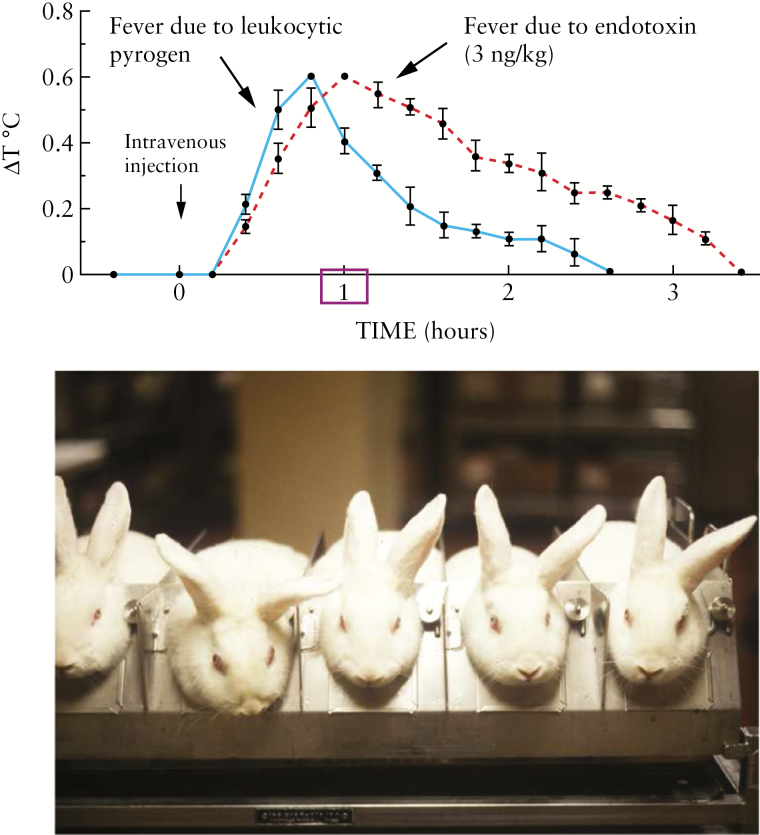
In the year 50 A.D., Celsus, a Roman physician, described calor (fever) as one of the cardinal signs of inflammation. However, it was not until 1977 that Dinarello, Renfer and Wolff isolated the responsible molecule: the endogenous pyrogen, better known as interleukin-1 [IL-1] (Figure 1).1 This discovery opened the field of cytokine biology, eventually leading to the characterization of many other cytokines (e.g. tumour necrosis factor [TNF], IL-12, IL-23). Further understanding the role of cytokines in the pathogenesis of inflammatory bowel disease [IBD] and a bit of serendipity1,2 gave us infliximab in 1998,3 the first biological that targets the pro-inflammatory cytokine TNF, for the treatment of IBD.
Figure 1.
The isolation of interleukin-1. Images provided by Charles Dinarello.
The history of the second major development for IBD therapy [targeting the dysregulated recruitment of leukocytes to the site of inflammation] is much shorter, but not less remarkable. In 1959, James L. Gowans described the recirculation of lymphocytes in the rat.4 Irving Weissman was in the audience at the New York Academy of Sciences meeting in 1962 when he heard Jim Gowans speak about lymphocyte recirculation. When Gowans concluded, Weissman describes how he joined the audience in a standing ovation, the only one he had witnessed for a scientific talk.5 Weissman then took nine months off from medical school in 1964 to work in Gowans’ laboratory. Subsequently, it took around two decades for Weissman and his postdocs, Gallatin, Siegelman, Holzmann and Butcher, to identify the homing receptors for the peripatetic cells.1,6–9
For us physicians, who care for patients with IBD, the next major development happened when Butcher, Scollay and Weissman described the organ specificity of lymphocyte migration,1 later called the intestinal address code. The concept that gut-homing lymphocytes express specific surface molecules that allow them to recognize specific microenvironments gave us the first prototype drug in 1998 [natalizumab], followed by the gut selective vedolizumab [2014], based on the discoveries of Butcher and his trainees, over the years. With two currently approved drugs for the treatment of both ulcerative colitis and Crohn’s disease and several others in phase 3 trials, the blockade of trafficking molecules has firmly emerged as a new era in the history of IBD therapy.
In this supplement of the JCC, we commissioned several of our colleagues who have had direct involvement with diverse basic or clinical aspects of the development of these molecular targets, to discuss those whose efficacy have been examined in clinical trials: Salas and Panes provide an overall review,10 while Trivedi and Adams cover the chemokines and their receptors that have been targeted therapeutically ([e.g. IP10, CCR9].11 Lamb and colleagues cover the most prolific topic of integrin-targeting [e.g. natalizumab, AJM300, vedolizumab and etrolizumab],12 whereas Reinisch and colleagues discuss the targeting of integrin endothelial ligands [anti-MAdCAM-1, anti-ICAM-1].13 Finally, Danese and colleagues describe a novel strategy that interferes with lymphocyte recirculation, at least in part by blocking lymphocyte egress from lymph nodes (sphingosine-1-phosphate receptor agonists [e.g. ozanimod, etrasimod, amiselimod]).14
Twenty centuries passed from the empirical observation of fever by Celsus [50 A.D.], to the isolation of the fever molecule [IL-1, 1977], to the development of infliximab [1998].3 However, the development of drugs that interfere with lymphocyte traffic went much faster: Gowans describes lymphocyte recirculation [1959], while Butcher and Weissman described the organ (intestinal) specificity of the process [1980] to the first prototype drug [1998]. Twenty centuries compared with around 40 years. So, there is hope that perhaps within our lifespans we may see the cure for these diseases.
Funding
Supported by US National Institutes of Health Grant DK 108670.
Conflict of Interest
None.
Acknowledgments
This paper was published as part of a supplement supported by an educational grant from F. Hoffmann-La Roche Ltd & Takeda Development Center Americas, Inc.
References
- 1. Butcher EC, Scollay RG, Weissman IL. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol 1980;10:556–61. [DOI] [PubMed] [Google Scholar]
- 2. Derkx B, Taminiau J, Radema S, et al. Tumour-necrosis-factor antibody treatment in Crohn’s disease. Lancet 1993;342:173–4. [DOI] [PubMed] [Google Scholar]
- 3. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 4. Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol 1959;146:54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissman I. Lymphocytes, Jim Gowans and in vivo veritas. Nat Immunol 2010;11:1073–5. [DOI] [PubMed] [Google Scholar]
- 6. Gallatin M, St John TP, Siegelman M, Reichert R, Butcher EC, Weissman IL. Lymphocyte homing receptors. Cell 1986;44:673–80. [DOI] [PubMed] [Google Scholar]
- 7. Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 1983;304:30–4. [DOI] [PubMed] [Google Scholar]
- 8. Siegelman MH, van de Rijn M, Weissman IL. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science 1989;243:1165–72. [DOI] [PubMed] [Google Scholar]
- 9. Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer’s patch–specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4α. Cell 1989;56:37–46. [DOI] [PubMed] [Google Scholar]
- 10.Salas J, Panes A. Past, Present and Future of Therapeutic Interventions Targeting Leukocyte Trafficking in Inflammatory Bowel Disease. J Crohns Colitis 2018;12:S633–S640. [DOI] [PubMed]
- 11.Trivedia PJ, Adams DH. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. J Crohns Colitis 2018;12:S641–S652 [DOI] [PMC free article] [PubMed]
- 12.Lamb CA, O’Byrnec S, Keird ME, Butcher EC. Gut-Selective Integrin-Targeted Therapies for Inflammatory Bowel Disease. J Crohns Colitis 2018;12:S653–S668 [DOI] [PubMed]
- 13.Reinisch W, Hung K, Hassan-Zahraee M, Cataldi F. Targeting Endothelial Ligands: ICAM-1/alicaforsen, MAdCAM-1. J Crohns Colitis 2018;12:S669–S677 [DOI] [PubMed]
- 14.Danese S, Furfaro F, Vetrano S. Targeting S1P in Inflammatory Bowel Disease: New Avenues for Modulating Intestinal Leukocyte Migration. J Crohns Colitis 2018;12:S678–S686 [DOI] [PubMed]