Highlights
-
•
HAM can be a useful, potential scaffold for treatment of infected, irregular, deep wounds due to the promotion of epithelial regeneration, elastic and antibacterial characteristics.
-
•
Clinical case of chronic wound with an entero-cutaneous fistula was successfully treated with HAM dressings after 8 weeks.
-
•
Histological analysis showed partial re-epithelialisation and muscle cells recovery 4 weeks after the treatment.
-
•
The preliminary results support the initiation of prospective trial and registry according to IDEAL.
Keywords: Case report, Amniotic membrane, Chronic wound, Dressing, IDEAL recommendations
Abstract
Introduction
Infected wounds are difficult to treat and there are no standardized protocols.
Presentation of case
We report a case of infected postoperative wound and entero-cutaneous fistula in a 83 years-old woman. An innovative treatment protocol for Human amniotic membrane (HAM)-assisted dressing of infected wound as the Idea Stage following the IDEAL recommendations is presented. The development of amnion preparation and the involved treatment steps are described. No adverse events and no graft rejection have been detected.
Discussion
Favorable results confirm the technical simplicity, safety and efficacy of this procedure. HAM has been shown to promote wound healing and to have antibacterial characteristics, which was supported by the presented case.
Conclusion
We are able to report a successful treatment of an infected wound caused by entero-cutaneous fistula with HAM dressing. Following the IDEAL recommendations, consecutive prospective cohort trials are justified.
1. Introduction
Chronic wounds have a significant impact on public health in that they lead to increased disability, morbidity and risk of mortality and hence result in longer ambulatory and hospital treatments and higher costs. Especially diabetic wounds or postoperative infected wounds are difficult to treat due to insufficient tissue perfusion and persistent infection. Chronic wounds are often defined as wounds that have not achieved a 50% reduction in wound size after 4 weeks of standard wound care [1]. However, standardized treatment guidelines are lacking and the outcome/efficiency of the on the market available bioengineered skin substitutes, topical growth factors and stromal matrices is poor.
Human amniotic membrane (HAM) possesses many interesting key properties (e.g. immune tolerance, promotion of epithelialization, anti-inflammatory, anti-angiogenetic, anti-fibroblastic and anti-microbial characteristics), making it a potential scaffold for tissue engineering. It contains multiple soluble growth factors that promote epithelial wound healing and limit the inflammatory reaction. Especially the stromal matrix of the HAM has a considerable influence on this anti-fibrotic development [2]. Its antibacterial effects stem from enzymes like elastase. First reports of amnion application into the pelvic region have shown fast epithelialization and improved functionality in vaginal and bladder reconstruction [[3], [4], [5]].
A standardized procedure for the treatment of wound infections is not available to date. Innovative patient-tailored innovative surgical approaches are difficult to standardize and recommendations cannot easily be generalized. A new approach, the IDEAL method has been proposed in 2009 by Mc Culloch et al. [6]. The IDEAL procedure clearly provides stages of surgical innovations, which allow for the ability to assign a new method to its specific level of development and evidence. For the first time, we present a detailed treatment protocol with amniotic membrane for an infected wound as the “Idea level” following the IDEAL recommendations in an academic hospital setting. The case report is compliant with the SCARE Guidelines [7]. The presented study was registered at Research Registry (http://www.researchregistry.com; registration number 4111).
2. Case report
A retired 83-year-old Caucasian female, BMI 21, non-smoker, hypertensive, underwent a radical cystectomy with cutaneous ureterostomy for locally invasive bladder cancer (pT2a, G3, N0, R0). Two weeks after surgery she was admitted again to our hospital with a severe infection of the laparotomy wound. Treatment consisted of wide resection of subcutaneous tissue with vacuum sealing followed by a displacement plastic skin repair. A week later the patient presented a cutaneous fistula with purulent secretion of 30–50 ml/day. A CT scan revealed communication with a small bowel loop. The patient underwent parenteral nutrition and octreotid injections for one week, which reduced fistula secretion to 20–30 ml/day. Due to the complex situation, we discussed an individualized treatment strategy consisting of off-label use of HAM dressing. The wound was measured and cleaned. Two-layer amnion dressing was applied weekly after cleaning and debridement for 4 weeks. A nonadherent inner dressing with wound gauze Cuticell (BSN medical, Hamburg, Germany) covered by a standard dry dressing was then applied over the graft. The patient was advised not to remove the dressing and to keep it dry until the next visit. Wound size and secretion were documented by taking photographs every week. A small biopsy of the wound margin was performed at week four. The patient provided written consent for treatment, biopsy and the use of photographs.
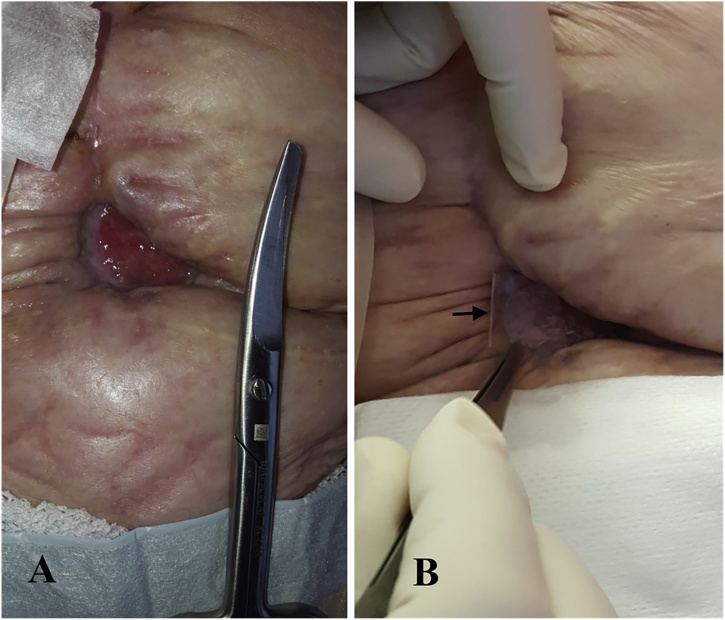
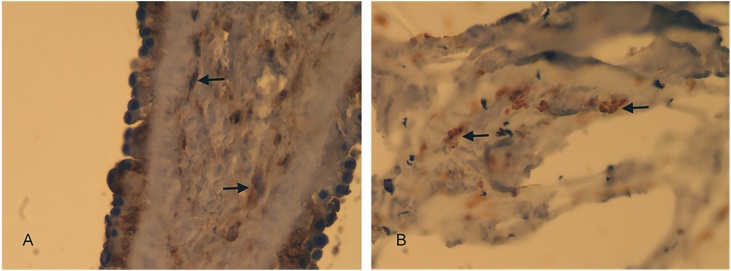
The wound size at the first HAM application was 3 cm × 2 cm × 1 cm (Fig. 1). Dressings and off-loading were conducted as described above. After 2 weeks, the secretion stopped and the ulcer diminished in size by 20% (Fig. 1). The ulcer was healed at 8 weeks after 4 HAM applications and the wound remained healed at 6-month and 9-month follow up. No complications occurred during the follow-up and patient reported no adverse events. Patient was satisfied with the treatment and would undergo the treatment again retrospectively. Histological analysis showed re-epithelialization and muscle cells recovery 4 weeks after the treatment; HAM had not degraded and was still detectable as a smooth layer on the surface. Moderate signs of inflammation but no graft rejection were observed (Fig. 2).
Fig. 1.
A. Initial presentation with entero-cutaneous fistula and wound ulceration. B. After 2 weeks the secretion stopped and the wound was reduced by 20% and contracted. HAM dressing is marked with an arrow.
Fig. 2.
Histological analysis of wound healing. High magnification (400×) Vimentin-stained sections. A. shows epidermis formation and dermis composition with muscle cell formation (arrows). B. shows muscle cells (arrows) adhering to the scattered pieces of HAM.
3. Discussion
Several randomized trials have demonstrated the effectiveness of dehydrated human amnionic and chorionic membrane (dHACM) in the treatment of chronic diabetic foot ulcers (DFUs) [8]. In a prospective, randomized, single-center clinical trial, Zelen et al. compared wound reduction and rates of complete healing in patients with diabetic foot ulcers treated with dHACM versus standard of care. Significant differences in wound reduction were observed at 4 and 6 weeks, with wounds reducing in size by a mean of 32.0% ± 47.3% in the standard of care group (n = 12) versus 97.1% ± 7.0% (P < 0.001) for the patients receiving dHACM (n = 13) [9]. Another prospective randomized trial of 40 patients with chronic DFUs, in whom dHACM allografts were applied weekly, reported a mean time until complete healing of 2.4 weeks until complete healing and 90% of DFUs, dHACM allografts were applied weekly [10]. In the first week, the external dressings were changed every other day due to initial infection of the wound. The amnion acts as a physical barrier against bacterial contamination and also creates the moist environment required for healing [11]. However, HAM and dHACM applications were analyzed in clinically uninfected cases in most of the reported studies. Only one other study randomized a tissue-engineered form of wound dressing containing acellular human amniotic collagen membrane vs. wet dressing in patients with partially infected wounds. The complete healing rate was significantly higher in the amnion group (40.7%) compared to the control group (16.7%) [12]. Moreover, HAM was found to be cost-effective in the treatment of chronic wounds and to reduce hospitalization [10,12].
The limitations of the study is the outcome of one wound in one patient which is insufficient to draw strong conclusions by now. However, we present a structured implementation of a new method for the secondary wound care with amnion following the IDEAL recommendations [6]. Considering the positive results of this first case we plan consecutive prospective developmental studies in an animal model and cohort studies.
4. Conclusion
In conclusion, we report for the first time successful treatment of an entero-cutaneous fistula and infected wound. HAM is simple to use, covers larger and irregular surface area wounds and can also be applied into deep tunneling wounds. Future studies with larger numbers of patients with infected wounds are warranted and inclusion of amnion dressings in the treatment protocol of secondary wound care should be considered and further evaluated.
Conflict of interest
No.
Funding
No.
Ethical approval
No ethical approval was requested due to a case report form. However, the clinical case was planned and published according to the SCARE Guidelines (Agha et al. Int. J. Surg. 34 (2016) 180–186, Oct.) and registered at Research Registry (international study registration platform, Registration number 4111).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
DB, MB and TO: experimental design.
DB and TO: performance of the procedure and follow up.
DB: data collection and writing of the paper.
DB, HG, TE, GV, MB, JPT, IP and TO: data analysis and interpretation.
HG, TE, MB, JPT and TO: revision of the manuscript and proof reading.
Registration of research studies
Registration number 4111.
Guarantor
Dimitri Barski.
References
- 1.Warriner R.A., Snyder R.J., Cardinal M.H. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int. Wound J. 2011;8(6):632–637. doi: 10.1111/j.1742-481X.2011.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koizumi N., Inatomi T., Quantock A.J., Fullwood N.J., Dota A., Kinoshita S. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000;19(1):65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Fishman I.J., Flores F.N., Scott F.B., Spjut H.J., Morrow B. Use of fresh placental membranes for bladder reconstruction. J. Urol. 1987;138(5):1291–1294. doi: 10.1016/s0022-5347(17)43586-5. [DOI] [PubMed] [Google Scholar]
- 4.Barski D., Gerullis H., Ecke T., Varga G., Boros M., Pintelon I. Repair of a vesico-vaginal fistula with amniotic membrane – step 1 of the IDEAL recommendations of surgical innovation. Cent. Eur. J. Urol. 2015;68(4):459–461. doi: 10.5173/ceju.2015.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barski D., Gerullis H., Ecke T., Yang J., Varga G., Boros M. Bladder reconstruction with human amniotic membrane in a xenograft rat model: a preclinical study. Int. J. Med. Sci. 2017;14(4):310–318. doi: 10.7150/ijms.18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 7.McCulloch P., Altman D.G., Campbell W.B., Flum D.R., Glasziou P., Marshall J.C. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 8.Laurent I., Astère M., Wang K.R., Cheng Q.F., Li Q.F. Efficacy and time sensitivity of amniotic membrane treatment in patients with diabetic foot ulcers: a systematic review and meta-analysis. Diabetes Ther. 2017;8(October (5)):967–979. doi: 10.1007/s13300-017-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelen C.M., Serena T.E., Denoziere G., Fetterolf D.E. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int. Wound J. 2013;10(5):502–507. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelen C.M., Serena T.E., Snyder R.J. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int. Wound J. 2014;11(2):122–128. doi: 10.1111/iwj.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao Y., Singh-Varma A., Hoffman T., Dhall S., Danilkovitch A., Kohn J. The effect of cryopreserved human placental tissues on biofilm formation of wound-associated pathogens. J. Funct. Biomater. 2018;9(1) doi: 10.3390/jfb9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohajeri-Tehrani M.R., Variji Z., Mohseni S., Firuz A., Annabestani Z., Zartab H. Comparison of a bioimplant dressing with a wet dressing for the treatment of diabetic foot ulcers: a randomized, controlled clinical trial. Wounds. 2016;28(7):248–254. [PubMed] [Google Scholar]