Abstract
Traditionally, antibiotics are included in animal feed at subtherapeutic levels for growth promotion and disease prevention. However, recent links between in-feed antibiotics and a rise in antibiotic-resistant pathogens have led to a ban of all antibiotics in livestock production by the European Union in January 2006 and a removal of medically important antibiotics in animal feeds in the United States in January 2017. An urgent need arises for antibiotic alternatives capable of maintaining animal health and productivity without triggering antimicrobial resistance. Host defense peptides (HDP) are a critical component of the animal innate immune system with direct antimicrobial and immunomodulatory activities. While in-feed supplementation of recombinant or synthetic HDP appears to be effective in maintaining animal performance and alleviating clinical symptoms in the context of disease, dietary modulation of the synthesis of endogenous host defense peptides has emerged as a cost-effective, antibiotic-alternative approach to disease control and prevention. Several different classes of small-molecule compounds have been found capable of promoting HDP synthesis. Among the most efficacious compounds are butyrate and vitamin D. Moreover, butyrate and vitamin D synergize with each other in enhancing HDP synthesis. This review will focus on the regulation of HDP synthesis by butyrate and vitamin D in humans, chickens, pigs, and cattle and argue for potential application of HDP-inducing compounds in antibiotic-free livestock production.
Keywords: Host defense peptides, Antibiotic alternatives, Butyrate, Vitamin D, Antibiotics
1. Introduction
In-feed supplementation of antibiotics was firstly reported to promote growth in chickens (Moore et al., 1946) and subsequently in pigs (Jukes et al., 1950, Luecke et al., 1950). Since then, antibiotics have been routinely used in livestock production at subtherapeutic levels for disease prevention and growth promotion and are more commonly known as antibiotic growth promoters (Dibner and Richards, 2005). Unfortunately, continuous long-term exposure of gut commensals and pathogens to low-dose antibiotics in livestock animals has been linked to increased emergence of antibiotic-resistant pathogens (Dibner and Richards, 2005, Lhermie et al., 2016, Thanner et al., 2016). Consequently, the European Union has banned the use of all antibiotics for the purpose of growth promotion since January 2006 (Seal et al., 2013), and the United States has taken a similar measure to phase out the application of medically important antibiotics for livestock production starting January 2017. A shift in governmental policy and public opinion has resulted in an urgent need for alternatives to in-feed antibiotics in order to ensure animal health and production efficiency with a minimum risk of triggering antimicrobial resistance.
Antibiotic alternatives such as organic acids, probiotics, prebiotics, and essential oils have been used for decades, but with limited success (Seal et al., 2013, Stanton, 2013). More effective antibiotic alternatives are, therefore, needed. Host defense peptides (HDP), also known as antimicrobial peptides, possess direct antimicrobial, immunomodulatory and often barrier protective activities (Hancock et al., 2012, Hilchie et al., 2013, Mansour et al., 2014, Robinson et al., 2015). A great majority of these HDP are expressed by mucosal epithelial cells of the digestive, respiratory, and urogenital tracts (Lyu et al., 2015). The strategies to increase HDP accumulation on mucosal surfaces will conceivably enhance animal immunity, health and disease resistance without relying on antibiotics. Consistently, accumulating evidence suggests the potential for HDP to be developed as novel antibiotic alternatives (Sang and Blecha, 2008, Robinson et al., 2015, Xiao et al., 2015, Wang et al., 2016). While dietary supplementation of exogenous HDP have shown promise in swine and chickens (Xiao et al., 2015, Wang et al., 2016), modulation of endogenous HDP to ensure animal growth and immunity is just beginning to be explored, with multiple compounds being identified with the ability to induce endogenous HDP synthesis (Campbell et al., 2012, van der Does et al., 2012, Lyu et al., 2015). The goal of this review is to summarize the relevant work regarding induction of HDP by dietary compounds, focusing specifically on butyrate and vitamin D.
2. Host defense peptides as effectors of innate immunity
2.1. Classification of HDP
Host defense peptides consist of a large group of diverse small peptides with direct microbicidal activities that exist in virtually all species of life and constitute an important mechanism of the first-line defense (Mansour et al., 2014). In vertebrates, a majority of HDP belong to either the defensin or cathelicidin family. Members of the defensin family contain 3 conserved disulfide bridges that form 2-3 antiparallel β-sheets (Selsted and Ouellette, 2005). Based upon the explicit spacing pattern of the cysteine residues, defensins are further categorized into α-, β-, and θ-defensins. While θ-defensins have only been found in primates, β-defensins are present in virtually all vertebrate animals, with only certain mammalian species harboring α-defensins (Selsted and Ouellette, 2005). Mammalian β-defensin genes are located in several separate clusters on different chromosomes, with the α- and θ-defensin genes, if any, existing within one of the β-defensin clusters (Patil et al., 2004, Patil et al., 2005). The human genome harbors 6 functional α-defensins and 5 pseudogenes in one cluster, 39 β-defensins in 5 different clusters, and one θ-defensin pseudogene (Schutte et al., 2002, Patil et al., 2004, Patil et al., 2005). On the other hand, at least 29 β-defensins are clustered on 4 different chromosomes in pigs (Choi et al., 2012), whereas the bovine genome encodes a minimum of 57 β-defensins in 4 separate clusters (Meade et al., 2014). In chickens, a total of 14 β-defensin genes are located in tandem in a single cluster (Xiao et al., 2004, Lynn et al., 2007).
Cathelicidins are identified by the presence of a highly conserved cathelin domain that is cleaved off to release a biologically active, mature peptide with diverse sequences (Zanetti, 2004). The human genome only contains one cathelicidin gene, known as human cationic antimicrobial peptide -18 (hCAP-18), cyclic Adenosine monophosphate (CAMP) or LL-37 (Gudmundsson et al., 1996). Four cathelicidin genes have been identified in chickens, namely fowlicidins 1-3 (or CATH1-3) (Xiao et al., 2006) and cathelicidin-B1 (Goitsuka et al., 2007) while 10 cathelicidin genes have been reported in cattle (Whelehan et al., 2014) and 11 cathelicidin genes in pigs (Sang and Blecha, 2009).
Defensins, and β-defensins in particular, are expressed in epithelial and phagocytic cells in a variety of different tissues, primarily on the mucosal surfaces lining the gastrointestinal, respiratory, and urogenital tracts along with the skin (Selsted and Ouellette, 2005). Cathelicidins are also present in various epithelial cells, but with more abundant expression in mammalian neutrophils (Selsted and Ouellette, 2005) or avian heterophils (Cuperus et al., 2013, Zhang and Sunkara, 2014). Additionally, chicken cathelicidin-B1 is expressed in the M cells of the bursa of Fabricius (Goitsuka et al., 2007).
2.2. Pleiotropic effects of HDP
Host defense peptides are characterized by their direct antimicrobial activities against a range of pathogens. Cathelicidins and β-defensins are active against Gram-negative and Gram-positive bacteria along with certain viruses, fungi, and protozoa (Zasloff, 2002, Takahashi et al., 2010, Wang, 2014). Host defense peptides are generally positively charged and amphipathic enabling them to bind to negatively charged phospholipids on bacterial membranes through physical electrostatic interactions. Once bound, hydrophobic regions of the HDP are inserted into the membrane bilayers to form pores mainly through ‘barrel-stave’, ‘carpet’ or ‘toroidal-pore’ mechanisms (Brogden, 2005, Takahashi et al., 2010). Certain HDP such as human α-defensin human neutrophil peptides-1 (HNP-1) and porcine cathelicidin prolinearginine rich-39 (PR-39) are also able to enter the bacterial cells and interfere with DNA and protein synthesis, protein folding, or cell wall synthesis (Brogden, 2005). These modes of action make it extremely difficult for bacteria to develop resistance to HDP even through millions of years of co-evolution, although certain bacteria are able to resist HDP killing by reducing the negative charge on the membrane (Peschel and Sahl, 2006). This is exemplified by the ability of Salmonella enterica and Pseudomonas aeruginosa to reduce their membrane affinity for HDP by incorporating positively charged aminoarabinose into lipid A (Ernst et al., 2001, Miller et al., 2005).
Besides the antimicrobial activities, HDP possess profound immunomodulatory and barrier protective functions as well. Many HDP are chemotactic to neutrophils (or heterophils in chickens), monocytes, lymphocytes, or dendritic cells, with the ability to further activate macrophages and dendritic cells (Hilchie et al., 2013, Mansour et al., 2014). Chicken CATH-1 has the capacity to recruit neutrophils and activate macrophages in mice (Bommineni et al., 2014), while protecting mice from infection (Bommineni et al., 2010, Bommineni et al., 2014). Three bovine β-defensins were also found to be chemotactic to immature dendritic cells (Mackenzie-Dyck et al., 2011). Several, but not all human defensins, were recently shown to have mucosal barrier protective properties by directly upregulating mucin and tight junction protein expression (Robinson et al., 2015). Porcine cathelicidin PR-39 is also capable of improving angiogenesis and wound repair with the ability to suppress nicotinamide adenine dinucleotide phosphate (NADPH) oxidase assembly and activation (Gallo et al., 1994, Shi et al., 1996).
2.3. Impact of exogenous HDP on growth and disease prevention
Consistent with their antimicrobial, immunomodulatory, and barrier protective activities, dietary supplementation of synthetic or recombinant HDP has been found to enhance animal growth and gut health, particularly in the context of disease (Xiao et al., 2015), highlighting the potential of HDP to be developed as antibiotic alternatives for disease control and prevention. Weanling pigs challenged with enterotoxic Escherichia coli show an improvement in growth performance with a decrease in the incidence of diarrhea when supplemented with colicin E1 (a bacteriocin) (Cutler et al., 2007), recombinant cecropin A/D (a silkworm HDP) (Wu et al., 2012), recombinant lactoferrin (a bovine HDP) (Tang et al., 2012), or a mixture of three recombinant HDP (Xiong et al., 2014). In healthy piglets, presence of a synthetic HDP is also capable of improving growth performance along with intestinal morphology and digestibility (Yoon et al., 2013). Similar results have also been observed in broilers. Dietary inclusion of a synthetic HDP or recombinant cecropin A/D resulted in enhanced growth performance associated with improved intestinal morphology and nutrient digestibility (Wen and He, 2012, Choi et al., 2013a, Choi et al., 2013b).
Although direct feeding of synthetic or recombinant HDP show their potential to be used as antibiotic alternatives, this approach is not expected to be efficient as a majority of exogenous HDP would be digested in the stomach and upper intestinal tract without efficiently reaching the lower intestinal tract where most pathogens reside. Moreover, it remains unclear whether this strategy is cost-effective and commercially viable for livestock applications as the cost of producing synthetic or recombinant HDP is expected to be substantial. Instead, dietary modulation of the synthesis of endogenous HDP may be a better approach. Currently, a range of small-molecule compounds have been found to induce HDP synthesis in humans and livestock animals (Campbell et al., 2012, van der Does et al., 2012, Lyu et al., 2015, Yedery and Jerse, 2015). Of these compounds, butyrate and vitamin D are among the earliest identified and most potent, so they will be the focus of this review.
3. Butyrate
3.1. Physiological roles of butyrate
Short-chain fatty acids (SCFA) are a group of fatty acids consisting of less than 6 carbons that are produced by bacterial fermentation of non-digestible carbohydrates in the large intestine of animals as well as the rumen of ruminant animals (Hamer et al., 2008, Canani et al., 2011). Acetate, propionate, and butyrate are the primary SCFA produced, with acetate comprising the greatest percentage. Butyrate encompasses the smallest percentage of SCFA produced, but is the primary energy source for colonocytes in humans (Canani et al., 2011). The amount of butyrate produced through bacterial fermentation varies greatly with animal species, age, gastrointestinal location, and diet (Rehman et al., 2007). In chickens, for a typical corn and soybean meal diet, the butyrate concentration in small intestine (duodenum, jejunum, and ileum) is typically 0.1 to 0.2 mmol/L of the digesta, while it can reach 7 to 17 mmol/L in the cecum, depending upon the bird age (Rehman et al., 2007). In weanling pigs, butyrate concentrations in the jejunum, ileum, and cecum was reported to be 2.2, 2.8, and 10.4 mmol/L of the digesta, respectively (Franklin et al., 2002). In cattle, butyrate amounts to 10 to 15 mmol/L in the rumen fluid (Balch and Rowland, 1957). Short-chain fatty acids produced in the large intestine are estimated to contribute 5% to 15% of the total caloric requirements of humans (Bergman, 1990), while providing approximately 24% of the energy for heat production in pigs (Yen et al., 1991). Short-chain fatty acids production in the cecum of steers provides approximately 8% of metabolizable energy, while ruminal SCFA contribute 50% to 75% (Siciliano-Jones and Murphy, 1989).
Apart from being an important energy source for the intestinal cells, SCFA and butyrate in particular exert a range of mucosal protective effects by suppressing carcinogenesis, inflammation, and barrier permeability (Hamer et al., 2008, Canani et al., 2011). Butyrate is capable of preventing colorectal cancers through inhibition of tumor proliferation, stimulation of differentiation, and induction of apoptosis in cancerous cells (Scheppach et al., 1995, Canani et al., 2011). Paradoxically, butyrate stimulates cell proliferation and differentiation while inhibiting apoptosis in normal intestinal cells in humans, cattle, and pigs (Scheppach et al., 1995, Kotunia et al., 2004, Mazzoni et al., 2008, Guilloteau et al., 2010). Butyrate promotes intestinal barrier function by facilitating tight junction assembly and inducing the expression and synthesis of multiple tight junction proteins and mucin-2 (Ploger et al., 2012). Moreover, butyrate displays potent anti-inflammatory effects through suppression of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-12, and tumor necrosis factor-α (TNF-α) (Säemann et al., 2000, Nancey et al., 2002, Fukae et al., 2005), while inducing anti-inflammatory cytokine, IL-10, in humans (Säemann et al., 2000). In chickens, butyrate has a minimum effect on the expression of IL-1β, IL-6, interferon-γ (IFN- γ) or nitric oxide, but suppresses the induction of these cytokines by lipopolysaccharides (LPS) both in vitro and in vivo (Sunkara et al., 2011, Zhang et al., 2011, Zhou et al., 2014). Similarly, dietary supplementation of weanling pigs with sodium butyrate reduces serum levels of IL-6 and TNF-α (Wen et al., 2012).
3.2. Role of butyrate in gut health and animal performance
Consistent with a plethora of beneficial effects of butyrate, an improvement in animal health and performance is often observed following butyrate supplementation. Intestinal morphology of broilers fed 0.5, 1 or 2 g/kg of unprotected sodium butyrate shows an increased villus height to crypt depth ratio (Hu and Guo, 2007), while oral gavage of sodium butyrate at 3 g/kg of the daily intake of milk dry matter during the suckling phase improves apparent digestibility of dry matter, organic matter, and nitrogen of growing pigs (Le Gall et al., 2009). Inclusion of 2 g/kg sodium butyrate in calf milk replacer or starter diets stimulates rumen development resulting in increased dry matter intake, metabolizable energy, and crude protein intake along with a decrease in the incidence of scours (Górka et al., 2011). Multiple studies have reported improved growth performance, particularly in the context of infections, in broilers when supplemented with 4 g/kg butyrate glycerides or 1 g/kg sodium butyrate (Leeson et al., 2005, Zhang et al., 2011). Similarly, the growth performance of growing and weanling pigs were also improved when fed 1.7 or 2 g/kg sodium butyrate (Galfi and Bokori, 1990, Xiong et al., 2016). This is also the case in dairy calves when supplemented with 3 g/kg of dry matter or 2 g/kg of the diet (Guilloteau et al., 2009, Górka et al., 2011). However, butyrate supplementation appears to provide no obvious benefits in growth performance in healthy animals (Leeson et al., 2005, Biagi et al., 2007, Guilloteau et al., 2010, Zhang et al., 2011, Fang et al., 2014).
4. Role of butyrate in HDP expression
In addition to the positive effects of butyrate on energy supply, inflammation, barrier integrity, and gut health, recent research has indicated the ability of butyrate to modulate intestinal immunity through induction of endogenous HDP in humans and multiple livestock species.
4.1. Humans
Butyrate is capable of enhancing LL-37 transcription and translation in a time- and dose-dependent manner in a variety of human cell lines (Hase et al., 2002, Schauber et al., 2003, Schauber et al., 2004). In addition to butyrate, other SCFA and their structural analogs such as propionate, isobutyrate, and 4-phenylbutyrate are also capable of stimulating LL-37 synthesis in human cells (Schauber et al., 2003, Steinmann et al., 2009). A direct comparison of saturated free fatty acids for their HDP-inducing activity revealed that fatty acids with 4-6 carbons are the most potent (Jiang et al., 2013). Consistently, oral administration of sodium butyrate or 4-phenylbutyrate reverses Shigella-mediated downregulation of cathelicidin in rabbits (Raqib et al., 2006, Sarker et al., 2011). Furthermore, an enema of sodium butyrate (80 mmol/L), twice daily for 3 days, increased both LL-37 mRNA levels in the rectal epithelia and LL-37 protein concentrations in the stools of human shigellosis patients (Raqib et al., 2012).
Besides LL-37, human β-defensin 1 (HBD-1) and HBD-2 are also induced by butyrate, albeit in a cell type- and gene-specific manner (Schauber et al., 2006, Schwab et al., 2008). For example, butyrate shows a minimum effect on the expression of HBD-1 and HBD-2 in primary human keratinocytes, but enhances strongly the expression of both defensins in HT-29 colonic epithelial cells, with preferential induction of HBD-2, but not HBD-1, in U937 monocytes (Schauber et al., 2006).
4.2. Poultry
In chickens, 1 to 4 mmol/L sodium butyrate increases the expression of multiple, but not all, HDP in different cell types, showing a clear gene- and cell type-specific induction pattern (Sunkara et al., 2011). Among all 14 chicken β-defensins and cathelicidins, 6 β-defensins (AvBD3, AvBD4, AvBD8, AvBD9, AvBD10, and AvBD14) and 1 cathelicidin (CATH-B1) are induced to different extents, with AvBD9 showing the highest magnitude of induction. Among several chicken cell types examined, butyrate increased AvBD9 gene expression by thousands, hundreds, and tens of times in HD11 macrophage cells, primary monocytes, and intestinal explants, respectively (Sunkara et al., 2011). Despite an inability to detect the HDP protein expression levels due to a lack of specific antibodies, an increase in the bacterial killing activity of chicken monocytes has been observed following butyrate treatment, which is very likely to be mediated through HDP induction as butyrate at the concentrations used has neither a direct antimicrobial activity nor the capacity to increase oxidative burst or phagocytosis of monocytes (Sunkara et al., 2011). Consistently, dietary supplementation of 1 or 2 g/kg of sodium butyrate augments AvBD9 expression throughout the intestinal tract of chickens and the clearance of Salmonella enteritidis following an experimental infection (Sunkara et al., 2011). Among short-, medium-, and long-chain fatty acids, SCFA are the most potent, with butyrate giving the highest fold induction (Sunkara et al., 2012). Several butyrate analogs such as benzyl butyrate and glyceryl tributyrate also display an ability to increase AvBD9 expression, albeit at a lesser magnitude (Sunkara et al., 2014).
4.3. Cattle
In cattle, studies with mammary epithelial cells have demonstrated the ability of 0.25, 0.5, 1 mmol/L butyrate, 1 mmol/L propionate, and 1 mmol/L hexanoate to reduce Staphylococcus aureus internalization by 50% to 60% (Ochoa-Zarzosa et al., 2009, Alva-Murillo et al., 2012). Stimulation of mammary epithelial cells with sodium butyrate followed by S. aureus infection showed a significant increase in bovine β-defensin and tracheal antimicrobial peptide (TAP) expression. Increased HDP expression was seen with 0.5 mmol/L butyrate which was associated with the greatest inhibition of S. aureus internalization (Ochoa-Zarzosa et al., 2009). These observations, along with evidence that butyrate is not toxic to S. aureus, indicate that butyrate-mediated upregulation of HDP rather than butyrate itself is likely responsible for inhibition of S. aureus internalization (Ochoa-Zarzosa et al., 2009, Alva-Murillo et al., 2012). However, it is important to note that stimulation of cells with butyrate and S. aureus also produced a 10-fold induction in nitric oxide (Ochoa-Zarzosa et al., 2009), suggesting that butyrate enhances bovine mammary gland defense by triggering both oxidative and non-oxidative mechanisms. Nevertheless, there is no increase in nitric oxide production in bovine mammary epithelial cells in response to 1 mmol/L sodium propionate or hexanoate (Alva-Murillo et al., 2012).
4.4. Swine
In porcine IPEC-J2 intestinal epithelial cells and 3D4/2 or 3D4/31 lung alveolar macrophage cells, 2 to 8 mmol/L butyrate induces the expression of multiple, but not all, porcine β-defensins and cathelicidins (PG1-5) in a time- and dose-dependent manner (Sunkara et al., 2014, Xiong et al., 2016). However, PBD1 fails to be induced in any cell type. Moreover, the magnitude of induction and optimal butyrate dose vary among different genes and cell types. For example, PBD2 is the most highly induced HDP in IPEC-J2 cells with an approximately 80-fold increase in response to 8 mmol/L butyrate (Zeng et al., 2013), but shows only a marginal induction in 3D4/31 cells at all doses applied, while PBD3 displays an impressive 200-fold induction when stimulated with 8 mmol/L butyrate (Zeng et al., 2013).
As in humans and chickens, the hydrocarbon chain length of free fatty acids is also correlated with their ability to induce HDP expression in swine. All free fatty acids with a hydrocarbon chain length of 1 to 18 are capable of inducing PBD2, PBD3, and EP2C in a dose-dependent manner (Zeng et al., 2013). However, free fatty acids with a length of four carbons or less display an increased magnitude of HDP induction with an increasing carbon number, while a length-dependent decrease in HDP induction was observed for the carbon length greater than four (Zeng et al., 2013). Additionally, 2 g/kg sodium butyrate shows the ability to alleviate the clinical symptoms of E. coli O157:H7 infection in weanling pigs and enhance bacterial clearance in 3D4/2 cells, which is associated with an increased HDP expression (Xiong et al., 2016).
5. Vitamin D
5.1. Physiological functions of vitamin D
Vitamin D is taken up directly from the diet or converted naturally from cholesterol in the skin in response to ultraviolet-B sunlight exposure. Biologically active 1α,25-dihydroxyvitamin D3 (1,25D3) is formed by activation of pre-vitamin D3 consecutively in the liver and kidney through 2 hydroxylation reactions (Svensson et al., 2016, Dimitrov and White, 2017). 1,25D3 exerts its biological activities through binding to the vitamin D receptor (VDR), which forms a heterodimer with retinoid X receptors to regulate the expression of a large group of genes (Svensson et al., 2016, Dimitrov and White, 2017). Vitamin D is well-known to be critical in calcium homeostasis and bone mineralization. Accumulating evidence has suggested a number of extra-skeletal effects on innate immunity and cancer inhibition (Svensson et al., 2016, Dimitrov and White, 2017). First, vitamin D exerts anti-inflammatory effects by reducing the expression of pro-inflammatory cytokines such as IL-1β, 1L-6, IL-8, CCL2, TNF-α, and IFN-γ after pathogenic challenge (Khoo et al., 2011, Di Rosa et al., 2012, Zhang et al., 2012). Vitamin D is also capable of promoting macrophage activation and phagocytosis, while steering monocyte differentiation toward macrophages rather than dendritic cells (Xu et al., 1993, Zhu et al., 2002, Griffin et al., 2003).
5.2. Vitamin D in growth performance and disease prevention
National Research Council (NRC) recommends dietary inclusion of vitamin D at 200, 150 to 220, and 250 IU/kg for broilers, growing pigs, beef cattle, respectively, where 1 IU is equivalent to 25 ng vitamin D3. The effects of vitamin D on growth and disease prevention in livestock has not been well studied. Inclusion of vitamin D at 5,000 IU/kg in piglet diets improved intestinal morphology and the serum concentration of IFN-β, while decreasing IL-2 and IL-6 serum concentrations, in the context of porcine rotavirus infection (Zhao et al., 2014). As a result, growth performance (average daily gain and average daily feed intake) of rotavirus-challenged piglets was improved in response to 5,000 IU/kg of vitamin D (Zhao et al., 2014). Similarly in dairy cattle, intramammary injection of 100 μg of 25-hydroxyvitamin D3 twice daily for 5 days has been shown to increase bacterial clearance and decrease mastitis severity, milk somatic cell counts, and body temperature, while restoring feed intake and loss of milk production in a mastitis model (Lippolis et al., 2011).
6. Impact of vitamin D on HDP expression
6.1. Humans
Besides its role in calcium homeostasis and anti-inflammation, vitamin D was recently shown to have a strong ability to induce LL-37 and HBD-2 expression in multiple human cell lines and primary cell cultures (Wang et al., 2004, Gombart et al., 2005, Hansdottir et al., 2008, Karlsson et al., 2008, Lee et al., 2012, Lowry et al., 2014). Induction of LL-37 is shown to be critical in vitamin D-mediated protection against Mycobacterium tuberculosis (Liu et al., 2007). Replication of rhinovirus is suppressed in primary bronchial epithelial cells pretreated with vitamin D, which is likely due to induction of LL-37 (Schogler et al., 2016). Consistently, a positive correlation between serum concentrations of 25-hydroxyvitamin D3 and LL-37 has been observed (Jeng et al., 2009, Dixon et al., 2012). Furthermore, oral supplementation of vitamin D increases plasma concentrations of LL-37 in both healthy (Raftery et al., 2015) and mechanically ventilated, critically ill individuals (Han et al., 2017). Vitamin D supplementation also promotes the anti-mycobacterial activity of macrophages in type 2 diabetes patients (Lopez-Lopez et al., 2014). It is noted that vitamin D-mediated induction of cathelicidin is not conserved in rodents, since murine cathelicidin gene lacks a vitamin D response element in the promoter (Dimitrov and White, 2016); however, cathelicidins and defensins can still be induced by vitamin D in livestock animals as detailed below.
6.2. Poultry
In poultry, 20 and 200 ng/mL (equivalent to 48 and 480 nmol/L) vitamin D3 has been shown to induce AvBD3, AvBD6, and AvBD9 in chicken embryonic intestinal epithelial cells, while AvBD1, AvBD6, and AvBD9 were induced in a dose-dependent manner in primary monocytes (Zhang et al., 2016). Consistently, CATH-1 and CATH-B1 were dose-dependently induced by dietary vitamin D in the spleen of chickens and that induction was further enhanced by low calcium and phosphorus in the diet, although CATH-3 was suppressed (Rodriguez-Lecompte et al., 2016). However, only CATH-1 was significantly induced in response to 9,800 IU/kg of vitamin D3 supplementation in the bursa of Fabricius, while no cathelicidins were enhanced by any of the treatments in peripheral blood mononuclear cells (Rodriguez-Lecompte et al., 2016). Intraperitoneal injection of chickens with 5,000 IU/kg of body weight of vitamin D failed to upregulate HDP expression in the chicken intestinal tract, but multiple HDP were synergistically induced in response to both vitamin D and LPS (Lu et al., 2015).
6.3. Cattle
Cholecalciferol, a 1,25D3 precursor, induces several β-defensins such as lingual antimicrobial peptide and bovine β-defensin 1 in bovine mammary epithelial cells, and further inhibits S. aureus internalization at 50 nmol/L (Tellez-Perez et al., 2012). S. aureus also induces all HDP investigated, but cholecalciferol inhibits the induction of HDP except for bovine neutrophil β-defensins (BNBD)10 (Tellez-Perez et al., 2012). Vitamin D up-regulates the expression of multiple BNBD including BNBD3, BNBD4, BNBD6, BNBD7, and BNBD10 in both a time- and dose-dependent manner in bovine monocytes with 1 nmol/L observed as the optimal dose (Merriman et al., 2015). However, 4 ng/mL vitamin D alone fails to induce the expression of bovine cathelicidins 4-6 in monocytes (Nelson et al., 2010). Expression of β-defensins is further increased when monocytes are stimulated with both 1 nmol/L cholecalciferol and LPS (Merriman et al., 2015). Intramammary administration of 10 μg of 1,25D3 every 12 h for 2 days results in an induction of BNBD7 in mammary somatic cells and mammary macrophage cells (Merriman et al., 2015, Merriman et al., 2017). Along with BNBD7, repeated injection of 1,25D3 in bovine mammary glands with subclinical mastitis also leads to an induction of BNBD4 (Merriman et al., 2017).
6.4. Swine
Very little work has been done to elucidate the effect of vitamin D on HDP expression in pigs. However, porcine cathelicidin PR-39 is induced and viral replication reduced in response to 0.5 μmol/L 25-hydroxyvitamin D3 in porcine IPEC-J2 and 5,000 IU/kg in jejunal and ileal epithelial cells of piglets challenged with rotavirus (Tian et al., 2016). Additionally, stimulation of IPEC-J2 cells with 0.5 μmol/L 25-hydroxyvitamin D3 followed by rotavirus infection increases the expression of two other porcine cathelicidins (PG1-5 and PMAP23) (Tian et al., 2016). However, it should be noted that both antiviral RIG-I (Zhao et al., 2014) and autophagy signaling pathways (Tian et al., 2016) appear to be activated by vitamin D treatment as well; therefore, relative contribution of vitamin D-mediated HDP synthesis in limiting rotavirus infection remains to be investigated.
7. Signaling mechanisms in HDP induction by butyrate and vitamin D
7.1. Butyrate induces HDP expression through histone acetylation and mitogen-activated protein kinase (MAPK) signaling pathways
Histone acetylation is an important chromatin epigenetic modification mechanism that regulates gene transcription. Histone deacetylases (HDAC) function by removing acetyl groups from histone proteins causing chromatin condensation and reduced binding of transcription factors to the gene promoter and subsequent gene transcription, whereas the opposite is true with histone acetylases (HAT) (Chen et al., 2015). The balanced action between HDAC and HAT is critical in controlling the transcription of a large group of genes. HDAC inhibitors act by suppressing HDAC enzymes, thereby facilitating histone acetylation and gene transcription (Schotterl et al., 2015). In fact, transcription of as many as 7% to 10% of human genes is affected by HDAC inhibition (Glaser et al., 2003).
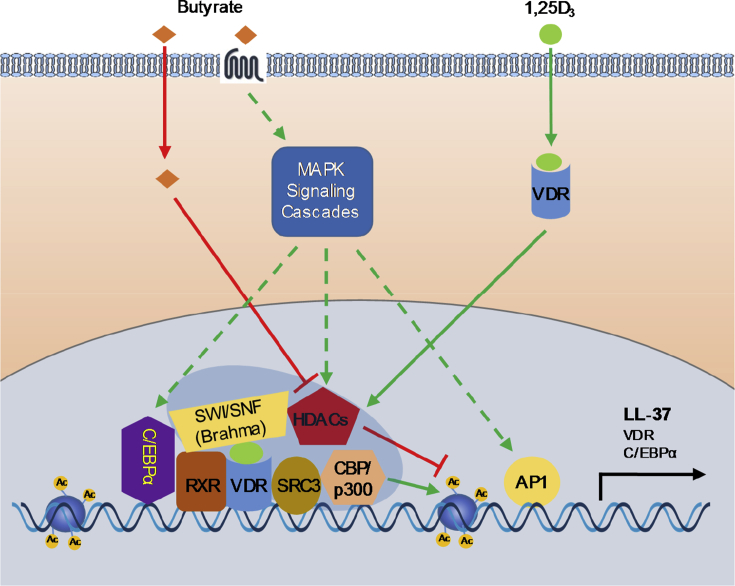
Butyrate is a well-known natural HDAC inhibitor (Riggs et al., 1977, Davie, 2003) and has been shown to induce HDP gene expression primarily by acting as a HDAC inhibitor (Yedery and Jerse, 2015) (Fig. 1). Butyrate-mediated HDP induction is associated with increased acetylation of histone proteins in human EBC-1 lung epithelial cells (Kida et al., 2006), HT-29 colonic carcinoma cells, HepG2 hepatocellular carcinoma cells, and 23132/87 gastric carcinoma cells (Schauber et al., 2004), as well as chicken HD11 macrophage cells (Sunkara et al., 2012) and porcine 3D4/2 macrophage cells (Xiong et al., 2016). Consistently, structurally unrelated, but functionally similar HDAC inhibitors such as trichostatin A (Kallsen et al., 2012), sulforaphane (Schwab et al., 2008), resveratrol and its precursor or analog (Park et al., 2013, Ravagnan et al., 2013, Guo et al., 2014), curcumin (Guo et al., 2013), apicidin (Kallsen et al., 2012), and MS-275/entinostat (Kallsen et al., 2012, Miraglia et al., 2016) are all capable of inducing HDP expression. However, the HDAC inhibitory activity of an HDAC inhibitor does not always directly correlate with its ability to induce HDP gene expression. For example, a potent HDAC inhibitor such as trichostatin A is less efficient in HDP induction than a weak HDAC inhibitor such as butyrate (Schauber et al., 2004). Secondly, high concentrations of a HDAC inhibitor, which normally results in stronger HDAC inhibition, often show reduced HDP induction (Schauber et al., 2004, Sunkara et al., 2011, Sunkara et al., 2012). These results imply the involvement of other regulatory mechanisms beyond epigenetic control. In accordance with this, the MAPK and VDR pathways have been implicated in the upregulation of HDP by butyrate (Fig. 1).
Fig. 1.
Induction of human cathelicidin LL-37 gene expression by butyrate and vitamin D. Butyrate induces LL-37 expression primarily by acting as a histone deacetylases (HDAC) inhibitor and by activation of mitogen-activated protein kinase (MAPK) signaling cascades, whereas 1α,25-dihydroxyvitamin D3 (1,25D3) enhances LL-37 expression by interacting with vitamin D receptor (VDR), which subsequently dimerizes with retinoid X receptor (RXR) and binds to the vitamin D response element (VDRE) site on the LL-37 gene promoter. Inhibition of HDAC by butyrate will lead to hyper-acetylation of histones, relaxation of chromatin, and subsequent LL-37 gene expression. Butyrate-mediated LL-37 expression involves activator protein 1 (AP-1) activation by MAPK and also requires VDR, while vitamin D enhances LL-37 expression through VDR/ retinoid X receptor (RXR) dimerization and subsequent recruitment of transcriptional coactivators such as steroid receptor coactivator-3 (SRC-3) and CBP/p300, which have the histone acetylase (HAT) activity resulting in hyper-acetylation of histones and subsequent LL-37 expression. CCAAT-enhancer-binding protein α (C/EBPα) and Brahma, which is a component of the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex, are also critically involved in vitamin D induction of LL-37. Vitamin D amplifies its own effects by inducing C/EBPα. Butyrate and vitamin D synergize with each other mainly by cooperatively acting on chromatin remodeling and histone acetylation. Butyrate also transcriptionally induces VDR expression. Activation of MAPK signaling cascades further exerts profound influence on C/EBPα and components of chromatin remodeling complex and histone acetylation enzymes; however, whether and which butyrate receptors are involved in MAPK activation remain elusive. Ac = acetyl group; CBP = cyclic AMP-response element-binding protein (CREB) binding protein.
Mitogen-activated protein kinase signaling cascades are comprised of three major canonical pathways including the extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 kinase pathways (Whitmarsh and Davis, 1996, Karin et al., 1997, Robinson and Cobb, 1997), with the specific pathway involved in butyrate regulation varying by cell type and gene. Inhibition of the ERK1/2 pathway using specific inhibitors has been shown to consistently attenuate butyrate-mediated induction of LL-37 in human lung epithelial cells (Kida et al., 2006) and several colonic cell lines (Schauber et al., 2003, Schauber et al., 2004, Schwab et al., 2007). However, suppression of the ERK1/2 pathway plays no role in butyrate-induced bovine HDP expression in mammary cells (Alva-Murillo et al., 2015) and surprisingly further potentiates butyrate induction of AvBD9 in chicken HD11 macrophage (Sunkara et al., 2014). Similarly, the ERK1/2 pathway is also involved in HDP induction by butyrate derivatives such as 4-phenylbutyrate (Steinmann et al., 2009). In contrast, the p38 pathway is essential in butyrate-mediated HDP induction in human lung and Caco-2 colonic epithelial cells (Kida et al., 2006, Schwab et al., 2007), bovine mammary cells (Alva-Murillo et al., 2015), and chicken HD11 cells (Sunkara et al., 2014), but has a minimal role in LL-37 induction in human SW620 colonic epithelial cells (Schauber et al., 2003). Involvement of JNK signaling in butyrate-induced HDP expression has been shown in human lung cells (Kida et al., 2006) and chicken HD11 cells (Sunkara et al., 2014), but appears to be dispensable in bovine mammary cells (Alva-Murillo et al., 2015).
The transcription factor, AP-1, is regulated by all three canonical MAPK signaling pathways (Whitmarsh and Davis, 1996, Karin et al., 1997) and putative binding sites for AP-1 have been found in the LL-37 promoter (Kida et al., 2006) and the promoters of bovine HDP (Alva-Murillo et al., 2015). In both cases, butyrate treatment was found to activate AP-1, indicating its involvement in the regulation of HDP expression. In Caco-2 cells, VDR inhibition significantly decreased LL-37 induction by butyrate or 4-phenylbutyrate (Schwab et al., 2007), suggesting that VDR is involved in the induction of HDP by butyrate. As the p38 pathway is required for butyrate induction of the VDR and Caco-2 cell differentiation (Daniel et al., 2004), it is possible that butyrate may induce LL-37 through p38 phosphorylation. Butyrate is also known to achieve some of its biological effects through interacting with its major receptors such as FFA2/GPR43 and FFA3/GPR41 (Bolognini et al., 2016); however, the role of these receptors in butyrate-mediated HDP induction remains to be determined. It is also noted that PU.1 (Termen et al., 2008) and TGF-β1 receptor kinase (Schwab et al., 2007), but not peroxisome proliferators-activated receptors γ (PPARγ) (Schwab et al., 2007), have been implicated in butyrate-mediated up-regulation of LL-37 in human HT-29 or Caco-2 cells.
7.2. Vitamin D induces HDP expression via VDR
Vitamin D is known to elicit its effects through binding and activation of VDR, which subsequently binds to the vitamin D response element (VDRE) in the promoter of target genes to increase transcription (Svensson et al., 2016). Indeed, inhibition of the VDR or deletion of the VDRE diminishes LL-37 induction in response to vitamin D in human keratinocytes (Schauber et al., 2003, Schauber et al., 2006), bronchial epithelial cells (Yim et al., 2007), and pulmonary epithelial cells (Dhawan et al., 2015) (Fig. 1). While a VDRE is present in the promoter of LL-37 and HBD-2, bovine β-defensins may not be directly targeted by vitamin D, as cycloheximide, a protein translation inhibitor, can block vitamin D-mediated β-defensin induction in bovine monocytes (Merriman et al., 2015). In addition to VDR, Schauber et al. (2006) found that inhibition of the ERK1/2 pathway also significantly reduced LL-37 induction by vitamin D, indicating the involvement of the ERK1/2 pathway in regulation of the VDR.
Furthermore, Dhawan et al. (2015) found that vitamin D increases the CCAAT/enhancer-binding protein α (C/EBPα) synthesis, which subsequently induces LL-37 expression in pulmonary epithelial cells. Chromatin immunoprecipitation assays in these cells found that vitamin D recruits C/EBPα and VDR to their respective binding sites in the LL-37 promoter and that C/EBPα binding is associated with recruitment of Brahma, an ATPase of the SWI/SNF chromatin remodeling complex (Dhawan et al., 2015). Moreover, inhibition of SRC3, a major transcriptional cofactor of VDR in keratinocytes, attenuated vitamin D induction of LL-37, indicating its role in the HDP response as well (Schauber et al., 2003).
7.3. Butyrate and vitamin D signaling pathways converge to synergistically induce HDP
In addition to inducing HDP individually, concomitant stimulation with butyrate and vitamin D has been shown to produce a synergistic induction of HDP gene transcription in multiple cell lines (Fig. 1). In human keratinocytes, butyrate alone has a minimum effect on LL-37 expression, but in combination with vitamin D leads to a synergistic induction (Schauber et al., 2008). The synergy is associated with increased histone acetylation and activation of SRC3 and VDR (Schauber et al., 2008). It is not surprising that 4-phenylbutyrate also synergizes with vitamin D in LL-37 induction in human bronchial epithelial cells (Steinmann et al., 2009, Kulkarni et al., 2015) and macrophages (Rekha et al., 2015), and the VDR is critically involved in the synergy (Steinmann et al., 2009, Kulkarni et al., 2015). However, additional studies are warranted to identify other mechanisms such as the involvement of the MAPK pathways in the synergy between butyrate and vitamin D.
8. Conclusions
The discovery of links between subtherapeutic antibiotic use in livestock animals and antibiotic resistance has created a shift in public opinion and governmental policy regarding the use of antibiotics in livestock production. In order to maintain animal health and productivity, effective and reliable antibiotic alternatives are needed. Modulation of endogenous HDP synthesis by dietary compounds such as butyrate and vitamin D has emerged as a potentially viable alternative to fill this need. It is noted that, although butyrate is produced endogenously through bacterial fermentation to the millimolar concentrations in the rumen, cecum or colon, which should be sufficient in HDP induction, butyrate production in the small intestine is well below the HDP-inducing concentrations. Dietary supplementation of protected or unprotected butyrate at 1 to 2 g/kg often provides benefits to animals, particularly in the context of stress or infection. Similarly, although vitamin D3 is routinely included in the animal diet to 150 to 250 IU/kg, much higher doses (e.g., 2,000 to 5,000 IU/kg) are needed in order to elicit a HDP-inducing and health-promoting effect. It has become clear that the molecular mechanisms involved in butyrate- and vitamin D-mediated HDP synthesis vary by gene, cell type and animal species, but are primarily accomplished through chromatin remodeling, histone acetylation, and activation of the MAPK signaling pathways, leading to activation of transcription factors such as VDR and AP-1. Synergistic induction of HDP synthesis by butyrate and vitamin D appears to occur through the cooperative activation of chromatin remodeling complex and HAT and suppression of HDAC, resulting in hyper-acetylated histones and much relaxed chromatin for subsequent enhancement in gene transcription. Overall, these HDP-inducing compounds, particularly their combinations, have the ability to augment animal innate immunity and protect animals from diseases demonstrates their potential for further development as novel alternatives to antibiotics.
Conflicts of interest
The authors declare that they have no competing conflicts of interest.
Acknowledgements
This work was supported in part by Oklahoma Center for the Advancement of Science and Technology grants (AR12.2-077, HR12-051, and AR15.049), Oklahoma Agricultural Experiment Station Project (H-3025), and National Science Foundation of China grant (31528018). Kelsy Robinson was supported by a USDA-NIFA National Needs Fellowship grant (2013-38420-20500).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Alva-Murillo N., Medina-Estrada I., Baez-Magana M., Ochoa-Zarzosa A., Lopez-Meza J.E. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol Immunol. 2015;68:445–455. doi: 10.1016/j.molimm.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Alva-Murillo N., Ochoa-Zarzosa A., Lopez-Meza J.E. Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet Microbiol. 2012;155:324–331. doi: 10.1016/j.vetmic.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Balch D.A., Rowland S.J. Volatile fatty acids and lactic acid in the rumen of dairy cows receiving a variety of diets. Br J Nutr. 1957;11:288–298. doi: 10.1079/bjn19570046. [DOI] [PubMed] [Google Scholar]
- Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Biagi G., Piva A., Moschini M., Vezzali E., Roth F.X. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J Anim Sci. 2007;85:1184–1191. doi: 10.2527/jas.2006-378. [DOI] [PubMed] [Google Scholar]
- Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- Bommineni Y.R., Achanta M., Alexander J., Sunkara L.T., Ritchey J.W., Zhang G. A fowlicidin-1 analog protects mice from lethal infections induced by methicillin-resistant Staphylococcus aureus. Peptides. 2010;31:1225–1230. doi: 10.1016/j.peptides.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Bommineni Y.R., Pham G.H., Sunkara L.T., Achanta M., Zhang G. Immune regulatory activities of fowlicidin-1, a cathelicidin host defense peptide. Mol Immunol. 2014;59:55–63. doi: 10.1016/j.molimm.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Campbell Y., Fantacone M.L., Gombart A.F. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur J Nutr. 2012;51:899–907. doi: 10.1007/s00394-012-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani R.B., Costanzo M.D., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.P., Zhao Y.T., Zhao T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015;20:35–47. doi: 10.1615/critrevoncog.2015012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.K., Le M.T., Nguyen D.T., Choi H., Kim W., Kim J.H. Genome-level identification, gene expression, and comparative analysis of porcine beta-defensin genes. BMC Genet. 2012;13:98. doi: 10.1186/1471-2156-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Ingale S., Kim J., Park Y., Kwon I., Chae B. Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim Feed Sci Technol. 2013;185:78–84. [Google Scholar]
- Choi S.C., Ingale S.L., Kim J.S., Park Y.K., Kwon I.K., Chae B.J. An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br Poult Sci. 2013;54:738–746. doi: 10.1080/00071668.2013.838746. [DOI] [PubMed] [Google Scholar]
- Cuperus T., Coorens M., van Dijk A., Haagsman H.P. Avian host defense peptides. Dev Comp Immunol. 2013;41:352–369. doi: 10.1016/j.dci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Cutler S.A., Lonergan S.M., Cornick N., Johnson A.K., Stahl C.H. Dietary inclusion of colicin e1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob Agents Chemother. 2007;51:3830–3835. doi: 10.1128/AAC.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Schroder O., Zahn N., Gaschott T., Stein J. p38 MAPK signaling pathway is involved in butyrate-induced vitamin D receptor expression. Biochem Biophys Res Commun. 2004;324:1220–1226. doi: 10.1016/j.bbrc.2004.09.191. [DOI] [PubMed] [Google Scholar]
- Davie J.R. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- Dhawan P., Wei R., Sun C., Gombart A.F., Koeffler H.P., Diamond G. C/EBPalpha and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J Cell Physiol. 2015;230:464–472. doi: 10.1002/jcp.24729. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Malaguarnera G., De Gregorio C., Palumbo M., Nunnari G., Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dimitrov V., White J.H. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol. 2016;164:246–253. doi: 10.1016/j.jsbmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Dimitrov V., White J.H. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol. 2017;453:68–78. doi: 10.1016/j.mce.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Dixon B.M., Barker T., McKinnon T., Cuomo J., Frei B., Borregaard N. Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults. BMC Res Notes. 2012;5:575. doi: 10.1186/1756-0500-5-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst R.K., Guina T., Miller S.I. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 2001;3:1327–1334. doi: 10.1016/s1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- Fang C.L., Sun H., Wu J., Niu H.H., Feng J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J Anim Physiol Anim Nutr (Berl) 2014;98:680–685. doi: 10.1111/jpn.12122. [DOI] [PubMed] [Google Scholar]
- Franklin M.A., Mathew A.G., Vickers J.R., Clift R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J Anim Sci. 2002;80:2904–2910. doi: 10.2527/2002.80112904x. [DOI] [PubMed] [Google Scholar]
- Fukae J., Amasaki Y., Yamashita Y., Bohgaki T., Yasuda S., Jodo S. Butyrate suppresses tumor necrosis factor α production by regulating specific messenger RNA degradation mediated through a cis-acting AU-rich element. Arthritis Rheumatol. 2005;52:2697–2707. doi: 10.1002/art.21258. [DOI] [PubMed] [Google Scholar]
- Galfi P., Bokori J. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet Hung. 1990;38:3–17. [PubMed] [Google Scholar]
- Gallo R.L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser K.B., Staver M.J., Waring J.F., Stender J., Ulrich R.G., Davidsen S.K. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Canc Ther. 2003;2:151–163. [PubMed] [Google Scholar]
- Goitsuka R., Chen C.L., Benyon L., Asano Y., Kitamura D., Cooper M.D. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci U S A. 2007;104:15063–15068. doi: 10.1073/pnas.0707037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Górka P., Kowalski Z.M., Pietrzak P., Kotunia A., Jagusiak W., Holst J.J. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J Dairy Sci. 2011;94:5578–5588. doi: 10.3168/jds.2011-4166. [DOI] [PubMed] [Google Scholar]
- Griffin M.D., Xing N., Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G.H., Agerberth B., Odeberg J., Bergman T., Olsson B., Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Savary G., Jaguelin-Peyrault Y., Rome V., Le Normand L., Zabielski R. Dietary sodium butyrate supplementation increases digestibility and pancreatic secretion in young milk-fed calves. J Dairy Sci. 2010;93:5842–5850. doi: 10.3168/jds.2009-2751. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Zabielski R., David J.C., Blum J.W., Morisset J.A., Biernat M. Sodium-butyrate as a growth promoter in milk replacer formula for young calves1. J Dairy Sci. 2009;92:1038–1049. doi: 10.3168/jds.2008-1213. [DOI] [PubMed] [Google Scholar]
- Guo C., Rosoha E., Lowry M.B., Borregaard N., Gombart A.F. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem. 2013;24:754–759. doi: 10.1016/j.jnutbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Sinnott B., Niu B., Lowry M.B., Fantacone M.L., Gombart A.F. Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res. 2014;58:528–536. doi: 10.1002/mnfr.201300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Troost F.J., Brummer R.J. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Han J.E., Alvarez J.A., Jones J.L., Tangpricha V., Brown M.A., Hao L. Impact of high-dose vitamin D3 on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition. 2017;38:102–108. doi: 10.1016/j.nut.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R.E., Nijnik A., Philpott D.J. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K., Eckmann L., Leopard J.D., Varki N., Kagnoff M.F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchie A.L., Wuerth K., Hancock R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- Hu Z., Guo Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim Feed Sci Technol. 2007;132:240–249. [Google Scholar]
- Jeng L., Yamshchikov A.V., Judd S.E., Blumberg H.M., Martin G.S., Ziegler T.R. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Sunkara L.T., Zeng X., Deng Z., Myers S.M., Zhang G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides. 2013;50:129–138. doi: 10.1016/j.peptides.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Jukes T.H., Stokstad E.L.R., Taylor R.R., Cunha T.J., Edwards H.M., Meadows G.B. Growth-promoting effect of aureomycin on pigs. Arch Biochem. 1950;26:324–325. [PubMed] [Google Scholar]
- Kallsen K., Andresen E., Heine H. Histone deacetylase (HDAC) 1 controls the expression of beta defensin 1 in human lung epithelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Liu Z., Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Carlsson G., Larne O., Andersson M., Putsep K. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J Leukoc Biol. 2008;84:1279–1286. doi: 10.1189/jlb.0607437. [DOI] [PubMed] [Google Scholar]
- Khoo A.L., Chai L.Y., Koenen H.J., Oosting M., Steinmeyer A., Zuegel U. Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine. 2011;55:294–300. doi: 10.1016/j.cyto.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Kida Y., Shimizu T., Kuwano K. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol. 2006;43:1972–1981. doi: 10.1016/j.molimm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Kotunia A., Wolinski J., Laubitz D., Jurkowska M., Rome V., Guilloteau P. Effect of sodium butyrate on the small intestine. J Physiol Pharmacol. 2004;55:59–68. [PubMed] [Google Scholar]
- Kulkarni N.N., Yi Z., Huehnken C., Agerberth B., Gudmundsson G.H. Phenylbutyrate induces cathelicidin expression via the vitamin D receptor: linkage to inflammatory and growth factor cytokines pathways. Mol Immunol. 2015;63:530–539. doi: 10.1016/j.molimm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Le Gall M., Gallois M., Seve B., Louveau I., Holst J.J., Oswald I.P. Comparative effect of orally administered sodium butyrate before or after weaning on growth and several indices of gastrointestinal biology of piglets. Br J Nutr. 2009;102:1285–1296. doi: 10.1017/S0007114509990213. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Cha H.W., Sohn M.Y., Lee S.J., Kim do W. Vitamin D increases expression of cathelicidin in cultured sebocytes. Arch Dermatol Res. 2012;304:627–632. doi: 10.1007/s00403-012-1255-z. [DOI] [PubMed] [Google Scholar]
- Leeson S., Namkung H., Antongiovanni M., Lee E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult Sci. 2005;84:1418–1422. doi: 10.1093/ps/84.9.1418. [DOI] [PubMed] [Google Scholar]
- Lhermie G., Grohn Y.T., Raboisson D. Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front Microbiol. 2016;7:2114. doi: 10.3389/fmicb.2016.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippolis J.D., Reinhardt T.A., Sacco R.A., Nonnecke B.J., Nelson C.D. Treatment of an intramammary bacterial infection with 25-hydroxyvitamin D(3) PLoS One. 2011;6 doi: 10.1371/journal.pone.0025479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Tang D.H., Modlin R.L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez N., Gonzalez-Curiel I., Castaneda-Delgado J., Montoya-Rosales A., Gandara-Jasso B., Enciso-Moreno J.A. Vitamin D supplementation promotes macrophages' anti-mycobacterial activity in type 2 diabetes mellitus patients with low vitamin D receptor expression. Microbes Infect. 2014;16:755–761. doi: 10.1016/j.micinf.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Lowry M.B., Guo C., Borregaard N., Gombart A.F. Regulation of the human cathelicidin antimicrobial peptide gene by 1alpha,25-dihydroxyvitamin D3 in primary immune cells. J Steroid Biochem Mol Biol. 2014;143:183–191. doi: 10.1016/j.jsbmb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li S.M., Zhang L., Liu X.Q., Li D.Y., Zhao X.L. Expression of beta-defensins in intestines of chickens injected with vitamin D3 and lipopolysaccharide. Genet Mol Res. 2015;14:3330–3337. doi: 10.4238/2015.April.13.12. [DOI] [PubMed] [Google Scholar]
- Luecke R.W., McMillen W.N., Thorp F., Jr. Effect of vitamin B12, animal protein factor and streptomycin on the growth of young pigs. Arch Biochem. 1950;26:326–327. [PubMed] [Google Scholar]
- Lynn D.J., Higgs R., Lloyd A.T., O'Farrelly C., Herve-Grepinet V., Nys Y. Avian beta-defensin nomenclature: a community proposed update. Immunol Lett. 2007;110:86–89. doi: 10.1016/j.imlet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lyu W., Curtis A.R., Sunkara L.T., Zhang G. Transcriptional regulation of antimicrobial host defense peptides. Curr Protein Pept Sci. 2015;16:672–679. doi: 10.2174/1389203716666150630133432. [DOI] [PubMed] [Google Scholar]
- Mackenzie-Dyck S., Attah-Poku S., Juillard V., Babiuk L.A., van Drunen Littel-van den Hurk S. The synthetic peptides bovine enteric beta-defensin (EBD), bovine neutrophil beta-defensin (BNBD) 9 and BNBD 3 are chemotactic for immature bovine dendritic cells. Vet Immunol Immunopathol. 2011;143:87–107. doi: 10.1016/j.vetimm.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Mansour S.C., Pena O.M., Hancock R.E. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Le Gall M., De Filippi S., Minieri L., Trevisi P., Wolinski J. Supplemental sodium butyrate stimulates different gastric cells in weaned pigs. J Nutr. 2008;138:1426–1431. doi: 10.1093/jn/138.8.1426. [DOI] [PubMed] [Google Scholar]
- Meade K.G., Cormican P., Narciandi F., Lloyd A., O'Farrelly C. Bovine beta-defensin gene family: opportunities to improve animal health? Physiol Genom. 2014;46:17–28. doi: 10.1152/physiolgenomics.00085.2013. [DOI] [PubMed] [Google Scholar]
- Merriman K.E., Kweh M.F., Powell J.L., Lippolis J.D., Nelson C.D. Multiple beta-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol. 2015;154:120–129. doi: 10.1016/j.jsbmb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Merriman K.E., Poindexter M.B., Kweh M.F., Santos J.E., Nelson C.D. Intramammary 1,25-dihydroxyvitamin D3 treatment increases expression of host-defense genes in mammary immune cells of lactating dairy cattle. J Steroid Biochem Mol Biol. 2017;173:33–41. doi: 10.1016/j.jsbmb.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Miller S.I., Ernst R.K., Bader M.W. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Miraglia E., Nylen F., Johansson K., Arner E., Cebula M., Farmand S. Entinostat up-regulates the CAMP gene encoding LL-37 via activation of STAT3 and HIF-1alpha transcription factors. Sci Rep. 2016;6:33274. doi: 10.1038/srep33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P.R., Evenson A., Luckey T.D., McCoy E., Elvehjem C.A., Hart E.B. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J Biol Chem. 1946;165:437–441. [PubMed] [Google Scholar]
- Nancey S., Bienvenu J., Coffin B., Andre F., Descos L., Flourié B. Butyrate strongly inhibits in vitro stimulated release of cytokines in blood. Dig Dis Sci. 2002;47:921–928. doi: 10.1023/a:1014781109498. [DOI] [PubMed] [Google Scholar]
- Nelson C.D., Reinhardt T.A., Thacker T.C., Beitz D.C., Lippolis J.D. Modulation of the bovine innate immune response by production of 1alpha,25-dihydroxyvitamin D(3) in bovine monocytes. J Dairy Sci. 2010;93:1041–1049. doi: 10.3168/jds.2009-2663. [DOI] [PubMed] [Google Scholar]
- Ochoa-Zarzosa A., Villarreal-Fernandez E., Cano-Camacho H., Lopez-Meza J.E. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog. 2009;47:1–7. doi: 10.1016/j.micpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Park K., Elias P.M., Hupe M., Borkowski A.W., Gallo R.L., Shin K.O. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol. 2013;133:1942–1949. doi: 10.1038/jid.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil A., Hughes A.L., Zhang G. Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genom. 2004;20:1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- Patil A.A., Cai Y., Sang Y., Blecha F., Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genom. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- Peschel A., Sahl H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- Ploger S., Stumpff F., Penner G.B., Schulzke J.D., Gabel G., Martens H. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- Raftery T., Martineau A.R., Greiller C.L., Ghosh S., McNamara D., Bennett K. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn's disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J. 2015;3:294–302. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Sarker P., Bergman P., Ara G., Lindh M., Sack D.A. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Sarker P., Mily A., Alam N.H., Arifuzzaman A.S., Rekha R.S. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis. 2012;12:111. doi: 10.1186/1471-2334-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan G., De Filippis A., Carteni M., De Maria S., Cozza V., Petrazzuolo M. Polydatin, a natural precursor of resveratrol, induces beta-defensin production and reduces inflammatory response. Inflammation. 2013;36:26–34. doi: 10.1007/s10753-012-9516-8. [DOI] [PubMed] [Google Scholar]
- Rehman H.U., Vahjen W., Awad W.A., Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr. 2007;61:319–335. doi: 10.1080/17450390701556817. [DOI] [PubMed] [Google Scholar]
- Rekha R.S., Rao Muvva S.S., Wan M., Raqib R., Bergman P., Brighenti S. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–1699. doi: 10.1080/15548627.2015.1075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M.G., Whittaker R.G., Neumann J.R., Ingram V.M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Robinson K., Deng Z., Hou Y., Zhang G. Regulation of the intestinal barrier function by host defense peptides. Front Vet Sci. 2015;2:57. doi: 10.3389/fvets.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.J., Cobb M.H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lecompte J.C., Yitbarek A., Cuperus T., Echeverry H., van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult Sci. 2016;95:2547–2556. doi: 10.3382/ps/pew186. [DOI] [PubMed] [Google Scholar]
- Säemann M.D., Böhmig G.A., Österreicher C.H., Burtscher H., Parolini O., Diakos C. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- Sang Y., Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev. 2008;9:227–235. doi: 10.1017/S1466252308001497. [DOI] [PubMed] [Google Scholar]
- Sang Y., Blecha F. Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol. 2009;33:334–343. doi: 10.1016/j.dci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sarker P., Ahmed S., Tiash S., Rekha R.S., Stromberg R., Andersson J. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J., Dorschner R.A., Yamasaki K., Brouha B., Gallo R.L. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J., Iffland K., Frisch S., Kudlich T., Schmausser B., Eck M. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Schauber J., Oda Y., Buchau A.S., Yun Q.C., Steinmeyer A., Zugel U. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Schauber J., Svanholm C., Termen S., Iffland K., Menzel T., Scheppach W. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W., Bartram H.P., Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995;31:1077–1080. doi: 10.1016/0959-8049(95)00165-f. [DOI] [PubMed] [Google Scholar]
- Schogler A., Muster R.J., Kieninger E., Casaulta C., Tapparel C., Jung A. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J. 2016;47:520–530. doi: 10.1183/13993003.00665-2015. [DOI] [PubMed] [Google Scholar]
- Schotterl S., Brennenstuhl H., Naumann U. Modulation of immune responses by histone deacetylase inhibitors. Crit Rev Oncog. 2015;20:139–154. doi: 10.1615/critrevoncog.2014012393. [DOI] [PubMed] [Google Scholar]
- Schutte B.C., Mitros J.P., Bartlett J.A., Walters J.D., Jia H.P., Welsh M.J. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Reynders V., Loitsch S., Steinhilber D., Schroder O., Stein J. The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology. 2008;125:241–251. doi: 10.1111/j.1365-2567.2008.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Reynders V., Shastri Y., Loitsch S., Stein J., Schroder O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol Immunol. 2007;44:2107–2114. doi: 10.1016/j.molimm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Seal B.S., Lillehoj H.S., Donovan D.M., Gay C.G. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim Health Res Rev. 2013;14:78–87. doi: 10.1017/S1466252313000030. [DOI] [PubMed] [Google Scholar]
- Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Shi J., Ross C.R., Leto T.L., Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox. Proc Natl Acad Sci U S A. 1996;93:6014–6018. doi: 10.1073/pnas.93.12.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano-Jones J., Murphy M.R. Production of volatile fatty acids in the rumen and cecum-colon of steers as affected by forage:concentrate and forage physical form. J Dairy Sci. 1989;72:485–492. doi: 10.3168/jds.S0022-0302(89)79130-X. [DOI] [PubMed] [Google Scholar]
- Stanton T.B. A call for antibiotic alternatives research. Trends Microbiol. 2013;21:111–113. doi: 10.1016/j.tim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Steinmann J., Halldorsson S., Agerberth B., Gudmundsson G.H. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53:5127–5133. doi: 10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L.T., Achanta M., Schreiber N.B., Bommineni Y.R., Dai G., Jiang W. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L.T., Jiang W., Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L.T., Zeng X., Curtis A.R., Zhang G. Cyclic AMP synergizes with butyrate in promoting beta-defensin 9 expression in chickens. Mol Immunol. 2014;57:171–180. doi: 10.1016/j.molimm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Svensson D., Nebel D., Nilsson B.O. Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production. Inflamm Res. 2016;65:25–32. doi: 10.1007/s00011-015-0884-z. [DOI] [PubMed] [Google Scholar]
- Takahashi D., Shukla S.K., Prakash O., Zhang G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie. 2010;92:1236–1241. doi: 10.1016/j.biochi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Tang X.S., Fatufe A.A., Yin Y.L., Tang Z.R., Wang S.P., Liu Z.Q. Dietary supplementation with recombinant lactoferrampin-lactoferricin improves growth performance and affects serum parameters in piglets. J Anim Vet Adv. 2012;11:2548–2555. [Google Scholar]
- Tellez-Perez A.D., Alva-Murillo N., Ochoa-Zarzosa A., Lopez-Meza J.E. Cholecalciferol (vitamin D) differentially regulates antimicrobial peptide expression in bovine mammary epithelial cells: implications during Staphylococcus aureus internalization. Vet Microbiol. 2012;160:91–98. doi: 10.1016/j.vetmic.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Termen S., Tollin M., Rodriguez E., Sveinsdottir S.H., Johannesson B., Cederlund A. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Mol Immunol. 2008;45:3947–3955. doi: 10.1016/j.molimm.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Thanner S., Drissner D., Walsh F. Antimicrobial resistance in agriculture. MBio. 2016;7 doi: 10.1128/mBio.02227-15. e02227–02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G., Liang X., Chen D., Mao X., Yu J., Zheng P. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J Steroid Biochem Mol Biol. 2016;163:157–163. doi: 10.1016/j.jsbmb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- van der Does A.M., Bergman P., Agerberth B., Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol. 2012;92:735–742. doi: 10.1189/jlb.0412178. [DOI] [PubMed] [Google Scholar]
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 2014;7:545–594. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zeng X., Yang Q., Qiao S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Wen L.F., He J.G. Dose-response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br J Nutr. 2012;108:1756–1763. doi: 10.1017/S0007114511007240. [DOI] [PubMed] [Google Scholar]
- Wen Z.-S., Lu J.-J., Zou X.-T. Effects of sodium butyrate on the intestinal morphology and DNA-binding activity of intestinal nuclear factor-kappaB in weanling pigs. J Anim Vet Adv. 2012;11:814–821. [Google Scholar]
- Whelehan C.J., Barry-Reidy A., Meade K.G., Eckersall P.D., Chapwanya A., Narciandi F. Characterisation and expression profile of the bovine cathelicidin gene repertoire in mammary tissue. BMC Genom. 2014;15:128. doi: 10.1186/1471-2164-15-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh A.J., Davis R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Wu S., Zhang F., Huang Z., Liu H., Xie C., Zhang J. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides. 2012;35:225–230. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Xiao H., Shao F., Wu M., Ren W., Xiong X., Tan B. The application of antimicrobial peptides as growth and health promoters for swine. J Anim Sci Biotechnol. 2015;6:19. doi: 10.1186/s40104-015-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Cai Y., Bommineni Y.R., Fernando S.C., Prakash O., Gilliland S.E. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281:2858–2867. doi: 10.1074/jbc.M507180200. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Hughes A.L., Ando J., Matsuda Y., Cheng J.F., Skinner-Noble D. A genome-wide screen identifies a single beta-defensin gene cluster in the chicken: implications for the origin and evolution of mammalian defensins. BMC Genom. 2004;5:56. doi: 10.1186/1471-2164-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Guo B., Gan Z., Song D., Lu Z., Yi H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep. 2016;6:27070. doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Yang H.S., Li L., Wang Y.F., Huang R.L., Li F.N. Effects of antimicrobial peptides in nursery diets on growth performance of pigs reared on five different farms. Livest Sci. 2014;167:206–210. [Google Scholar]
- Xu H., Soruri A., Gieseler R.K., Peters J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535–540. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Yedery R.D., Jerse A.E. Augmentation of cationic antimicrobial peptide production with histone deacetylase inhibitors as a novel epigenetic therapy for bacterial infections. Antibiotics (Basel) 2015;4:44–61. doi: 10.3390/antibiotics4010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J.T., Nienaber J.A., Hill D.A., Pond W.G. Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J Anim Sci. 1991;69:2001–2012. doi: 10.2527/1991.6952001x. [DOI] [PubMed] [Google Scholar]
- Yim S., Dhawan P., Ragunath C., Christakos S., Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Ingale S.L., Kim J.S., Kim K.H., Lohakare J., Park Y.K. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J Sci Food Agric. 2013;93:587–592. doi: 10.1002/jsfa.5840. [DOI] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zeng X., Sunkara L.T., Jiang W., Bible M., Carter S., Ma X. Induction of porcine host defense Peptide gene expression by short-chain Fatty acids and their analogs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Sunkara L.T. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals (Basel) 2014;7:220–247. doi: 10.3390/ph7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lu L., Li S., Zhang G., Ouyang L., Robinson K. 1,25-Dihydroxyvitamin-D3 induces avian beta-defensin gene expression in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.H., Jiang Y., Zhu Q.F., Gao F., Dai S.F., Chen J. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Leung D.Y., Richers B.N., Liu Y., Remigio L.K., Riches D.W. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yu B., Mao X., He J., Huang Z., Zheng P. Dietary vitamin D supplementation attenuates immune responses of pigs challenged with rotavirus potentially through the retinoic acid-inducible gene I signalling pathway. Br J Nutr. 2014;112:381–389. doi: 10.1017/S000711451400097X. [DOI] [PubMed] [Google Scholar]
- Zhou Z.Y., Packialakshmi B., Makkar S.K., Dridi S., Rath N.C. Effect of butyrate on immune response of a chicken macrophage cell line. Vet Immunol Immunopathol. 2014;162:24–32. doi: 10.1016/j.vetimm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Zhu K., Glaser R., Mrowietz U. Vitamin D(3) and analogues modulate the expression of CSF-1 and its receptor in human dendritic cells. Biochem Biophys Res Commun. 2002;297:1211–1217. doi: 10.1016/s0006-291x(02)02357-4. [DOI] [PubMed] [Google Scholar]