Abstract
Background
Anti-inflammatory mediators such as mucin-domain containing-3 (TIM-3) and IL-37 play an important role in the regulation of Th1-mediated immunity. This study was designed to investigate the proportions of various T cell subsets and monocytes in the peripheral blood of rheumatoid arthritis (RA) patients, as well as the level of TIM-3 on these cells and serum cytokine levels.
Material/Methods
We enrolled 59 RA patients and 46 age- and sex-matched healthy controls in this study. The proportion of T cells and TIM-3 expression on these T cells were determined by flow cytometry. Cytokine levels in serum were determined by ELISA.
Results
Compared with the healthy controls, the proportions of CD3+CD4+ T cells and CD3+CD4+CD25+CD127low T cells in the peripheral blood were significantly higher in RA patients. However, RA patients had significantly lower proportions of CD3+CD8+ T cells and CD3+CD4−CD8− T cells. TIM-3 was highly expressed on CD3+CD4+, CD3+CD8+, CD3+CD4+CD25+CD127low, and CD3+CD4−CD8− T cells, as well as CD14+ monocytes, in RA patients. Nevertheless, no correlation between TIM-3 level and an RA disease activity score of 28 was found. The elevated serum levels of IL-6 and IL-37 were positively correlated with tumor necrosis factor-α (TNF-α).
Conclusions
Both pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory mediators (TIM-3 and IL-37) simultaneously contribute to the pathogenesis of RA. TIM-3 and IL-37 may be used as potential biomarkers of active RA.
MeSH Keywords: Interleukins; T-Lymphocyte Subsets; Arthritis, Rheumatoid
Background
Rheumatoid arthritis (RA) is an autoimmune disease featuring synovial hyperplasia and progressive bone destruction [1]. Multiple immune cells, including CD4+ T cells [2], CD8+ T cells [3], regulatory T cells [4], and monocytes/macrophages [5], as well as various cytokines such as IL-6, TNF-α, and IL-17 [6], are involved in the mechanism of RA. The imbalance between anti-inflammatory and pro-inflammatory cytokines, which are generally attributed to either the increase of pro-inflammatory cytokines and positive regulatory cells or the decrease of anti-inflammatory cytokine and negative regulatory cells, can result in the onset of autoimmune disorders such as RA [7,8] and systemic lupus erythematosus (SLE) [9,10]. However, the exact mechanisms remain unclear [11].
T cell immunoglobulin and mucin-domain containing-3 (TIM-3) are novel transmembrane proteins involved in the regulation of T-helper 1 (Th1) cell-mediated immunity. TIM-3 and its ligand galectin-9 can inhibit Th1-mediated auto- and allo-immune responses and promote immunological tolerance [12,13]. Previous studies indicate that TIM-3 on the peripheral lymphocytes from RA patients increased and were negatively correlated with the disease progression [14,15]. Nevertheless, they did not clarify the differences in TIM-3 levels on various T cell subsets and monocytes in RA patients.
Interleukin-37 (IL-37) is a recently identified member of the interleukin-1 (IL-1) family and is an important anti-inflammatory cytokine involved in inflammatory regulation [16–19]. Generally, serum IL-37 levels in RA patients are significantly higher than those in healthy controls and are significantly positively correlated with serum levels of pro-inflammatory cytokines and clinical parameters of disease activity [20–22]. In addition, IL-37 levels in active RA patients were significantly enhanced as compared with those in patients in remission [21,22].
It has been reported that the percentage of Tim-3+ cells in peripheral blood mononuclear cells and Tim-3 expression on CD8+ T and NKT cells negatively correlates with pro-inflammatory cytokine TNF-α level in plasma from RA patients [14]. In contrast to TNF-α, IL-37 is a newly identified anti-inflammatory cytokine and is involved in the pathogenesis of RA [16,19,20]. Both IL-37 and TIM-3 have negative regulation roles in RA [14,15,19], but whether IL-37 and TIM-3 correlate and synergize with each other remains unclear in RA patients. Therefore, this study assessed whether there is a special association between IL-37 and TIM-3. We investigated the proportions of T cell subsets and monocytes in the peripheral blood of RA patients, as well as the expression of TIM-3 on these cells and serum cytokine levels. The relationship of TIM-3 and IL-37 with RA disease progression was also investigated.
Material and Methods
Patients
Fifty-nine RA patients from Qilu Hospital of Shandong University were enrolled in the study between 2013 and 2017. RA was diagnosed according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria [23]. Patients who had other diseases or were pregnant were excluded. The clinical disease activity was evaluated using the disease activity score 28 (DAS28) [24]. Forty-six age- and sex-matched healthy people were also enrolled as healthy controls (HCs). The clinical characteristics of the study cohort are shown in Table 1. Fresh peripheral blood was collected from RA patients and HCs. Serum and peripheral blood mononuclear cells were isolated. Prior written and informed consent were obtained from every patient. This study conformed to the approved institutional guidelines and was approved by the Ethics Committee of Qilu Hospital of Shandong University (No. 12126).
Table 1.
The demographic and clinical characteristics of the study cohort.
| Items | Rheumatoid arthritis patients | Healthy controls |
|---|---|---|
| N | 59 | 46 |
| Age, yr | 50.4±14.7 | 44.7±13.7 |
| Female, n (%) | 52 (91.3) | 29 (89.3) |
| Male, n (%) | 7 (8.7) | 17 (10.7) |
| CRP, mg/L | 40.2±45.0 | – |
| ESR, mm/h | 54.8±28.7 | – |
| HAQ | 1.1±0.6 | – |
| DAS28 | 4.8±1.0 | – |
| WBCs (×109/L) | 8.1±2.9# | 6.1±1.1 |
| Lymphocytes, %* | 15.9±6.6# | 23.2±6.8 |
| IL-37 (ng/L) | 139.5±57.76# | 114.2±22.47 |
| TNF-α (ng/L) | 4.84±1.64# | 3.62±0.83 |
| IL-6 (ng/L) | 9.07±13.35# | 0.74±0.58 |
CRP – c-reactive protein; ESR – erythrocyte sedimentation rate; HAQ – Health Assessment Questionnaire; DAS28 – Disease Activity Score-28; SDAI – Simplified Disease Activity Index; CDAI – Clinical Disease Activity Index; WBCs – white blood counts; IL – interleukin; TNF-α – tumor necrosis factor-α. Values are shown as mean ±SD, unless otherwise noted.
Percentage of total white blood cells in peripheral blood.
p<0.05 compared to healthy controls.
Flow cytometry
Peripheral blood mononuclear cells was stained using the following fluorochrome-conjugated monoclonal antibodies: anti-human CD3 APC-eFluor780, anti-human CD8 FITC, anti-human CD127 PE-Cyanine7, anti-human CD14 PerCP− Cyanine 5.5 or isotype matched controls (all from eBioscience, San Diego, CA, USA); anti-human CD4 Alexa Fluor 700, anti-human CD25 PE/Dazzle 594 or isotype matched control (all from BioLegend, San Diego, CA, USA); and anti-human Tim-3 PE or isotype matched control (R&D, Minneapolis, MN, USA). Data were obtained from multicolor analysis using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and were analyzed using FlowJo software 7.6.2 (Tree Star, Ashland, OR, USA).
Enzyme-linked immunosorbent assay (ELISA)
IL-6 (BD OptEIA™ Human IL-6 ELISA Set, BD Biosciences, San Joes, USA), TNF-α (BD OptEIA™ Human TNF ELISA Set, BD Biosciences, San Joes, USA) and IL-37 (Invitrogen™ IL-37 Human Uncoated ELISA Kit in serum of RA patients and HCs were determined by ELISA according to the manufacturer’s protocol.
Statistical analysis
All values are shown as mean ±SD or median (range) unless otherwise stated. Inter-group differences were assessed for significance using the independent-samples t test or Mann-Whitney U test. Correlations between variables were assessed by Pearson or Spearman’s rank correlation coefficient. A value of p<0.05 indicated statistical significance. All statistical analyses were carried out using GraphPad Prism 5.00 (San Diego, CA, USA).
Results
Demographic and clinical characteristics of study cohort
There were no significant differences in age and sex distribution between RA patients and HCs (Table 1). Among RA patients, 3 patients had erythrocyte sedimentation rate (ESR) ≤15 mm/h and 11 patients had c-reactive protein (CRP) ≤8 mg/l. When recruited, only 2 patients (3.4%) were in clinical remission (DAS28 ≤2.6) and the remaining 57 patients (96.6%) were assessed as being in the active period of RA according to DAS28 scoring. The average number of peripheral white blood cells (WBCs) in circulation was significantly higher in RA patients than in HCs (8.1±2.9 vs. 6.1±1.1 cells/L), while the proportion of lymphocytes in WBCs was significantly lower in RA patients than in HCs (P<0.05).
Correlations of serum IL-37, IL-6 and TNF-α in RA patients
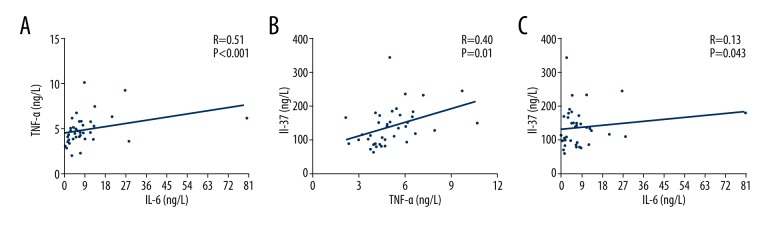
Serum levels of IL-37, IL-6, and TNF-α were tested by ELISA. As shown in Table 1, the levels of these cytokines in RA patients were remarkably higher compared to those in HCs. The serum levels of IL-6 and IL-37 correlated positively with TNF-α (Figure 1A, 1B). However, there was no significant correlation between the serum levels of IL-6 and IL-37 (Figure 1C). This result indicates that the pro-inflammatory cytokines TNF-α and IL-6 and the anti-inflammatory cytokine IL-37 contribute to the occurrence of RA.
Figure 1.
The correlation of IL-6 and TNF-α with IL-37 in RA patients. (A) Correlation between TNF-α and IL-6 in RA patients. (B) Correlation between IL-37 and TNF-α in RA patients. (C) Correlation between IL-37 and IL-6 in RA patients. Correlations between two cytokines were analyzed by Pearson or Spearman rank correlation test. A value of p<0.05 was considered to be statistically significant.
Clinical characteristics of T cell subsets and monocytes in RA patients
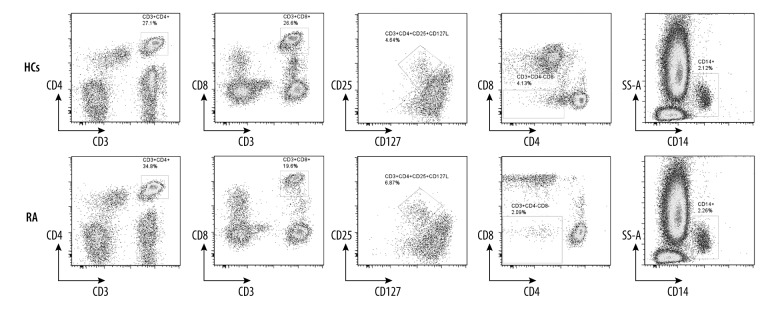
To determine the proportion of T cell subsets and monocytes, flow cytometry was performed. Representative flow cytometry results are shown in Figure 2. Statistically, RA patients had a remarkably higher percentage of peripheral CD3+CD4+ T cells than HCs (35.10 (10.40, 54.10) vs. 29.85 (13.70, 44.20), p=0.0200), and CD3+CD4+CD25+CD127low T cells (regulatory T cells) (5.02 (1.99, 10.20) vs. 3.95 (2.00, 6.70), p=0.0018) (Table 2). Meanwhile, RA patients presented a significantly lower percentage of CD3+CD8+ T cells than HCs (19.10 (6.82, 49.40) vs. 24.30 (6.82, 50.30), p=0.0052), as well as CD3+CD4−CD8− T cells (2.97 (0.72, 25.80) v 4.53 (1.86, 24.60), p=0.0019). Nevertheless, the proportion of CD14+ cells (monocytes) was similar in the 2 groups. This result suggests that the imbalance of T cell subsets contributes to the onset of RA.
Figure 2.
Representative flow cytometry results. The percentage of different T cell subsets in RA patients and HCs was analyzed by flow cytometry. The T cell subsets included CD3+CD4+ T subsets, CD3+CD8+ T subsets, CD3+CD4+CD25+CD127LowCD4+ T subsets, CD3+ CD4−CD8− T subsets, and CD14+ monocytes.
Table 2.
Comparisons of T cell subsets and monocyte between rheumatoid arthritis patients and healthy controls.
| Cell subsets | Rheumatoid arthritis patients | Healthy controls | P value |
|---|---|---|---|
| CD3+CD4+ T cells | 35.10 (10.40, 54.10) | 29.85 (13.70, 44.20) | 0.0200 |
| CD3+CD8+ T cells | 19.10 (6.82, 49.40) | 24.30 (6.82, 50.30) | 0.0052 |
| CD3+CD4+CD25+ CD127low T cells |
5.02 (1.99, 10.20) | 3.95 (2.00, 6.70) | 0.0018 |
| CD3+CD4−CD8− T cells | 2.97 (0.72, 25.80) | 4.53 (1.86, 24.60) | 0.0019 |
| CD14+ T cells | 2.82 (0.65, 34.70) | 2.64 (1.36, 5.82) | 0.2900 |
Values are shown as median (range), unless otherwise noted.
TIM-3 level on different T cell subsets and monocytes in RA patients
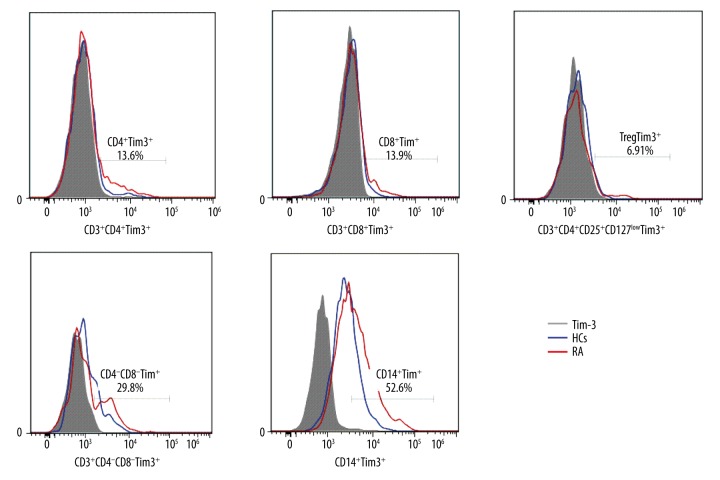
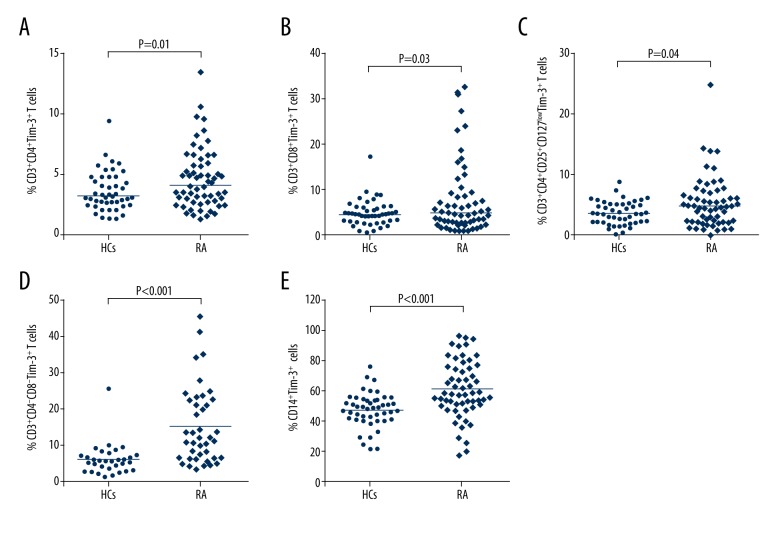
Populations of varied T cell subsets and monocytes in RA patients and HCs were gated using FACS, and the surface expressions of TIM-3 on these cells were subsequently evaluated. Representative flow cytometry results are shown in Figure 3. As shown in Figure 4, surface expressions of TIM-3 on CD3+CD4+ T cells (Figure 4A), CD3+CD8+ T cells (Figure 4B), CD3+CD4+CD25+CD127low T cells (Figure 4C), CD3+CD4−CD8− T cells (Figure 4D), and CD14+ cells (Figure 4E) were all higher in RA patients than those on the corresponding cells of HCs (p<0.05), indicating that the increase of TIM-3 expression on various T cell subsets may be a compensatory reaction against the elevation of pro-inflammation cytokines.
Figure 3.
Representative flow cytometry results. The expression of TIM-3 on different T cell subsets was analyzed by flow cytometry. The results of RA patients and HCs are shown.
Figure 4.
TIM-3 expressions on different T cell subsets and monocyte in RA patients. (A) Proportions of CD3+CD4+TIM-3+ T cells in RA compared to HCs. (B) Proportions of CD3+CD8+TIM-3+ T cells in RA patients compared to HCs. (C) Proportions of CD3+CD4+CD25+ CD127lowTIM-3+ T cells in RA patients compared to HCs. (D) Proportions of CD3+CD4−CD8− TIM-3+ T cells in RA patients compared to HCs. (E) Proportions of CD14+TIM-3+ cells in RA patients compared to HCs. The horizontal indicates the median values of the different groups. The differences between the 2 groups were analyzed by Mann-Whitney U test. A value of p<0.05 was considered to be statistically significant.
Correlation between TIM-3 expression and RA disease activity
To analyze how TIM-3 expression relates to RA disease activity, correlation analysis was performed. No correlation was found between DAS28 score and TIM-3 expression on CD3+CD4+ T cells (r=0.113, p=0.383), CD3+CD8+ T cells (r=−0.142, p=0.270), CD3+CD4+CD25+CD127low T cells (r=0.083, p=0.522), CD3+CD4−CD8− T cells (r=−0.006, p=0.968), and CD14+ cells (r=0.149, p=0.250). Similarly, no correlation was found between CRP and TIM-3 expression on CD3+CD4+ T cells (r=0.239, p=0.061), CD3+CD8+ T cells (r=−0.052, p=0.690), CD3+CD4+CD25+CD127low T cells (r=0.149, p=0.247), CD3+CD4−CD8− T cells (r=−0.143, p=0.355), and CD14+ cells (r=0.213, p=0.100). This was also the case between ESR and TIM-3 expression on CD3+CD4+ T cells (r=0.116, p=0.371), CD3+CD8+ T cells (r=−0.162, p=0.209), CD3+CD4+CD25+CD127low T cells (r=0.179, p=0.164), CD3+CD4−CD8− T cells (r=0.061, p=0.693), and CD14+ cells (r=0.247, p=0.055).
Correlation between TIM-3 expression and IL-37
To analyze how TIM-3 expression affects IL-37, correlation analysis was performed. No correlation was found between IL-37 and TIM-3 expression on CD3+CD4+ T cells (r=−0.237, p=0.147), CD3+CD8+ T cells (r=−0.098, p=0.555), CD3+CD4+CD25+CD127low T cells (r=−0.072, p=0.664), CD3+CD4−CD8− T cells (r=0.152, p=0.361), and CD14+ cells (r=−0.101, p=0.624).
Discussion
RA is an autoimmune inflammatory disease featuring articular synovial proliferation with or without systemic inflammatory reaction [25]. The imbalance between pro- and anti-inflammatory cytokine activities [6] is involved in the pathogenesis of RA. Therefore, promoting the expression of anti-inflammatory cytokines and/or inhibiting the expression of pro-inflammatory cytokines [26] may be a promising tactic for RA therapy.
As a newly identified anti-inflammatory cytokine, IL-37 can inhibit the expression, production, and function of pro-inflammatory cytokines [19] and is involved in the development of autoimmune diseases [14,27]. As shown in our present study, IL-37, TNF-α, and IL-6 in RA patients were remarkably higher compared to levels in HCs. The serum level of IL-37 was positively correlated with TNF-α, but no correlation was found between IL-37 and IL-6. This result is in accord with a previous study [22]. Both TNF-α and IL-6 contribute to the pathogenesis of RA [28,29], but the why IL-37 was positively correlated with TNF-α rather than IL-6 remains unclear. In this study, we speculate that increased IL-37 is likely to be stimulated by pro-inflammatory cytokines such as IL-6 and TNF-α. IL-37 suppresses excessive inflammatory reaction by mediating a negative feedback mechanism in RA patients. Nevertheless, increased IL-37 could not completely neutralize the deteriorating effects of pro-inflammatory cytokines in active RA.
Besides cytokines, multiple T cell subsets also contribute to the pathogenesis of RA [30,31]. In the present study, we found that the proportion of CD3+CD4+ T cells was significantly higher and the percentage of CD3+CD8+ T cells was significantly lower in RA patients compared to HCs. These findings are identical with those of previous studies [32,33]. CD4+ T cells are necessary for disease initiation [34]. The depletion of CD4v T cells suppresses autoantibody production and reduces disease severity in the collagen- and antigen-induced arthritis models in rodents [35,36]. In contrast, depletion of CD8+ T cells enhanced the onset of arthritis in severe combined immune deficiency (SCID) mice [35] or did not significantly affect the transfer of arthritis into SCID mice [36]. These results demonstrate that CD4+ T cells are essential in the pathogenesis of antigen-induced arthritis (AIA), whereas CD8+ T cells seem to not be required for the induction of this disease [36] or might have a suppressive role in the etiology [35].
Regulatory T cells have been found in synovial tissue and peripheral blood of RA patients [37–40]. However, there is a controversy regarding the relative number and function of regulatory T cells in RA [4,37,40]. Here, we found that the proportions of regulatory T cells were significantly higher in RA patients compared to HCs. This result is in accordance with the study by van Amelsfort et al. [4]. Depletion of CD25+ cells with anti-CD25 antibody led to the aggravation of joint inflammation [41,42] and adoptive transfer of regulatory T cells during the initiation phase of arthritis decreased the severity of disease [42,43]. Therefore, increased numbers of regulatory T cells benefit the body in regulating the overactive inflammation reaction and function as the compensatory response to the pro-inflammatory mediators or cells, but the compensation is always incomplete.
Similarly, with regulatory T cells, double-negative (CD4−CD8−) T (DNT) cells represent a novel suppressor cell population negatively regulating immune responses. It has been reported that DNT cells participate in multiple autoimmune diseases [44–46]. The proportion of DNT cells was considerably increased in SLE [44,45] and Behcet’s disease patients [46]. However, the character of DNT cells in RA remains unclear. In our present study, the proportion of DNT cells (CD3+CD4−CD8− T cells) was remarkably lower in RA patients compared to HCs. This result is consistent with another study, which reported that RA patients presented considerably lower levels of gamma delta DNT cells in peripheral blood than in control subjects, which means that gamma delta DNT cells are probably relevant to RA and take part in the mechanism of RA [47]. The weakness of negative regulation caused by the decrease of DNT cells partially results in the onset of RA.
TIM-3 is expressed primarily on the surface of activated CD3+CD4+CD8− T cells and other immune cells such as CD3+CD8− IL-17+ T lymphocytes, CD3+CD4−CD8+ T lymphocytes, macrophages, natural killer T cells, and dendritic cells [48,49]. TIM-3 negatively regulates the T cell response by inducing T cell apoptosis [50] and serves as a phagocytic receptor to eliminate apoptotic cells [51]. In the present case-control research, the expression of TIM-3 on various T cell subsets and monocytes between RA patients and the matched HCs were assessed and compared. The results showed that the surface expressions of TIM-3 on CD3+CD4+ T cells, CD3+CD8+ T cells, regulatory T cells, DNT cells, and monocytes were remarkably higher in RA patients compared with healthy controls. These results are in accordance with previous studies that suggested the dysregulation of TIM-3 expression on CD8+ T cells and CD4+ T cells is partially due to autoimmune diseases [48,52]. These data show that TIM-3 is probably involved in the mechanism of RA and functions as the compensatory response to synovial inflammation and proliferation. However, in the present study, the results of differences between the expression of Tim-3 in various T cell subsets and DAS28 scores were inconsistent with previous studies [14,15], which may be attributed to the different status of patients. In our study, the enrolled patients included not only newly diagnosed patients, but also treated patients. Even though both IL-37 levels in plasma and the surface expressions of TIM-3 on various T cells from RA patients significantly increased, no correlation was found between TIM-3 and IL-37, which means that TIM-3 and IL-37 may have no synergetic effect and may play independent roles via different signal transduction pathways.
Conclusions
In conclusion, the surface expressions of TIM-3 on different T cell subsets and monocytes, as well as the serum concentration of IL-37, were significantly higher in RA patients compared to HCs. These findings suggest that pro-inflammatory cytokines such as TNF-α and IL-6 and anti-inflammatory mediators such as TIM-3 and IL-37 simultaneously play an important role in the pathogenesis of RA. TIM-3 and IL-37 are likely to be the potential biomarkers of active RA.
Footnotes
Conflicts of interests
None.
Source of support: This study was supported by the Shenzhen Science and Technology Innovation Committee Fund (JCYJ20160331173652555) and the Natural Science Foundation of Shandong Province (ZR2013HQ044)
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Poulter LW, Duke O, Panayi GS, et al. Activated T lymphocytes of the synovial membrane in rheumatoid arthritis and other arthropathies. Scand J Immunol. 1985;22:683–90. doi: 10.1111/j.1365-3083.1985.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 3.Berner B, Akca D, Jung T, et al. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–35. [PubMed] [Google Scholar]
- 4.van Amelsfort JM, Jacobs KM, Bijlsma JW, et al. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 5.Davignon JL, Hayder M, Baron M, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:590–98. doi: 10.1093/rheumatology/kes304. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 7.Mateen S, Zafar A, Moin S, et al. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;455:161–71. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Brzustewicz E, Bryl E. The role of cytokines in the pathogenesis of rheumatoid arthritis – Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine. 2015;76:527–36. doi: 10.1016/j.cyto.2015.08.260. [DOI] [PubMed] [Google Scholar]
- 9.Yap DY, Lai KN. The role of cytokines in the pathogenesis of systemic lupus erythematosus – from bench to bedside. Nephrology. 2013;18:243–55. doi: 10.1111/nep.12047. [DOI] [PubMed] [Google Scholar]
- 10.Davis LS, Hutcheson J, Mohan C. The role of cytokines in the pathogenesis and treatment of systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:781–89. doi: 10.1089/jir.2011.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Shu Q, Gao L, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–95. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Oh JM, Hwang JW, et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis. Scand J Rheumatol. 2011;40:334–40. doi: 10.3109/03009742.2010.547871. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello CA, Nold-Petry C, Nold M, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–81. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu WD, Zhao Y, Liu Y. Insights into IL-37, the role in autoimmune diseases. Autoimmun Rev. 2015;14:1170–75. doi: 10.1016/j.autrev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Quirk S, Agrawal DK. Immunobiology of IL-37: Mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 2014;10:1703–9. doi: 10.1586/1744666X.2014.971014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Zhang J, Tao J, Lu T. Elevated serum levels of Interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS. 2015;123:1025–31. doi: 10.1111/apm.12467. [DOI] [PubMed] [Google Scholar]
- 21.Xia T, Zheng XF, Qian BH, et al. Plasma Interleukin-37 is elevated in patients with rheumatoid arthritis: Its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis Markers. 2015;2015:795043. doi: 10.1155/2015/795043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao PW, Jiang WG, Wang L, et al. Plasma levels of IL-37 and correlation with TNF-alpha, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9:e95346. doi: 10.1371/journal.pone.0095346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–88. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 25.Biswas S, Sharma S, Saroha A, et al. Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach. PLoS One. 2013;8:e56246. doi: 10.1371/journal.pone.0056246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang QL, Zhou FJ, Wu CB, et al. Circulating biomarkers for predicting infliximab response in rheumatoid arthritis: A systematic bioinformatics analysis. Med Sci Monit. 2017;23:1849–55. doi: 10.12659/MSM.900897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Qiu F, Fan Y, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33:111–17. doi: 10.1007/s10875-012-9791-z. [DOI] [PubMed] [Google Scholar]
- 28.Nawata Y, Eugui EM, Lee SW, Allison AC. IL-6 is the principal factor produced by synovia of patients with rheumatoid arthritis that induces B-lymphocytes to secrete immunoglobulins. Ann NY Acad Sci. 1989;557:230–38. doi: 10.1111/j.1749-6632.1989.tb24016.x. discussion 239. [DOI] [PubMed] [Google Scholar]
- 29.Brennan FM, Maini RN, Feldmann M. TNF alpha – a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992;31:293–98. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 30.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrens EJ, Prakken BJ, van Wijk F. T cells out of control – impaired immune regulation in the inflamed joint. Nat Rev Rheumatol. 2013;9:34–42. doi: 10.1038/nrrheum.2012.149. [DOI] [PubMed] [Google Scholar]
- 32.Kuryliszyn-Moskal A. Comparison of blood and synovial fluid lymphocyte subsets in rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 1995;14:43–50. doi: 10.1007/BF02208083. [DOI] [PubMed] [Google Scholar]
- 33.Pawlowska J, Mikosik A, Soroczynska-Cybula M, et al. Different distribution of CD4 and CD8 T cells in synovial membrane and peripheral blood of rheumatoid arthritis and osteoarthritis patients. Folia Histochem Cytobiol. 2009;47:627–32. doi: 10.2478/v10042-009-0117-9. [DOI] [PubMed] [Google Scholar]
- 34.Wong PK, Quinn JM, Sims NA, et al. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158–68. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 35.Kadowaki KM, Matsuno H, Tsuji H, Tunru I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin Exp Immunol. 1994;97:212–18. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrow PK, Thoss K, Katenkamp D, Brauer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: Role of CD4+ and CD8+ T cells. Immunol Invest. 1996;25:341–53. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- 37.Lawson CA, Brown AK, Bejarano V, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 2006;45:1210–17. doi: 10.1093/rheumatology/kel089. [DOI] [PubMed] [Google Scholar]
- 38.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao D, Borjesson O, Larsson P, et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444–52. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 40.Mottonen M, Heikkinen J, Mustonen L, et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–67. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan ME, Sutmuller RP, Witteveen HJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 42.Frey O, Petrow PK, Gajda M, et al. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005;7:R291–301. doi: 10.1186/ar1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan ME, Flierman R, van Duivenvoorde LM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 44.Anand A, Dean GS, Quereshi K, et al. Characterization of CD3+ CD4− CD8− (double negative) T cells in patients with systemic lupus erythematosus: Activation markers. Lupus. 2002;11:493–500. doi: 10.1191/0961203302lu235oa. [DOI] [PubMed] [Google Scholar]
- 45.Dean GS, Anand A, Blofeld A, et al. Characterization of CD3+ CD4− CD8− (double negative) T cells in patients with systemic lupus erythematosus: Production of IL-4. Lupus. 2002;11:501–7. doi: 10.1191/0961203302lu234oa. [DOI] [PubMed] [Google Scholar]
- 46.Ling E, Shubinsky G, Press J. Increased proportion of CD3+CD4−CD8− double-negative T cells in peripheral blood of children with Behcet’s disease. Autoimmun Rev. 2007;6:237–40. doi: 10.1016/j.autrev.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Liu MF, Yang CY, Chao SC, et al. Distribution of double-negative (CD4− CD8−, DN) T subsets in blood and synovial fluid from patients with rheumatoid arthritis. Clin Rheumatol. 1999;18:227–31. doi: 10.1007/s100670050089. [DOI] [PubMed] [Google Scholar]
- 48.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: Emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–62. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 49.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–80. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 50.Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama M, Akiba H, Takeda K, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–30. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 52.Liberal R, Grant CR, Holder BS, et al. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012;56:677–86. doi: 10.1002/hep.25682. [DOI] [PubMed] [Google Scholar]