Abstract
An optimally functioning gastrointestinal tract (GIT) clearly is of importance to the overall metabolism, physiology, disease status and performance of pigs of all stages of growth and development. Recently, the ‘health’ of the GIT (‘gut health’) has attracted much attention despite the lack of a clear definition to the term or its aetiology, although in broad terms, ‘gut health’ encompasses a number of physiological and functional features including nutrient digestion and absorption, host metabolism and energy generation, a stable and appropriate microbiota/microbiome, defence mechanisms including barrier function and mucosal immune mechanisms, and the interactions between these components. ‘Gut health’ in the newly-weaned (young) pig is of obvious interest due to changes in GIT structure and function associated with the post-weaning transition, and more recently to the upsurge in interest in different feed additives as dietary alternatives/replacements caused by bans/reductions in certain antimicrobial compounds being available in some parts of the world. In the presence of enteric disease(s) after weaning, a deterioration in ‘gut health’ may be synonymous to the overall health of the pig, and although some direct relationships can be drawn between pig performance and efficiency and a ‘healthy’ GIT, sometimes this connection is subtler and less obvious, especially in the absence of overt enteric disease(s). The factors and conditions involved in ‘gut health’ are multifactorial, complex, often poorly described and sometimes incorrectly interpreted, although it is evident that perturbations of the GIT can cause an imbalance and disturb the generalized homeostasis. In addition to any enteric diseases or conditions that might arise as a result of these disturbances, other influences will also impact such as the responses occurring in the GIT in the period immediately after weaning, any changes that might occur after a change in diet, and (or) disruptions to meal patterns and hence the flow of nutrients. Ultimately, ‘gut health’ represents the outcome of the GIT in response to its capacity and ability to respond and adapt to the insults and challenges it encounters.
Keywords: Gastrointestinal tract, Gut health, Structure and function, Pig, Piglet, Weaning
1. Introduction
Gastrointestinal tract (GIT) health (‘gut health’) is a term used very commonly, and is the subject of enormous interest currently throughout the world, yet generally lacks a precise and unifying meaning or aetiology. Several recent articles and reviews (e.g., Adewole et al., 2016, Bischoff, 2011, Celi et al., 2017, Jayaraman and Nyachoti, 2017, Kogut and Arsenault, 2016, Moeser et al., 2017, Pluske, 2013) have summarized valuable and timely information relating to this particular topic in a number of different species, and it is not our intention to reiterate all details included in these publications. With regard to a working definition of gut health, however, Kogut and Arsenault (2016) defined it as the ‘absence/prevention/avoidance of disease so that the animal is able to perform its physiological functions in order to withstand exogenous and endogenous stressors’. Whilst not disagreeing with this interpretation, we consider that gut health is more general and can be described as a generalized condition of homeostasis in the GIT, with respect to its overall structure and function. In accordance with the World Health Organisation (WHO) definition of ‘health’ from 1948 (cited by Bischoff, 2011), which proposed a positive definition instead of ‘the absence of diseases’. Bischoff (2011) commented that ‘gut health’ is ‘a state of physical and mental well-being in the absence of GI (gastrointestinal) complaints that require the consultation of a doctor, in the absence of indications of or risks for bowel disease and in the absence of confirmed bowel disease’. This clearly pertains to human health; nonetheless, Bischoff (2011) argued that although the WHO defined health as being more than just the absence of disease, prevention or avoidance of GIT disease forms an integral part of our understanding of the overall issue.
These definitions typically associate gut health with pathogens that cause, either clinically or sub clinically, illness, mortality and (or) morbidity to pigs, and subsequent economic losses. However, and in agreement with the definition of Bischoff (2011), we propose that gut health in pigs can be compromised even in the absence of any overt disease(s) in the GIT. The low feed intake after weaning (Dong and Pluske, 2007), for example, means an absence of luminal nutrition (Diamond and Karasov, 1983), and stressors and challenges associated with weaning also cause changes to the structure and function of the GIT (Celi et al., 2017, Kim et al., 2012, Jayaraman and Nyachoti, 2017, Moeser et al., 2017, Pluske et al., 1997). Together, the immediate post-weaning period in pigs not only causes marked structural and functional changes to the small intestine (e.g., Kelly et al., 1991a, Kelly et al., 1991b, Pluske et al., 1996a, Pluske et al., 1996b), but also contributes to an intestinal inflammatory status that in turn compromises villous-crypt architecture (e.g., McCracken et al., 1999, Spreeuwenberg et al., 2001, Pié et al., 2004), GIT barrier function (e.g., Camilleri et al., 2012, Kim et al., 2012, Moeser et al., 2017, Wijtten et al., 2011), and disruption of the microbiota (e.g., Fouhse et al., 2016, Gresse et al., 2017, Schachtschneider et al., 2013).
Bischoff (2011) further defined 5 major criteria that could form the basis of an overarching definition of gut health, with these being: 1) effective digestion and absorption of food, 2) absence of GI illness, 3) normal and stable intestinal microbiome, 4) effective immune status, and 5) status of well-being. In general agreement, Celi et al. (2017) remarked that the key components of GIT functionality are diet, effective structure and function of the gastrointestinal barrier, host interaction with the gastrointestinal microbiota, effective digestion and absorption of feed, and effective immune status. Nevertheless, and whilst correct, the functions of the GIT extend beyond the processes associated with processes such as feed intake, digestion, and the subsequent active or passive absorption and barrier function, as the GIT plays a major role in regulating epithelial and immune functions of vital importance for normal biological functioning and homeostasis in both the GIT and the body. The association between the enteric nervous system (ENS) and the higher centres via the parasympathetic nervous system and (or) endocrine system also plays a key role in animal well-being, health, and structure and function of the GIT (Moeser et al., 2017, Fig. 1). For example, a study in germ-free mice reported that the GIT microbiome directly influenced not only functions of the GIT but also the development of behaviour and corresponding neurochemical changes in the brain (Neufeld et al., 2011). The precise mechanisms of how the GIT microbiome contributes to gut health, however, are less clear, although there is considerable recent work attempting to unravel such mechanisms (e.g., reviews by Carabotti et al., 2015, Foster et al., 2017).
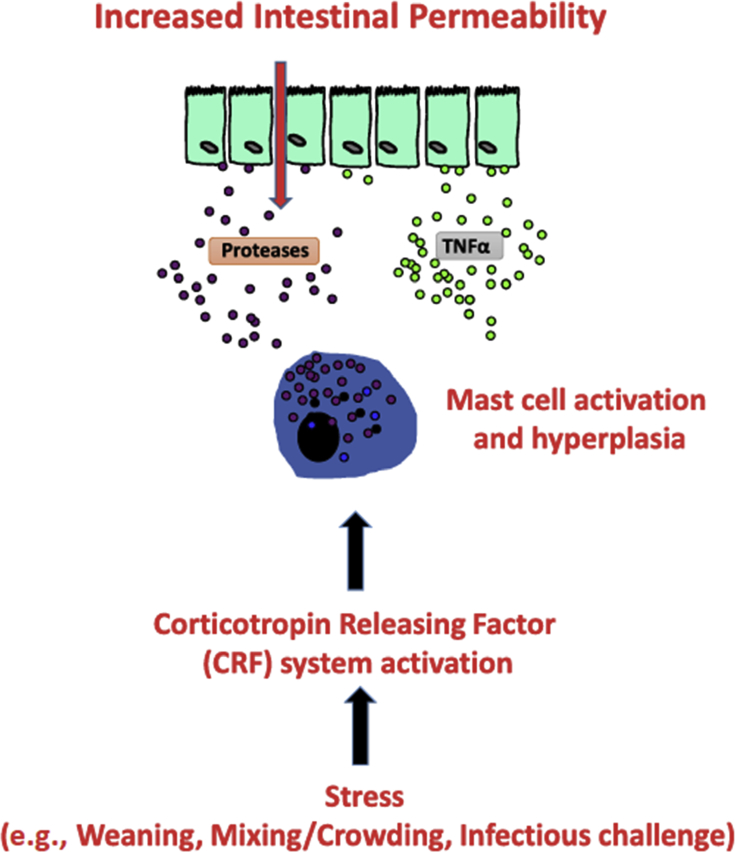
Fig. 1.
A generalized overview of the impacts of weaning stressors on the developmental trajectory of gastrointestinal tract barrier function, after Moeser et al. (2017). TNFα = tumor necrosis factor α.
2. Underlying biological mechanisms associated with a healthy GIT
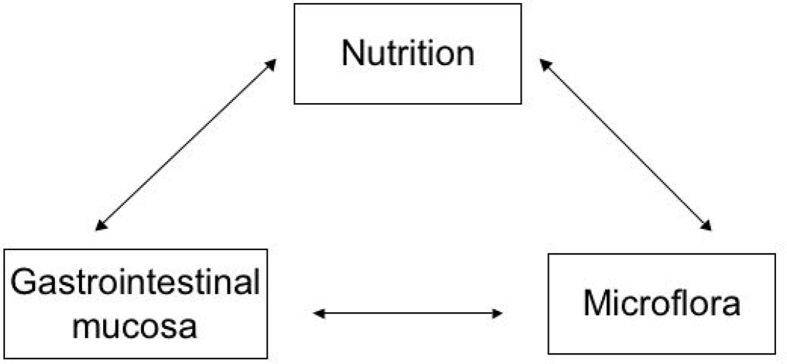
The GIT is a very complex, dynamic and ever-changing organ, with for example the GIT of young pigs at weaning undergoing rapid changes in size, protein turnover rates, microbiota mass and composition, and quick and marked alterations in digestive, absorptive, barrier and immune functions (e.g., Boudry et al., 2004, Cranwell, 1995, Hampson, 1986a, Hampson, 1986b, Lallès et al., 2004, Lallès et al., 2007a, Lallès et al., 2007b, Moeser et al., 2017, Pluske, 2013, Pluske, 2016, Pluske et al., 1996a, Pluske et al., 1996b, Pluske et al., 1997, Pluske et al., 2003). In effect, the complex interactions occurring in the GIT between nutrition, the mucosa (epithelium) of the GIT, and the microbiota are key in impacting gut health (Fig. 2). Whilst there has been a very large body of research conducted in increasing our understanding of the various factors and influences on morphological, anatomical, microbial, enzymatic and immunological changes occurring at key stages during development, less emphasis has been placed on more functional characteristics of the GIT in regard to gut health (and arguably the management of the GIT at critical life stages and/or during critical production impositions) and how this may be affected, for example by nutrition and feeding. Bischoff (2011) commented that the 2 prime functional entities key to achieving a healthy GIT system, in accord with the 5 criteria listed previously, are the GIT microbiota/microbiome (e.g., Frese et al., 2015, Gaskins, 2001, Gresse et al., 2017) and the function of the GIT barrier (e.g., Camilleri et al., 2012, Moeser et al., 2017, Wijtten et al., 2011), and the interaction between the two. Moreover, the impacts of nutrition on these functions must also be considered (Fig. 2).
Fig. 2.
A schematic representation of the complex mutual interactions that occur between nutrition, the gastrointestinal mucosa (incorporating barrier function), and the microflora (microbiota/microbiome), adapted from Niewold (2006).
Of course, and in context, the pork industry's attention, understanding and appreciation of these 2 factors has increased considerably in the last 10 to 15 years due predominately to changes reducing the use of antimicrobials, especially antibiotic growth promoters (AGP; substances that affect intestinal bacteria and digestive function that are administered at a low, sub-therapeutic dose) and (or) heavy minerals, such as zinc (ZnO) and copper (CuSO4). It is simply outside the scope of this review to discuss the various issues posed for and against such bans/restrictions on AGP and (or) heavy metals, nor to discuss the varied purported modes of action of AGP (see reviews, for example, by Anderson et al., 1999, Dibner and Richards, 2005, Gaskins et al., 2002, Kil and Stein, 2010, Thacker, 2013), but merely to highlight that such changes have caused a marked shift in the nature and volume of the research being conducted pertaining to gut health. In this regard, discussion relating to the microbiota/microbiome, barrier function and the immune systems of the GIT, is highly germane to this topic.
There is a wide array of products, such as feed additives, and feeding/management strategies available that influence, or purport to influence, different aspects of gut health (e.g., Adewole et al., 2016, Cheng et al., 2014, de Lange et al., 2010, Heo et al., 2013, Jayaraman and Nyachoti, 2017, Pluske, 2013, Pluske et al., 2002). The large number of feed and (or) water additives available to pork producers to use as alternatives or replacements to AGP that have been evaluated are generally aimed at: 1) enhancing the pigs' immune responses (e.g., immunoglobulin, ω-3 fatty acids, yeast derived β-glucans), 2) reducing pathogen load in the pig's GIT (e.g., organic and inorganic acids, high levels of zinc oxide, essential oils, herbs and spices, some types of prebiotics, bacteriophages, anti-microbial peptides), 3) stimulating establishment of beneficial GIT microbes (e.g., probiotics and some types of prebiotics), and (or) 4) stimulating digestive function (e.g., butyric acid, gluconic acid, lactic acid, glutamine, threonine, cysteine, and nucleotides) (de Lange et al., 2010). These products are characterized predominately not only by their different modes of action, but also by the variation in responses seen when offered to pigs. This variation is presumably a consequence, in part, of the many different conditions of management that pigs are under, that in turn influences factors such as composition of the microbiota and mucosal immunity. Ultimately, the cost-benefit of adopting such practices to influence GIT health requires consideration.
2.1. Microbiota/microbiome of the gastrointestinal tract: Implications for gut health
It is not our intention to describe the generalized features of the GIT microbiota because these have been adequately portrayed in numerous papers and review articles. Nevertheless, it is evident that myriad of factors influence the diversity and activity of the GIT microbiota, including colonization and associated succession of microbial populations, the age of the pig and the environment it inhabits, antimicrobial agents, dietary composition, feed additives, feed processing, feeding methods, disease load, weaning, season, environment, stress and genetics. Furthermore, the intestinal microbiota (or microbiome, representing the genomic information of the microbiota) represents a compromise between helpful barrier functionality, synthesis of beneficial nutrients and proteins and improved energy harvest from dietary components with low inherent potential, and the deleterious effects of inflammation and sub-clinical (and clinical) pathologies (Celi et al., 2017).
Expectedly, there is a varied assemblage of bacteria that diverges in population density and diversity in different compartments of the GIT and at different stages in the life of a pig (Holman et al., 2017, Leser et al., 2002, Richards et al., 2005, Zhao et al., 2015). Additionally, the microbiota is intimately involved in cross talk between the enteric bacteria and the host, with the chemistry and distribution of bacterial binding sites on gut mucosal surfaces playing key roles in determining host and tissue susceptibility and in triggering host responses, especially in young animals (e.g., Kelly and King, 2001, Montagne et al., 2003, Celi et al., 2017). Some of the discussion with regard to the microbiota and gut health focusses simplistically on ‘good’ versus ‘bad’ bacteria and their impact on GIT structure and function, however Hillman (2004) proposed that emphasis in relation to the composition and diversity of the GIT microbiota should be placed on an optimal microbiota being present in the GIT rather than a normal microbiota, because commensal and pathogenic co-exist normally, even in the absence of overt disease, for much of a pig's productive life. Hence, the presence or absence of a pathogenic organism may not necessarily predict that disease will occur unless numbers proliferate to such an extent to overwhelm the general microbial population in the GIT, or more specifically a specific region of the GIT (Hopwood et al., 2005). This will vary according to production sites (e.g., indoor or outdoor sites; Schmidt et al., 2011), genetics, diets and so on. In this respect, Hillman (2001) showed that lactobacilli having anti-pathogenic activity against pathogenic F4 Escherichia coli were unevenly distributed across 19 Scottish pig farms, likely explaining variations seen in the efficacy of probiotics and other additives. The addition of an antimicrobial to the GIT where there is a population of bacteria already possessing a high indigenous antimicrobial activity is likely to be less effective than adding it where there is a population that shows little or no pre-existing antimicrobial activity (Hillman, 2001). The consistency of activity (e.g., across farms, across diets, across seasons) rather than the degree of activity of selected feed additives that modulate the GIT bacteria, that in turn may impact on gut health, is currently poorly understood with respect to the large number of antibiotic replacements/alternatives on the market at present. Arguably, in this regard, a focus for gut health should be on supporting the animal to regulate shifts in the intestinal microbiome such that rapid population swings are avoided and equilibrium can be maintained.
2.2. Gut microbiota disruptions and post-weaning diarrhoea
Any discussion of gut health in the post-weaning period must include the potential impacts of enterotoxigenic E. coli (ETEC) strains (serotypes) associated with infections and disease, i.e., post-weaning colibacillosis (or post-weaning diarrhoea; PWD) (e.g., Fairbrother et al., 2005, Francis, 2002, Lallès et al., 2007b, Pluske et al., 2002), and the means to prevent or control it. Amongst the physiological and GIT factors impacted by the weaning transition, microbiota disruption in the GIT is likely a key influence leading to PWD. Most of the studies conducted during the weaning transition have reported a decrease in bacteria of the Lactobacillus spp. group and a loss of microbial diversity, whereas Clostridium spp., Prevotella spp. or facultative anaerobes such as Proteobacteriaceae, including E. coli, were positively impacted (Gresse et al., 2017). The piglets' change from sows' milk to a solid diet of different composition and form undeniably plays a major role in the predisposition to diarrhoea after weaning, both of microbial and dietary origin. Furthermore, in-feed and (or) in-water antibiotics also cause differences in the GIT microbiota at weaning due to their wide spectrum of activity and thus their potential ability to kill or prevent the growth of both pathogenic and beneficial microbes (Gresse et al., 2017). The diversity of the microbiota may be even more decreased (Looft et al., 2012) with the extended use of AGP, which can increase opportunities for pathogenic microorganisms to colonize and trigger diseases (Fouhse et al., 2016). In this regard, antimicrobial resistance is also key to any discussions pertaining to the use of antibiotics.
Consequently, weaning under commercial conditions has been associated with a disrupted state of the microbiota, or a dysbiosis (Lallès et al., 2007a). However, the precise underlying characteristics allowing the prediction of such a state are not completely clear. Recent work, however, by Dou et al. (2017) assessed whether the GIT bacterial community diversity and composition during the suckling period were associated with differences in susceptibility of pigs to PWD. Using a molecular characterisation of faecal microbiota with CE-SSCP fingerprinting, next generation sequencing and qPCR, Dou et al. (2017) showed that diarrheic and healthy pigs, i.e., with respect to having PWD or not, were mainly discriminated as early as post-natal day 7, i.e., 4 weeks before the occurrence of PWD. At post-natal day 7, the healthy pigs (no PWD) displayed a lower evenness and a higher abundance of Prevotellaceae, Lachnospiraceae, Ruminocacaceae and Lactobacillaceae compared with the diarrheic pigs (after weaning). Regression analyses indicated that these bacterial families were strongly correlated to a higher Bacteroidetes abundance observed in the healthy pigs (no PWD) 1 week before diarrhoea occurred. These authors emphasized the potential of early microbiota diversity and composition, in lactation, as being an indicator of susceptibility to PWD.
As alluded to previously, a feature of the GIT after weaning is an inflammatory response. Zeng et al. (2017) remarked that perturbations of the microbiota are commonly observed in diseases involving inflammation in the GIT, with the inflamed microenvironment being particularly conducive to overgrowth of Enterobacteriaceae, which acquire fitness benefits while other families of symbiotic bacteria succumb to environmental changes inflicted by inflammation. Gastrointestinal tract inflammatory host-response mechanisms produce reactive species such as nitric oxide that when released into the GIT lumen is rapidly transformed into nitrate. This nitrate-rich environment confers growth advantages on some strains of E. coli, which possess nitrate reductase genes that are absent in species of Clostridia or Bacteroidia (Gresse et al., 2017). A recent study by Wei et al. (2017) reported an increased concentration of reactive oxygen species in the intestine coupled with an expansion of the E. coli population 7 days after weaning. Consequently, there is much interest in the use of assessing antioxidant status (Buchet et al., 2017) and compounds (Zhu et al., 2012) to mitigate this aspect of gut health.
3. Barrier function and mucosal immune aspects of the GIT
An integral issue when discussing gut health, interconnected to the effects of the microbiota/microbiome and the host, is that of epithelial barrier function and the mucosal immune system (Burkey et al., 2009, Pluske et al., 2018). The mucosal immune system is continuously challenged by external (e.g., diet, aerosols) and internal (e.g., the microbiota) factors, hence numerous cell types such as dendritic cells, lymphocytes (adaptive immune system), macrophages and cytokines (innate immune system) have evolved to play important functions in the regulation of the communication between the GIT microbiome and its mucosal immune system. As described by Moeser et al. (2017), the intestinal epithelial cells act as immune sentinel cells by recognizing pathogenic signal molecules and secreting interleukins (IL) and growth factors (e.g., IL-17A, IL-33, IL-23 and transforming growth factor-β), which have important immunomodulatory properties. The resident immune cells and related gut-associated lymphoid tissue constitute the largest immune organ in the body. Given the massive antigenic luminal environment and continual exposure to luminal products, the GIT immune system is tightly regulated via a number of molecular mechanisms, to prevent excessive activation and inflammation in response to these factors. Conversely, the GIT immune system must also rapidly and strongly respond to any violation in barrier function or in the event of a pathogenic/antigenic challenge, to mobilize innate and adaptive immune responses, which is critical in preventing the systemic spread of infection and inflammation (Moeser et al., 2017, Pluske et al., 2018).
As alluded to previously, epithelial barrier function is compromised in the immediate post-weaning period, where weaning causes a more permeable small intestine (e.g., Spreeuwenberg et al., 2001; Moeser et al., 2007; Pohl et al., 2017). Inflammation of the intestine is associated with increased permeability that may lead to translocation of toxins, allergens, viruses or even bacteria. If and when bacteria cross this first line of defence and reach the lamina propria, their metabolites or mediators liberated from epithelial cells may cause an inflammatory response, and in this case the measurement of pro-inflammatory cytokines provides some information as to the degree of local inflammation (Johnson, 1997). Therefore, weaning per se, and essentially the period of anorexia that occurs immediately after weaning, causes an inflammatory response (e.g., McCracken et al., 1999, Pié et al., 2004) that initiates perturbations to gut health; in this case, simply encouraging pigs to eat more feed after weaning assists in ameliorating these responses (Pluske et al., 1997).
Moeser et al. (2017) have provided a comprehensive review of the interactions and associations between weaning stress and GIT barrier development and function, with a discussion on implications for lifelong gut health in pigs. Again, it is not our intention to re-examine this, but to reiterate that early life stressors such as weaning alter the developmental trajectory of GIT barrier functions leading potentially to long-lasting deleterious consequences on gut health, disease susceptibility, and production consequences. As concluded by Moeser et al. (2017), the concept of early life origins of GIT disease susceptibility in the pig is supported by paradigms in humans where early life adverse events (e.g., psychological trauma, inflammation, infection) are risk factors for GIT inflammatory and functional diseases later in life. Greater understanding and appreciation of how early-life stressors and challenges, such as weaning, mould long-term epithelial, immune, and ENS functions, are needed to discover new targets and (or) management interventions to promote optimal GIT development and longer-term gut health. In this regard, products such as cromolyn, a mast cell stabilizing agent, may impart benefits for improved barrier function and performance in the post-weaning period (Mereu et al., 2015, Table 1).
Table 1.
The post-weaning performance of piglets injected i.p. with either saline (Control) or 20 mg/kg BW sodium cromolyn (cromolyn) at 0.5, 8 and 16 h relative to weaning.1
| Item | Treatments |
SEM | P-value | |
|---|---|---|---|---|
| Control | Cromolyn | |||
| Body weight, kg | ||||
| Initial (d 1) | 6.3 | 6.6 | 0.2 | 0.418 |
| Final (d 36) | 16.5 | 17.9 | 0.2 | 0.002 |
| ADG, g | 235 | 283 | 11.7 | 0.006 |
| ADFI2, g | 313 | 369 | 13.6 | 0.009 |
| Gain:feed2 | 0.40 | 0.59 | 0.2 | 0.042 |
ADG = average daily gain; ADFI = average daily feed intake.
Adapted from Mereu et al. (2015). Values are least squares means ± SEM.
Calculated using data until d 29.
4. Impacts of pre-weaning and post-weaning nutrition and management on gut health after weaning
There is a plethora of studies and reviews concerning the nutritional management and control of the GIT to influence gut health, even in the absence of manifest enteric disease(s), and we will not repeat the major outcomes of those studies in this review. Nevertheless, it would be remiss not to reiterate the conclusions of Jayaraman and Nyachoti (2017) in that husbandry practices relating to feeding and nutrition, animal welfare, biosecurity and disease prevention are important determinants of gut health and piglet performance, and subsequently adopting high husbandry practices is critical in implementation of strategies aimed at raising pigs in environments with reduced use of antibiotics, or without the use of AGP and (or) minerals such as Zn and Cu. The following discussion commences with an examination of supplementary feeding and pre-weaning management strategies as possible agents to influence gut health after weaning, and concludes with a section related to aspects of a diet fed after weaning that could be considered in the context of enhancing gut health.
4.1. Supplementary feeding of piglets in lactation to enhance gut health after weaning
Creep feeding is a long-established practice of offering a solid diet (a ‘creep’ feed) to piglets while they are still sucking the sow. Traditionally this feed was presented in a sectioned-off area (the ‘creep’) of the farrowing crate in order to prevent the mother from gaining access to the feed, however now the term is simply used for feed offered to piglets in lactation (English, 1981). Providing dry (creep) diets to piglets in lactation presents opportunities for improving weaning weights and post-weaning pig performance, ostensibly through the stimulation of digestive enzymes associated with carbohydrate and protein digestion, and (or) tolerance to antigens present in the diets fed after weaning. It has been proposed that creep feeding becomes more important and beneficial as weaning age increases, because as piglets grow, their demand for nutrients similarly grows and with increasing age this demand outstrips the capacity of the sow to supply them, as the sow's milk yield peaks at around 3 weeks and then slowly declines (Pluske et al., 1995). English et al. (1980) suggested that a piglet should consume, on average, 600 g of creep feed before weaning to become prepared for the process and mitigate the post-weaning growth check. At current weaning ages, this level of individual intake is unattainable.
Not surprisingly, therefore, this area of feeding and management has attracted a plethora of research and development given its potential impacts on post-weaning GIT structure and function, and hence performance. However, evidence to support the notion that creep feeding in lactation prepares the GIT for the post-weaning challenges is equivocal. Chapple et al. (1989) reported that the variation in amylolytic activity in the pancreas of piglets was more a function of the sow (litter of origin) than of the intake of solid feed during lactation and immediately after weaning. Similarly, Lindemann et al. (1986) and de Passille et al. (1989) found that pepsin and maltase activities in the GIT could not be related to weaning weight or to the duration of creep feeding during lactation. Bruininx et al. (2004) reported the lack of any association between creep feed intake before weaning and gut structure at 5 days after weaning. A likely reason for these findings is the generally low amount of dry matter piglets consume whilst sucking the sow (Brooks and Tsourgiannis, 2003, King and Pluske, 2003, Pluske et al., 1995).
Nevertheless, studies in which piglets were categorized into eaters and non-eaters of creep feed have provided some new insights on the value of creep feeding after weaning (e.g., Bruininx et al., 2002, Pluske et al., 2007, Sulabo et al., 2010). These studies have generally shown that only a certain proportion of pigs (about 45% to 65%) within the litter consume creep feed and that eaters (i.e., piglets in the litter that positively consumed creep feed) have better initial post-weaning exploratory behaviours, feed intake and growth performance than non-eaters (i.e., piglets that did not consume creep feed). Increasing the proportion of individual pigs consuming creep feed within litters may elicit positive effects on nursery performance. Therefore, it is important to identify factors that may create more eaters of creep feed in whole litters. However, Carstensen et al. (2005) reported that intestinal function associated with a voluntary low creep feed contact during the suckling period led to decreased feed intake just after weaning, and in turn reduced the intestinal proliferation of E. coli O149 in these piglets.
Despite the general lack of positive associations between creep (solid) feed, weaning weight and gut health after weaning, there is good evidence that providing supplemental feed in liquid or gruel form before and (or) after weaning increases dry matter intake during lactation (e.g., reviews by King and Pluske, 2003, Pluske et al., 1995, Pluske et al., 2005), increases weaning weight, and can reduce the severity of the growth check after weaning. Studies by Pluske et al. (1997) clearly demonstrated that aspects of GIT structure and function could be maintained in the immediate post-weaning period by offering milk liquid diets to newly-weaned pigs, commensurate with little deviation in villous and crypt architecture and function compared with sow-suckled counterparts. However, pigs offered a solid weaner diet at the same level of intake as pigs offered a milk liquid diet showed atrophy of the epithelial structure. These changes can be viewed as a compromise of gut health in these pigs, however despite these morphological changes, the performance of pigs at the same level, of dry matter intake was mostly similar (Table 2). These data highlight some of the ambiguity in discussions concerning gut health, i.e., the pigs grew very well after weaning, the structural and functional aspects of their GIT were negatively impacted. Furthermore, and in the absence of any intestinal diseases in these pigs, these data reinforce the need to define the context of any discussions pertaining to the subject.
Table 2.
The performance of pigs up until weaning at 28 days of age and for 5 days after weaning when fed cows' liquid milk at calculated maintenance (Ma), 2.5 times Ma or ad libitum levels, or in pigs fed a solid starter diet (Starter).1
| Item | Treatments |
SED | P-value | ||||
|---|---|---|---|---|---|---|---|
| SR | Starter | Ma | 2.5 Ma | Ad libitum | |||
| BW, kg | |||||||
| Weaning | 8.9 | 9.0 | 9.1 | 9.2 | 9.2 | 0.86 | >0.05 |
| After 5 days | – | 10.5 | 9.4 | 10.5 | 11.7 | 0.86 | >0.05 |
| Empty BW, kg | |||||||
| Weaning | 8.7 | 8.8 | 8.9 | 9.0 | 9.0 | 0.85 | >0.05 |
| After 5 days | – | 10.0 | 9.2 | 10.3 | 11.3 | 0.84 | >0.05 |
| Daily gain, g | |||||||
| Live weight | – | 288 | 58 | 272 | 514 | 80.1 | <0.001 |
| Empty BW | – | 231 | 49 | 253 | 463 | 74.2 | <0.001 |
| Voluntary feed intake, g DM/d | – | 286 | 102 | 234 | 400 | 41.0 | <0.001 |
| Energy intake, MJ GE/d | – | 5.1 | 2.3 | 5.2 | 8.9 | 0.76 | <0.001 |
SR = sow reared.
Adapted from Pluske et al. (1996b).
4.2. Management approaches
An alternative, or complementary, approach to improving adaptation time to weaning, with possible impacts on gut health, is to mimic the gradual weaning processes that occur in pigs in their natural environment. This may address piglet needs in such a way that it brings about long term benefits with respect to performance and welfare. In nature, a sow will isolate herself before farrowing and return to the group to allow her litter to mix with other piglets at approximately 10 days after birth (Jensen, 1986). There is no particular point where the sow begins to wean her young as it is a gradual process that occurs over 13 to 19 weeks, with the sow predominately controlling the initiation and termination of suckling bouts (Jensen, 1988, Jensen and Recén, 1989). As the benefits of suckling begin to decrease for the piglets (i.e., too much energy expenditure for not enough reward), piglet solid food intake from exploration of the environment begins to increase (Bøe, 1991). Interestingly, there is no drop in daily weight gain during weaning under natural conditions (Bøe, 1991).
A system of lactation housing, where a sow can leave her piglets at will by stepping over a barrier, provides an option for the sow to gradually wean her piglets in a commercial setting. In such systems, sows will generally choose to spend less time with their litter at the end of lactation, resulting in an increase in creep feed consumption by the piglets compared with conventionally housed piglets (Pajor et al., 2002, Rantzer et al., 1995a, Weary et al., 2002). However, this effect is often coupled with a decrease in growth rate suggesting that the increase in creep feed consumption is not enough to compensate for the loss of milk consumption as a result of the sow spending less time with the piglets (Pajor et al., 2002, Pluske et al., 1995, Rantzer et al., 1995a, Weary et al., 2002). Further to this, improvements in post-weaning performance vary with some studies reporting immediate (i.e., 24 h after weaning; Pajor et al., 2002) or short-term (i.e., within the first week after weaning; Pajor et al., 1999, Weary et al., 2002) improvements in weight gain and feed intake, which are in contrast to results from Rantzer et al. (1995a), where no differences between treatment groups for post-weaning performance were reported. Data pertaining to indices of gut health, however, were not reported. Interestingly, group lactation systems, where there is freedom for sows to spend less time with the piglets and opportunity for piglets to socialize with non-littermates before weaning, seem to have longer-lasting improvements in performance with higher weight gains in pigs exposed to group lactation reported up to 5 weeks after weaning, compared with conventionally housed pigs (Kutzer et al., 2009, van Nieuwamerongen et al., 2015, van Nieuwamerongen et al., 2017). Despite varying production benefits associated with sow-controlled housing, there is a risk that the variation in how much time sows chose to spend with their piglets may result in a failure to benefit from the system at all (Pitts et al., 2002).
Sows range in attitudes and behaviour from those that spend almost all of their time away from the litter (especially towards the end of lactation) to those that rarely leave the nest area (Bøe, 1991, Pajor et al., 1999, Pajor et al., 2000, Pajor et al., 2002, Rantzer et al., 1995b). The results of Pajor et al. (1999) illustrated a general trend for post-weaning weight gain and food consumption of litters of “early leavers” (sows that spent more than 60% of their time away from the litter by the third week of lactation) to be higher than those of late leavers, however this was often compensating for a depression in piglet weight gain in lactation. Furthermore, from a behavioural point of view, pigs from “early leavers” manipulated pen-mates more often than pigs from conventionally-housed sows or from “late leavers” (Pajor et al., 1999), possibly due to a lack of maternal care, but this is contradicted by Pitts et al. (2002) who observed that sows that used the get-away area in a pen more responded more strongly to the piglet-need related stimuli. Nevertheless, the variability in the amount sows use their piglet-free area has been shown to weaken the benefits of sow-controlled housing, therefore prompting the investigation into housing systems that allow for more frequent and consistent use of a sow get-away area. This, in turn, may allow for greater pre-weaning feed intake to affect GIT structure and function after weaning.
Similar in concept to sow controlled housing, intermittent suckling (IS) involves the daily separation of sows and piglets for a specified period of time during the last part of lactation. Intermittent suckling regimens have traditionally been examined for their potential to induce an oestrus in lactation, allowing for possible mating (Downing, 2015, Gerritsen et al., 2008), however studies have also highlighted welfare benefits for the piglets as evidenced by improvements in post-weaning performance (Berkeveld et al., 2009, Kuller et al., 2004, Kuller et al., 2007b, Turpin et al., 2017a, Turpin et al., 2017b) and behaviour (de Ruyter et al., 2017). In contrast, there is a concern that enforced, repeated episodes of sow-piglet separation are detrimental to piglet and sow welfare with possible implications for post-weaning gut health. However, physiological and behavioural studies that have examined this further have only reported a transient increase in piglet cortisol (Downing, 2015, Turpin et al., 2016a) and activity (Berkeveld et al., 2007a; Turpin, 2017) on the first 1 to 2 days of separation, after which time the levels stabilize to baseline or to the same levels as their conventionally-weaned counterparts. Turpin (2017) did not report any adverse effects of this rise on aspects of gut health (mast cell degradation, CRF-1 receptor density) after weaning, in contrast to other reports linked to weaning stress (see Moeser et al., 2017). A difference in weaning age in these studies (16 to 18 days) than in the former study (>24 days of age) may have been responsible for this difference. Furthermore, no behavioural patterns indicative of piglet distress during IS have been reported (Berkeveld et al., 2007a; Kearns et al., 2011; Turpin et al., 2017a, Turpin et al., 2017b).
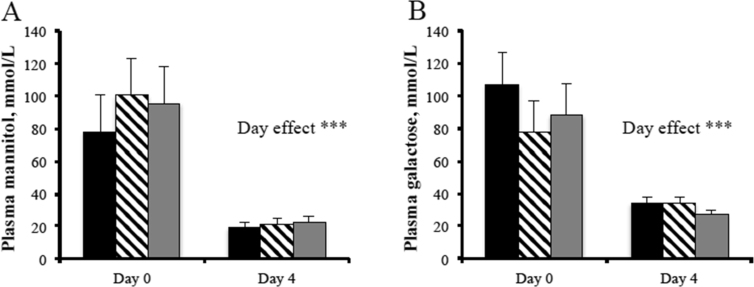
Similar to sow-controlled housing, however, IS studies often document an initial growth check at the start of IS in lactation due to a lower milk intake from reduced nursing opportunity (Berkeveld et al., 2009, Kuller et al., 2004, Kuller et al., 2007a, Thompson et al., 1981). This growth check is generally less than that observed after conventional weaning and is compensated for during the first week after weaning with higher feed intakes and weight gains compared with abruptly weaned pigs (Berkeveld et al., 2007b, Berkeveld et al., 2009, Kuller et al., 2004, Kuller et al., 2007b, Turpin et al., 2016b). In this regard, one might postulate that having a reduced period of growth when the piglet is still supported by maternal antibodies, growth factors and hormones in the milk (Cera et al., 1987) rather than after weaning, when antibody synthesis and cellular immunity are reduced (Blecha and Kelley, 1981, Blecha et al., 1983), is more advantageous especially with respect to disease risk. This is further supported by the fact that IS can prevent weaning-associated villous atrophy in the immediate post-weaning period (Berkeveld et al., 2009, Nabuurs et al., 1996). However, using sugar absorption tests to assess in vivo the overall absorptive capacity of the small intestine, Turpin (2017) found no improvements before and after weaning when pigs were subjected to IS for 16 or 8 h (Fig. 3). The exception with IS, however, seems to be in primiparous litters where improvements in growth, feed consumption and GIT morphology have been modest (Turpin et al., 2016a, Turpin et al., 2017b) or absent (Turpin et al., 2016b), most likely due to lower colostrum and milk production by primiparous sows and hence lower consumption of milk by the piglets. This is exacerbated by a further reduction in consumption when separation occurs.
Fig. 3.
Plasma mannitol concentration (A) and plasma galactose concentration (B) in control pigs (black bar), piglets that underwent intermittent suckling (IS; separated from the sow in lactation) for 16 h (IS16; black stripe bar), and piglets that underwent IS for 8 h (IS8; grey bar), when euthanized either at weaning (d 0) or 4 days after weaning (d 4). *** indicates a significant (P < 0.01) effect of day. Adapted from Turpin (2017).
Collectively, studies examining IS regimes suggest that combining IS with an extended lactation length (33 to 45 days) eliminates the post-weaning growth check and villous atrophy in both primiparous (Turpin et al., 2016b) and multiparous (Berkeveld et al., 2007b, Berkeveld et al., 2009) progeny. As discussed previously, many studies (without IS) have reported low creep feed consumption in the first 3 weeks of age followed by a marked increase in the fourth and fifth week of age (Pajor et al., 1991, Pluske et al., 2007, Puppe and Tuchscherer, 2000). Therefore, delaying IS and weaning presumably gives piglets more time to become familiar with solid feed and the GIT is better adapted to digesting and absorbing nutrients (Pluske et al., 2003), causing an improvement in ‘gut health’.
4.3. Formulating a commercial diet after weaning for optimum gut health
To complete this review, we have attempted to define the aspects of a diet for a newly-weaned pig that should be considered for optimum gut health, with references as appropriate. In light of the plethora of literature past and present and with an eye on commercial practice and reality, and in the absence of AGP, a diet to enhance gut health in the post-weaning period may incorporate (but not be limited to) the following features:
-
1)Protein/amino acid management
-
a)Reduce protein content as much as possible (up to the level that does not compromise least cost and performance), balance with essential amino acids, and use highly digestible protein source (or sources) immediately after weaning (e.g., Heo et al., 2013).
-
b)Consider the use of phytase super-dosing and xylanase to improve protein digestibility (e.g., Cowieson et al., 2017).
-
c)Increase the sulfur amino acid to lysine ratio to 60% to 65%, the Trp to Lys ratio to 21% to 22%, and the Thr to Lys ratio to 70% (e.g., Capozzalo et al., 2017, Goodband et al., 2014).
-
d)Maximize the Trp to large neutral amino acids ratio to increase serotonin levels (e.g., Shen et al., 2012, Sterndale et al., 2017).
-
e)Where appropriate, use a source of plasma protein (e.g., Crenshaw et al., 2017, Pérez-Bosque et al., 2016).
-
a)
-
2)Carbohydrate management
-
a)Minimize the soluble non-starch polysaccharide (NSP) inclusion, use a processed starch source, and if possible, grind grains coarser rather than finer (e.g., Flis et al., 2017, Jha and Berrocoso, 2015).
-
b)Include a source (or sources) of insoluble NSP (e.g., wheat bran, oat hulls, barley hulls, lignocellulose) (e.g., Flis et al., 2017, Jha and Berrocoso, 2016, Molist et al., 2016).
-
c)Use carbohydrases (xylanase, β-glucanase), where appropriate, to increase in vivo availability of short-chain oligosaccharides (Bedford and Walk, 2017).
-
a)
-
3)Fat/fatty acid management
-
a)Minimize the n-6 to n-3 PUFA ratio, within the level that does not compromise diet cost (e.g., Shin et al., 2017).
-
b)Consider using medium-chain triglycerides/fatty acids (e.g., Zentek et al., 2011).
-
a)
-
4)Mineral management
-
a)Reduce Ca levels by 10% to 20% (to reduce buffering capacity in the stomach and ensure activity of endogenous (pepsin) and exogenous (phytase) enzymes (Stein, 2007).
-
b)Use pharmacological levels of Zn and (or) Cu if allowed and where appropriate (e.g., Pluske, 2013).
-
a)
-
5)
Supplement 150 to 200 IU vitamin E and other antioxidants (e.g., essential oils and phytogenics with antioxidant capacity; Kim et al., 2016).
-
6)
Consider other additives that will have impacts on mitigating inflammation and immune function (e.g., review by Brufau et al., 2015), such as aspirin (Kim et al., 2016).
5. Conclusions
The compromised state of the young pig after weaning makes it an ideal candidate for the range of dietary and (or) management interventions that might beneficially influence gut health, especially in an era of reduced/banned AGP and (or) heavy metal use. Important progress has been made in a relatively short period of time in relation to our understanding in this field, with reference to the mechanisms underpinning the physiology and morphology, microbiota/microbiome, and localised immune system, and then in turn their interactions with nutrition and management. In this regard, generalized criteria (or indices) for the assessment of gut health in young pigs could include: 1) effective digestion and absorption of food and excretion of wastes), 2) a functional and protective gut barrier, 3) a stable and appropriate microbial population, 4) effective functioning of the gut immune system, 5) minimal activation/stimulation of stress/neural pathways, and 6) the absence of disease(s). Collectively, these should allow for optimal functioning of the GIT concomitant with optimal production performance. However, and somewhat ambiguously, some studies pertaining to gut health have examined changes in these criteria (indices) in the absence of any overt disease; this makes overarching recommendations (e.g., dietary, management) more difficult. Nevertheless, some concepts for enhanced gut health in the post-weaned pig have emerged, for example, the notion of stimulating/nullifying specific groups of bacteria in the GIT to modify the GIT environment, preserving epithelial barrier function, manipulating dietary composition (e.g., protein type and level), and careful attention to management procedures. In a more general context, gut health represents the outcomes of the GIT in response to its capacity and ability to respond and adapt to the insults and challenges it encounters in its attempts to maintain homeostasis.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adewole D.I., Kim I.H., Nyachoti C.M. Gut health of pigs: challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives — a review. Asian-Australas J Anim Sci. 2016;29:909–924. doi: 10.5713/ajas.15.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.B., McCracken V.J., Aminov R.I., Simpson J.M., Mackie R.I., Verstegen M.W.A. Gut microbiology and growth-promoting antibiotics in swine. Pig News Inf. 1999;20:115N–122N. [Google Scholar]
- Bedford M.R., Walk C.L. The use of exogenous enzymes to improve feed efficiency in pigs. In: Wiseman J., editor. Achieving sustainable production of pig meat. Vol. 2 Animal Breeding and Nutrition. Burleigh Dodds Science Publishing; Cambridge, UK: 2017. pp. 209–229. [Google Scholar]
- Berkeveld M., Langendijk P., Bolhuis J.E., Koets A.P., Verheijden J.H., Taverne M.A. Intermittent suckling during an extended lactation period: effects on piglet behavior. J Anim Sci. 2007;85:3415–3424. doi: 10.2527/jas.2007-0223. [DOI] [PubMed] [Google Scholar]
- Berkeveld M., Langendijk P., van Beers-Schreurs H.M., Koets A.P., Taverne M.A., Verheijden J.H. Postweaning growth check in pigs is markedly reduced by intermittent suckling and extended lactation. J Anim Sci. 2007;85:258–266. doi: 10.2527/jas.2006-143. [DOI] [PubMed] [Google Scholar]
- Berkeveld M., Langendijk P., Soede N.M., Kemp B., Taverne M.A., Verheijden J.H. Improving adaptation to weaning: effect of intermittent suckling regimens on piglet feed intake, growth, and gut characteristics. J Anim Sci. 2009;87:3156–3166. doi: 10.2527/jas.2008-1764. [DOI] [PubMed] [Google Scholar]
- Bischoff S.C. ‘Gut health’: a new objective in medicine? BMC Med. 2011;9:24. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecha F., Kelley K.W. Effects of cold and weaning stressors on the antibody-mediated immune response of pigs. J Anim Sci. 1981;53:439–447. doi: 10.2527/jas1981.532439x. [DOI] [PubMed] [Google Scholar]
- Blecha F., Pollman D., Nichols D. Weaning pigs at an early age decreases cellular immunity. J Anim Sci. 1983;56:396–400. doi: 10.2527/jas1983.562396x. [DOI] [PubMed] [Google Scholar]
- Bøe K. The process of weaning in pigs: when the sow decides. Appl Anim Behav Sci. 1991;30:47–59. [Google Scholar]
- Boudry G., Péron V., Le Huëron-Luron I., Lallès J.P., Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Brooks P.H., Tsourgiannis C.A. Factors affecting the voluntary feed intake of the weaned pig. In: Pluske J.R., Le Dividich J., Verstegen M.W.A., editors. The weaner pig: concepts and consequences. Wageningen Academic Publishers; Wageningen, The Netherlands: 2003. pp. 81–116. [Google Scholar]
- Brufau J., Esteve E., Tarradas J. Review of immune stimulator substances/agents that are susceptible of being used as feed additives: mode of action and identification of end-points for efficacy assessment. EFSA Supporting Publ. 2015 EN-905, 267 pages. [Google Scholar]
- Bruininx E., Binnendijk G., Van der Peet-Schwering C., Schrama J., Den Hartog L., Everts H. Effect of creep feed consumption on individual feed intake characteristics and performance of group-housed weanling pigs. J Anim Sci. 2002;80:1413–1418. doi: 10.2527/2002.8061413x. [DOI] [PubMed] [Google Scholar]
- Bruininx E.M.A.M., Schellingerhout A.B., Binnendijk G.P., van der Peet-Schwering C.M.C., Schrama J.W., den Hartog L.A. Individually assessed creep feed consumption by suckled piglets: influence on post-weaning food intake characteristics and indicators of gut structure and hind-gut fermentation. Anim Sci. 2004;78:67–75. [Google Scholar]
- Buchet A., Belloc C., Leblanc-Maridor M., Merlot E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey T.E., Skjolaas K.A., Minton J.E. Board-Invited Review: porcine mucosal immunity of the gastrointestinal tract. J Anim Sci. 2009;87:1493–1501. doi: 10.2527/jas.2008-1330. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Madsen K., Spiller R., van Meerveld B.G., Verne G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastrol and Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzalo M.M., Kim J.C., Htoo J.K., de Lange C.F.M., Mullan B.P., Hansen C.F. Pigs experimentally infected with an enterotoxigenic strain of Escherichia coli have improved feed efficiency and indicators of inflammation with dietary supplementation of tryptophan and methionine in the immediate post-weaning period. Anim Prod Sci. 2017;57:935–947. [Google Scholar]
- Carabotti M., Scirocco A., Maselli A.M., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Carstensen L., Ersbøll A.K., Jensen K.H., Nielsen J.P. Escherichia coli post-weaning diarrhoea occurrence in piglets with monitored exposure to creep feed. Vet Microbiol. 2005;110:113–123. doi: 10.1016/j.vetmic.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.-M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol. 2017;234:88–100. [Google Scholar]
- Cera K., Mahan D., Simmen F. In vitro growth-promoting activity of porcine mammary secretions: initial characterization and relationship to known peptide growth factors. J Anim Sci. 1987;65:1149–1159. doi: 10.2527/jas1987.6541149x. [DOI] [PubMed] [Google Scholar]
- Chapple R.P., Cuaron J.A., Easter R.A. Effect of glucocorticoids and limited nursing on the carbohydrate digestive capacity and growth rate of piglets. J Anim Sci. 1989;67:2956–2973. doi: 10.2527/jas1989.67112956x. [DOI] [PubMed] [Google Scholar]
- Cheng G., Hao H., Xe S., Wang X., Dai M., Huang L. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol. 2014;5:1–15. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson A.J., Ruckebusch J.-P., Sorbara J.O.B., Wilson J.W., Guggenbuhl P., Roos F.F. A systemic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim Feed Sci Technol. 2017;225:182–194. [Google Scholar]
- Cranwell P.D. Development of the neonatal gut and enzyme systems. In: Varley M.A., editor. The neonatal pig: development and survival. CAB International; Wallingford, Oxon, UK: 1995. pp. 99–154. [Google Scholar]
- Crenshaw J.D., Campbell J.M., Polo J., Stein H.H. Effects of specialty proteins as alternatives to bovine or porcine spray-dried plasma in non-medicated diets fed to weaned pigs housed in an unsanitary environment. Transl Anim Sci. 2017;1 doi: 10.2527/tas2017.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange C.F.M., Pluske J.R., Gong J., Nyachoti M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134:124–134. [Google Scholar]
- de Passille A.M.B., Pelletier G., Menard J., Morrisset J. Relationships of weight gain and behavior to digestive organ weight and enzyme activities in piglets. J Anim Sci. 1989;67:2921–2929. doi: 10.2527/jas1989.67112921x. [DOI] [PubMed] [Google Scholar]
- de Ruyter E.M., van Wettere W.H., Lines D.S., Plush K.J. Gradually reducing sow contact in lactation is beneficial for piglet welfare around weaning. Appl Anim Behav Sci. 2017;193:43–50. [Google Scholar]
- Diamond J.M., Karasov W.H. Trophic control of the intestinal mucosa. Nat (Lond) 1983;304:18. doi: 10.1038/304018a0. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dong G.Z., Pluske J.R. The low feed intake in newly-weaned pigs: problems and possible solutions. Asian Aust J Anim Sci. 2007;20:440–452. [Google Scholar]
- Dou S., Gadonna-Widehem P., Rome V., Hamoudi D., Rhazi L., Lakhal L. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J.A. Development of a lactational oestrus induction protocol that can be implemented in confinement free sow housing systems. Anim Prod Sci. 2015;55:1411–1423. [Google Scholar]
- English P. Establishing the early weaned pig. Pig Vet Soc Proc. 1981;7:29–37. [Google Scholar]
- English P.R., Robb C.M., Bias M.F.M. Evaluation of creep feeding using a highly-digestible diet for litters weaned at four weeks of age. Anim Prod. 1980;30:496. [Google Scholar]
- Fairbrother J.M., Nadeau É., Gyles C.L. Escherichia coli in postweaning diarrhoea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Flis M., Sobotka W., Antoszkiewicz Z. Fiber substrates in the nutrition of weaned piglets-a review. Ann Anim Sci. 2017;17:627–643. [Google Scholar]
- Foster J.A., Rinamin L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017 doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouhse J.M., Zijlstra R.T., Willing B.P. The role of gut microbiota in the health and disease of pigs. Anim Front. 2016;6:30–36. [Google Scholar]
- Francis D.H. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J Swine Health Prod. 2002;10:171–175. [Google Scholar]
- Frese S.A., Parker K., Calvert C.C., Mills D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins H.R. Intestinal bacteria and their influence on swine growth. In: Lewis A.J., Southern L.L., editors. Swine nutrition. 2nd ed. CRC Press; Florida, USA: 2001. pp. 585–608. [Google Scholar]
- Gaskins H.R., Collier C.T., Anderson D.B. Antibiotics as growth promotants: mode of action. Anim Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- Gerritsen R., Soede N., Langendijk P., Hazeleger W., Kemp B. The intermittent suckling regimen in pigs: consequences for reproductive performance of sows. Reprod Dom Anim. 2008;43:29–35. doi: 10.1111/j.1439-0531.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Goodband R., Tokach M., Dritz S., DeRouchey J., Woodworth J. Practical starter pig amino acid requirements in relation to immunity, gut health and growth performance. J Anim Sci Biotechnol. 2014;5:12. doi: 10.1186/2049-1891-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hampson D.J. Alterations in piglet small intestinal structure at weaning. Res Vet Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Hampson D.J. Attempts to modify changes in the piglet small intestine after weaning. Res Vet Sci. 1986;40:313–317. [PubMed] [Google Scholar]
- Heo J.M., Opapeju F.O., Pluske J.R., Kim J.-C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hillman K. An analysis of gut microbes. Pig Int. 2004;34(6):27–29. [Google Scholar]
- Hillman K. Bacteriological aspects of the use of antibiotics and their alternatives in the feed of non-ruminant animals. In: Garnsworthy P.C., Wiseman J., editors. Recent advances in animal nutrition. Nottingham University Press; Loughborough, UK: 2001. pp. 107–134. [Google Scholar]
- Holman D.B., Brunelle B.W., Trachsel J., Allen H.K. Meta-analysis to define a core microbiota in the swine gut. mSystems. 2017;2:e00004–17. doi: 10.1128/mSystems.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D.E., Pluske J.R., Hampson D.J. Dietary manipulation of infectious bowel disease. In: Mosenthin R., Zentek J., Zebrowska E., editors. Biology of nutrition in growing animals. vol. 2005. Elsevier Limited; Amsterdam, The Netherlands: 2005. pp. 365–385. [Google Scholar]
- Jayaraman B., Nyachoti C.M. Husbandry practices and gut health outcomes in weaned piglets: a review. Anim Nutr. 2017;3:205–211. doi: 10.1016/j.aninu.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. Observations on the maternal behaviour of free-ranging domestic pigs. Appl Anim Behav Sci. 1986;16:131–142. [Google Scholar]
- Jensen P. Maternal behaviour and mother—young interactions during lactation in free-ranging domestic pigs. Appl Anim Behav Sci. 1988;20:297–308. [Google Scholar]
- Jensen P., Recén B. When to wean—observations from free-ranging domestic pigs. Appl Anim Behav Sci. 1989;23:49–60. [Google Scholar]
- Jha R., Berrocoso J.D. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9:1441–1452. doi: 10.1017/S1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.D. Review: dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. 2016;212:18–26. [Google Scholar]
- Johnson R.W. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J Anim Sci. 1997;75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Kearns E., Macnamara G., Giles L.R., Downing J. The behaviour of piglets and lactating sows during overnight separation. In: Van Barneveld R.J., editor. Manipulating pig production XIII. Proceedings of the Thirteenth Biennial Conference of the Australasian Pig Science Association (APSA), Melbourne, Australia. 2011. p. 241. [Google Scholar]
- Kelly D., King T.P. Luminal bacteria: regulation of gut function and immunity. In: Piva A., Bach Knudsen K.E., Lindberg J.E., editors. Gut environment of pigs. Nottingham University Press; Loughborough, UK: 2001. pp. 113–131. [Google Scholar]
- Kelly D., Smyth J.A., McCracken K.J. Digestive development of the early-weaned pig. 1. Effect of continuous nutrient supply on the development of the digestive tract and on changes in digestive enzyme activity during the first week post-weaning. Br J Nutr. 1991;65:169–180. doi: 10.1079/bjn19910078. [DOI] [PubMed] [Google Scholar]
- Kelly D., Smyth J.A., McCracken K.J. Digestive development of the early-weaned pig. 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br J Nutr. 1991;65:181–188. doi: 10.1079/bjn19910079. [DOI] [PubMed] [Google Scholar]
- Kil D.Y., Stein H.H. Board Invited Review: management and feeding strategies to ameliorate the impact of removing antibiotic growth promoters from diets fed to weanling pigs. Can J Anim Sci. 2010;90:447–460. [Google Scholar]
- Kim J.C., Hansen C.F., Mullan B.P., Pluske J.R. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2012;173:3–16. [Google Scholar]
- Kim J.C., Mullan B.P., Black J.L., Hewit J.E., van Barneveld R.J., Pluske J.R. Acetylsalicylic acid supplementation improves protein utilization efficiency while vitamin E supplementation reduces markers of the inflammatory response in weaned pigs challenged with enterotoxigenic E. coli. J Anim Sci Biotechnol. 2016;7:58. doi: 10.1186/s40104-016-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R.H., Pluske J.R. Nutritional management of the weaner pig. In: Pluske J.R., Le Dividich J., Verstegen M.W.A., editors. The weaner pig: concepts and consequences. Wageningen Academic Publishers; Wageningen, The Netherlands: 2003. pp. 37–51. [Google Scholar]
- Kogut M.H., Arsenault R.J. Editorial: gut health: the new paradigm in food animal production. Front Vet Sci. 2016;3:71. doi: 10.3389/fvets.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller W., Soede N., van Beers-Schreurs H., Langendijk P., Taverne M., Verheijden J. Intermittent suckling: effects on piglet and sow performance before and after weaning. J Anim Sci. 2004;82:405–413. doi: 10.2527/2004.822405x. [DOI] [PubMed] [Google Scholar]
- Kuller W., van Beers-Schreurs H., Soede N., Langendijk P., Taverne M., Kemp B. Creep feed intake during lactation enhances net absorption in the small intestine after weaning. Livest Sci. 2007;108:99–101. [Google Scholar]
- Kuller W., Soede N., van Beers-Schreurs H., Langendijk P., Taverne M., Kemp B. Effects of intermittent suckling and creep feed intake on pig performance from birth to slaughter. J Anim Sci. 2007;85:1295–1301. doi: 10.2527/jas.2006-177. [DOI] [PubMed] [Google Scholar]
- Kutzer T., Bünger B., Kjaer J.B., Schrader L. Effects of early contact between non-littermate piglets and of the complexity of farrowing conditions on social behaviour and weight gain. Appl Anim Behav Sci. 2009;121:16–24. [Google Scholar]
- Lallès J.P., Bosi P., Smidt H., Stokes C.R. Nutritional management of gut health in pigs around weaning. Proc Nutr Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- Lallès J.-P., Bosi P., Smidt H., Stokes C.R. Weaning-a challenge to gut physiologists. Livest Sci. 2007;108:82–93. [Google Scholar]
- Lallès J.P., Boudry G., Favier C., Le Floc’h N., Luron I., Montagne L. Gut function and dysfunction in young pigs: physiology. Anim Res. 2004;53:301–316. [Google Scholar]
- Leser T.D., Amenuvor J.Z., Jensen T.K., Lindecrona R.H., Boye M., Møller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol. 2002;68:673–690. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann M.D., Cornelius S.G., El Kandelgy S.M., Moser R.L., Pettigrew J.E. Effect of age, weaning and diet on digestive enzyme levels in the piglet. J Anim Sci. 1986;62:1298–1307. doi: 10.2527/jas1986.6251298x. [DOI] [PubMed] [Google Scholar]
- Looft T., Johnson T.A., Allen H.K., Bayles D.O., Alt D.P., Stedtfeld R.D. In-feed antibiotic effects on the swine intestinal microbiome. Proc Nat Acad Sci. 2012;109:1691–1696. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken B.A., Spurlock M.E., Roos M.A., Zuckerman F.A., Gaskins H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 1999;129:613–619. doi: 10.1093/jn/129.3.613. [DOI] [PubMed] [Google Scholar]
- Mereu A., Tedó G., Moeser A.J., Rimbach G., Ipharraguerre I.R. Cromolyn-mediated improvement of intestinal barrier function is associated with enhanced piglet performance after weaning. BMC Vet Res. 2015;11:274. doi: 10.1186/s12917-015-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A.J., Vander Klok C., Ryan K.A., Wooten J.G., Little J.G., Cook V.L. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol: Gastro Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Pohl C.S., Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. 2017;3(4):313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molist F., van Oostrum M., Pérez J.F., Mateos G.G., Nyachoti C.M., van der Aar P.J. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim Feed Sci Technol. 2016;189:1–10. [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95–117. [Google Scholar]
- Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogast and Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Niewold T.W. Intestinal genomics for the evaluation of alternatives to AGPs: current situation and perspectives. In: Barug D., de Jong J., Kies A.K., Verstegen M.W.A., editors. Antimicrobial growth promoters. Wageningen Academic Publishers; Wageningen, The Netherlands: 2006. pp. 361–367. [Google Scholar]
- Nabuurs M., Hoogendoorn A., Van Zijderveld-Van Bemmel A. Effect of supplementary feeding during the sucking period on net absorption from the small intestine of weaned pigs. Res Vet Sci. 1996;61:72–77. doi: 10.1016/s0034-5288(96)90114-9. [DOI] [PubMed] [Google Scholar]
- Pajor E., Kramer D., Fraser D. Regulation of contact with offspring by domestic sows: temporal patterns and individual variation. Ethology. 2000;106:37–51. [Google Scholar]
- Pajor E.A., Fraser D., Kramer D.L. Consumption of solid food by suckling pigs: individual variation and relation to weight gain. Appl Anim Behav Sci. 1991;32:139–155. [Google Scholar]
- Pajor E.A., Weary D.M., Fraser D., Kramer D.L. Alternative housing for sows and litters: 1. Effects of sow-controlled housing on responses to weaning. Appl Anim Behav Sci. 1999;65:105–121. [Google Scholar]
- Pajor E.A., Weary D.M., Caceres C., Fraser D., Kramer D.L. Alternative housing for sows and litters: Part 3. Effects of piglet diet quality and sow-controlled housing on performance and behaviour. Appl Anim Behav Sci. 2002;76:267–277. [Google Scholar]
- Pérez-Bosque A., Polo J., Torrallardona D. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc Health Manag. 2016;2:16. doi: 10.1186/s40813-016-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J.P., Blazy F., Laffitte J., Seve B., Oswald I.P. Weaning is associated with an upregulation of expression if inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Pitts A.D., Weary D.M., Fraser D., Pajor E.A., Kramer D.L. Alternative housing for sows and litters: Part 5. Individual differences in the maternal behaviour of sows. Appl Anim Behav Sci. 2002;76:291–306. [Google Scholar]
- Pluske J.R. Feed and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. 2013;4:1. doi: 10.1186/2049-1891-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluske J.R. Invited review: aspects of gastrointestinal tract growth and maturation in the pre- and postweaning period of pigs. J Anim Sci. 2016;94:399–411. [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236. [Google Scholar]
- Pluske J.R., Kerton D.J., Cranwell P.D., Campbell R.G., Mullan B.P., King R.H. Age, sex and weight at weaning influence the physiological and gastrointestinal development of weanling pigs. Aust J Agric Res. 2003;54:515–527. [Google Scholar]
- Pluske J.R., Kim J.C., Black J.L. Manipulating the immune system for pigs to optimise performance. Anim Prod Sci. 2018 [Google Scholar]
- Pluske J.R., Kim J.C., Hansen C.F., Mullan B.P., Payne H.G., Hampson D.J. Piglet growth before and after weaning in relation to a qualitative estimate of solid (creep) feed intake during lactation: a pilot study. Arch Anim Nutr. 2007;61:469–480. doi: 10.1080/17450390701664249. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Payne H.G., Williams I.H., Mullan B.P. Early feeding for lifetime performance of pigs. In: Cronje P.B., Richards N., editors. Recent advances in animal nutrition in Australia, volume 15. Animal science. University of New England; Armidale, NSW: 2005. pp. 171–181. [Google Scholar]
- Pluske J.R., Pethick D.W., Hopwood D.E., Hampson D.J. Nutritional influences on some major enteric bacterial diseases of pigs. Nutr Res Rev. 2002;15:333–371. doi: 10.1079/NRR200242. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim Sci. 1996;62:131–144. [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Villous height and crypt depth in piglets in response to increases in the intake of cows' milk after weaning. Anim Sci. 1996;62:145–158. [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Nutrition of the neonatal pig. In: Varley M.A., editor. The neonatal pig - development and survival. CAB International; Wallingford UK: 1995. pp. 187–235. [Google Scholar]
- Pohl C.S., Medland J.E., Mackey E., Edwards L.L., Bagley K.D., DeWilde M.P. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neuro Gastroenterol Motil. 2017 doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppe B., Tuchscherer A. The development of suckling frequency in pigs from birth to weaning of their piglets: a sociobiological approach. Anim Sci. 2000;71:273–279. [Google Scholar]
- Rantzer D., Svendsen J., Weström B. Weaning of pigs raised in sow-controlled and in conventional housing systems, 1: description of systems, production and bacteriology. Swed J Agric Res. 1995;25:37–46. [Google Scholar]
- Rantzer D., Svendsen J., Weström B. Weaning of pigs raised in sow-controlled and in conventional housing systems, 2: behaviour studies and cortisol levels. Swed J Agric Res. 1995;25:61–71. [Google Scholar]
- Richards J.D., Gong J., de Lange C.F.M. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: current understanding, possible modulations, and new technologies for ecological studies. Can J Anim Sci. 2005;85:421–435. [Google Scholar]
- Schachtschneider K.M., Yeoman C.J., Isaacson R.E., White B.A., Schook L.B., Pieters M. Modulation of systemic immune responses through commensal gastrointestinal microbiota. PLoS One. 2013;8(1):e53969. doi: 10.1371/journal.pone.0053969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B., Mulder I.E., Musk C.C., Aminov R.I., Lewis M., Stokes C.R. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS One. 2011;6(12):e28284. doi: 10.1371/journal.pone.0028284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.B., Voilque G., Odle J., Kim S.W. Dietary L-Tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases stress hormone secretion in nursery pigs under social-mixing stress. J Nutr. 2012;142:1540–1546. doi: 10.3945/jn.112.163824. [DOI] [PubMed] [Google Scholar]
- Shin T.K., Yi Y.J., Kim J.C., Pluske J.R., Cho H.M., Wickramasuriya S.S. Reducing the dietary omega-6 to omega-3 polyunsaturated fatty acid ratio attenuated inflammatory indices and sustained epithelial tight junction integrity in weaner pigs housed in a poor sanitary condition. Anim Feed Sci Technol. 2017;234:312–320. [Google Scholar]
- Spreeuwenberg M.A., Verdonk J.M., Gaskins H.R., Verstegen M.W.A. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J Nutr. 2001;131:1520–1527. doi: 10.1093/jn/131.5.1520. [DOI] [PubMed] [Google Scholar]
- Stein H.H. Proceedings of the 7th London swine conference – Today's challenges, Tomorrow's opportunities. London, ON, Canada. 2007. Feeding the pigs' immune system and alternatives to antibiotics; pp. 65–82. [Google Scholar]
- Sterndale S.O., Miller D.W., Mansfield J.P., Kim J.C., Pluske J.R. Increasing dietary tryptophan and decreasing other large neutral amino acids increases weight gain and feed intake in weaner pigs infected with Escherichia coli. Anim Prod Sci. 2017;57:2410. doi: 10.1093/jas/skaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulabo R.C., Tokach M.D., Dritz S.S., DeRouchey J.M., Nelssen J.L. Effects of varying creep feeding duration on the proportion of pigs consuming creep feed and neonatal pig performance. J Anim Sci. 2010;88:3154–3162. doi: 10.2527/jas.2009-2134. [DOI] [PubMed] [Google Scholar]
- Thacker P.A. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol. 2013;4:35. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L.H., Hanford K.J., Jensen A.H. Estrus and fertility in lactating sows and piglet performance as influenced by limited nursing. J Anim Sci. 1981;53:1419–1423. doi: 10.2527/jas1982.5361419x. [DOI] [PubMed] [Google Scholar]
- Turpin D.L. Murdoch University; Murdoch WA, Australia: 2017. The Gradual weaning of piglets: the influence of intermittent suckling and co-mingling on performance, the stress response, behaviour, and function and morphology of the gastrointestinal tract. [PhD Thesis] [Google Scholar]
- Turpin D.L., Langendijk P., Chen T.-Y., Lines D., Pluske J.R. Intermittent suckling causes a transient increase in cortisol that does not appear to compromise selected measures of piglet welfare and stress. Animals. 2016;6:24. doi: 10.3390/ani6030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D.L., Langendijk P., Chen T.-Y., Pluske J.R. Intermittent suckling in combination with an older weaning age improves growth, feed intake and aspects of gastrointestinal tract carbohydrate absorption in pigs after weaning. Animals. 2016;6:66. doi: 10.3390/ani6110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D.L., Langendijk P., Plush K., Pluske J.R. Intermittent suckling with or without co-mingling of non-littermate piglets before weaning improves piglet performance in the immediate post-weaning period when compared with conventional weaning. J Anim Sci Biotechnol. 2017;8:14. doi: 10.1186/s40104-017-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D.L., Langendijk P., Sharp C., Pluske J.R. Improving welfare and production in the peri-weaning period: effects of co-mingling and intermittent suckling on the stress response, performance, behaviour and, gastrointestinal tract carbohydrate absorption in young pigs. Livest Sci. 2017;203:82–91. [Google Scholar]
- van Nieuwamerongen S., Soede N., van der Peet-Schwering C., Kemp B., Bolhuis J. Development of piglets raised in a new multi-litter housing system vs. conventional single-litter housing until 9 weeks of age. J Anim Sci. 2015;93:5442–5454. doi: 10.2527/jas.2015-9460. [DOI] [PubMed] [Google Scholar]
- van Nieuwamerongen S., Soede N., van der Peet-Schwering C., Kemp B., Bolhuis J. Gradual weaning during an extended lactation period improves performance and behavior of pigs raised in a multi-suckling system. Appl Anim Behav Sci. 2017 [Google Scholar]
- Weary D.M., Pajor E.A., Bonenfant M., Fraser D., Kramer D.L. Alternative housing for sows and litters: Part 4. Effects of sow-controlled housing combined with a communal piglet area on pre-and post-weaning behaviour and performance. Appl Anim Behav Sci. 2002;76:279–290. [Google Scholar]
- Wei H.K., Xue H.X., Zhou Z.X., Peng J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal. 2017;11:193–201. doi: 10.1017/S1751731116001397. [DOI] [PubMed] [Google Scholar]
- Wijtten P.J.A., van der Meulen J., Verstegen M.W.A. Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Zeng M.Y., Inohara N., Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Ferrara F., Vahjen W., Van Kessel A., Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim Health Res Rev. 2011;12:83–93. doi: 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang Y., Liu S., Huang J., Zhai Z., He C. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]