC is the minimal concentration of an antimicrobial compound that inhibits the growth of a microorganism. The MIC of an antimicrobial compound has been widely used in laboratory to measure the activity of an antimicrobial compound against a microorganism. Individual essential oil's MIC can be different from bacterium to bacterium and from strain to strain. Laboratory conditions for MIC assays may also affect results. Some popularly used essential oils and their MIC values on several bacterial pathogens are described in Table 2.
Table 2.
Minimum inhibition concentration (MIC) of essential oils against various bacterial pathogens (Adapted from Yang et al., 2015).
| Product | Pathogenic microbe | Gram | MIC (unit) | MIC (#) | Reference |
|---|---|---|---|---|---|
| Thymol | Lactococcus piscicum | + | mg/L | 320 | Navarrete et al., 2010 |
| Streptococcus phocae | + | mg/L | 640 | Navarrete et al., 2010 | |
| Flavobacteriaum psychrophilum | − | mg/L | 320 | Navarrete et al., 2010 | |
| Vibrio anguillarum | − | mg/L | 80 | Navarrete et al., 2010 | |
| Vibrio parahaemolyticus | − | mg/L | 320 | Navarrete et al., 2010 | |
| Pseudomonus sp. | − | mg/L | 640 | Navarrete et al., 2010 | |
| Brachyspira hyodysenteriae | − | mmol/L | 1.25 | Vande Maele et al., 2016 | |
| Escherichia coli 0157:H7 | − | μg/mL | 166 | Si et al., 2006 | |
| Salmonellatyphimurium DT104 | − | μg/mL | 233 | Si et al., 2006 | |
| Escherichia coli K88 | − | μg/mL | 100 | Si et al., 2006 | |
| Lactococcus lactis | + | mg/L | 1,280 | Navarrete et al., 2010 | |
| Eugenol | Vibrio sp. | − | μg/mL | 156 | Seongwei et al., 2009 |
| Escherichia coli | − | μg/mL | 625 | Seongwei et al., 2009 | |
| Salmonella | − | μg/mL | 156 | Seongwei et al., 2009 | |
| Pseudomonas sp. | − | μg/mL | 325 | Seongwei et al., 2009 | |
| Edwardsiella tarda | − | μg/mL | 56 to 125 | Seongwei et al., 2009 | |
| Aeromonas hydrophilla | − | μg/mL | 625 | Seongwei et al., 2009 | |
| Brachyspira hyodysenteriae | − | mmol/L | 2.5 | Vande Maele et al., 2016 | |
| Escherichia coli 0157:H7 | − | μg/mL | 466 | Si et al., 2006 | |
| Salmonellatyphimurium DT104 | − | μg/mL | 400 | Si et al., 2006 | |
| Escherichia coli K88 | − | μg/mL | 300 | Si et al., 2006 | |
| Carvacrol | Listonella anguillarum | − | μg/mL | 25 | Volpatti et al., 2013 |
| Brachyspira hyodysenteriae | − | mmol/L | 1.25 | Vande Maele et al., 2016 | |
| Escherichia coli 0157:H7 | − | μg/mL | 283 | Si et al., 2006 | |
| Salmonellatyphimurium DT104 | − | μg/mL | 167 | Si et al., 2006 | |
| Escherichia coli K88 | − | μg/mL | 100 | Si et al., 2006 | |
| Cinnamaldehyde | Brachyspira hyodysenteriae | − | mmol/L | 0.31 | Vande Maele et al., 2016 |
| Escherichia coli 0157:H7 | − | μg/mL | 133 | Si et al., 2006 | |
| Salmonellatyphimurium DT104 | − | μg/mL | 100 | Si et al., 2006 | |
| Escherichia coli K88 | − | μg/mL | 133 | Si et al., 2006 |
3.3. Synergism of essential oils and organic acids
Most G+ bacterial cell wall (approximately 90% to 95%) are comprised of peptidoglycan. This feature can allow hydrophobic compounds to easily penetrate the cells and then act on both cell wall and cytoplasm (Karatzas et al., 2001, Trombetta et al., 2005). After entering the cell, these compounds can not only affect several enzymes involved in energy production at lower concentrations, but also denature proteins at higher concentrations. G− bacteria only have a 2 to 3 mm peptidoglycan layer comprising about 20% of the dry weight of bacterial cells and an outer-membrane is located in the outside of this peptidoglycan layer and this outer-membrane is composed of a double layer of phospholipids firmly linked to the inner membrane by Braun's lipoprotein. Basically, G− bacteria are more resistant to essential oils when compared with G+ bacteria. The thick outer membrane in G− bacteria reduces permeability and provides an extra layer to protect cells from essential oils.
Organic acids have a better efficacy against G− bacteria than essential oils (Zhou et al., 2007, Souza et al., 2009, Mahmoud, 2014). A study has shown that grape seed extract had a much higher MIC value at 10 mg/mL against Vibrio parahaemolyticus in sucked oysters when compared to citric acids (5 mg/mL) and lactic acids (1 mg/mL). Small hydrophilic organic acids are believed to be able to pass through the membrane via porin protein but not the hydrophobic polyphenol compounds. There are several factors contributing to the microorganism inhibition by organic acids, including reduction in pH, the ratio of non-disassociated form of organic acids, chain length, level of branching and cell physiology/metabolism (Booth, 1985). The lipophilic nature of weak organic acids allows them to easily enter plasma membrane and thus reduce the pH of cell's interior, eventually leading to the death of bacterium (Wang et al., 2013).
There are several studies demonstrating the additive effects of some essential oils and organic acids (Zhou et al., 2007, Souza et al., 2009, Hulánková and Bořilová, 2011). Zhou et al. (2007) reported that an essential oil (carvacrol or thymol) in combination with acetic acid or citric acid but not with lactic acid had a better efficacy against G− bacteria (S. typhimurium), when compared with individual essential oil or organic acids alone. The mechanisms underlying this potential synergism between some essential oils and organic acids are still not clear. However, it is well-known that phenols in essential oil can change the structure and functions of bacterial cell membrane, leading to swelling and thus increased membrane permeability. The compromised cell membrane might explain the observed synergism, since the phenolic compounds could cause significant damages to the cell membranes, increasing the susceptibility of the bacteria to organic acids. Moreover, the hydrophobicity of an essential oil is increased at low pH, enabling it to more easily pass through the lipids of the bacterial cell membrane (Karatzas et al., 2001). In recent studies, the results have been clearly shown in vivo efficacy of such synergistic dietary strategies in pigs and poultry (Diao et al., 2015, Balasubramanian et al., 2016, Walia et al., 2017, Liu et al., 2017). The inclusion of essential oils blend with organic acids in pig diets before slaughter can hinder Salmonella shedding and seroprevalence (Noirrit and Philippe, 2016; Walia et al., 2017).
3.4. Synergy of essential oils and medium chain fatty acids (MCFA)
As shown in Table 3, MCFA include lauric acid (C12), capric acid (C10), caprylic acid (C8), carboxylic acids (C7 and C9) and caproic acid (C6) and their derivatives are also another of the alternatives to antibiotics for piglets (Boyen et al., 2008, Zentek et al., 2011, Zentek et al., 2012, Hanczakowska et al., 2013, Zentek et al., 2013, De Smet et al., 2016). Medium chain fatty acids have the capacity to fight against microbial activity of Salmonella and E. coli (Dierick et al., 2002, Rossi et al., 2010). Research carried out by Han et al. (2011) shows that the performance of pig fed eucalyptus MCFA blend was the same as that of antibiotics. Medium chain fatty acids are shown to have a good effect on G− and G+ bacteria. The effectiveness of the antimicrobial activity of MCFA towards some groups of bacteria is different based on their chain lengths (Rossi et al., 2010). Caprylic acid may have similar mode of action with short chain fatty acids because MCFA may inactivate bacteria by creating an acidic environment or by a direct impact on the expression of virulence factors necessary for Salmonella colonization. At low dietary levels, MCFA may be regarded as modulators of the gastric microbiota in weaned piglets. The additive effects were observed with multiple strains of Salmonella, Listeria monocytogenes, E. coli and Streptococcus aureaus when treated with a combination of oregano oil and caprylic acid (Hulánková and Bořilová, 2011). Similar effects of cinnamaldehyde and lauric acid against Brachyspira hyodysenteriae, the causative pathogen of swine dysentery, were observed in vitro (Maele et al., 2016). Medium chain fatty acids are generally recognized as safe (GRAS) by the Food and Drug Administration (de Los Santos et al., 2008). Some MCFA and their derivatives have strong and unpleasant smells that can reduce feed palatability and feed intake of pigs (Zentek et al., 2011). These may be overcomed by using the combination of essential oils and MCFA. However, there is no information on the in vivo application of the combination of essential oils and MCFA in swine production.
Table 3.
Molecular structure and physico-chemical properties of medium chain fatty acids used in pig feed.
| Name | Systemic name | Formula | Skeletal structure | Melting point, °C | Boiling point, °C | Density, g/mL |
|---|---|---|---|---|---|---|
| Caproic acid | Hexanoic acid | C6H12O2 | 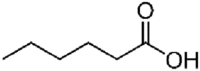 |
−3.4 | 205.8 | 0.929 |
| Caprylic acid | Octanoic acid | C8H16O2 | 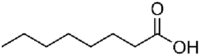 |
16.7 | 239 | 0.910 |
| Capric acid | Decanoic acid | C10H20O2 | 27 to 32 | 268 to 270 | 0.893 | |
| Lauric acid | Dodecanoic acid | C12H24O2 | 43.8 | 225 to 297 | 1.007 |
4. Effect of essential oils on intestinal inflammation
The gut has various important functions including absorption of nutrient, electrolytes and water, secretion of water, electrolytes, immunoglobulin, cytokines and mucin, and selective barrier protection against harmful antigens, toxins and pathogens (Lallès et al., 2004). However, it has been believed that except its absorption, secretion and barrier properties, the gut also plays a dynamic role in organ integrity, immunity and body defense (Eckmann et al., 1995, Pitman and Blumberg, 2000). Gut epithelial cells play an important role in immune system as “watch dogs” and they can detect the onset of immune responses or inflammation through cytokines. These cytokines are vital for recruitment and activation of different types of immune cells including neutrophils, macrophages, T and B cells and dendritic cells (Eckmann et al., 1995, Pitman and Blumberg, 2000). The gut immune system is dramatically different from systemic immune system. The gut immune system must balance 2 opposite functions: not only mounting an immune response to pathogens, but also maintaining tolerance to antigens derived from commensal bacteria and compounds from feeds (Pitman and Blumberg, 2000).
The balance of those 2 opposite functions could cause feed intolerance, inflammation and diseases (Yang et al., 2015). Gut inflammation is associated with compromised gut growth and development and reduced efficiency of nutrient utilization. It has been reported that gut acute and chronic inflammatory diseases often lead to gut morphological changes, mucosa damage, increased mucosal permeability, compromised gut development and poor nutrient absorption capacity (Waters et al., 1999, Nagura et al., 2001, Podolsky, 2002, Strober et al., 2002). Generally, 3 types of gut inflammation have been observed in pigs related to pathogens, nutrition and management and they are pathogen infection-associated, diet allergen-associated and weaning-associated gut inflammation (Yang et al., 2015). Although the inflammation does not cause full-blown clinical symptoms, it leads to a significant reduction of growth performance and causes considerable economic loss in pig production.
When immune response is initiated, macrophages are recruited in the tissues to produce an inflammatory reaction, and then T cells are also involved in the inflammation in later stages of immune response. Nuclear factor kappa B (NF-κB) is a transcriptional factor and plays very important roles during the above process (Rogler et al., 1998). After activated by several inducers such as pro-inflammatory cytokines, reactive oxygen species (ROS) and lipopolysaccharides, NF-kB is translocated from the cytoplasma to the nucleus and then induces the expression of numerous pro-inflammatory proteins including cytokines, chemokines, adhesion molecules and enzymes that are involved in inflammation, cell apoptosis and proliferation (Barnes and Karin, 1997). It has been evidenced that there is potential cross-talks between the nuclear factor-erythroid 2-related factor-2 (Nrf2) and NF-κB pathways and Nrf2 gene dysfunction could lead to increased susceptibility to inflammatory stresses (Khor et al., 2006). Given that gut inflammation in pigs does not only impair function and integrity of the gut but also affect growth performance, dietary strategies are necessary to inhibit the inflammatory process in the gut. Essential oils have been shown to manipulate both Nrf2 and NF-κB transcription factors to suppress inflammation (Kroismayr et al., 2008, Wondrak et al., 2010, Zou et al., 2016, Fang et al., 2017). Essential oils and avilamycin significantly reduced the expression of the NF-κB, tumor necrosis factor-α (TNF-α) and the size of Peyer's patches in the intestine of weaned piglets, as well as the cyclin D1 in the colon, mesenteric lymph nodes and spleen (Kroismayr et al., 2008). Several studies have demonstrated that essential oils including cinnamaldehyde (Wondrak et al., 2010) and oregano oil (Zou et al., 2016) increased the expression and translocation of Nrf2 and prevented the activation of NF-κB. These results suggest that these essential oils can reduce inflammation, and eventually lead to the improvement of pig health and growth performance through modifying the Nrf2 and NF-κB pathways. The supplementation of cinnamon oil in feeds attenuated lipopolysaccharide (LPS)-induced injury by suppressing inflammation (Wang et al., 2015). There is experimental evidence showing that oral administration of essential oils significantly reduces and limits the severity and development of experimental autoimmune encephalomyelitis, mainly through the modulation of Th1/Treg immune balance (Alberti et al., 2014). Therefore, it is clearly demonstrated that essential oils can modulate pig immune responses through different cell signaling pathways to enhance pig health.
5. Effect of essential oils on oxidative stress
Oxidative stress represents an important chemical mechanism that leads to biological damage, which in turn can affect growth performance and health in pigs, especially in modern high-performance swine production systems. Pigs are frequently exposed to several stressors including weaning, malnutrition, disease challenge, heat stress, in-feed mycotoxin contaminations, transportation and overcrowding. These stressors are known to increase the production of ROS and when the antioxidant system is overwhelmed by the production of ROS, oxidative stress occurs. The oxidative stress might be associated with a drop in performance, compromised immunity, muscle degeneration, increased risk of stroke in fast growing pigs, mulberry heart disease, reduced appetite, diarrhea, destruction of liver tissue, and increased risk of abortion of gestation sows.
As an important counterpart of NF-κB, Nrf2 is a redox sensitive transcription factor and can be sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1) under normal conditions. Nuclear factor-erythroid 2-related factor-2 can be dissociated from Keap1 and translocated into the nucleus, activating the expression of genes containing an antioxidant response element (ARE) (Nair et al., 2008). The Nrf2-ARE pathway positively regulates the expression of antioxidant and detoxification enzymes in cells such as glutathione peroxidase (GPx), catalase (CAT), glutathione S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD), NADH(P)H-Quinone-Oxidoreductase 1 (NQO1), Heme oxygenase (HO1) and the glutathione (GSH) precursor gamma-glutamyl cysteine synthetase (γ-GCS) and these enzymes can help to re-establish cellular redox homeostasis (Dhakshinamoorthy et al., 2001, Lee and Johnson, 2004, Nguyen et al., 2009, Mine et al., 2015).
Synthetic antioxidants such as ethoxyquin, butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are commonly used as effective feed additives in pig diets in order to increase the stability of feed and protect nutrients (e.g., fat and vitamins) from oxidation. However, such synthetic antioxidants do not have biological effects in vivo, and their toxicological safety is also a concern. This has driven the search for natural compounds that could not only replace synthetic antioxidants in pig feed, but also provide additional zootechnical benefits (Yang et al., 2015). It is well known that essential oils and plant extracts have anti-oxidative effects (Baschieri et al., 2017) and they have been used successfully in animal diets (Mueller et al., 2012, Akbarian et al., 2014, Placha et al., 2014, Tan et al., 2015, Zou et al., 2016, Liu et al., 2017). Zou et al. (2016) investigated the antioxidative effects of oregano essential oil in pig small intestinal epithelial cells (IPEC-J2) and demonstrated that ROS and malondialdehyde (MDA) induced by H2O2 were dramatically suppressed by oregano essential oil by inducing Nrf2 and several antioxidant enzymes (superoxide dismutase and g-glutamylcysteine ligase). Kang et al. (2015) also found that schisandrae semen essential oil attenuated cell damage induced by oxidative stress in C2C12 murine skeletal muscle cells through the upregulation of HO-1 mediated by Nrf2. Another in vitro study also indicated that lemongrass essential oil reduced DNA damage and oxidative stress induced by benzo(a)pyrene in human embryonic lung fibroblast cells. Zeng et al. (2015) indicated that pigs fed an essential oil diet had higher levels of albumin, immunoglobulin A (IgA), IgG, total antioxidant capacity and lower fecal score when compared to pigs fed control diets. A recent in vivo pig study has shown that dietary supplementation with 100 mg/kg mixture of carvacrol and thymol (1:1) decreased the weaning associated intestinal oxidative stress through decreasing TNF-α mRNA (Wei et al., 2017).
Increased oxidative stress leads to impaired milk production, reproductive performance, and longevity of sows (Berchieri-Ronchi et al., 2011, Zhao et al., 2013). Compromised ability to produce milk could directly impair the health and growth of nursing piglets. It may also have a long-term effect on health and growth throughout the life of pigs. An increase in systemic oxidative stress was observed in late gestation and early lactation of sows and the supplementation of oregano essential oil improved performance of their piglets through reducing oxidative stress (Tan et al., 2015, Tan, 2015). Therefore, supplementation of essential oils is a promising method to ameliorate the negative effects of oxidative stress induced by different stressors in modern high-performance swine production systems.
6. Effect of essential oil on quorum sensing
6.1. Bacterial cell signaling
Quorum sensing is referred to a regulatory system depending on population density in bacteria and it regulates gene expression in response to cell density through accumulation of diffusible signaling molecules (De Kievit and Iglewski, 2000). Quorum sensing has an important role to regulate various important physiological processes, particularly the expression of virulence factors which play important roles during the process of pathogenic microbe-host interactions (Greenberg, 2003, Xavier and Bassler, 2003, Vendeville et al., 2005, Defoirdt et al., 2013, Kantas et al., 2015, Joshi et al., 2016). There are three major types of signaling components that have been used for QS, including acyl-homoserine lactones (AHL), small polypeptides and autoinducer-2 (AI-2). Acyl-homoserine lactones are biosynthesized by the LuxI family members of AHL synthases and mainly used in G− bacteria (Parsek et al., 1999). However, G+ bacteria utilize modified oligopeptides as autoinducer molecules because G+ bacteria do not have LuxI or LuxR homologs (Rocha-Estrada et al., 2010). The G− and G+ bacteria can share AI-2 and the gene, luxS that is responsible for the synthesis of AI-2 in bacteria (Schauder et al., 2001). It has been reported that QS plays significant roles in the regulation of virulence factors expression in numerous enteric pathogens (Ohtani et al., 2002, Vendeville et al., 2005, Khan et al., 2009, Zhu et al., 2011). Therefore, it can be a promising tool to control enteric pathogens in pig production through interfering QS by small molecules or QS quenching enzymes. However, identification of QS inhibitors for pathogenic pathogens and studies on their mechanisms and applications in swine production are still required.
6.2. Disrupting quorum sensing
Each QS circuit used by a specific bacterium is different. However, all kinds of QS systems share a common mechanism comprised of signal synthesis, signal accumulation and signal detection (LaSarre and Federle, 2013). Therefore, there are 3 steps that QS inhibitors can target: QS signal biosynthesis, QS signal degradation and inactivation, and signal detection (Czajkowski and Jafra, 2009, LaSarre and Federle, 2013). Extensive studies have been investigated the application of small molecules to disrupt the expression of virulence genes that are regulated by QS, and numinous natural and synthetic small molecules that can inhibit QS have been identified (Galloway et al., 2010). Eugenol and carvacrol are the most intensely studied QS-disrupting essential oils (Zhou et al., 2013, Burt et al., 2014, Mith et al., 2015). Except the use of small molecules, another strategy for inhibiting QS is to degrade QS autoinducers enzymatically (Czajkowski and Jafra, 2009). There are 3 known classes of enzymes including lactonases, acylases and oxidoreductases, hydrolyzing AHL to produce products that are no longer active signaling agents (Hong et al., 2011). It has been recently reported that Ruminococcus obeum could decrease Vibrio cholerae growth and colonization through increasing LuxS/AI-2-based QS of R. obeum (Hsiao et al., 2014). In conclusion, it may be feasible to disrupt QS by using synthesized or natural small molecules, QS quenching enzymes and probiotics bacteria, which could limit the growth and colonization of enteric pathogens in swine production.
Numerous studies have shown that various essential oils can disrupt the QS of pathogenic bacteria (Bjarnsholt et al., 2005, Choo et al., 2006, Szabó et al., 2010, Zhou et al., 2013). However, all evidence has come from in vitro studies and studies on food microbiology (Kerekes et al., 2013, Alvarez et al., 2014). It is an unexplored strategy to control bacterial infections in animals by using inhibition of QS (Defoirdt et al., 2004). However, it is an exception in aquaculture. It has been reported that Cinnamaldehyde and its derivatives are effective against Vibrio harveyi in brine shrimp (Niu et al., 2006, Brackman et al., 2008) and the potential mechanism is to disrupt protein-DNA interactions of the QS-responsive master regulatory protein LuxR. A most recent study strongly support that carvacrol and eugenol inhibit specific virulence determinants in Pectobacteria through disputing the QS machinery and these 2 volatiles directly inhibit AHL production, potentially via direct interaction with ExpI/ExpR proteins (Joshi et al., 2016). Similar results were also found in a study indicating that essential oil (carvacrol and thymol) suppresses biofilm (Oh et al., 2017). Although the application of QS inhibition in pig pathogens are still lacking, it is expected that this strategy will receive significant attentions in swine production in the coming years.
7. Effect of essential oils on intestinal microbiota and microbiome
The pig gut is generally considered to be sterile prior to birth, but rapidly becomes colonized with microbes from the environment and diet (Jensen, 1998, Kim and Isaacson, 2015). The colonizing microbes subsequently develop into a highly diverse microbiota with varying microbial density and composition among different gut compartments. Our understanding of gut microbiota composition and function has been significantly improved following the application of molecular and “omics” methodologies in combination with bioinformatics and statistical tools. For example, Firmicutes and Bacteroidetes were shown to be the most dominant phyla in pigs regardless of age, followed by Proteobacteria, Actinobacteria and Spirochaetes (Kim et al., 2012, Kim et al., 2015, Lu et al., 2014, Slifierz et al., 2015, Zhao et al., 2015). Nevertheless, there are still some dynamic shifts in the composition of gut microbiota with age. The phylum Proteobacteria was found to be more abundant in the pig gut prior to weaning (Zhao et al., 2015). In general, the gut microbiota becomes increasingly stable during animal growth and consequently it becomes more resistant to dietary perturbations (Kim and Isaacson, 2015). This explains why piglets are more susceptible to pathogen infection than adult pigs. It also demonstrates the importance in modulating the gut microbiota of young animals in order to have a healthy microbiota developed for better animal performance.
Several in vivo studies indicated that essential oils increased the Lactobacillus group and decreased E. coli or total coliforms in piglets (Namkung et al., 2004, Castillo et al., 2006, Li et al., 2012, Zeng et al., 2015, Wei et al., 2017). These results were consistent with the results observed in several poultry studies with the supplementation of essential oils (Oviedo-Rondón et al., 2006, Tiihonen et al., 2010, Amerah et al., 2011, Basmacioglu-Malayoglu et al., 2016, Cetin et al., 2016, Liu et al., 2017), suggesting that essential oil treatment led to some fundamental changes within gut microbiota mainly in the number of observed Lactobacillus species. However, studying the effects of essential oils on the gut microbiome of pigs with integrated approaches (e.g., various molecular and “omics” technologies as well as bioinformatics and statistical analyses) is still needed in order to comprehensively monitor the shifts in composition and functionality of the microbiota in response to dietary essential oils treatments.
8. Effect of essential oils on feed palatability and digestibility and nutrient metabolism
Essential oils can increase feed palatability and intake with the enhanced flavor and odor (Kroismayr et al., 2006). However, the observed effect of supplemented essential oils to pig diets on feed intake is not consistent (Neill et al., 2006, Stelter et al., 2013, Zeng et al., 2015). It is believed that the increased feed palatability associated with the supplementation of essential oils could also be due to their antioxidative properties that can preserve the qualities of diets and prevent the release of unfavorable odors from the diets (Franz et al., 2010, Solà-Oriol et al., 2011). Therefore, it might be interesting to replace chemical antioxidants (e.g., ethoxyquin and butylated hydroxytoluene) commonly used in the animal diet with enough amounts of essential oils (natural antioxidants), particularly when chemical antioxidants are prohibited (Yang et al., 2015).
The gastrointestinal tract is the largest and most vulnerable surface to the outside world in the body and it not only absorbs nutrients but also senses luminal nutrients, chemicals and microbes through a chemosensory system with many nerve and receptors (Furness et al., 2013). Recently, gut chemosensory system has received a lot of attention due to the fact that the system can regulate digestion, absorption and metabolism, with potential nutritional and pharmacological applications to improve gut growth, development and health (Mace and Marshall, 2013). Approximately 90% cells in the gut epithelium are absorptive epithelial cells and the cells express various nutrient transporters (Henning et al., 1994). The gut epithelium has also small amount of enteroendocrine cells that can secret gut hormone peptides, such as glucoinsulinotropic polypeptide, glucagon-like peptides 1 and 2 (GLP-1 and 2) and peptide YY (PYY) (Murphy et al., 2006). It has been reported that several taste receptors including the sweet taste receptor T1R1 + T1R2, the umami taste receptor T1R1 + T1R3, bitter taste-sensing type 2 receptors (T2Rs) and other taste receptors are not only expressed in taste buds but also located in gut (Jeon et al., 2011, Daly et al., 2013, Shirazi-Beechey et al., 2014). Moreover, there are also other nutrient receptors including calcium sensing receptor (CaSR) that is also called Kokumi receptor and lipid receptors in the gut (Reimann et al., 2012, Huang et al., 2016). All these receptors are considered to belong to a group of G protein coupled receptors (GPCR) (Reimann et al., 2012, Huang et al., 2016). The major function of nutrient transporters is to absorb luminal nutrients. However, it has been reported that nutrient transporters also contribute to the detection of nutrients in the lumen as transceptors (Hundal and Taylor, 2009). Therefore, amino acids, peptides, glucose, and lipids can be detected by these chemosensors. It can be assumed that other chemicals (e.g., essential oils) in feeds might also be detected by gut chemosensors that have not been identified. Studies indicate that fasting, refeeding and high protein diets can affect the expression of the taste signaling molecule α-transducin throughout the pig gastrointestinal tract, providing further support to the concept that taste receptors contribute to luminal chemosensing in gut (Mazzoni et al., 2013, De Giorgio et al., 2016). The chemosensors transduce information regarding the nutrient profile and concentration of the lumen to regulate intestinal gene expression (e.g., transporters), digestive enzyme and gut peptide secretions, eventually to control feed intake, digestion, absorption and metabolism. Several studies have been shown that phytogenic compounds can regulate the gene expression profile of ileal mucosa (Liu et al., 2013, Liu et al., 2014) and stimulate digestive secretions for improving nutrient digestibility (Janz et al., 2007, Maenner et al., 2011, Li et al., 2012, Ahmed et al., 2013). However, the mechanisms of regulating gene expression relating to immune and digestive functions are still not fully clear. It is very important to identify specific receptors for essential oils, which will help us to understand of the underlying mechanisms.
9. Considerations of the use of essential oils in swine nutrition
Viable alternatives to in-feed antibiotics should have several features: be safe to the public, cost-effective in swine production, and environmentally friendly (Gong et al., 2013). Due to these multiple requirements, so far there is no single alternative that has been identified to completely substitute antibiotics in feeds. It is also challenging to use comprehensive and systematic studies to evaluate the efficacy, cost-effectiveness, and safety of essential oils in swine production. Moreover, other challenges in using essential oils as antibiotic alternatives are some potential side effects (e.g., unpleasant odor/taste and toxic), regulatory concerns and possible interactions with other feed ingredients (e.g., fats) (Lambert et al., 2001, Friedman et al., 2002). The traceability of essential oils in feeds and pig tissues and feasible analytical methods are also important. A complete assessment (e.g., in vitro cell and in vivo animal models) on the toxicity and safety of essential oils is still needed before the compounds can be used extensively in pig feeds.
It is very important to fully understand the mechanism on why antibiotics can promote animal growth, which will help to develop effective alternatives to antibiotics in feeds. Several hypotheses on the potential mechanisms have been proposed: 1) reducing infection by the inhibition of pathogens; 2) making more energy and nutrients available for animals through reduction of total bacterial burden in the gut; 3) increasing nutrient absorption by thinning of the gut mucosal layer; and 4) reducing inflammation though modulation of the immune system (Niewold, 2007, Allen et al., 2013, Yang et al., 2015). Although the above hypotheses are supported by some studies, the mechanisms behind are still not fully understood. This has limited our effort to develop effective antibiotic alternatives including essential oils.
The gastrointestinal tract is a well-organized and complicated ecosystem and organ. It is mainly composed of epithelial cells, the mucosal immune system and microbiome. The gut microbiota has commensal and beneficial bacteria as well as bacterial pathogens. The ecosystem normally stays in homeostasis and the disruption of the homeostasis would affect gut functions and thus compromise gut health, animal growth and well-being. Essential oils have multiple functions as a whole, including antimicrobial, anti-inflammatory, anti-oxidative and disruption of QS as well as enhancing digestion and immunity. It is very critical to define the specific effects and target sites (either animal host or its microbiota) of individual components in essential oils, which will facilitate the application of essential oils in feeds.
Essential oils have been investigated as alternatives to antibiotic in animal production. However, the results obtained from previous studies are highly inconsistent. There are 3 potential reasons associated with the inconsistency: 1) variable dosages that may not be efficacious (Cross et al., 2007), 2) different trial conditions (e.g., environment, animal age, genetics, feeds and health status), and 3) the lack of definition of the essential oils used in the study and the proper characterization of the active compounds involved. Moreover, most essential oils have the MIC values that are significantly higher than the levels that could be acceptable in swine production in terms of cost-effectiveness. The acceptance by the industry to use antibiotic alternatives to optimize the animal performance and health is also dependent on the cost of alternatives.
With their lipophilic characteristics, essential oil compounds may raise concerns about their potential toxicity and possible negative impact on animal health (Ambrosio et al., 2017). Most essential oils are also very volatile and can evaporate rapidly during feed processing and storage, resulting in varied amount of essential oils that are in the feeds and delivered to animals (Lambert et al., 2001). It has been shown that most or all thymol, carvacrol, eugenol and trans-cinnamaldehyde after oral injection were disappeared in the stomach and the upper small intestine in piglets (Michiels et al., 2008). Additionally, essential oils may interact with other components in feeds, leading to compromised antimicrobial activity (Si et al., 2006). Therefore, if not properly protected, majority of essential oils will be lost during feed processing, storage and delivery to the animal gut and thus may fail to reach the lower intestine of animals where most pathogens locate. It will affect the profitability of feed mills and farmers eventually and become one of major barriers for essential oil application in swine production.
A combination of different alternatives to antibiotic may hold the most promising method to substitute antibiotics in animal feeds. There are 3 major reasons: 1) one antibiotic alternative fail to cover all the performance-enhancing properties that antibiotics have; 2) there is a synergistic effect among different alternatives that will reduce effective dosages required to combat pathogens (e.g., organic acids and essential oils); and 3) an integrated approach should be taken to replace antibiotics, including nutrition, biosecurity and management rather than a supplementation of antibiotic alternatives alone (Yang et al., 2015). Several recently published studies have shown that the combined use of different antibiotic alternatives had better effects on the performance and health of weaned pigs when compared with single compounds (Zeng et al., 2015, Walia et al., 2017). It is very important to understand the effects and mechanisms of action of various alternatives, which will help the design for more effective essential oils products to promote animal growth and improve feed efficiency in swine production.
A method that can effectively and practically deliver essential oils is very important for the use of essential oils in swine production. Enteric protections (e.g., microencapsulation and coating) have become the most promising method to solve the problem (Gauthier, 2012). A widely used method is to microencapsulate essential oils in a lipid matrix that could release essential oils as it passes along small intestine (Gauthier, 2012). It has been also reported that alginate-whey protein microparticles is a good carrier for enhancing the gut delivery of carvacrol in pigs (Zhang et al., 2016). Therefore, obviously proper protection technologies can reduce the required effective dosage of essential oils in feeds and reduce the cost of swine production. However, there are still some research possibilities to fully understand protection technologies and optimize delivery of essential oils into the lower gut. Thus, it would be for instance very interesting to investigate the physicochemical and molecular characterization of microparticles, which will bring more knowledge on the mechanisms underlying the phenomenon of stability or release of essential oils and then help optimize microencapsulation techniques to better protect and deliver essential oils. In this regard, the advantages of biodegradable polymer-encapsulated essential oil nanoparticles have made nanotechnology as well an exciting essential oil delivery method in animal gut (Aytac et al., 2017, Manukumar et al., 2017).
10. Conclusions
The ban of AGP in swine production has been implemented in the European Union since 2006. There are more countries that are expected to follow in the coming years. A number of challenges (e.g., enteric infections and compromised growth performance) are associated with the withdrawal of antibiotics from feeds. The development of cost-effective antibiotic alternatives is the largest challenge, which is crucial for the long-term sustainability and profitability of swine production. Essential oils have a number of active ingredients and thus are one of the most promising antibiotic alternatives. However, the applications of essential oils in swine production have been increased slowly, mainly due to their variable outcomes and unclear modes of action. An improved understanding of the mechanisms underlying the functions of essential oils, including the effects on the 3 components in the gut ecosystem: gut microbiota, gut physiology and immunology, will allow us to make the best use of essential oils in swine production. Finally, microencapsulation and nanotechnology provide promising tools to effectively deliver essential oils to the animal gut and improve the efficacy of essential oils in swine production.
Acknowledgments
This work was supported by Natural Sciences and Engineering Council of Canada (NSERC) (CRDPJ 503580-16) Collaborative Research and Development Grants (C. Yang), the University of Manitoba Start-Up Grant (46561), Manitoba Pork Council (47370) and Jefo Nutrition Inc (47369). The authors are grateful to W. Li and Brayden Schindell, who were summer undergraduate students in the Department of Animal Science at the University of Manitoba, for their help in the manuscript preparation.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbaszadeh S., Sharifzadeh A., Shokri H., Khosravi A., Abbaszadeh A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Med Mycol. 2014;24(2):e51–e56. doi: 10.1016/j.mycmed.2014.01.063. [DOI] [PubMed] [Google Scholar]
- Ahmed S.T., Hossain M.E., Kim G.M., Hwang J.A., Ji H., Yang C.J. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas J Anim Sci. 2013;26(5):683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Golian A., Buyse J., Wang Y., De Smet S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult Sci. 2014;93(8):1930–1941. doi: 10.3382/ps.2014-03896. [DOI] [PubMed] [Google Scholar]
- Alberti T.B., Marcon R., Bicca M.A., Raposo N.R., Calixto J.B., Dutra R.C. Essential oil from Pterodon emarginatus seeds ameliorates experimental autoimmune encephalomyelitis by modulating Th1/Treg cell balance. J Ethnopharmacol. 2014;155(1):485–494. doi: 10.1016/j.jep.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21(3):114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Alvarez M.V., Ortega-Ramirez L.A., Gutierrez-Pacheco M.M., Bernal-Mercado A.T., Rodriguez-Garcia I., Gonzalez-Aguilar G.A. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front Microbiol. 2014;5:699. doi: 10.3389/fmicb.2014.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio C.M., de Alencar S.M., de Sousa R.L., Moreno A.M., Da Gloria E.M. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind Crops Prod. 2017;97:128–136. [Google Scholar]
- Amerah A.M., Péron A., Zaefarian F., Ravindran V. Influence of whole wheat inclusion and a blend of essential oils on the performance, nutrient utilisation, digestive tract development and ileal microbiota profile of broiler chickens. Br Poult Sci. 2011;52(1):124–132. doi: 10.1080/00071668.2010.548791. [DOI] [PubMed] [Google Scholar]
- Aytac Z., Ipek S., Durgun E., Tekinay T., Uyar T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017;233:117–124. doi: 10.1016/j.foodchem.2017.04.095. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B., Park J.W., Kim I.H. Evaluation of the effectiveness of supplementing micro-encapsulated organic acids and essential oils in diets for sows and suckling piglets. Ital J Anim Sci. 2016;15(4):626–633. [Google Scholar]
- Barnes P.J., Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Baschieri A., Ajvazi M.D., Tonfack J.L.F., Valgimigli L., Amorati R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017;232:656–663. doi: 10.1016/j.foodchem.2017.04.036. [DOI] [PubMed] [Google Scholar]
- Basmacioğlu-Malayoğlu H., Ozdemir P., Bağriyanik H. Influence of an organic acid blend and essential oil blend, individually or in combination, on growth performance, carcass parameters, apparent digestibility, intestinal microflora and intestinal morphology of broilers. Br Poult Sci. 2016;57(2):227–234. doi: 10.1080/00071668.2016.1141171. [DOI] [PubMed] [Google Scholar]
- Bedford M., Cowieson A. Exogenous enzymes and their effects on intestinal microbiology. Anim Feed Sci Technol. 2012;173(1):76–85. [Google Scholar]
- Bengtsson B., Wierup M. Antimicrobial resistance in Scandinavia after a ban of antimicrobial growth promoters. Anim Biotechnol. 2006;17(2):147–156. doi: 10.1080/10495390600956920. [DOI] [PubMed] [Google Scholar]
- Berchieri-Ronchi C., Kim S., Zhao Y., Correa C., Yeum K.-J., Ferreira A. Oxidative stress status of highly prolific sows during gestation and lactation. Animal. 2011;5(11):1774–1779. doi: 10.1017/S1751731111000772. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Jensen P.Ø., Rasmussen T.B., Christophersen L., Calum H., Hentzer M. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol. 2005;151(12):3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- Booth I.R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49(4):359. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen F., Haesebrouck F., Vanparys A., Volf J., Mahu M., Van Immerseel F. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet Microbiol. 2008;132(3):319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Brackman G., Defoirdt T., Miyamoto C., Bossier P., Van Calenbergh S., Nelis H. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8(1):149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci Technol. 2010;158(1):1–14. [Google Scholar]
- Burt S.A., Ojo-Fakunle V.T., Woertman J., Veldhuizen E.J. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One. 2014;9(4):e93414. doi: 10.1371/journal.pone.0093414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M., Martín-Orúe S., Roca M., Manzanilla E., Badiola I., Perez J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J Anim Sci. 2006;84(10):2725–2734. doi: 10.2527/jas.2004-556. [DOI] [PubMed] [Google Scholar]
- Cetin E., Yibar A., Yesilbag D., Cetin I., Cengiz S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br Poult Sci. 2016;57:780–787. doi: 10.1080/00071668.2016.1214682. [DOI] [PubMed] [Google Scholar]
- Chitprasert P., Sutaphanit P. Holy basil (Ocimum sanctum Linn.) essential oil delivery to swine gastrointestinal tract using gelatin microcapsules coated with aluminum carboxymethyl cellulose and beeswax. J Agric Food Chem. 2014;62(52):12641–12648. doi: 10.1021/jf5019438. [DOI] [PubMed] [Google Scholar]
- Choi S., Ingale S., Kim J., Park Y., Kwon I., Chae B. An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br Poult Sci. 2013;54(6):738–746. doi: 10.1080/00071668.2013.838746. [DOI] [PubMed] [Google Scholar]
- Choo J., Rukayadi Y., Hwang J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- Conner D., Beuchat L. Effects of essential oils from plants on growth of food spoilage yeasts. J Food Sci. 1984;49(2):429–434. [Google Scholar]
- Cooke C.J., Nanjee M.N., Dewey P., Cooper J.A., Miller G.J., Miller N.E. Plant monoterpenes do not raise plasma high-density-lipoprotein concentrations in humans. Am J Clin Nutr. 1998;68(5):1042–1045. doi: 10.1093/ajcn/68.5.1042. [DOI] [PubMed] [Google Scholar]
- Cox S., Gustafson J., Mann C., Markham J., Liew Y., Hartland R. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett Appl Microbiol. 1998;26(5):355–358. doi: 10.1046/j.1472-765x.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Cross D., McDevitt R., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Bri Poult Sci. 2007;48(4):496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Czajkowski R., Jafra S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol. 2009;56(1):1–16. [PubMed] [Google Scholar]
- Daly K., Al-Rammahi M., Moran A., Marcello M., Ninomiya Y., Shirazi-Beechey S.P. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304(3):G271–G282. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgio R., Mazzoni M., Vallorani C., Latorre R., Bombardi C., Bacci M.L. Regulation of α-transducin and α-gustducin expression by a high protein diet in the pig gastrointestinal tract. PLoS One. 2016;11(2):e0148954. doi: 10.1371/journal.pone.0148954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kievit T.R., Iglewski B.H. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68(9):4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange C., Pluske J., Gong J., Nyachoti C. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134(1):124–134. [Google Scholar]
- de Los Santos F.S., Donoghue A., Venkitanarayanan K., Dirain M., Reyes-Herrera I., Blore P. Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult Sci. 2008;87(4):800–804. doi: 10.3382/ps.2007-00280. [DOI] [PubMed] [Google Scholar]
- De Smet S., Michiels J., Ovyn A., Dierick N., Laget M., Cools A. Gut antibacterial effects of C7 and C9 carboxylic acids in the diet of piglets. J Anim Sci. 2016;94(supplement 3):54–57. [Google Scholar]
- Defoirdt T., Boon N., Bossier P., Verstraete W. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquac. 2004;240(1):69–88. [Google Scholar]
- Defoirdt T., Brackman G., Coenye T. Quorum sensing inhibitors: how strong is the evidence? Trends Microbiol. 2013;21(12):619–624. doi: 10.1016/j.tim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Delaquis P.J., Stanich K., Girard B., Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol. 2002;74(1):101–109. doi: 10.1016/s0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S., Long D.J., Jaiswal A.K. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2001;36:201–216. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Diao H., Zheng P., Yu B., He J., Mao X., Yu J. Effects of benzoic Acid and thymol on growth performance and gut characteristics of weaned piglets. Asian-Australas J Anim Sci. 2015;28(6):827. doi: 10.5713/ajas.14.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick N., Decuypere J., Molly K., Van Beek E., Vanderbeke E. The combined use of triacylglycerols containing medium-chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition: I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Livest Prod Sci. 2002;75(2):129–142. [Google Scholar]
- Duke J. CRC Press, Inc; Boca Raton: 1986. Handbook of medicinal herbs. Florida. [Google Scholar]
- Eckel B., Kirchgessner M., Roth F. Influence of formic acid on daily weight gain, feed intake, feed conversion rate and digestibility, 1: investigations about the nutritive efficacy of organic acids in the rearing of piglets. J Anim Physiol Anim Nutr. 1992;67:93–100. [Google Scholar]
- Eckmann L., Kagnoff M.F., Fierer J. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 1995;3(3):118–120. doi: 10.1016/s0966-842x(00)88894-0. [DOI] [PubMed] [Google Scholar]
- Faleiro M. vol. 2. Formatex Research Center; Badajoz, Spain: 2011. The mode of antibacterial action of essential oils; pp. 1143–1156. (Science Against Microbial Pathogens: Communicating Current Research and Technological Advances). [Google Scholar]
- Fang, Li H., Qin T., Li M., Ma S. Thymol improves high-fat diet-induced cognitive deficits in mice via ameliorating brain insulin resistance and upregulating NRF2/HO-1 pathway. Metab Brain Dis. 2017;32(2):385–393. doi: 10.1007/s11011-016-9921-z. [DOI] [PubMed] [Google Scholar]
- Franz C., Baser K., Windisch W. Essential oils and aromatic plants in animal feeding–a European perspective. A review. Flavour Frag J. 2010;25(5):327–340. [Google Scholar]
- Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Furness J.B., Rivera L.R., Cho H.J., Bravo D.M., Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10(12):729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- Galloway W.R., Hodgkinson J.T., Bowden S.D., Welch M., Spring D.R. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2010;111(1):28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- Gauthier R. To protect or not to protect? That is the question! Pig progress. Altern Growth Promot. 2012 Special Issue: 2–3. [Google Scholar]
- Gill A.O., Holley R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol. 2004;70(10):5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill A., Holley R. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol. 2006;108(1):1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gong J., Yin F., Hou Y., Yin Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: potential and challenges in application. Can J Anim Sci. 2013;94(2):223–241. [Google Scholar]
- Greenberg E.P. Bacterial communication and group behavior. J Clin Investig. 2003;112(9):1288–1290. doi: 10.1172/JCI20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.-K., Hwang I.H., Thacker P.A. Use of a micro-encapsulated eucalyptus-medium chain fatty acid product as an alternative to zinc oxide and antibiotics for weaned pigs. J Swine Health Prod. 2011;19(1):34–43. [Google Scholar]
- Hanczakowska E., Szewczyk A., Swiatkiewicz M., Okon K. Short-and medium-chain fatty acids as a feed supplement for weaning and nursery pigs. Pol J Vet Sci. 2013;16(4):647–654. doi: 10.2478/pjvs-2013-0092. [DOI] [PubMed] [Google Scholar]
- Helander I.M., Alakomi H.-L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E.J. Characterization of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem. 1998;46(9):3590–3595. [Google Scholar]
- Henning S., Rubin D., Shulman R. Ontogeny of the intestinal mucosa. Physiol Gastrointest Tract. 1994;1:571–610. [Google Scholar]
- Heo J.S., Park J.W., Lee K.W., Lee S.K., Joh J.W., Kim S.J. Posttransplantation lymphoproliferative disorder in pediatric liver transplantation. Transpl Proc. 2004;36(8):2307–2308. doi: 10.1016/j.transproceed.2004.08.138. [DOI] [PubMed] [Google Scholar]
- Heo J., Opapeju F., Pluske J., Kim J., Hampson D., Nyachoti C. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97(2):207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hong K.-W., Koh C.-L., Sam C.-K., Yin W.-F., Chan K.-G. Quorum quenching revisited—from signal decays to signalling confusion. Sensors. 2011;12(4):4661–4696. doi: 10.3390/s120404661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao A., Ahmed A.S., Subramanian S., Griffin N.W., Drewry L.L., Petri W.A. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature. 2014;515(7527):423–426. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulánková R., Bořilová G. In vitro combined effect of oregano essential oil and caprylic acid against Salmonella serovars, Escherichia coli O157: H7, Staphylococcus aureus and Listeria monocytogenes. Acta Vet Brno. 2011;80(4):343–348. [Google Scholar]
- Huang B., Xiao D., Tan B., Xiao H., Wang J., Yin J. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J Agric Food Chem. 2016;64(1):245–252. doi: 10.1021/acs.jafc.5b05195. [DOI] [PubMed] [Google Scholar]
- Hundal H.S., Taylor P.M. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296(4):E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz J., Morel P., Wilkinson B., Purchas R. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007;75(2):350–355. doi: 10.1016/j.meatsci.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Jensen B.B. The impact of feed additives on the microbial ecology of the gut in young pigs. J Anim Feed Sci. 1998;7(Suppl. 1):45–64. [Google Scholar]
- Jeon T.-I., Seo Y.-K., Osborne T.F. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438(1):33–37. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi J.R., Khazanov N., Senderowitz H., Burdman S., Lipsky A., Yedidia I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci Rep. 2016;6:38126. doi: 10.1038/srep38126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.S., Han M.H., Kim G.Y., Kim C.M., Chung H.Y., Hwang H.J. Schisandrae semen essential oil attenuates oxidative stress-induced cell damage in C2C12 murine skeletal muscle cells through Nrf2-mediated upregulation of HO-1. Int J Mol Med. 2015;35(2):453–459. doi: 10.3892/ijmm.2014.2028. [DOI] [PubMed] [Google Scholar]
- Kantas D., Papatsiros V.G., Tassis P.D., Athanasiou L.V., Tzika E.D. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. 2015;86(1):92–98. doi: 10.1111/asj.12240. [DOI] [PubMed] [Google Scholar]
- Karatzas A., Kets E., Smid E., Bennik M. The combined action of carvacrol and high hydrostatic pressure on Listeria monocytogenes Scott A. J Appl Microbiol. 2001;90(3):463–469. doi: 10.1046/j.1365-2672.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- Kerekes E.B., Deák É., Takó M., Tserennadmid R., Petkovits T., Vágvölgyi C. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J Appl Microbiol. 2013;115(4):933–942. doi: 10.1111/jam.12289. [DOI] [PubMed] [Google Scholar]
- Khan M.S.A., Zahin M., Hasan S., Husain F.M., Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009;49(3):354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- Khor T.O., Huang M.-T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.-N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006;66(24):11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013;26(01):71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Isaacson R.E. The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. 2015;177(3):242–251. doi: 10.1016/j.vetmic.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Kim J., Marshall M.R., Wei C.-i. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43(11):2839–2845. [Google Scholar]
- Kim S., Fan M., Applegate T. Nonruminant nutrition symposium on natural phytobiotics for health of young animals and poultry: mechanisms and application. J Anim Sci. 2008;86(14 Suppl.):E138–E139. doi: 10.2527/jas.2007-0769. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Borewicz K., White B.A., Singer R.S., Sreevatsan S., Tu Z.J. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci. 2012;109(38):15485–15490. doi: 10.1073/pnas.1205147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Nguyen S.G., Guevarra R.B., Lee I., Unno T. Analysis of swine fecal microbiota at various growth stages. Arch Microbiol. 2015;197(6):753–759. doi: 10.1007/s00203-015-1108-1. [DOI] [PubMed] [Google Scholar]
- Kroismayr A., Steiner T., Zhang C. Proc. J. Anim. Sci. Am. Soc. Anim. Sci. 1111 North Dunlap Ave, Savoy, IL 61874 USA. 2006. Influence of a phytogenic feed additive on performance of weaner piglets. 270–270. [Google Scholar]
- Kroismayr A., Sehm J., Pfaffl M., Schedle K., Plitzner C., Windisch W. Effects of avilamycin and essential oils on mRNA expression of apoptotic and inflammatory markers and gut morphology of piglets. Czech J Anim Sci. 2008;53(53):377–387. [Google Scholar]
- Lallès J.-P., Boudry G., Favier C., Le Floc'h N., Luron I., Montagne L. Gut function and dysfunction in young pigs. Physiol Anim Res. 2004;53(4):301–316. [Google Scholar]
- Lambert R., Skandamis P.N., Coote P.J., Nychas G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91(3):453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- LaSarre B., Federle M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77(1):73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B., Reynolds R. Progress in essential oils. Perfum Flavorist. 1983;8(6):69–79. [Google Scholar]
- Lee J.M., Johnson J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. BMB Rep. 2004;37(2):139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Everts H., Beynen A.C. Essential oils in broiler nutrition. Int J Poult Sci. 2004;3(12):738–752. [Google Scholar]
- Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J Anim Sci. 2012;25(11):1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T., Almeida J., Lee J., Bravo D. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic. J Anim Sci. 2013;91(11):5294–5306. doi: 10.2527/jas.2012-6194. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T., Bravo D., Maddox C., Pettigrew J. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J Anim Sci. 2014;92(8):3426–3440. doi: 10.2527/jas.2013-6496. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim Sci J. 2017;88(9):1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Lu X.-M., Lu P.-Z., Zhang H. Bacterial communities in manures of piglets and adult pigs bred with different feeds revealed by 16S rDNA 454 pyrosequencing. Appl Microbiol Biotechnol. 2014;98(6):2657–2665. doi: 10.1007/s00253-013-5211-4. [DOI] [PubMed] [Google Scholar]
- Mace O., Marshall F. Digestive physiology of the pig symposium: gut chemosensing and the regulation of nutrient absorption and energy supply. J Anim Sci. 2013;91(5):1932–1945. doi: 10.2527/jas.2012-5906. [DOI] [PubMed] [Google Scholar]
- Maele L.V., Heyndrickx M., Dominiek M., Nele D., Maxime M., Verlinden M. In vitro susceptibility of Brachyspira hyodysenteriae to organic acids and essential oil components. J Vet Med Sci. 2016;78(2):325–328. doi: 10.1292/jvms.15-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner K., Vahjen W., Simon O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. J Anim Sci. 2011;89(7):2106–2112. doi: 10.2527/jas.2010-2950. [DOI] [PubMed] [Google Scholar]
- Mahmoud B.S. The efficacy of grape seed extract, citric acid and lactic acid on the inactivation of Vibrio parahaemolyticus in shucked oysters. Food Control. 2014;41:13–16. [Google Scholar]
- Manukumar H., Umesha S., Kumar H.N. Promising biocidal activity of thymol loaded chitosan silver nanoparticles (TC@ AgNPs) as anti-infective agents against perilous pathogens. Int J Biol Macromol. 2017;102:1257–1265. doi: 10.1016/j.ijbiomac.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., De Giorgio R., Latorre R., Vallorani C., Bosi P., Trevisi P. Expression and regulation of alpha-transducin in the pig gastrointestinal tract. J Cell Mol Med. 2013;17(4):466–474. doi: 10.1111/jcmm.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J. Ghent University; Belgium: 2009. Effect of essential oils on gut bacteria and functionality in the pig. [PhD thesis] 282p. [Google Scholar]
- Michiels J., Missotten J., Dierick N., Fremaut D., Maene P., De Smet S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J Sci Food Agric. 2008;88(13):2371–2381. [Google Scholar]
- Mine Y., Young D., Yang C. Antioxidative stress effect of phosphoserine dimers is mediated via activation of the Nrf2 signaling pathway. Mol Nutr Food Res. 2015;59(2):303–314. doi: 10.1002/mnfr.201400381. [DOI] [PubMed] [Google Scholar]
- Mith H., Clinquart A., Zhiri A., Daube G., Delcenserie V. The impact of oregano (Origanum heracleoticum) essential oil and carvacrol on virulence gene transcription by Escherichia coli O157: H7. FEMS Microbiol Lett. 2015;362(1):1–7. doi: 10.1093/femsle/fnu021. [DOI] [PubMed] [Google Scholar]
- Mueller K., Blum N.M., Kluge H., Mueller A.S. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic-and antioxidant enzymes in broiler chickens. Br J Nutr. 2012;108(04):588–602. doi: 10.1017/S0007114511005873. [DOI] [PubMed] [Google Scholar]
- Murphy K.G., Dhillo W.S., Bloom S.R. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27(7):719–727. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- Musa, H. H. and H. Seri. 2009. The potential benefits of probiotics in animal production and health.
- Nagura H., Ohtani H., Sasano H., Matsumoto T. The immuno-inflammatory mechanism for tissue injury in inflammatory bowel disease and Helicobacter pylori-infected chronic active gastritis. Digestion. 2001;63(Suppl. 1):12–21. doi: 10.1159/000051905. [DOI] [PubMed] [Google Scholar]
- Nair S., Doh S., Chan J., Kong A., Cai L. Regulatory potential for concerted modulation of Nrf2-and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99(12):2070–2082. doi: 10.1038/sj.bjc.6604703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung H., Gong M.L.J., Yu H., Cottrill M., de Lange C. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84(4):697–704. [Google Scholar]
- Navarrete P., Toledo I., Mardones P., Opazo R., Espejo R., Romero J. Effect of thymus vulgaris essential oil on intestinal bacterial microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum) and bacterial isolates. Aquac Res. 2010;41:e667–e678. [Google Scholar]
- Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill C.R., Nelssen J.L., Tokach M.D., Goodband R.D., DeRouchey J.M., Dritz S.S. Effects of oregano oil on growth performance of nursery pigs. J Swine Health Prod. 2006;14(6):312–316. [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci. 2007;86(4):605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- Niu C., Afre S., Gilbert E. Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett Appl Microbiol. 2006;43(5):489–494. doi: 10.1111/j.1472-765X.2006.02001.x. [DOI] [PubMed] [Google Scholar]
- Noirrit M., Philippe F. Reduction of salmonella prevalence on sows and finishing pigs by use of a protected mix of organic acids and essential oils in the feed of lactating sows and weaned piglets. Journees Recherché Porcine. 2016;48:351–352. [Google Scholar]
- Oh S., Yun W., Lee J., Lee C., Kwak W., Cho J. Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J Anim Sci Technol. 2017;59(1):4. doi: 10.1186/s40781-017-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K., Hayashi H., Shimizu T. The luxS gene is involved in cell–cell signalling for toxin production in Clostridium perfringens. Mol Microbiol. 2002;44(1):171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E., Hume M., Hernandez C., Clemente-Hernández S. Intestinal microbial ecology of broilers vaccinated and challenged with mixed Eimeria species, and supplemented with essential oil blends. Poult Sci. 2006;85(5):854–860. doi: 10.1093/ps/85.5.854. [DOI] [PubMed] [Google Scholar]
- Oyen L., Dung N.X. vol. 19. Backhuys Publ; 1999. (Plant resources of South-East Asia). [Google Scholar]
- Parsek M.R., Val D.L., Hanzelka B.L., Cronan J.E., Greenberg E.P. Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci. 1999;96(8):4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.S., Blumberg R.S. First line of defense: the role of the intestinal epithelium as an active component of the mucosal immune system. J Gastroenterol. 2000;35(11):805–814. doi: 10.1007/s005350070017. [DOI] [PubMed] [Google Scholar]
- Placha I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci. 2014;55(1):105–114. doi: 10.1080/00071668.2013.873772. [DOI] [PubMed] [Google Scholar]
- Podolsky D.K. Inflammatory bowel disease. New Eng J Med. 2002;347(6):417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Puvača N., Stanaćev V., Glamočić D., Lević J., Perić L., MILIĆ D. Beneficial effects of phytoadditives in broiler nutrition. World's Poult Sci J. 2013;69(01):27–34. [Google Scholar]
- Randrianarivelo R., Danthu P., Benoit C., Ruez P., Raherimandimby M., Sarter S. Novel alternative to antibiotics in shrimp hatchery: effects of the essential oil of Cinnamosma fragrans on survival and bacterial concentration of Penaeus monodon larvae. J Appl Microbiol. 2010;109(2):642–650. doi: 10.1111/j.1365-2672.2010.04694.x. [DOI] [PubMed] [Google Scholar]
- Reimann F., Tolhurst G., Gribble F.M. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15(4):421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Rocha-Estrada J., Aceves-Diez A.E., Guarneros G., de la Torre M. The RNPP family of quorum-sensing proteins in Gram-positive bacteria. Appl Microbiol Biotechnol. 2010;87(3):913–923. doi: 10.1007/s00253-010-2651-y. [DOI] [PubMed] [Google Scholar]
- Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Roller S., Seedhar P. Carvacrol and cinnamic acid inhibit microbial growth in fresh-cut melon and kiwifruit at 4° and 8° C. Lett Appl Microbiol. 2002;35(5):390–394. doi: 10.1046/j.1472-765x.2002.01209.x. [DOI] [PubMed] [Google Scholar]
- Rossi R., Pastorelli G., Cannata S., Corino C. Recent advances in the use of fatty acids as supplements in pig diets: a review. Anim Feed Sci Technol. 2010;162(1):1–11. [Google Scholar]
- Schauder S., Shokat K., Surette M.G., Bassler B.L. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41(2):463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- Seongwei L., Najiah M., Wendy W., Nadirah M. Chemical composition and anti-microbial activity of the essential oil Syzygium aromaticum flower bud (clove) against fish systemic bacteria isolated from aquaculture sites. Front Agric China. 2009;3:332–333. [Google Scholar]
- Shirazi-Beechey S.P., Daly K., Al-Rammahi M., Moran A.W., Bravo D. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr. 2014;111(S1):S8–S15. doi: 10.1017/S0007114513002286. [DOI] [PubMed] [Google Scholar]
- Si W., Gong J., Chanas C., Cui S., Yu H., Caballero C. In vitro assessment of antimicrobial activity of carvacrol, thymol and cinnamaldehyde towards Salmonella serotype Typhimurium DT104: effects of pig diets and emulsification in hydrocolloids. J Appl Microbiol. 2006;101(6):1282–1291. doi: 10.1111/j.1365-2672.2006.03045.x. [DOI] [PubMed] [Google Scholar]
- Slifierz M.J., Friendship R.M., Weese J.S. Longitudinal study of the early-life fecal and nasal microbiotas of the domestic pig. BMC Microbiol. 2015;15(1):184. doi: 10.1186/s12866-015-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solà-Oriol D., Roura E., Torrallardona D. Feed preference in pigs: effect of selected protein, fat, and fiber sources at different inclusion rates. J Anim Sci. 2011;89(10):3219–3227. doi: 10.2527/jas.2011-3885. [DOI] [PubMed] [Google Scholar]
- Souza E.L.d., Barros J.C.d., Conceição M.L.d., Gomes Neto N.J., Costa A.C.V.d. Combined application of Origanum vulgare L. essential oil and acetic acid for controlling the growth of Staphylococcus aureus in foods. Braz J Microbiol. 2009;40(2):387–393. doi: 10.1590/S1517-838220090002000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelter K., Frahm J., Paulsen J., Berk A., Kleinwächter M., Selmar D. Effects of oregano on performance and immunmodulating factors in weaned piglets. Arch Anim Nutr. 2013;67(6):461–476. doi: 10.1080/1745039X.2013.858897. [DOI] [PubMed] [Google Scholar]
- Strober W., Fuss I.J., Blumberg R.S. The immunology of mucosal models of inflammation 1. Annu Rev Immunol. 2002;20(1):495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Szabó M.Á., Varga G.Z., Hohmann J., Schelz Z., Szegedi E., Amaral L. Inhibition of quorum-sensing signals by essential oils. Phytother Res. 2010;24(5):782–786. doi: 10.1002/ptr.3010. [DOI] [PubMed] [Google Scholar]
- Tan E.C.-H. Web-based and telephone surveys to assess public perception toward the national health insurance in Taiwan: a comparison of cost and results. J Med Internet Res. 2015;17(5) doi: 10.2196/ijmr.4090. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C., Wei H., Sun H., Ao J., Long G., Jiang S. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed Res Int. 2015;2015 doi: 10.1155/2015/525218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen K., Kettunen H., Bento M.H.L., Saarinen M., Lahtinen S., Ouwehand A. The effect of feeding essential oils on broiler performance and gut microbiota. Br Poult Sci. 2010;51(3):381–392. doi: 10.1080/00071668.2010.496446. [DOI] [PubMed] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49(6):2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A., Kets E., Smid E. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65(10):4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A., Bennik M., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68(4):1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Grijalva N.V., Pinelli-Saavedra A., Muhlia-Almazan A., Domínguez-Díaz D., González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol. 2017;59(1):8. doi: 10.1186/s40781-017-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Maele L., Heyndrickx M., Maes D., De Pauw N., Mahu M., Verlinden M. In vitro susceptibility of Brachyspira hyodysenteriae to organic acids and essential oil components. J Vet Med Sci. 2016;78(2):325–328. doi: 10.1292/jvms.15-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V.H. Carvacrol and thymol reduce swine waste odor and pathogens: stability of oils. Curr Microbiol. 2002;44(1):38–43. doi: 10.1007/s00284-001-0071-z. [DOI] [PubMed] [Google Scholar]
- Vendeville A., Winzer K., Heurlier K., Tang C.M., Hardie K.R. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3(5):383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- Volpatti D., Bulfon C., Tulli F., Galeotti M. Growth parameters, innate immune response and resistance to Listonella (Vibtio) anguillarum of Dicentrarchus labrax fed carvacrol supplemented diets. Aquac Res. 2013;45:31–44. [Google Scholar]
- Walia K., Argüello H., Lynch H., Leonard F.C., Grant J., Yearsley D. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev Vet Med. 2017;137:28–35. doi: 10.1016/j.prevetmed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang W., Jin Z., Du B., Ding Y., Ni T. Screening and diversity of plant growth promoting endophytic bacteria from peanut. Afr J Microbiol Res. 2013;7(10):875–884. [Google Scholar]
- Wang H., Fang H., Cai D. Opinions matter: a general approach to user profile modeling for contextual suggestion. Inf Retr J. 2015;18(6):586–610. [Google Scholar]
- Waters W., Sacco R., Dorn A., Hontecillas R., Zuckermann F., Wannemuehler M. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Vet Immunol Immunopathol. 1999;69(1):75–87. doi: 10.1016/s0165-2427(99)00043-4. [DOI] [PubMed] [Google Scholar]
- Wei H.K., Xue H.X., Zhou Z., Peng J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal. 2017;11(2):193–201. doi: 10.1017/S1751731116001397. [DOI] [PubMed] [Google Scholar]
- Wendakoon C.N., Sakaguchi M. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Prot. 1995;58(3):280–283. doi: 10.4315/0362-028X-58.3.280. [DOI] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86(14_suppl):E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Wondrak G.T., Villeneuve N.F., Lamore S.D., Bause A.S., Jiang T., Zhang D.D. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15(5):3338–3355. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier K.B., Bassler B.L. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol. 2003;6(2):191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M., Huo Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4(1):137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap P.S.X., Yiap B.C., Ping H.C., Lim S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. 2014;8(1) doi: 10.2174/1874285801408010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Xu X., Zhang Q., Li P., Zhao P., Li Q. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim Sci J. 2015;86(3):279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Ferrara F., Vahjen W., Van Kessel A., Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim Health Res Rev. 2011;12(01):83–93. doi: 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Männer K., Pieper R., Vahjen W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch Anim Nutr. 2012;66(1):14–26. doi: 10.1080/1745039x.2011.644916. [DOI] [PubMed] [Google Scholar]
- Zentek J., Ferrara F., Pieper R., Tedin L., Meyer W., Vahjen W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J Anim Sci. 2013;91(7):3200–3210. doi: 10.2527/jas.2012-5673. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Q.C., Yu H., Zhu J., de Lange K., Yin Y. Evaluation of alginate–whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. J Sci Food Agric. 2016;96(8):2674–2681. doi: 10.1002/jsfa.7385. [DOI] [PubMed] [Google Scholar]
- Zhao J., Harper A., Estienne M., Webb K., McElroy A., Denbow D. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J Anim Sci. 2007;85(5):1302–1310. doi: 10.2527/jas.2006-434. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Flowers W., Saraiva A., Yeum K.-J., Kim S. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J Anim Sci. 2013;91(12):5848–5858. doi: 10.2527/jas.2013-6388. [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang Y., Liu S., Huang J., Zhai Z., He C. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One. 2015;10(2):e0117441. doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Ji B., Zhang H., Jiang H., Yang Z., Li J. Synergistic effect of thymol and carvacrol combined with chelators and organic acids against Salmonella Typhimurium. J Food Prot. 2007;70(7):1704–1709. doi: 10.4315/0362-028x-70.7.1704. [DOI] [PubMed] [Google Scholar]
- Zhou L., Zheng H., Tang Y., Yu W., Gong Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett. 2013;35(4):631–637. doi: 10.1007/s10529-012-1126-x. [DOI] [PubMed] [Google Scholar]
- Zhu J., Yin X., Yu H., Zhao L., Sabour P., Gong J. Involvement of quorum sensing and heat-stable enterotoxin a in cell damage caused by a porcine enterotoxigenic Escherichia coli strain. Infect Immun. 2011;79(4):1688–1695. doi: 10.1128/IAI.01281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Wang J., Peng J., Wei H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 Cells. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/5987183. 5987183. [DOI] [PMC free article] [PubMed] [Google Scholar]


