Abstract
C. auris is an emerging fungal pathogen with high prevalence of resistance to current antifungal agents. Central nervous system infection with C. auris has been infrequently described. We describe here an adult with nosocomial CSF shunt infection due to multi drug resistant C. auris. Systemic therapy with echinocandin and flucytosine failed. Fortunately, administration of daily intraventricular caspofungin 10 mg for 10 days in conjunction with systemic voriconazole resulted in both clinical and microbiological cure.
Keywords: C. auris, CSF shunt, Intraventricular caspofungin
1. Introduction
C. auris was first described in an external ear sample of a Japanese patient in 2009 [1]. Since then, the fungus has been reported from numerous countries across the five continents as causing invasive infections and outbreaks in health care facilities [2], [3]. Chakrabarti et al. have reported 5.2% of candidemia in Indian ICU's due to C. auris [4]. In the author's own institution, four of seven cases of nosocomial candidemia in 2017 were due to C. auris (unpublished data). CDC (Centers for Disease Control & Prevention) of USA, Public Health of England, ECDC (European Centre for Disease Prevention and Control) of Europe and Indian Council of Medical Research have issued advisories, which is the first for any fungal disease [5], [6], [7].
C. auris is phylogenetically related to C. haemulonii and C. ruelliae. It is commonly misidentified as Candida haemulonii, C. famata, C. lusitaniae, C. parapsilosis, C. sake, Cryptococcus laurentii, Rhodotorula glutinis, Saccharomyces cerevisiae by conventional laboratory testing [5]. Accurate identification is possible by the VITEK MS or MALDI TOF MS systems or by DNA sequencing. The VITEK 2 system's database has now been upgraded to identify C. auris [8]. In the absence of these systems, growth of the fungus at 37–42 °C, inhibition by cycloheximide, utilization of dextrose, dulcitol and mannitol, formation of pink colonies on chromogenic agar and multidrug resistance should make one suspect C. auris [2]. Risk factors for C. auris infection are similar to those for other candida species. In a study in Indian ICU's by Rudramurthy et al. patients with C. auris were more likely to have longer prior hospitalization, vascular surgery, antifungal exposure, low APACHE 2 scores and coexistent respiratory disease as compared to patients with other Candida species [9]. C. auris mostly causes fungemia but sometimes other nosocomial invasive infections. Tentative breakpoints for susceptibility testing for C. auris have been proposed by the CDC [5]. The fungus is multi drug resistant with almost universal resistance to fluconazole and variable susceptibility to other azoles and amphotericin B. Echinocandins are the drugs of choice for empiric and definitive therapy but resistance due to mutations in the FKS gene have been reported [9]. Four percent of all isolates have been reported as resistant to all available antifungal agents [8]. Mortality rates have ranged from 28% to 66% across different studies [2]. C. auris has this unique ability to colonize hosts, health care workers and hospital environment for prolonged periods thus causing outbreaks [8]. Control measures involve screening for colonization in close contacts of infected patients, placing colonized and infected patients under strict contact isolation thorough environmental cleaning with disinfectants such as hypochlorite and decolonization of infected/ colonized hosts with chlorhexidine soap [10].
We describe here a patient who developed a recalcitrant C. auris CNS shunt infection was successfully treated with intraventricular caspofungin when conventional systemic therapy failed.
2. Case
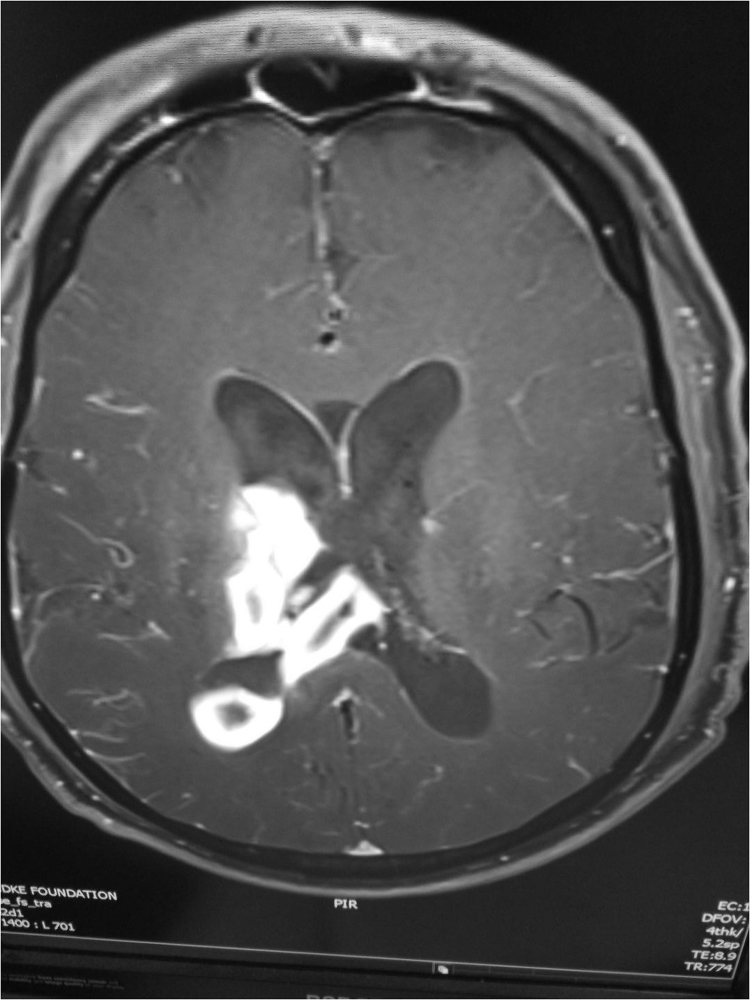
A 58 year old with history of ischemic heart disease and bypass grafting 15 years ago presented with sudden onset of altered sensorium following a right basal ganglia bleed with intraventricular extension. He underwent a right burr hole craniotomy and an external ventricular drainage (EVD) of the right ventricle. Three days later he developed high fever and was empirically put on meropenem and vancomycin for suspected meningitis. EVD fluid was sterile on culture. When fever refused to abate colistin was added, and he was referred to our hospital (Day 0). At admission he was febrile with a GCS of 6/15, stable vitals, a tracheostomy in situ, a central line and an indwelling urinary catheter. A possibility of meningitis and or ventriculitis was considered and the patient was appropriately investigated. The WBC count and CRP were elevated and lumbar CSF showed 980 cells with 80% polymorphs, sugar of 48 mg% (random blood sugar of 125 mg%) and a protein of 114 mg%. Blood and CSF cultures were sterile. The MRI showed enhancement of the ventricular lining (Fig. 1). Treatment with colistin, meropenem and vancomycin was continued for the next 3 weeks over which time the fever resolved, sensorium improved, the WBC count and CRP declined and the CT showed reduction in ventricular size. Neurorehabilitative therapy was started. The urine and tracheal cultures grew carbapenem resistant Klebsiella pneumoniae. Around 4 weeks after admission (Day +28), there was a sudden decline in the patient's sensorium and the repeat CT showed an increase in ventricular size. A ventriculo peritoneal shunt was inserted with perioperative prophylaxis regime of colistin, meropenem and vancomycin for 48 h.
Fig. 1.
Contrast MRI showing meningitis, ventriculitis and intraventricular hemorrhage.
Seven weeks after admission (day +49) and nearly 3 weeks after insertion of shunt, the patient again developed fever and decline in sensorium. A shunt tap was done which was suggestive of a shunt infection (445 cells, 76% polys; Sugar 33; Protein 56) and the gram stain showed budding yeast. Treatment with liposomal amphotericin B and flucytosine was initiated, the shunt removed and an EVD placed (Day +50). Forty eight hours later the fungus was identified as C. auris on Vitek 2 (Day +52). The proximal and distal end of the shunt also grew the same organism. VITEK 2 (based on the MIC breakpoints of C. albicans) pronounced the isolate susceptible to caspofungin (MIC <0.25), micafungin (<0.12), flucytosine (MIC <1) and voriconazole (MIC = 1) while resistant to fluconazole (MIC >32) and amphotericin B (>16). Treatment was modified; intravenous caspofungin was initiated, flucytosine continued while liposomal amphotericin B stopped (Day +53). Despite this, the EVD fluid was continuously positive for C. auris. Therefore after taking informed consent, treatment with intraventricular caspofungin (CANCIDAS R, Merck & Co) was given as a single daily dose of 10 mg through the EVD followed by clamping of the tube for 6 h (Day +59). Treatment was well tolerated. Oral voriconazole was also added and systemic caspofungin and flucytosine continued. Five days later the CSF was negative for C. auris (Day +65). Seven days after initiation of intraventricular therapy systemic caspofungin was stopped and so was flucytosine due to GI toxicity (Day +67). Treatment with oral voriconazole and intraventricular caspofungin continued till day when intraventricular therapy was stopped and only voriconazole continued (Day +70). The CSF remained consistently negative for candida and a VP shunt was reinserted on Day +80. Voriconazole was continued for a total period of 6 weeks (Day +102). The patient made a remarkable recovery and now 6 months later is ambulatory and independent.
3. Discussion
This is the first report describing a shunt infection due to C. auris. The identification was based on the latest version of VITEK 2 whose database had been upgraded to include the biochemical characteristics of Candida auris. Phenotypically the isolate could not be differentiated from other candida species on the standard Sabouraud's agar. We did not systematically screen the patient for other sites for colonization but none of the other cultures from non sterile sites such as tracheal secretions or urine grew Candida. There were no other cases of invasive C. auris related to this case.
The treatment of the intracranial infection was a challenge since the most effective drugs against C. auris, the echinocandins do not have adequate CNS penetration [11]. This lead to the repeated isolation of Candida despite systemic echinocandin and flucytosine therapy. Interpretation of the antifungal susceptibility reports for the C. auris isolate is also difficult since no breakpoints have been established, there is poor agreement between various systems (broth microdilution, VITEK 2 and E test) in determining MICs and that voriconazole MICs have shown bimodal/ trimodal distributions [12], [13]. For these reasons voriconazole even with its MIC of 1 by VITEK 2 (with suggested MIC 50 of 0.5–2) and its good CNS penetration could not be relied upon for therapeutic success. Besides the azoles have very high MIC for biofilm organisms and are less effective when a foreign device (in this case the EVD) is in situ [14].
Treatment of intracranial infections with multidrug resistant pathogens with intrathecal/ intraventricular therapy is well established [15]. Therefore, treatment with local intraventricular caspofungin therapy was instituted which was safe, well tolerated and efficacious. However, the relative contribution of voriconazole and intraventricular caspofungin in therapeutic success in the index case cannot be delineated. Invitro synergy of echinocandins and azoles against has been recently reported [16].
Soman et al. reported a case of Candida albicans shunt associated meningitis which was refractory to therapy with liposomal amphotericin B and flucytosine despite invitro susceptibility where instillation of 10 mg of caspofungin through the Omaya reservoir for 3 weeks resulted in clinical/ microbiologic cure [17]. Mursch et al. have also reported successful therapy of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode with intraventricular caspofungin [18]. Williams et al. recently reported use of intraventricular caspofungin 5 mg in a patient with Scedosporium apiospermum CNS infection following spine surgery who failed therapy with voriconazole and terbinafine. Treatment was well tolerated and resulted in clinical improvement and sterilization of the CSF; unfortunately progression of disease occurred after withdrawal of therapy leading to death [19].
With the growing menace of C. auris, we are likely to see increase in intracranial infections due to this pathogen as well. Owing to inherent resistance of this fungus against antifungal agents with good CNS penetration, the safety, optimum dosage/formulation and efficacy of intraventricular echinocandin therapy needs to be investigated further.
Acknowledgements
Dr Arunaloke Chakrabarti. Head of Medical Microbiology, Post Graduate Institute of Medical Education and Research, Chandigarh, India for his input in clinical management of the case
Acknowledgments
Conflict of interest
There are none.
References
- 1.Kazuo Satoh, Koichi Makimura, Yayoi Hasumi, Yayoi Nishiyama, Katsuhisa Uchida, Hideyo Yamaguchi. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53(1):41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Sears D., Schwartz B.S. Candida auris: an emerging multidrug-resistant pathogen. Int J. Infect. Dis. 2017;63:95–98. doi: 10.1016/j.ijid.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart Shawn R., Etienne Kizee A., Snigdha Vallabhaneni, Joveria Farooqi, Anuradha Chowdhary, Govender Nelesh P. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti A., Sood P., Rudramurthy S.M., Chen S., Kaur H., Capoor M. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 5.CDC, 2017. Candida auris interim recommendations for healthcare facilities and laboratories, Fungal diseases, CDC. Available at: 〈https://www.cdc.gov/fungal/diseases/candidiasis/recommendations.html〉. (Accessed August 10, 2018).
- 6.European Center for Disease Prevention and Control, 2016. Candida auris in healthcare settings. 20th Dec 2016. Available at 〈https://ecdc.europa.eu/en/publications-data/candida-auris-healthcare-settings〉. (Accessed on August 10, 2018).
- 7.Indian Council of Medical Research, 2018. Candida auris in health care settings- India. Available at:〈https://icmr.nic.in/icmrnews/candida%20auris.pdf〉. (Accessed on August 10, 2018).
- 8.Chowdhary A., Sharma C., Meis J.F. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma C., Kumar N., Pandey R., Meis J.F., Chowdhary A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect. 2016;13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswal M., Rudramurthy S.M., Jain N., Shamanth A.S., Sharma D., Jain K., Yaddanapudi L.N., Chakrabarti A. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J. Hosp. Infect. 2017;97 doi: 10.1016/j.jhin.2017.09.009. (363–37) [DOI] [PubMed] [Google Scholar]
- 11.Dodds Ashley E.S., Lewis R., Lewis J.S., Martin C., Andes D. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 2006;43:S28–S39. [Google Scholar]
- 12.Arendrup M.C., Prakash A., Meletiadis J., Sharma C., Chowdhary A. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00485-17. (e00485-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kathuria S., Singh P.K., Sharma C., Prakash A., Masih A., Kumar A., Meis J.F., Chowdhary A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by vitek 2, CLSI broth microdilution, and Etest method. J. Clin. Microbiol. 2015;53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobudic S., Kratzer C., Lassnigg A., Presterl E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses. 2012;55:199–204. doi: 10.1111/j.1439-0507.2011.02076.x. [DOI] [PubMed] [Google Scholar]
- 15.Tunkel A.R., Hasbun R., Bhimraj A., Byers K., Kaplan S.L., Michael Scheld W. Infectious diseases society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin. Infect. Dis. 2017;2017(64):e34–e65. doi: 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J.F., Badali H. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.01056-17. (e01056-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mursch K., Trnovec S., Ratz H., Hammer D., Horré R., Klinghammer A., de Hoog S., Behnke-Mursch J. Successful treatment of multiple Pseudallescheria boydii brainabscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 2006;22:189–192. doi: 10.1007/s00381-005-1151-3. [DOI] [PubMed] [Google Scholar]
- 18.Soman R., Koparkar V., Shetty A., Rodrigues C. Innovative therapy with caspofungin. J. Assoc. Phys. India. 2017;65:116. [PubMed] [Google Scholar]
- 19.Williams J.R., Tenforde M.W., Chan J.D., Ko A., Graham S.M. Safety and clinical response of intraventricular caspofungin for Scedosporium apiospermum complex central nervous system infection. Med. Mycol. Case Rep. 2016;13:1–4. doi: 10.1016/j.mmcr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]