ABSTRACT
Objective:
To evaluate the effectiveness of a smoking cessation program, delivered by trained health care professionals, in patients hospitalized for acute respiratory disease (RD) or heart disease (HD).
Methods:
Of a total of 393 patients evaluated, we included 227 (146 and 81 active smokers hospitalized for HD and RD, respectively). All participants received smoking cessation treatment during hospitalization and were followed in a cognitive-behavioral smoking cessation program for six months after hospital discharge.
Results:
There were significant differences between the HD group and the RD group regarding participation in the cognitive-behavioral program after hospital discharge (13.0% vs. 35.8%; p = 0.003); smoking cessation at the end of follow-up (29% vs. 31%; p < 0.001); and the use of nicotine replacement therapy (3.4% vs. 33.3%; p < 0.001). No differences were found between the HD group and the RD group regarding the use of bupropion (11.0% vs. 12.3%; p = 0.92). Varenicline was used by only 0.7% of the patients in the HD group.
Conclusions:
In our sample, smoking cessation rates at six months after hospital discharge were higher among the patients with RD than among those with HD, as were treatment adherence rates. The implementation of smoking cessation programs for hospitalized patients with different diseases, delivered by the health care teams that treat these patients, is necessary for greater effectiveness in smoking cessation.
Keywords: Smoking, Smoking cessation, Hospitalization, Respiratory tract diseases, Heart diseases
RESUMO
Objetivo:
Avaliar a eficácia de um programa de cessação de tabagismo, oferecido por profissionais da saúde treinados, para pacientes hospitalizados por doença cardíaca (DC) ou doença respiratória (DR).
Métodos:
Foram avaliados 393 pacientes, sendo incluídos 227 (146 e 81 pacientes tabagistas ativos hospitalizados com DC e DR, respectivamente) que receberam intervenção para cessação tabágica durante a internação com seguimento de seis meses após a alta hospitalar.
Resultados:
Houve diferenças significativas entre os grupos DC e DR em relação à participação na intervenção cognitivo-comportamental após a alta hospitalar (13,0% vs. 35,8%; p = 0,003); cessação do tabagismo ao final do seguimento (29% vs. 31%; p < 0,001); e uso de terapia de reposição de nicotina (3,4% vs. 33,3%; p < 0,001). Em relação ao uso da bupropiona, não houve diferença entre os grupos DC e DR (11,0% vs. 12,3%; p = 0,92). A vareniclina foi usada em apenas 0,7% dos pacientes do grupo DC.
Conclusões:
Nesta amostra, os pacientes com DR apresentaram maior taxa de cessação tabágica após seis meses da alta hospitalar e maior adesão ao tratamento. A incorporação de programas de cessação do tabagismo para pacientes hospitalizados com patologias diversas, promovidos pelas equipes que os atendem, é necessária para que ocorra uma maior efetividade na cessação tabágica.
Descritores: Hábito de fumar, Abandono do hábito de fumar, Hospitalização, Doenças respiratórias, Cardiopatias
INTRODUCTION
Although the prevalence of smoking in Brazil has been declining in recent decades, with a rate of 12.1% in 2013 in Brazilian state capitals and the Federal District of Brasília, there remains a high prevalence of smokers who are hospitalized. 1 , 2 These rates range from 15% to 22% in public hospitals 3 - 5 and are usually associated with tobacco-related diseases, which provides a window of opportunity for smoking cessation interventions. 6 - 8
Specific strategies can increase adherence, reduce health care costs, and, most importantly, improve patient quality of life. 8 , 9 Studies have shown that smoking cessation treatment is effective when initiated at hospital admission and continuing for one month after hospital discharge, 10 which also results in a reduction in readmission costs for tobacco-related diseases when the hospital has a smoking cessation program for inpatients. 9
Despite scientific evidence of the benefits of smoking cessation treatment initiated during hospitalization, there are few health care facilities in Brazil that provide the necessary treatment resources. 8 For functioning of this in-hospital service, there is a need for integration of a qualified team into routine care in hospitals, to approach patients who smoke, together with provision of pharmacological and behavioral treatment. In addition, it is recommended that patients receive post-discharge follow-up for maintenance of cessation. 8 However, few studies in Brazil have described this in-hospital intervention or have reported results regarding smoking cessation for different tobacco-related chronic diseases in hospitalized patients. Therefore, the objective of the present study was to evaluate the effectiveness of a smoking cessation program, delivered by trained health care professionals, in patients hospitalized for respiratory disease (RD) or heart disease (HD).
METHODS
We evaluated a total of 393 patients-246 and 147 patients hospitalized for HD and RD, respectively-at the Botucatu School of Medicine Hospital das Clínicas, located in the city of Botucatu, Brazil, between March 2012 and June 2014. We included patients ≥ 18 years of age. The group of patients with HD was classified according to the primary diagnosis during hospitalization: acute myocardial infarction, in 166 patients; unstable angina, in 65; and heart failure, in 15. The RD group was also classified according to the primary diagnosis: COPD, in 58 patients; other causes (dyspnea), in 52; pulmonary thromboembolism, in 28; interstitial lung disease, in 5; and pneumonia, in 4. All patients who reported current smoking (at least one cigarette/day in the previous week) at admission were classified as active smokers. Patients who had ceased using tobacco products for more than 30 days before hospitalization were considered former smokers. The exclusion criteria were the impossibility of evaluating patients because of their unstable clinical condition, such as the requirement for mechanical ventilation, hemodynamic instability, or coma, and a lack of understanding on the part of patients regarding the objectives of the protocol.
The study was approved by the Research Ethics Committee of the Botucatu School of Medicine (Protocol no. 3403-2009), and all participating patients gave written informed consent.
All participants were evaluated by clinical history taking and underwent a thorough physical examination. They also completed a specific questionnaire addressing demographic characteristics, smoking history (including current smoking status), smoking habits in social activities, age at smoking initiation, and number of cigarettes smoked per day. In addition, all participants were administered the Hospital Anxiety and Depression scale, 9 the Fagerström Test for Nicotine Dependence, 10 and the stages-of-change model for smoking cessation devised by Prochaska & DiClemente. 11
Patient status as a smoker or former smoker during treatment was confirmed by measuring exhaled carbon monoxide (CO) levels (Micro CO; Micro Medical Ltd, Rochester, England), and exhaled CO levels ≥ 7 ppm were considered significantly indicative of recent smoking. 12 , 13
Smoking cessation protocol
All smoking cessation treatment (during hospitalization and after discharge) was conducted by one trained health care professional. All patients underwent two 15-min sessions of individual counseling during hospitalization. Smoking cessation medications were used at the physician’s discretion, in accordance with smoking cessation guidelines, 7 , 14 that is, all patients with a dependence score ≥ 5 or who experienced withdrawal symptoms during hospitalization were prescribed smoking cessation medications (nicotine replacement therapy, bupropion, or varenicline). 7 , 14 Pharmacological treatment was not prescribed for patients who did not experience withdrawal symptoms and/or did not want to use medications. Educational material containing information about nicotine dependence and behavioral counseling was distributed to all participants. All of the material was developed by the smoking cessation group at the Botucatu School of Medicine Hospital das Clínicas.
At hospital discharge, all patients were referred for continuing treatment in a cognitive-behavioral smoking cessation treatment program, attending 2-h weekly sessions in the first month. In the second month, patients returned every 15 days for a session; and, from the third month onward, they returned once a month until they completed six months of follow-up. In addition, patients were contacted via telephone before the scheduled reevaluations, in order to improve adherence to treatment. For the purposes of data analysis, participants who did not complete follow-up were considered to have experienced treatment failure and to be active smokers. Readmissions and outpatient visits were analyzed.
Statistical analysis
The study sample size was calculated based on a population estimate. The proportion of smokers among patients hospitalized with HD or RD and treated at the Botucatu Hospital das Clínicas was unknown. In addition, we considered a confidence interval of 90% and a maximum estimation error of 5%. On this basis, the sample size was set at 271 patients.
Descriptive statistics are presented as mean and standard deviation or as median and interquartile range for variables with normal and non-normal distribution, respectively. The difference between the groups was assessed using the chi-square test for categorical variables and using the t-test or the Mann-Whitney test for continuous variables according to their parametric or non-parametric distribution, respectively. All analyses were performed with SPSS Statistics, version 22.0 for Windows (IBM Corporation, Armonk, NY, USA), and values of p < 0.05 were considered significant.
RESULTS
We evaluated 393 hospitalized patients, of whom 227 were active smokers and were included in the study: 146 in the HD group and 81 in the RD group (Figure 1). Compared with the RD group, the HD group had a significantly higher number of males (72.6% vs. 32.1%; p < 0.001); a higher monthly income (in Brazilian reals [R$]: R$1,150 [R$677-2,000] vs. R$677 [R$360-1,040]; p = 0.02); a higher mean body weight (77.4 ± 17.1 kg vs. 68.7 ± 20.0 kg; p < 0.002); and greater height (1.65 ± 0.10 m vs. 1.60 ± 0.10 m; p < 0.001). Conversely, tobacco consumption (in pack-years) was greater in the RD group patients than in the HD group patients (55.6 ± 36.0 pack-years vs. 53.4 ± 25.1 pack-years; p = 0.62), although that difference was not significant (Table 1).
Figure 1. Flowchart of study participants. PTE: pulmonary thromboembolism; ILD: interstitial lung disease; and AMI: acute myocardial infarction.
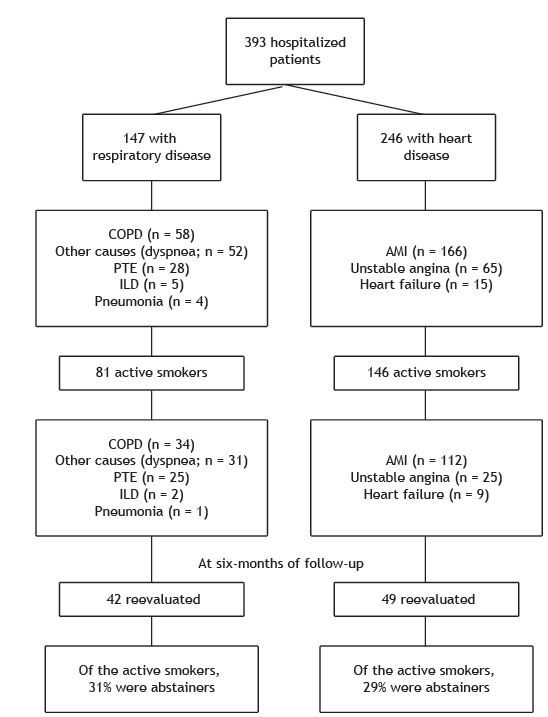
Table 1. Characteristics of smoking participants by study group.a .
| Variable | Respiratory disease | Heart disease | p* |
|---|---|---|---|
| (n = 81) | (n = 146) | ||
| Male gender | 26 (32.1) | 106 (72.6) | < 0.001 |
| Age, years | 59.7 ± 13.0 | 57.3 ± 17.1 | 0.13 |
| Income, R$b | 677 (360-1.040) | 1150 (677-2.000) | 0.02 |
| Weight, kg | 68.7 ± 20.0 | 78.4 ± 17.1 | 0.002 |
| Height, m | 1.60 ± 0.10 | 1.65 ± 0.10 | < 0.001 |
| BMI, kg/m² | 26.9 ± 7.6 | 28.1 ± 5.9 | 0.27 |
| Level of education | |||
| < 9 years of schooling | 45 (55.6) | 84 (57.5) | 0.29 |
| Age at smoking initiation, years | 14.1 ± 7.6 | 14.1 ± 5.9 | 0.79 |
| Smoking history, pack-years | 55.6 ± 36.0 | 53.4 ± 25.1 | 0.62 |
| Exhaled carbon monoxide, ppm | 3.6 ± 4.6 | 2.1 ± 1.8 | < 0.001 |
| Level of dependence | |||
| High | 38 (46.9) | 79 (54.1) | 0.29 |
| Low | 43 (53.1) | 67 (45.9) | |
| Motivational stage | |||
| Precontemplation | 14 (17.3) | 13 (8.9) | |
| Contemplation | 31 (38.3) | 52 (35.6) | 0.17 |
| Action | 36 (44.4) | 81 (55.5) | |
| Anxiety scale | |||
| Improbable | 45 (55.5) | 78 (53.4) | |
| Possible | 21 (26.0) | 36 (24.6) | 0.83 |
| Probable | 15 (18.5) | 32 (22.0) | |
| Depression scale | |||
| Improbable | 52 (64.1) | 110 (75.3) | |
| Possible | 17 (21.0) | 22 (15.0) | 0.20 |
| Probable | 12 (14.9) | 14 (9.7) |
BMI: body mass index. aValues expressed as n (%) or as mean ± SD, except where otherwise indicated. bValues expressed as median (interquartile range). *Chi-square test, t-test, or Mann-Whitney test
The causes of hospitalization among smokers in the RD group were, in decreasing order: other causes (dyspnea), in 34 patients; COPD, in 31; pulmonary thromboembolism, in 13; interstitial lung disease, in 2; and pneumonia, in 1. In the HD group, the causes of hospitalization among smokers were, also in decreasing order: acute myocardial infarction, in 112 patients; unstable angina, in 25; and heart failure, in 9.
The Fagerström Test for Nicotine Dependence scores were “high” among active smokers in the HD group and the RD group (54.1% vs. 46.9%; p = 0.29). The proportion of patients who were in the “action” stage of change in the HD group and the RD group was also similar (55.5% vs. 44.4%; p = 0.17). We also did not find statistically significant differences between the HD group and the RD group regarding the Hospital Anxiety and Depression Scale scores: “probable anxiety” (22.0% vs. 18.5%; p = 0.83) and “probable depression” (9.7% vs. 14.9%; p = 0.20).
Of the total number of active smokers at hospital discharge, only 19 patients (13.0%) in the HD group and 29 (35.8%) in the RD group participated in the cognitive-behavioral smoking cessation intervention (p = 0.003). Nicotine patch use occurred in 3.4% and 33.3% of the patients in the HD group and the RD group, respectively (p < 0.001), whereas bupropion use occurred in 11.0% and 12.3%, respectively (p = 0.92). Varenicline was used in 0.7% of the patients in the HD group.
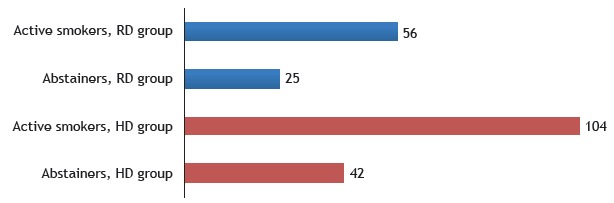
At six months after hospital discharge, 42 patients in the RD group and 49 patients in the HD group were reevaluated. The proportion of abstainers was higher in the RD group than in the HD group (Figure 2).
Figure 2. Number of patients in each group by smoking status at six months after hospital discharge. RD: respiratory disease; and HD: heart disease. p < 0.001.
There were 35 deaths within six months after hospital discharge: 18 in the RD group (13 patients still smoked) and 17 in the HD group (14 patients still smoked; p = 0.003). The primary cause of death was undetermined in 23.6% and 28.0% of the patients in the HR group and the RD group, respectively; followed by septicemia, in 23.6% and 11.1%; pneumonia, in 11.7% and 16.7%; and acute respiratory failure; in 11.7% and 16.7%. We observed that 19 (12.3%) and 27 (10.9%) of the patients in the RD group and the HD group, respectively, had further hospitalizations over the follow-up period, and most of them were active smokers (89% and 92% in the RD group and the HD group, respectively).
During the follow-up period, 75% of the total of 81 active smokers with RD and 78% of the total of 146 active smokers with HD had at least one outpatient visit with a medical specialist (pulmonologist or cardiologist, respectively).
DISCUSSION
The objective of the present study was to evaluate the rate of smoking cessation in patients hospitalized for HD or RD. At six months after discharge, the rates of smoking abstinence were 29% and 31% in the HD group and the RD group, respectively. In addition, we found that the highest proportion of patients adhering to the post-discharge smoking cessation protocol (35.8%) was seen in the RD group, as was the highest proportion patients using the medications provided through the protocol (23.3%).
Our study showed a similar rate of smoking cessation in the RD group at six months after hospital discharge similar to that reported in the literature. An early study, 15 in which 74 hospitalized patients with RD were randomized into a control or intervention group, showed that the rate of smoking cessation in the intervention group was 33.3% at six months after hospital discharge. The intervention group subjects were provided with 15- to 20-min smoking cessation counseling sessions every two days, whereas the control group subjects were simply advised to quit smoking. 15 Unlike our findings, those of a recent study conducted at three public hospitals in Australia showed that the sustained smoking cessation rate for a cohort of 600 patients hospitalized for different tobacco-related diseases was 11.6% at six months of follow-up. 16 In contrast, when the rate of smoking cessation in the group of patients with RD is compared with data from outpatient treatment of patients with RD, we observe that the rate found in our study was lower than those reported in two previous studies (40.5% and 52%, respectively). 17 , 18
A systematic review has shown that the effectiveness of smoking cessation interventions in hospitalized patients with RD did not differ by type of treatment, and none of the treatments studied had any significant effect. 19 However, in the present study, the rate of smoking cessation was higher in the RD group than in the HD group, and the rate of abstinence in the HD group was lower than that reported in the literature (range, 21.3-25.0%). 20 - 24
Our study showed a high prevalence of active smokers among patients hospitalized for RD or HD (57.8%), which is consistent with the literature showing that smoking is associated with 51.5% of hospitalizations. 4 , 14 A previous study conducted at our institution demonstrated that, of 348 patients hospitalized for tobacco-related diseases, 41% were active smokers. 3 Therefore, on the basis of treatment recommendations for hospitalized smokers, 25 our study showed that post-discharge treatment adherence was poor, being in contrast with a study showing that only 33% of patients were noncompliant with the recommended cognitive-behavioral intervention during follow-up. 26 Similarly, in a recent study of 109 participants who received pharmacological smoking cessation treatment with outpatient follow-up for four weeks, the dropout rate was 33.1%. 27
In our study, the RD group showed greater adherence to the post-discharge smoking cessation protocol compared with the HD group; this appears to be associated with the development of a relationship between patients and respiratory care professionals. The protocol required that the smoking cessation treatment always be provided by the same health care professional, even after hospital discharge. The better treatment adherence the RD group might have reflected the fact that, during hospitalization, respiratory care professionals directly assess smoking withdrawal symptoms on a daily basis, which was not true for the cardiac care team. These findings confirm that counseling and follow-up by the team treating smokers with non-respiratory, tobacco-related diseases are essential. 6 , 28 , 29
The rates of additional hospitalizations and mortality were comparable between the RD and HD groups, being low in both groups. Our study showed that 12.3% of the patients with RD were hospitalized again during the follow-up period, and most of them were still smoking. One recent study reported a 27.7% rate of hospital readmission at six months after hospital discharge among patients who received smoking cessation treatment, a rate higher than that found in our study. 20 In line with those findings, a study of 254 patients with coronary disease reported that 30% of the patients who continued smoking had a higher number of comorbidities and a higher hospital readmission rate, 30 which increases hospitalization rates and health care expenditures. 1 With regard to reducing costs, the study by Berndt et al. 31 showed that the costs of hospital readmissions for smokers were lower when these smokers received a combined approach of usual smoking cessation treatment and telephone or face-to-face counseling.
The rate of medication use to control nicotine withdrawal symptoms was low in the total sample (14.5%); however, nicotine replacement therapy use was more common in the RD group compared with the HD group. Our finding is similar to those reported by Barreto et al. 4 and Regan et al., 32 who found rates of use of these medications of 28.1% and 37.6%, respectively. In contrast, there have been studies showing a greater frequency of use of pharmacological treatment in hospitalized patients. Simon et al. 33 showed that 48% of smokers hospitalized with HD used smoking cessation medications. Similarly, Rigotti et al. 34 reported the use of medications in 67% of all patients hospitalized with tobacco-related diseases. The limited use of medications in the present study may have influenced the low smoking cessation rate at six months after hospital discharge.
Maintaining patient motivation depends on professionals working constantly and multidisciplinarily. 2 However, the lack of knowledge and training of health care professionals is an important factor in treatment failure. In contrast, a study conducted in Greece showed that 76.7% of health care professionals believed they provided effective smoking cessation counseling/aids for their patients. 35 In our study, the lack of qualified health care professionals made it impossible to assess the effectiveness of the intervention in patients with other tobacco-related diseases; in fact, it is necessary that all patients who smoke receive treatment. A study of surgeons showed that most of them (60.9%) reported advising their patients to quit smoking. At the surgeon’s advice, 95.3% of the patients agreed to quit smoking before surgery, 53.6% would quit after surgery, and 70.6% had already quit smoking. 36 Thorndike et al. 37 evaluated physician practices regarding the counseling of patients to quit smoking, between 1991 and 1995, and found that, at 65% of visits, physicians asked patients about their smoking status; at 29%, they counseled patients to quit smoking; and, at only 1.3%, they prescribed a specific smoking cessation treatment.
The demographic data in the present study revealed a low level of education and a low per capita income in both groups; however, the RD group had a lower median monthly per capita income compared with the HD group. These data are similar to those found previously at our facility, where 61% of the outpatient smokers had not completed high school and 66% had a monthly income of less than two times the national minimum wage. 38 Studies have associated increased smoking prevalence with lower economic and educational levels, and poor motivation and a lack of resources are also believed to be associated with smoking cessation failure. 39 Therefore, continuous formulation of smoking prevention and control strategies remains a major challenge for developing countries.(2.40)
The present study observed a low rate of smoking cessation during the follow-up period in both groups. The proportion of patients who were lost to follow-up was high, and the rate of medication use to aid in smoking cessation was low in both groups. Nevertheless, most of the patients were monitored by a specialist during outpatient follow-up. It is certainly essential to maintain smoking cessation programs for hospitalized smokers to prevent complications of tobacco-related chronic diseases and to improve overall aspects of health.
Study carried out at the Hospital das Clínicas, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista - UNESP - Botucatu (SP) Brasil.
Financial support: Sílvia Aline dos Santos Andrade received financial support in the form of a Young Investigator Grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 2012/02582-4).
REFERENCES
- 1.Figueiredo VC. Um panorama do tabagismo em 16 capitais brasileiras e Distrito Federal: tendências e heterogeneidades. Rio de Janeiro: Universidade do Estado do Rio de Janeiro; 2007. [Google Scholar]
- 2.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde; Secretaria de Gestão Estratégica e Participativa . Vigitel Brasil 2012: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico. Brasília: Ministério da Saúde; 2013. [Google Scholar]
- 3.Tanni SE, Iritsu NI, Tani M, de Camargo PA, Sampaio MG, Godoy I. Risk perceptions and behavior among hospitalized patients with smoking-related diseases. Prev Chronic Dis. 2009;6(4):A138–A138. [PMC free article] [PubMed] [Google Scholar]
- 4.Barreto RB, Pincelli MP, Steinwandter R, Silva AP, Manes J, Steidle LJ. Smoking among patients hospitalized at a university hospital in the south of Brazil prevalence, degree of nicotine dependence, and motivational stage of change. J Bras Pneumol. 2012;38(1):72–80. doi: 10.1590/S1806-37132012000100011. [DOI] [PubMed] [Google Scholar]
- 5.Corrêa AP. Prevalência e perfil tabágico de pacientes adultos internados em unidades cirúrgicas de um hospital universitário. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2014. [Google Scholar]
- 6.Rigotti NA, Arnsten JH, McKool KM, Wood-Reid KM, Pasternak RC, Singer DE. Smoking by patients in a smoke-free hospital prevalence, predictors, and implications. Pt 1Prev Med. 2000;31(2):159–166. doi: 10.1006/pmed.2000.0695. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health; 2004. [Google Scholar]
- 8.Reichert J, Araújo AJ, Gonçalves CM, Godoy I, Chatkin JM, Sales MP. Diretrizes para cessação do tabagismo da SBPT 2008. J Bras Pneumol. 2008;34(10):845–880. doi: 10.1590/S1806-37132008001000014. [DOI] [PubMed] [Google Scholar]
- 9.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 10.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3-4):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037/0022-006X.51.3.390. [DOI] [PubMed] [Google Scholar]
- 12.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 13.Santos UP, Gannam S, Abe JM, Esteves PB, F M, Filho, Wakassa TB. Use of breath carbon monoxide as an indicator of smoking status [Article in Portuguese] J. Pneumol. 2001;27(5):231–236. doi: 10.1590/S0102-35862001000500001. [DOI] [Google Scholar]
- 14.2008 PHS Guideline Update Panel. Liaisons.and Staff Treating tobacco use and dependence 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- 15.Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease a randomized clinical trial. Int J Addict. 1991;26(1):107–119. doi: 10.3109/10826089109056242. [DOI] [PubMed] [Google Scholar]
- 16.Thomas D, Abramson MJ, Bonevski B, Taylor S, Poole SG, Paul E, et al. Integrating smoking cessation into routine care in hospitals--a randomized controlled trial. Addiction. 2016;111:714–723. doi: 10.1111/add.13239. [DOI] [PubMed] [Google Scholar]
- 17.Willemse B, Lesman-Leegte I, Timens W, Postma D, ten Hacken N. High cessation rates of cigarette smoking in subjects with and without COPD. Chest. 2005;128(5):3685–3687. doi: 10.1378/chest.128.5.3685. [DOI] [PubMed] [Google Scholar]
- 18.Chen J. Chen Y, Chen P, Liu Z, Lou H, Cai S Effectiveness of individual counseling for smoking cessation in smokers with chronic obstructive pulmonary disease and asymptomatic smokers. Exp Ther Med. 2014;7(3):716–720. doi: 10.3892/etm.2013.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients a systematic review. Arch Intern Med. 2008;168(18):1950–1960. doi: 10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chouinard MC, Robichaud-Ekstrand S. The effectiveness of nursing inpatients smoking cessation program in individuals with cardiovascular disease. Nurs Res. 2005;54(4):243–254. doi: 10.1097/00006199-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen L, Johansen S, Eksten L. Smoking cessation among patients with acute heart disease. A randomized intervention project [Article in Danish]. Ugeskr Laeger. 2005;167(33):3044–3047. [PubMed] [Google Scholar]
- 22.Croghan GA, Croghan IT, Frost MH, Sloan JA, Novotny PJ, Nelson MA, et al. Smoking cessation interventions and postoperative outcomes in esophageal and lung cancer patients [POS3-101]; Society for Research on Nicotine and Tobacco 11th Annual Meeting; Prague: Czech Republic: 2005. [Google Scholar]
- 23.Rigotti NA, Thorndike NA, Regan S, McKool K, Pasternak RC, Chang Y. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;119(12):1080–1087. doi: 10.1016/j.amjmed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Mohiuddin SM, Mooss NA, Hunter CB, Grollmes TL, Cloutier DA, Hillerman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131(2):446–452. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 25.Raupach T, Falk J, Vangeli E, Schiekirka S, Rustler C, Grassi MC. Structured smoking cessation training for health professional on cardiology wards a prospective study. Eur J Prev Cardiol. 2014;21(7):915–922. doi: 10.1177/2047487312462803. [DOI] [PubMed] [Google Scholar]
- 26.Sardinha A, Oliva AD, Augustin JD, Ribeiro F, Falcone EM. Cognitive-behavioral group therapy for smoking cessation [Article in Portuguese] Rev Bras Ter Cogn. Rio de Janeiro. 2005;1(1):1–10. [Google Scholar]
- 27.Mesquita AA. Evaluation of a program for smoking cessation [Article in Portuguese] Rev Bras Ter Comport. Cogn. 2013;15(2):35–44. [Google Scholar]
- 28.McIvor A, Kayser J, Assaad JM, Brosky G, Demarest P, Desmarais P. Best practices for smoking cessation interventions in primary care. Can Respir J. 2009;16(4):129–134. doi: 10.1155/2009/412385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo AC, Azevedo RC. Factors that motivate smokers to seek outpatient smoking cessation treatment at university general hospital. J Bras Pneumol. 2010;36(5):603–611. doi: 10.1590/S1806-37132010000500012. [DOI] [PubMed] [Google Scholar]
- 30.Mosleh SM, Darawad M. Patients' Adherence to Healthy Behavior in Coronary Heart Disease Risk Factor Management Among Jordanian Patients. J Cardiovasc Nurs. 2015;30(6):471–478. doi: 10.1097/JCN.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 31.Berndt N, Bolman C, Lechner L, Max W, Mudde A, de Vries H. Economic evaluation of a telephone- and face-to-face-delivered counseling intervention for smoking cessation in patients with coronary heart disease. Eur J Health Econ. 2016;17(3):269–285. doi: 10.1007/s10198-015-0677-x. [DOI] [PubMed] [Google Scholar]
- 32.Regan S, Viana JC, Reyen M, Rigotti NA. Prevalence and predictors of smoking by inpatients during a hospital stay. Arch Intern Med. 2012;172(21):1670–1674. doi: 10.1001/2013.jamainternmed.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive cessation versus minimal counseling among hospitalized smokers treated with transdermal replacement a randomized trial. Am J Med. 2003;114(7):555–562. doi: 10.1016/S0002-9343(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 34.Rigotti NA, Regan S, Levy DE, Japuntich S, Chang Y, Parke ER. Sustained care intervention and postdircharge smoking cessation among hospitalized adults a randomized clinical trial. JAMA. 2014;312(7):719–728. doi: 10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moysidou A, Farsalinos KE, Voudris V, Merakou K, Kourea K, Barbouni Knowledge and Perceptions about Nicotine, Nicotine Replacement Therapies and Electronic Cigarettes among Healthcare Professionals in Greece. Int J Environ Res Public Health. 2016;13(5):pii: E514–pii: E514. doi: 10.3390/ijerph13050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajjar WM, Al-Nassar AS, Alahmadi RM, Almohanna SM, Alhilali SM. Behavior, knowledge, and attitude of surgeons and patients toward preoperative smoking cessation. Ann Thorac Med. 2016;11(2):132–140. doi: 10.4103/1817-1737.180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorndike NA, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA. 1998;279(8):604–608. doi: 10.1001/jama.279.8.604. [DOI] [PubMed] [Google Scholar]
- 38.Caram LM, Ferrari R, Tanni SE, Coelho LS, Godoy Id, Martin Rdos S. Characteristics of smokers enrolled in a public smoking cessation program. J Bras Pneumol. 2009;35(10):980–985. doi: 10.1590/S1806-37132009001000006. [DOI] [PubMed] [Google Scholar]
- 39.Peixoto SV, Firmo JO, Lima-Costa MF. Factors associated with smoking cessation in two different adult populations (Bambuí and Belo Horizonte Projects) [Article in Portuguese] Cad Saude. Publica. 2007;23(6):1319–1328. doi: 10.1590/S0102-311X2007000600007. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt MI, Duncan BB, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM. Chronic non-communicable diseases in Brazil burden and current challenges. Lancet. 2011;377(9781):1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]


