ABSTRACT
Objective:
To evaluate the value of soluble urokinase-type plasminogen activator receptor (suPAR) in the diagnosis of acute exacerbation of COPD (AECOPD) and in monitoring treatment response, analyzing the relationship between suPAR and fibrinogen in AECOPD. AECOPD leads to increased airway inflammation, contributing to an exaggerated release of inflammatory mediators.
Methods:
We recruited 45 patients with AECOPD and 20 healthy control subjects. Medical histories were taken, and all subjects underwent clinical examination, chest X-ray, pulmonary function tests, and blood gas analysis. On day 1 (treatment initiation for the AECOPD patients) and day 14 (end of treatment), blood samples were collected for the determination of serum suPAR and plasma fibrinogen.
Results:
Serum levels of suPAR were significantly higher in the AECOPD group than in the control group. In the AECOPD patients, there was a significant post-treatment decrease in the mean serum suPAR level. The sensitivity, specificity, and accuracy of suPAR were 95.6%, 80.0%, and 93.0%, respectively. The Global Initiative for Chronic Obstructive Lung Disease stage (i.e., COPD severity) correlated positively and significantly with serum levels of suPAR and plasma levels of fibrinogen.
Conclusions:
Monitoring the serum suPAR level can be helpful in the evaluation of the COPD treatment response and might be a valuable biomarker for determining the prognosis of AECOPD. Because serum suPAR correlated with plasma fibrinogen, both markers could be predictive of AECOPD.
Keywords: Pulmonary disease, chronic obstructive/complications; Pulmonary disease, chronic obstructive/diagnosis; Receptors, urokinase plasminogen activator; Fibrinogen
RESUMO
Objetivo:
Avaliar o valor do soluble urokinase-type plasminogen activator receptor (suPAR, receptor do ativador de plasminogênio tipo uroquinase solúvel) no diagnóstico de exacerbação aguda da DPOC (EADPOC) e no monitoramento da resposta ao tratamento, analisando-se a relação entre o suPAR e o fibrinogênio na EADPOC. A EADPOC leva ao aumento da inflamação das vias aéreas, contribuindo para a liberação exagerada de mediadores inflamatórios.
Métodos:
Foram recrutados 45 pacientes com EADPOC e 20 controles saudáveis. Realizou-se anamnese, e todos os indivíduos foram submetidos a exame clínico, radiografia de tórax, provas de função pulmonar e gasometria arterial. No dia 1 (início do tratamento para os pacientes com EADPOC) e no dia 14 (final do tratamento), foram coletadas amostras de sangue para dosagem de suPAR sérico e de fibrinogênio plasmático.
Resultados:
Os níveis séricos de suPAR foram significativamente maiores no grupo EADPOC do que no grupo controle. Nos pacientes com EADPOC, houve diminuição significativa da média de suPAR sérico após o tratamento. A sensibilidade, a especificidade e a acurácia do suPAR foram, respectivamente, de 95,6%, 80,0% e 93,0%. O estágio da doença segundo a Global Initiative for Chronic Obstructive Lung Disease (isto é, a gravidade da DPOC) apresentou correlação positiva e significativa com os níveis séricos de suPAR e os níveis plasmáticos de fibrinogênio.
Conclusões:
O monitoramento do suPAR sérico pode ser útil na avaliação da resposta ao tratamento da DPOC e seria um biomarcador valioso para a determinação do prognóstico da EADPOC. Como o suPAR sérico apresentou correlação com o fibrinogênio plasmático, ambos os marcadores poderiam ser preditores da EADPOC.
Descritores: Doença pulmonar obstrutiva crônica/complicações, Doença pulmonar obstrutiva crônica/diagnóstico, Receptores de ativador de plasminogênio tipo uroquinase, Fibrinogênio
INTRODUCTION
Acute exacerbation of COPD (AECOPD) is characterized by deterioration of the respiratory symptoms that is beyond the normal day-to-day variations and leads to a change in medication. 1 , 2 Although exacerbations are the main determinants of COPD-related morbidity and mortality, their exact incidence remains unknown. Exacerbations have a major impact on the quality of life of COPD patients, resulting in multiple hospitalizations. 3 AECOPD leads to increased airway inflammation, provoking the exaggerated release of numerous inflammatory mediators. 4
The most commonly used marker of COPD severity is FEV1. However, FEV1 does not correlate well with symptoms and other factors that quantify the progression of COPD. 5 It is therefore important to seek other markers of COPD activity.
Urokinase-type plasminogen activator receptor and plasminogen activator inhibitor type 1 are the main urokinase-type plasminogen activators. They are considered important components of the immune system and the inflammatory response. 6 , 7 Elevated levels of soluble urokinase-type plasminogen activator receptor (suPAR) result from increased stimulation of the immune system by different types of infections or solid tumors. Therefore, serum suPAR levels are believed to indicate the degree of immune activation. 8 There have been many studies reporting elevated suPAR levels in patients with infection, cancer, inflammatory diseases, sepsis, or bacteremia. 9 - 12
Determination of the serum level of suPAR is a simple test that is easy to perform and, in comparison with determination of the plasma level of fibrinogen, requires fewer precautions related to sample collection and processing. 13 Determination of the serum level of suPAR and the plasma level of fibrinogen could play an important role in the evaluation of patients with stable COPD. 14 Fibrinogen has come to be a helpful biomarker in COPD and is being considered as a drug development tool for qualification by the U.S. Food and Drug Administration and the European Medicines Agency. Fibrinogen is synthesized in the liver and converted to fibrin by thrombin during blood coagulation; it is considered an acute phase plasma protein. 15
The objective of this study was to evaluate the value of suPAR as a biomarker in the diagnosis of AECOPD and as a tool for monitoring the treatment response. We also analyzed the relationship between suPAR and fibrinogen in patients with AECOPD.
METHODS
We enrolled 45 patients with AECOPD and 20 healthy control subjects. The patients were recruited from among those under treatment at the outpatient clinics or in the inpatient wards of the Chest Department of the Tanta University Hospitals, in the city of Tanta, Egypt, between August 2015 and January 2016. The study was performed in accordance with the ethical standards of the Tanta University Hospitals and was approved by the Research Ethics Committee of the Tanta University Faculty of Medicine. All of the participants gave written informed consent.
The diagnosis of COPD was made in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, 16 on the basis of smoking history, clinical manifestations, and pulmonary function test results showing airflow obstruction, with a post-bronchodilator FEV1/FVC ratio < 0.7. AECOPD was defined as prolonged (≥ 48 h) worsening of dyspnea, coughing, or the production of mucoid or purulent sputum, leading to increased use of rescue and maintenance medications. 17
Patients who had conditions that could alter their serum level of suPAR 18 -such as bronchial asthma, bronchiectasis, requiring mechanical ventilation, malignancies, liver failure, renal failure, heart failure, and uncontrolled diabetes mellitus-were excluded. Pneumonia was ruled out if a chest X-ray revealed no pulmonary infiltrate.
The patients were admitted and managed with supplemental oxygen at an optimum saturation of 90-92%. Bronchodilators (short-acting β2 agonists), with or without short-acting anticholinergic agents, were used for the treatment of exacerbations. Prednisolone (40 mg/day for 5 days) was prescribed. Antibiotics were given if there were clinical signs of a bacterial infection, such as purulent sputum. 19 Medical histories were taken, and all patients underwent the following: thorough clinical examination; chest X-rays, in posteroanterior and lateral views, at enrolment (on day 1, when treatment for AECOPD was initiated) and on day 14 (after the end of that treatment); laboratory tests, including a complete blood count, renal function tests, liver function tests, and determination of fasting blood glucose levels; arterial blood gas analysis (arterial blood samples were collected, with sterile, disposable plastic syringes containing heparin, on day 1); pulmonary function tests to determine FEV1 and the FEV1/FVC ratio, with a spirometer (CHESTGRAPH HI-101; Chest M.I., Inc., Tokyo, Japan); and determination of the levels of fibrinogen and suPAR in plasma and serum, respectively, peripheral blood samples having been collected on day 1 and on day 14.
Plasma and serum were obtained from peripheral blood samples by centrifugation for 15 min at 1,500 g. Plasma and serum samples were stored at ≤ −20°C until analysis. Plasma fibrinogen was measured with a commercial kit (Fibrinogen Human ELISA Kit, ab108842; Abcam/KEMET Medical, Cairo, Egypt), with a typical sensitivity of approximately 0.10 µg/mL, the intra- and inter-assay coefficients of variation being 4.0% and 9.7%, respectively. Serum suPAR was also measured with a commercial kit (Quantikine Human uPAR Immunoassay Kit, DUP00; R&D Systems Europe, Oxon, United Kingdom), with a sensitivity < 33 pg/mL, the intra- and inter-assay coefficients of variation being 4.1% and 5.1%, respectively.
Statistical analysis
We calculated means and standard deviations, to which we applied unpaired Student’s t-tests, paired t-tests, and chi-square tests, as well as determining linear correlation coefficients and constructing ROC curves. Data were analyzed with the Statistical Package for the Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA). Values of p < 0.05 were considered statistically significant.
RESULTS
We included 45 patients diagnosed with AECOPD and 20 healthy, age- and gender-matched control subjects. Patients with AECOPD were treated for 14 days and re-evaluated at the end of treatment. The characteristics of the patients and control subjects are presented in Table 1. The AECOPD patients were stratified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage of airflow limitation: stage I, in 7 patients (15.5%); stage II, in 16 (35.6%); stage III, in 13 (28.9%); and stage 4, in 9 (20.0%).
Table 1. Baseline characteristics, pulmonary function parameters, stages of COPD, and arterial blood gas analysis results in patients with acute exacerbations and healthy control subjects.
| Variable | AECOPD group | Control group | p |
|---|---|---|---|
| (n = 45) | (n = 20) | ||
| Age (years), mean ± SD | 56.65 ± 6.48 | 57.711 ± 5.723 | 0.510 |
| Male gender, n (%) | 13 (65.0) | 31 (68.9) | 0.758 |
| Current smoker, n (%) | 14 (70.0) | 32 (71.1) | 0.928 |
| Smoking history (pack-years), mean ± SD | 31.00 ± 6.15 | 39.62 ± 9.56 | 0.003 |
| FEV1 (% of predicted), mean ± SD | 87 ± 4.078 | 50.44 ± 19.83 | < 0.001 |
| FEV1/FVC ratio (% of predicted), mean ± SD | 88.2 ± 8.16 | 54.53 ± 10.43 | < 0.001 |
| pH, mean ± SD | 7.38 ± 0.016 | 7.332 ± 0.043 | < 0.001 |
| PaO2 (mmHg), mean ± SD | 75.75 ± 5.18 | 58.77 ± 4.96 | < 0.001 |
| PaCO2 (mmHg), mean ± SD | 41.8 ± 3.17 | 55.40 ± 6.62 | < 0.001 |
| SpO2, mean ± SD | 95.75 ± 1.65 | 88.02 ± 4.25 | < 0.001 |
| GOLD stage of COPD, n (%) | |||
| I | 7 (15.5) | ||
| II | 16 (35.6) | ||
| III | 13 (28.9) | ||
| IV | 9 (20.0) |
AECOPD: acute exacerbation of COPD; and GOLD: Global Initiative for Chronic Obstructive Lung Disease.
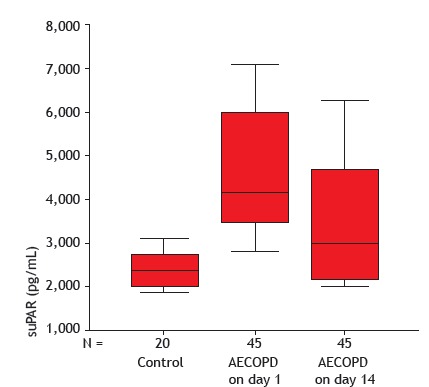
The serum levels of suPAR were significantly higher in the AECOPD patients than in the control subjects, on day 1 and day 14 (p < 0.001 for both). In the AECOPD group, there was a significant post-treatment decrease in the mean serum suPAR level-from 4,676.8 ± 1,478.9 pg/mL to 3,521.3 ± 1,382.9 pg /mL (p < 0.001)-as shown in Figure 1.
Figure 1. Serum levels of soluble urokinase-type plasminogen activator receptor (suPAR) in the control group, as well as in the acute exacerbation of COPD (AECOPD) group on day 1 and after 14 days of treatment.
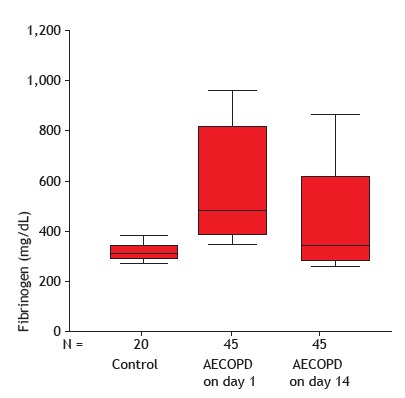
The plasma levels of fibrinogen were significantly higher in the AECOPD patients than in the control subjects, on day 1 and day 14 (p < 0.001 for both). In the AECOPD group, there was a significant post-treatment decrease in the mean plasma fibrinogen level-from 567.3 ± 216.6 mg/dL to 445.1 ± 190.8 mg/dL (p < 0.001)-as shown in Figure 2.
Figure 2. Plasma fibrinogen levels in the control group, as well as in the acute exacerbation of COPD (AECOPD) group on day 1 and after 14 days of treatment.
Serum levels of suPAR and plasma levels of fibrinogen increased in proportion to increases in the severity of COPD, being significantly higher in patients with GOLD stage III or IV than in those with GOLD stage I. Table 2 shows the comparison between suPAR and fibrinogen levels, by GOLD stage. The serum suPAR level was found to correlate negatively with FEV1 (% of predicted), the FEV1/FVC ratio (% of predicted), PaO2, and SpO2, whereas it correlated positively with the GOLD stage, both correlations being significant. Likewise, the plasma fibrinogen level correlated negatively with FEV1, the FEV1/FVC ratio, and SpO2, whereas it correlated positively with the GOLD stage, both correlations also being significant (p < 0.001). There was a significant positive correlation between the serum suPAR level and the plasma fibrinogen level (r = 0.715; p < 0.001).
Table 2. Comparison between serum levels of soluble urokinase-type plasminogen activator receptor and plasma levels of fibrinogen, by GOLD stage, in patients with acute exacerbation of COPD.
| Marker | GOLD stage | ANOVA | |||
|---|---|---|---|---|---|
| I or II | III | IV | |||
| Mean ± SD | Mean ± SD | Mean ± SD | F | p | |
| suPAR (pg/mL) | |||||
| Day 1 | 3,504.34 ± 542.53 | 5,309.23 ± 994.52 | 6,760.0 ± 502.81 | 78.232 | < 0.001 |
| Day 14 | 2,558.69 ± 607.38 | 4,084.61 ± 1,201.23 | 5,167.77 ± 1,054.14 | ||
| Fibrinogen (mg/dL) | |||||
| Day 1 | 443.47 ± 107.98 | 595.38 ± 229.98 | 843.33 ± 125.0 | 21.669 | < 0.001 |
| Day 14 | 337.82 ± 101.88 | 473.84 ± 201.31 | 677.77 ± 125.07 | ||
GOLD: Global Initiative for Chronic Obstructive Lung Disease; and suPAR: soluble urokinase-type plasminogen activator receptor.
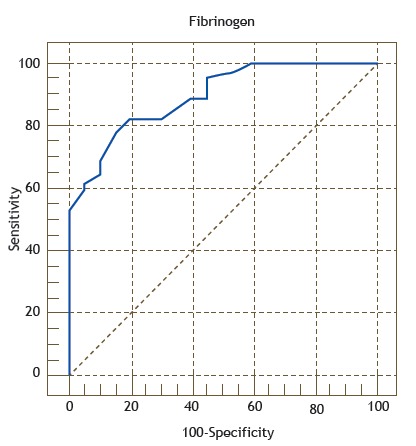
The suPAR and fibrinogen cut-off values for the diagnosis of AECOPD were obtained by calculating the maximum sum of sensitivity and specificity. The ROC curves for suPAR and fibrinogen are shown in Figures 3 and 4, respectively. For the diagnosis of AECOPD, the sensitivity, specificity, and accuracy of suPAR were 95.6%, 80.0%, and 93.0%, respectively, compared with 77.8%, 85.0%, and 89.5%, respectively, for fibrinogen.
Figure 3. ROC curve of the accuracy of soluble urokinase-type plasminogen activator receptor (suPAR) in identifying acute exacerbation of COPD, with an area under the curve of 0.93 (p <0.001). The curve was constructed by calculating the sensitivity versus the specificity for the different possible suPAR cut-off points.
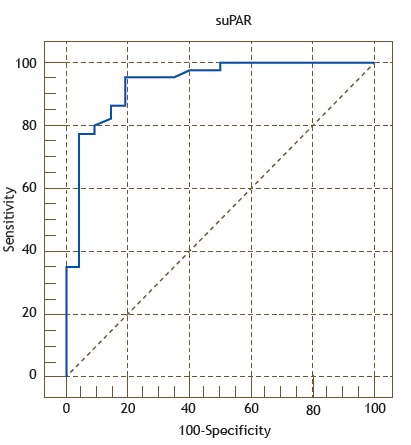
Figure 4. ROC curve of the accuracy of fibrinogen in identifying acute exacerbation of COPD, with an area under the curve of 0.89 (p < 0.005). The curve was constructed by calculating the sensitivity versus the specificity for the different possible fibrinogen cut-off points.
Of the 45 COPD patients, 9 (20.0%) did not recover from the exacerbation: 1 patient in GOLD stage II; 4 patients in GOLD stage III; and 4 patients in GOLD stage IV. Analyzing those 9 patients together, in comparison with the 36 patients who recovered, we found that the mean serum suPAR on day 1 had been slightly but significantly higher in the former group (5,551.1 ± 1,483.2 pg/mL vs. 4,462.71 ± 1,411.3 pg/mL; p = 0.046), as had the plasma fibrinogen levels on day 1 (685.5 ± 271.1 mg/dL vs. 522.5 ± 190.8 mg/dL; p = 0.048).
DISCUSSION
In patients with AECOPD, the deteriorating lung function and pronounced systemic inflammation worsen quality of life and reduce survival. 20 In the present study, suPAR and fibrinogen were evaluated as blood biomarkers of AECOPD. In accordance with our results, other studies have reported fibrinogen levels to be significantly higher in COPD patients than in control subjects. 21 - 23 Similarly, Portelli et al. 23 found that levels of serum suPAR were higher in patients with asthma or COPD than in control subjects. In another recent study, 14 fibrinogen was found to be higher in AECOPD patients than in healthy subjects. Therefore, determining the serum levels of suPAR and fibrinogen could be helpful in the evaluation of patients with stable COPD. 24
The presence of C-reactive protein and fibrinogen indicates systemic inflammation, and the levels of both of those markers increase during AECOPD. 25 In contrast, suPAR has been shown to be an independent marker of inflammation, because it is very stable and its serum concentration is unaffected by circadian changes. 26
In our study, serum suPAR levels were higher in the AECOPD patients than in the control subjects, and the difference was statistically significant. Our finding that serum suPAR levels were significantly higher before treatment than after is in agreement with the findings of another recent study 27 in which the suPAR levels of patients with stable COPD were compared with those of control subjects and were found to be significantly higher in the former, suggesting that there are inflammatory processes in stable COPD.
One recent study of patients with stable COPD 14 reported that serum suPAR levels were significantly higher on day 7 of treatment than on the day before treatment, and that levels of suPAR were higher in COPD patients than in healthy control subjects. Despite the fact that we measured serum suPAR after 14 days of treatment for AECOPD, that is in agreement with our results. Assessment of serum suPAR levels could play an important role in the evaluation of the inflammatory process in COPD. An increase in the serum suPAR level has been associated with GOLD stages III and IV, 18 which is also in agreement with our results.
Many studies have reported that fibrinogen levels are higher in COPD patients than in healthy control subjects. 28 - 32 As in our study, Gumus et al. 24 found a significant positive correlation between serum suPAR and fibrinogen. Those authors concluded that suPAR should be considered a marker of acute inflammation.
In the present study, a significant negative correlation was found between serum suPAR levels and FEV1 (% of predicted), which indicates the degree of airflow obstruction. That is in accordance with the findings of previous studies evaluating the relationship between inflammatory markers and lung function. 16 , 33 , 34 On the basis of these findings, suPAR can be considered an inflammatory marker in AECOPD.
Plasma fibrinogen appears to be an important blood biomarker of systemic inflammation. In COPD exacerbations, steroids might alter plasma fibrinogen through an effect on the inflammatory response, an effect not seen in patients with stable COPD. 15
Our study also showed a decrease in plasma fibrinogen and serum suPAR levels after 14 days of treatment for AECOPD. Analysis of the area under the ROC curve showed that suPAR was superior to fibrinogen in identifying patients with AECOPD on day 1 and day 14, which is in agreement with the findings of Gumus et al., 24 despite the fact that those authors evaluated their patients at 7 days of treatment.
We conclude that the determination of serum suPAR levels can be helpful in the follow-up of AECOPD and in the monitoring of the treatment response, potentially making suPAR a valuable biomarker in the prognosis of AECOPD. Because serum suPAR levels correlated with plasma fibrinogen levels, both markers have the potential to predict AECOPD. There is a need for further clinical studies including more patients in order to evaluate the diagnostic value of serum suPAR in comparison with that of other known markers of AECOPD.
Study carried out at the Faculty of Medicine, Tanta University, Tanta, Gharbia, Egypt.
Financial support: None.
REFERENCES
- 1.Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA. Characterization of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122–122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. doi: 10.1183/09031936.01.00229701. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Rennard S. Chronic obstructive pulmonary disease biomarker(s) for disease activity needed--urgently. Am J Respir Crit Care Med. 2010;182(7):863–864. doi: 10.1164/rccm.201004-0602ED. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Xiao W, Zhang Y, Xing Y. Urokinase-type plasminogen activator system and human cationic antimicrobial protein 18 in serum and induced sputum of patients with chronic obstructive pulmonary disease. Respirology. 2010;15(6):939–946. doi: 10.1111/j.1440-1843.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Xiao W, Jiang Y, Wang H, Xu X, Ma D. Levels of components of the urokinase-type plasminogen activator system are related to chronic obstructive pulmonary disease parenchymal destruction and airway remodelling. J Int Med Res. 2012;40(3):976–985. doi: 10.1177/147323001204000316. [DOI] [PubMed] [Google Scholar]
- 7.Eugen-Olsen J, Gustafson P, Sidenius N, Fischer TK, Parner J, Aaby P. The serum level of soluble urokinase receptor is elevated in tuberculosis patients and predicts mortality during treatment a community study from Guinea-Bissau. Int J Tuberc Lung Dis. 2002;6(8):686–692. [PubMed] [Google Scholar]
- 8.Wrotek A, Pavlik K, Jackowska T. Soluble receptor for urokinase plasminogen activator in community-acquired pneumonia in children. Adv Exp Med Biol. 2013;788:329–334. doi: 10.1007/978-94-007-6627-3_44. [DOI] [PubMed] [Google Scholar]
- 9.Loonen AJ, de Jager CP, Tosserams J, Kusters R, Hilbink M, Wever PC. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2014;9(1):e87315. doi: 10.1371/journal.pone.0087315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttunen R, Syrjänen J, Vuento R, Hurme M, Huhtala H, Laine J. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia a prospective cohort study. J Intern Med. 2011;270(1):32–40. doi: 10.1111/j.1365-2796.2011.02363.x. [DOI] [PubMed] [Google Scholar]
- 11.Eugen-Olsen J. suPAR - a future risk marker in bacteremia. J Intern Med. 2011;270(1):29–31. doi: 10.1111/j.1365-2796.2011.02372.x. [DOI] [PubMed] [Google Scholar]
- 12.Duvoix A, Dickens J, Haq I, Mannino D, Miller B, Tal-Singer R. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sin DD, Vestbo J. Biomarkers in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(6):543–545. doi: 10.1513/pats.200904-019DS. [DOI] [PubMed] [Google Scholar]
- 14.Can Ü, Güzelant A, Yerlikaya FH, Yosunkaya S. The role of serum soluble urokinase-type plasminogen activator receptor in stable chronic obstructive pulmonary disease. J Investig Med. 2014;62(7):938–943. doi: 10.1097/JIM.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 15.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 16.Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi: 10.2147/COPD.S34186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268(3):296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of COPD 2016. Bethesda: Global Initiative for Chronic Obstructive Lung Disease; http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016 [Google Scholar]
- 19.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 20.Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61(1):23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Rio F, Miravitlles M, Soriano JB, Mu-oz L, Duran-Tauleria E, Sánchez G. Systemic inflammation in chronic obstructive pulmonary disease a population-based study. Respir Res. 2010;11:63–63. doi: 10.1186/1465-9921-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portelli MA, Siedlinski M, Stewart CE, Potsma DS, Nieuwenhuis MA, Vonk JM. Genome-wide protein QTL mapping identifies human plasma kallikrein as a post-translational regulator of serum uPAR levels. FASEB J. 2014;28(2):923–934. doi: 10.1096/fj.13-240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumus A, Altintas N, Cinarka H, Kirbas A, Haziroglu M, Karatas M. Soluble urokinase-type plasminogen activator receptor is a novel biomarker predicting acute exacerbation in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:357–365. doi: 10.1183/13993003.congress-2015.PA3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moberg M, Vestbo J, Martinez G, Lange P, Ringbaek T. Prognostic value of C-reactive protein, leukocytes, and vitamin d in severe chronic obstructive pulmonary disease. ScientificWorldJournal. 2014;2014:140736–140736. doi: 10.1155/2014/140736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52(7):1284–1293. doi: 10.1373/clinchem.2006.067595. [DOI] [PubMed] [Google Scholar]
- 27.Ümmügülsüm C. The role of soluble urokinase-type plasminogen activator receptor (suPAR) in multiple respiratory diseases. Receptors Clin Invest. 2015;2(1):e473 [Google Scholar]
- 28.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation data from the Third National Health and Nutrition Examination. Am J Med. 2003;114(9):758–762. doi: 10.1016/S0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(12):1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 30.Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, Yenicerioglu Y. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis. 2008;26(2):97–102. doi: 10.1007/s11239-007-0073-1. [DOI] [PubMed] [Google Scholar]
- 31.Valipour A, Schreder M, Wolzt M, Saliba S, Kapiotis S, Eickhoff P. Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci (Lond) 2008;115(7):225–232. doi: 10.1042/CS20070382. [DOI] [PubMed] [Google Scholar]
- 32.Kunter E, Ilvan A, Ozmen N, Demirer E, Ozturk A, Avsar K. Effect of corticosteroids on hemostasis and pulmonary arterial pressure during chronic obstructive pulmonary disease exacerbation. Respiration. 2008;75(2):145–154. doi: 10.1159/000097748. [DOI] [PubMed] [Google Scholar]
- 33.Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(6):1008–1011. doi: 10.1164/ajrccm.164.6.2010067. [DOI] [PubMed] [Google Scholar]
- 34.Thorleifsson SJ, Margretardottir OB, Gudmundsson G, Olafsson I, Benediktsdottir B, Janson C. Chronic airflow obstruction and markers of systemic inflammation results from the BOLD study in Iceland. Respir Med. 2009;103(10):1548–1553. doi: 10.1016/j.rmed.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


