Abstract
Nitrate and manganese (Mn) are necessary elements for the growth and development of rice in paddy soil. Under physiological conditions, we previously reported that the uptake of Mn in roots can be improved by the addition of external nitrate but not ammonium. To investigate the mechanism(s) of this phenotype, we produced plant lines overexpressing OsNRT2.1 and assessed Mn uptake under alternating wet and dry (AWD) and waterlogged (WL) conditions. Under AWD condition, we observed a 31% reduction in grain yields of wild type (WT) plants compared to WL condition. Interestingly, the overexpression of OsNRT2.1 could recover this loss, as OsNRT2.1 transgenic lines displayed higher grain yields than WT plants. We also observed 60% higher grain Mn in the transgenic lines in AWD condition and approximately 30% higher Mn in the grain of transgenic lines in WL condition. We further found that the overexpression of OsNRT2.1 did not alter Mg and Fe in the seeds in either growth condition. The reasons for the increased Mn content in OsNRT2.1 transgenic seeds in AWD condition could be explained by the elevated expression of OsNRAMP family genes including OsNRAMP3, OsNRAMP5, and OsNRAMP6 in node I, the panicle-neck, and the flag leaves. The mechanism(s) underpinning the upregulation of these genes requires further investigation. Taken together, our results provide a new function of OsNRT2.1 in improving rice yields and grain Mn accumulation during water-saving cultivation patterns. This represents a new strategy for maintaining yield and improving food quality in a sustainable agricultural system.
Keywords: rice, OsNRT2.1, manganese uptake, yield, nitrate
Introduction
Trace elements play a vital role in plant growth and development (Yan et al., 2006). All organisms require trace levels of manganese (Mn) for survival due to its necessity during plant metabolism and its participation in several important pathways (Socha and Guerinot, 2014) including the oxygen-evolving complex (OEX) of photosystem II (PS II). In addition, Mn plays an important role during phosphoenolpyruvate carboxykinase activation and liquid metabolism (Dziwornu et al., 2018). Thus, it is required for photosynthesis indirectly by repressing thylakoid synthesis. In addition, manganese superoxide dismutase (MnSOD) is the major mitochondrial antioxidant defense enzyme (Shen, 2015) and Mn is a co-factor/activator of many enzymes involved in the catalysis of oxidation reduction, decarboxylation and hydrolytic reactions (Marschner, 1995; Xu et al., 2007).
Mn deficiency is a global problem in agriculture (Hebbern et al., 2005). Mn deficient plants are more vulnerable to cold stress and infections by pathogens, leading to decreased crop yields (Marschner, 1995; Hebbern et al., 2005). Addressing this issue is problematic as Mn2+ rapidly oxidizes when supplemented into fertilizers. In this regard, further knowledge of molecular mechanisms that can enhance Mn delivery are required. Several Mn transporters contribute to the uptake, transport and maintenance of Mn homeostasis in plants. The NRAMP family was shown to participate in Mn transport during early plant discoveries. In Arabidopsis, AtNRAMP1 localizes to the plasma membrane and displays root-specific expression where its function is to coordinate the absorption of Mn from soil (Cailliatte et al., 2010). OsNRAMP5 is mainly involved in Mn uptake and accumulation in rice and its silencing significantly reduces Mn accumulation in shoots (Yang et al., 2014). OsNRAMP3 is expressed in the node and regulates Mn transport and tissue distribution in response to environmental changes (Yamaji et al., 2013a). OsNRAMP6 distributes to the plasma membrane and transports Mn and Fe, maintaining their balance in cells (Peris-Peris et al., 2017). In rice, Mn homeostasis is controlled by the YSL2/6 gene. OsYSL2 can promote the long-distance transport of Mn (Koike et al., 2004; Ishimaru et al., 2010). OsYSL6 belongs to the Mn-nicotianamine (NA) transporter family and is required for the detoxification of high concentrations of Mn (Sasaki et al., 2011). In addition, the CAX proteins belong to the Ca2+/cation antiporter (CaCA) superfamily (Emery et al., 2012) and are potentially involved in Mn2+/H+ exchange to export Mn from the cytosol (Connorton et al., 2012).
Nitrogen (N) is an essential element for plant growth and development, especially for crops. Generally, N is absorbed by plants in the form of ammonium (NH4+) and nitrate (NO3-), but nitrate easily dissolves in water and is therefore lost to the environment (Jin et al., 2015). Roots acquire NO3- via transporters distributed throughout the whole plant (Xu et al., 2012). Plants adapt to the differing NO3- concentrations in soil by exploiting two forms of NO3- uptake, including low-affinity transporters (NRT1/NPF) and high-affinity NO3- transporters (NRT2) (Crawford and Glass, 1998). Particularly for rice plants, we previously identified a high-affinity NO3- transport system. The OsNRT2 gene family was found to play an important role during N uptake and translocation, requiring their partner protein NAR2 to perform this function, besides OsNRT2.3b (Tang et al., 2012; Xu et al., 2012; Chen et al., 2016b; Chen Z.C. et al., 2017; Fan et al., 2016; Chen J.G. et al., 2017).
Simultaneously, Mn can influence NO3- reductase activity and is associated with photosynthesis in plants (Botrill et al., 1970; Gong et al., 2011). Mn also influences N metabolism and regulates protein synthesis (Jiang, 2006). Studies have shown that the arabidopsis chl1-5 mutant lines display reduced NO3- uptake and a loss of AtIRT1 expression, which is responsible for Cd uptake into root cells (Muños et al., 2004; Lux et al., 2011). Fe deficiency was also shown to inhibit N metabolism in the roots and leaves of cucumber plants (Borlotti et al., 2012). These effects suggest that NO3- influences the uptake of trace elements in plants. In this study, we hypothesized that a close relationship between N and Mn in plants exists. We used transgenic rice over-expressing OsNRT2.1 to examine how the different forms of N influence Mn uptake and accumulation in grain.
Materials and Methods
Plant Materials and Growth Conditions
We amplified the OsNRT2.1 (AB008519) ORF (primers are displayed in Supplementary Table S1) using cDNA obtained from Oryza sativa L. ssp. Japonica cv. Nipponbare. PCR products were cloned into the pMD19-T vector (TaKaRa Biotechnology, Dalian, China) and the expression vector pTCK303 containing a ubiquitin promoter. Positive clones were verified by restriction digest analysis and DNA sequencing. Next, the binary vector pUbiquitin-OsNRT2.1 was introduced into A. tumefaciens (strain EHA105), which was used to transform the rice embryonic callus as previously described (Ai et al., 2009). Hygromycin-resistant T0 generation transgenic rice plants were transplanted to soil and grown to obtain seeds in fields (Tang et al., 2012). Three independent T4 generation lines overexpressing OsNRT2.1 were used for further experiments.
Firstly, rice seedlings were selected and cultured in 1 mM (NH4)2SO4 as the main source of N in nutrient solution (pH 5.5) for 1 month. Other elements and trace elements were supplied in IRRI (International Rice Research Institute) nutrient solution containing 0.35 mM K2SO4, 0.3 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 0.5 mM Na2SiO3, 20 μM H3BO3, 9 μM MnCl2, 20 μM EDTA-Fe, 0.77 μM ZnSO4, 0.32 μM CuSO4, and 0.39 μM (NH4)6Mo7O24. Rice were planted in a growth room (Thermoline Scientific Equipment Pty. Ltd., Smithfield, NSW, Australia) at 30°C during the day and 22°C at night with 16-h light/8-h of darkness. The light intensity was 400 μmol m-2 s-1 and the relative humidity was 65–70%. Wild type (WT) rice were then transferred to 0.25 or 1.25 mmol/L Ca(NO3)2 and 0.25 or 1.25 mmol/L (NH4)2SO4 nutrient solution, respectively, for 2 weeks (Figure 1). In Figures 2 and 4, WT and overexpression lines were transferred to 0.5 mM NH4+/NO3- nutrient solution for 2 weeks. For each line and treatment, four biological repeats were performed.
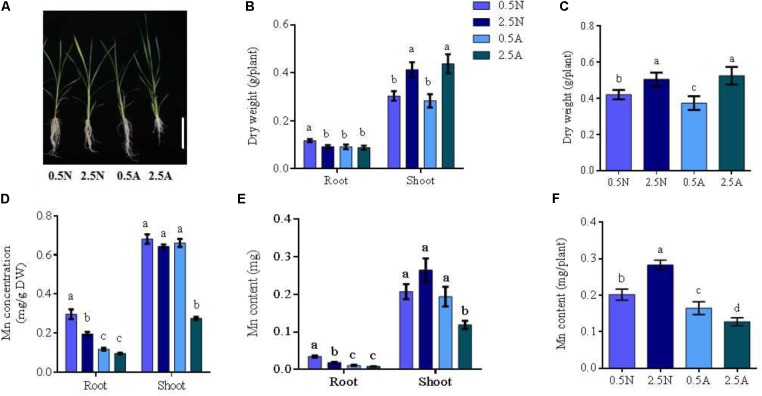
FIGURE 1.
Absorption of Mn elements under different N treatments; (A) the phenotype of wild type (WT) rice in different N treatments; (B) Root and shoot dry weight; (C) the dry weight of whole rice plants; (D) Mn concentrations; (E) Mn content in roots or shoots; (F) the Mn content in the whole plant. DW, dry weight; 0.5/2.5N: 0.5/2.5 mM NO3- as a nitrogen source; 0.5/2.5A: 0.5 mM/2.5 mM NH4+ as a nitrogen source (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA). Bars = 3 cm.
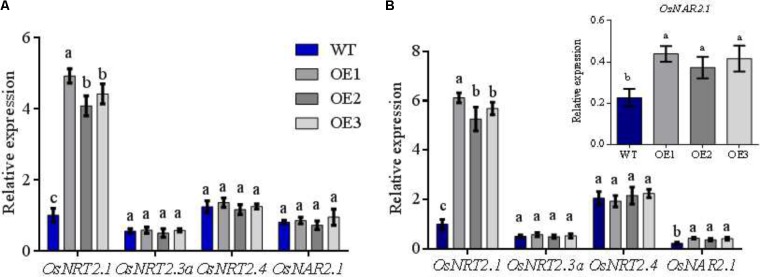
FIGURE 2.
Expression of OsNRT2s and OsNAR2.1 in transgenic lines. WT and transgenic lines seedlings were grown in the IRRI nutrient solution containing 1 mM NH4+ for 1 month and then treated with 0.5 mM NH4+/NO3- for 2 weeks. Extraction of total RNA from roots of WT and transgenic lines and qRT-PCR under (A) 0.5 mM NH4+, (B) 0.5 mM NO3-. WT, wild type; OE1/2/3 was performed for the three OsNRT2.1 transgenic lines, as below. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between transgenic and WT lines (P < 0.05, one-way ANOVA).
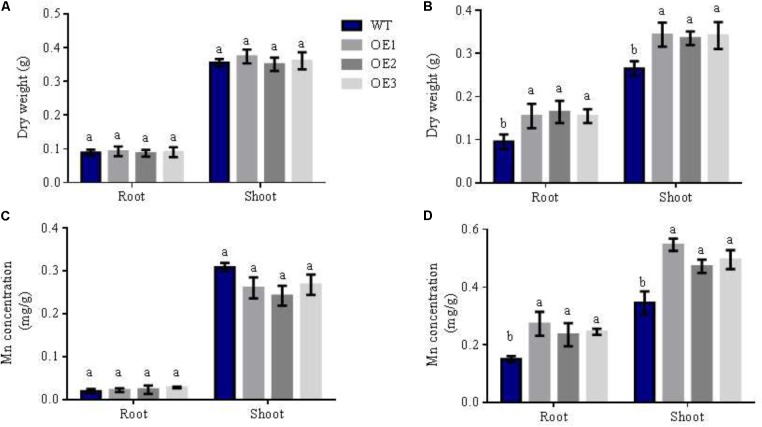
FIGURE 4.
Mn concentration of different lines in different N forms. WT and transgenic lines were treated as in Figure 2. Dry weight and Mn concentrations of shoots and roots in WT and OsNRT2.1 lines with (A,C) 0.5 mM NH4+, (B,D) 0.5 mM NO3-. Error bars: standard error (n = 4 plants). Data represent mean differences between plant lines under different N sources. Letters indicate statistically significant differences (P < 0.05, one-way ANOVA).
In field experiments, rice were planted in Nanjing, Jiangsu; a subtropical monsoon climate zone. The characteristics of the soil and N supply were as previously described (Chen et al., 2016b). For waterlogged (WL) treatment, rice fields were watered daily to maintain WT and transgenic lines in a flooded state. For alternating wet and dry treatment (AWD), lines were planted into fields and watered for a week, to keep the soil moist.
Southern Blot Analysis
Transgene cope numbers were identified by southern blot analysis. Briefly, genomic DNA was extracted from the leaves of WT and transgenic lines and digested with HindIII and EcoRI. Digested DNA was separated on 1% (w/v) agarose gels, transferred to a Hybond-N+ nylon membrane and hybridized using the hygromycin-resistant gene.
RNA Extraction and qPCR Analysis
Total RNA was extracted from 100 mg of tissue using TRIzol (Invitrogen, Carlsbad, CA, United States). Total RNA concentrations were assessed by UV spectrophotometry (Eppendorf, Bio-photometer, Germany). RNA (2 μg) was reverse transcribed into cDNA using HiScript Reverse Transcriptase (Vazyme, Nanjing, China) according to the manufacturers protocol. Four biological repeats were performed for each qPCR reaction, using OsActin as a reference gene. Primers were designed to detect OsNRT2.1, OsNRT2.3a, OsNRT2.4, OsNAR2.1, OsNRAMP3, OsNRAMP5, OsNRAMP6, OsIRT1, and OsMGT1 and are listed in Supplementary Table S2. PCR amplification was performed using SYBR qPCR Master Mix (Vazyme, Nanjing, China). PCR reactions were performed under the following parameters: 95°C for 30 s, followed by 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 10 s.
Determination of the 15N-NH4+/NO3- Influx Rate in Different Rice Lines
Rice seedlings of WT and OsNRT2.1 transgenic rice plants were planted in IRRI solution containing 1 mM NH4+ for 2 weeks and N starved for 3 days. Plants were first transferred into 0.1 mM CaSO4 for 1 min, then to complete nutrient solution containing either 0.5 mM 15NH4+ or 0.5 mM 15NO3- (atom% 15N: 99%) for 5 min and finally to 0.1 mM CaSO4 for 1 min (Duan et al., 2007). The 15N influx rate was calculated according to methods described by Tang et al. (2012).
Assessment of Dry Weight, Total N and Metal Ion Accumulation
To investigate the links between OsNRT2 function and metal ion uptake, we investigated the levels of metal elements using the ICP-OES method in OsNRT2.1 transgenic lines and Mn elements in OsNRT2.3a/b transgenic lines. The creation and identification processes of bO-1, bO-2, bO-8 for OsNRT2.3b transgenic lines and aO-1, aO-2 for OsNRT2.3a transgenic lines were performed as previously described (Fan et al., 2016).
Fresh WT or transgenic lines were harvested at the rice mature stage (n = 4) and heated at 105°C for 30 min. Panicles, flag leaves, second and third leaves, sheaths and stems were then dried for 3 days at 75°C. Rice obtained from hydroponic experiments was divided into shoots and roots only. Dry weights were recorded as biomass values.
Using the Kjeldahl method (Li et al., 2006), total N accumulation was assessed in the different plant areas through multiplying the N concentration by the corresponding biomass. Dried samples were wet-digested in concentrated HNO3 at 120°C until no brown nitrogen oxide gas was emitted. When the samples became transparent, they were further digested with HClO4 at 180°C. Samples were then diluted with ultrapure water and the concentrations of metal elements in the digestates were analyzed using ICP-OES (iCAP 6300).
Statistical Analysis
All data were analyzed using the Tukey’s test of one-way analysis of variance (ANOVA). Statistically significant differences at the p < 0.05 level (one-way ANOVA) between transgenic and WT and/or between other treatments were assessed. All statistical evaluations were performed using IBM SPSS Statistics version 20 software (SPSS Inc., Chicago, IL, United States).
Results
Assessment of Mn Absorption Under Different N Treatments
Wild type rice seedlings were planted under different conditions of N supply. Symptomatically, the roots of rice seedlings were better in 0.5 mM NO3- than in 0.5 mM MH4+, 2.5 mM NO3- and 2.5 mM NH4+ conditions (Figure 1A). Statistical analysis showed that the dry weights of the plant roots in 0.5 mM NO3- condition were significantly increased (Figure 1B). Low concentration NO3- could promote root elongation and increase root hairs (Kiba and Krapp, 2016). For the whole plant, the dry weight was best in 2.5 mM N, with no differences between 2.5 mM NO3- and 2.5 mM NH4+ observed (Figure 1C). Next, the total N in rice seedlings was investigated. Total N content in rice seedlings with 2.5 mM N supply was higher than that of the 0.5 mM N supply (Supplementary Figure S1). Rice seedlings planted in 2.5 mM NH4+ nutrient solution, displayed the best outcome (Supplementary Figure S1).
Simultaneously, the Mn concentration and content of rice roots in 0.5 mM NO3- was found to increase more than other conditions. However, shoots were lowest in 2.5 mM NH4+ solution (Figures 1D,E). The Mn content of rice seedlings in NO3- solution was higher than in NH4+ using the same N concentrations (Figure 1F). In addition, the expression of the nitrate transporters OsNRT2.1/OsNRT2.3 were up-regulated by external NO3- and the expression of OsNAR2.1 increased in 0.5 mM NO3-/2.5 mM NO3- compared to NH4+ treatments in the different tissues (Supplementary Figures S2A–C). The expression of Mn transporters OsNRAMP3/OsNRAMP5/OsNRAMP6 also increased following NO3- treatment compared with NH4+ treatment. Taken together, these results reveal that both Mn uptake and OsNRAMP3/OsNRAMP5/OsNRAMP6 expression are increased by NO3-. Therefore, NO3- positively regulates the absorption of Mn in rice.
Assessment of the Expression Patterns of OsNRT2s and OsNAR2.1 in the Roots of Transgenic Lines
Firstly, transgenic lines were identified by southern blot analysis and RT-PCR. The data showed that three transgenic lines were one copy insertions and OsNRT2.1 was overexpressed to approximately five-fold higher mRNA levels in roots and shoots under normal N conditions (1.25 mM NH4NO3 supply) (Supplementary Figure S4 and Chen et al., 2016a). WT and transgenic OsNRT2.1 lines were planted in 0.5 mM NO3-/NH4+ nutrition solution, respectively. RT-PCR was performed to confirm the gene expression patterns of the two families of NO3- transporters in WT and OsNRT2.1 transgenic lines under different N supplies. OsActin was used as a reference gene for comparison. Total RNA was extracted from the rice roots of the different lines. Under conditions of low concentration (0.5 mM) of NH4+ and NO3-, the expression of OsNRT2.1 in transgenic lines increased 4.5-fold and 5.7-fold, compared to WT (Figures 2A,B). No differences in the relative expression of other NO3- transporters OsNRT2.3a/OsNRT2.4 between transgenic and WT lines or between NO3- and NH4+ treatments were observed (Figures 2A,B). However, the expression levels of OsNAR2.1 increased approximately 80% in transgenic lines in 0.5 mM NO3-, but not in 0.5 mM NH4+ (Figures 2A,B). The total N content of the three transgenic lines was higher than WT in the roots and the shoots under 0.5 mM NO3- conditions, with no differences in the NH4+ solution observed (Supplementary Figure S5). Taken together, these results show that OsNRT2.1 expression is enhanced in the transgenic rice. In addition, the expression of OsNRT2.1 and OsNAR2.1 is enhanced in all transgenic lines, allowing an efficient transfer of NO3- in 0.5 mM NO3- conditions.
NH4+ and NO3- Influx Rates in WT and OsNRT2.1 Transgenic
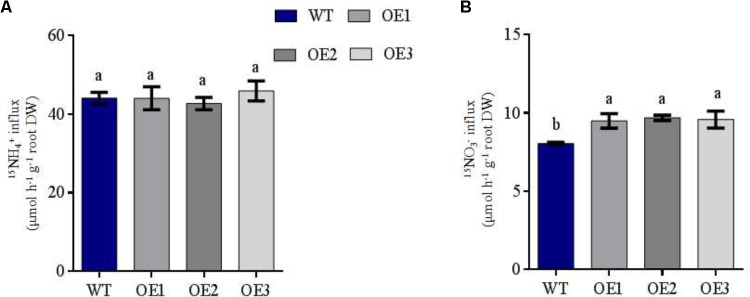
To confirm the influence of OsNRT2.1 on high-affinity root NO3- influx into intact plants, short-term nitrate absorption was assessed by transferring all the lines to either 0.5 mM 15NH4+ or 0.5 mM 15NO3- for 5 min. Under 0.5 mM 15NH4+ treatment condition, the three transgenic lines displayed no significant differences to WT (Figure 3A). However, OsNRT2.1 transgenic lines were enhanced by 19% compared to WT during NO3- influx (Figure 3B). In addition, the effects of overexpression on rice growth under different forms of N supply were studied by comparing the total N concentration and content in different parts of the rice plants. The total N of the transgenic lines did not significantly differ in the roots and shoots compared to WT lines in 0.5 mM NH4+ solution (Supplementary Figures S5A,B). However, the total N content of the roots and shoots of the transgenic rice plants was enhanced by 97% and 36%, respectively, compared to WT lines in 0.5 mM NO3- conditions (Supplementary Figure S5E). Total N concentrations in the shoots did not differ from WT (Supplementary Figure S5D). These results show that the overexpression of the high-affinity nitrate transporter OsNRT2.1 improves NO3- uptake in 0.5 mM NO3-, compared to WT.
FIGURE 3.
NH4+ and NO3- influx rates of WT and transgenic plants measured using 15N-enriched sources. WT and transgenic rice seedlings were grown in IRRI nutrient solution containing 1 mM NH4+ for 2 weeks and N-starved for 3 days. NO3- and NH4+ influx rates were then measured at (A) 0.5 mM 15NH4+ and (B) 0.5 mM 15NO3- for 5 min. DW: dry weight. Error bars: standard error (n = 4 plants). Significant differences between transgenic and WT lines are indicated by different letters (P < 0.05, one-way ANOVA).
Mn Concentration of Shoots and Roots of Transgenic Plants Under Different N Treatments
The transferability of Mn is weak. From hydroponic experiments (Figure 4), we tested the Mn concentration of shoots and roots in rice when planted in different nutritive forms of N. We found that the dry weight of roots and shoots increased by 66 and 29%, respectively, in transgenic lines relative to WT lines in 0.5 mM NO3- solution (Figure 4B). However, dry weights did not significantly differ in 0.5 mM NH4+ (Figure 4A). Simultaneously, Mn concentrations of roots and shoots in the overexpression lines were also enhanced by 43% and 47%, respectively, in 0.5 mM NO3- solution, but not in 0.5 mM NH4+ (Figures 4C,D). From Figure 4 and Supplementary Figure S3, we reasoned that this was due to the OsNRT2.1 gene transferring NO3- into the rice, increasing total N, Mn uptake and accumulation in 0.5 mM NO3- condition. These results indicate that Mn assimilation by OsNRT2.1 is NO3- uptake dependent, and that the overexpression of OsNRT2.1 does not only increase NO3- uptake to enhance total N, but also promotes Mn absorption in rice in low NO3- condition.
Effects of Different Irrigation Conditions on N and Mn Concentrations in Grain
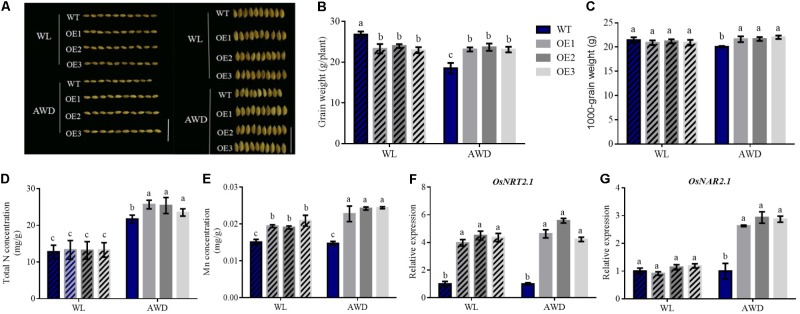
Rice typically grows in anaerobic flooded fields, which exist mainly in the form of NH4+-N. Conversely, NO3- is present mainly in aerobic uplands (Stitt, 1999). To simulate hydroponic conditions in the presence of different N treatments, we designed a field experiment under different irrigation conditions and investigated OsNRT2.1 function on rice grains in the field. From the assessment of seed morphology, seeds of WT under alternating wet and dry (AWD) condition were shorter than other seeds (Figure 5A and Supplementary Figure S6). Compared to other field treatments, we found that the grain weight of WT in AWD condition was approximately 31% lower than waterlogged (WL) condition, with no differences in the transgenic lines observed (Figure 5B).
FIGURE 5.
Effects of different irrigation conditions on N and Mn concentrations in grain. (A) Seed morphology images, (B) Grain weights, (C) 1000-grain weight, (D) total N concentration, (E) Mn concentration of seeds in WT and transgenic lines under different field experiments. Total RNA was extracted from the culm of WT and overexpression lines and the results of qPCR under different irrigation conditions was used to assess the relative expression of (F) OsNRT2.1 and (G) OsNAR2.1. Error bars: standard error (n = 4 plants). WL, waterlogged; AWD, alternating wet and dry conditions. Different letters indicate a significant difference between WT and overexpression lines or in differing irrigation conditions of all lines (P < 0.05, one-way ANOVA). Bars = 1 cm.
In addition, no evident differences in all lines in WL condition were observed. However, the grain weight of transgenic rice plants was approximately 26% higher compared to WT weights in AWD condition (Figure 5A). The 1000-grain weight displayed a similar pattern to the grain weights (Figure 5B). We also tested the total N concentration of the seeds under different field treatments. Interestingly, WT and transgenic lines were higher in AWD condition compared to waterlogged condition, and the total N concentration of the transgenic seeds also increased by 15% compared to WT in the AWD field (Figure 5C). However, the total N concentration of the husk in the overexpression lines was lower than that of WT in the AWD field, whilst no differences in all lines from the WL field were observed (Supplementary Figure S7A). As higher levels of N were transferred into the seeds of transgenic lines in the AWD field, their seed weights were higher than WT.
Simultaneously, the Mn concentrations in the seeds of transgenic lines in AWD condition were enhanced when compared to WL condition. No differences were observed for the different field conditions in WT lines (Figure 5D). In addition, the husk of grain displayed similar results in terms of Mn concentrations (Supplementary Figure S7B). The concentration of Fe and Mg in seeds and husk appeared to vary irregularly (Supplementary Figure S7). This presented the unity of the Mn element.
These results demonstrate that rice planted in AWD condition displays higher total N and Mn concentrations in grain, particularly for OsNRT2.1 transgenic lines. We extracted total RNA from the culm of all lines planted in the two types of irrigated field. From Figures 5F,G, the relative expression of OsNRT2.1 in transgenic lines was higher than WT lines in WL and AWD conditions. However, OsNAR2.1 expression was enhanced 2.8-fold only in AWD field relative to WT. Therefore, the soil of AWD primarily existed in NO3- form to enhance NO3- uptake through increased OsNRT2.1 expression, leading to the induction of OsNAR2.1 expression. As the relative expression of OsNRT2.1 and OsNAR2.1 increase following AWD treatment, NO3- uptake may further improve Mn uptake compared to the WL field.
Assessment of the Expression of Related Genes, Total N and Mn Accumulation During Maturity Stages in AWD Conditions
To understand mechanism(s) of how OsNRT2.1 improves total N and Mn accumulation at the mature stage in AWD field, we extracted total RNA from the different areas of rice (Supplementary Figure S9) and assessed the expression of OsNRT2.1, OsNAR2.1, and Mn transporters-OsNRAMP3, OsNRAMP5, and OsNRAMP6 (Yamaji et al., 2013a; Yang et al., 2014; Peris-Peris et al., 2017).
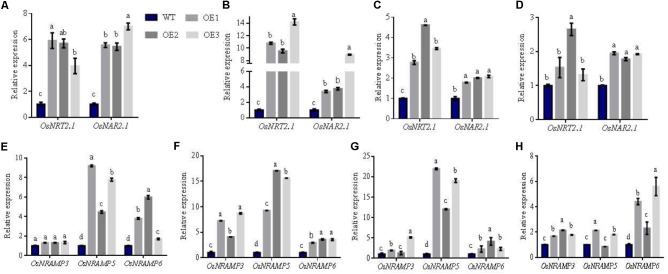
From Figure 6, the expression of the related nitrate genes-OsNRT2.1 and OsNAR2.1 in the three transgenic lines were higher in the panicle-neck, flag leaves, flag leaves sheaths and node I compared to WT rice. The panicle-neck connects vegetative and reproductive organs. Flag leaves are functional leaves for transferring nutrients. Studies have reported that high-affinity nitrate OsNRT2.1 requires its partner protein OsNAR2.1 to transfer nitrate (Feng et al., 2011; Yan et al., 2011; Tang et al., 2012). Accordingly, the expression of OsNRT2.1 and its partner protein OsNAR2.1 increased in the Panicle-neck and in the functional leaves at maturity. NO3- was transferred to the panicle to enhance total N accumulation in seeds, and further improve grain yields.
FIGURE 6.
Relative expression of related genes in different arears of WT and transgenic plants in AWD fields. Total RNA was isolated from (A,E) panicle-neck, (B,F) flag leaves, (C,G) flag leaves sheaths, and (D,H) Node I of WT and transgenic lines. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Interestingly, we found that the expression of the Mn transporters: OsNRAMP3, OsNRAMP5, and OsNRAMP6 were also upregulated in transgenic lines. These genes displayed similar expression patterns to NO3- transporters (Figures 6E–G). In particular, the expression of OsNRAMP3 and OsNRAMP6 in the transgenic lines increased by 87 and 311% in comparison to WT in the node I, respectively (Figure 6H). Node I represents the junction of the vascular system connecting the leaves, stems and panicles. Therefore, Mn transporter genes-OsNRAMP3 and OsNRAMP6 preferentially transport Mn to flag leaves and the panicle during the late stages of plant growth in rice. We found that the biomass of transgenic and WT lines displayed no significant differences at maturity (Supplementary Figure S10A). The NO3- concentrations of the different plant areas (except for leaves in the overexpression lines) were higher than WT (Supplementary Figure S10D). However, total N accumulation did not differ in various parts of the plants, and Mn showed an irregular trend without flag leaves (Supplementary Figure S10). These results suggest that total N and Mn are transferred to grains from vegetative organs at maturity. We further assessed Fe and Mg content in various parts of the different lines, in which we observed no differences (Supplementary Figure S11). When the relative expression of OsIRT1 and OsMGT1 that represent Fe and Mg related genes (Lee and An, 2009; Chen Z.C. et al., 2017) were analyzed, the expression patterns were also inconsistent in diverse areas of the transgenic rice plants (Figure 7).
FIGURE 7.
Relative expression of other related metal genes in the different lines under AWD conditions. Total RNA was isolated from (A) panicle-neck, (B) flag leaves, (C) flag leaves sheaths and (D) Node I of WT and overexpression lines. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and transgenic lines (P < 0.05, one-way ANOVA).
Taken together, these data suggest that the improvement of Mn concentration in OsNRT2.1 lines was due to the increased expression of Mn transporters, but no effects on other metal elements were observed.
Discussion
Nitrate and Mn are essential nutrients in plants, and it has been reported that Mn deficiency decreases N uptake and metabolism (Gong et al., 2011). Excessive NO3- was shown to enhance Cd uptake in Thlaspi caerulescens (Xie et al., 2009) and wheat (Li et al., 2011). In addition, crosstalk between mineral elements exists. Wang et al. (2015) reported that Al improves Mn uptake and accumulation in rice roots, However, these tactics do not enhance the security of crops for human consumption because they do not increase the accumulation of beneficial elements in plant. In this study, the main objective was to investigate how interactions between Mn and NO3- influence rice growth and nutrient accumulation in roots, leaves tissues and grain.
We found that NO3- improves Mn uptake in rice (Figure 2). When Mn concentrations were assessed in OsNRT2.1/OsNRT2.3a/b transgenic lines planted in normal field which was WL condition, respectively (Supplementary Figure S3), we observed increased Mn in the seeds and husk of OsNRT2.1 (Supplementary Figures S3A,D), but no enhanced uptake in OsNRT2.3a/b transgenic lines (Supplementary Figures S3B,C,E,F). Given these data, we investigated the pattern of Mn accumulation in OsNRT2.1 transgenic lines in further detail, under differing conditions of N supply and field conditions. This information is important as rice typically grows in anaerobic flooded fields, in which N exists mainly in the form of NH4+, as opposed to aerobic uplands where the major form of N is NO3- (Stitt, 1999). We found that OsNRT2.1-regulates NO3- uptake in roots, which in turn increases Mn root entry. This increases the Mn concentration in rice grain in the presence of low concentrations of NO3- and under AWD condition. Thus, enhancing NRT2.1-mediated NO3- uptake represents an attractive mechanism of increasing Mn accumulation in food.
Effects of NO3- and NH4+ Nutrition on Mn Accumulation
We demonstrate that NO3- nutrition promotes Mn assimilation in plants to higher levels than NH4+ nutrition (Figure 1). Thus Mn availability in nutrient solutions is influenced by the type of N-nutrient treatments. In 0.5 mM NH4+, Mn uptake in the roots did not differ in OsNRT2.1 transgenic lines (Figure 4C). However, Mn uptake drastically increased in the transgenic lines in 0.5 mM NO3- (Supplementary Figure S5 and Figure 4D). It was recently shown that NO3- uptake induces external alkalization, reducing Fe/Mn concentrations by enhancing the levels of H2O2 in rice (Chen et al., 2018). In this study, we performed hydroponic experiments in MES buffered nutrient medium (to control pH) and nutrient treatments were replaced every 2 days. Furthermore, the Mn content in grain from field experiments should not be influenced by pH as the rhizosphere ranges from pH 5.5 to 6.0 in paddy soil (Pan et al., 2016). Thus, any effects of soil alkalization were excluded. We thus hypothesize that NO3- upregulates the expression of Mn transporters, including OsNRAMP3, OsNRAMP5, and OsNRAMP6 to increase Mn uptake and accumulation (Figure 1 and Supplementary Figure S2). We verified the expression of OsNRT2.1 and OsNAR2.1 under NH4+ and NO3- conditions in transgenic OsNRT2.1 lines and found that OsNRT2.1 was unaffected by the different N forms. However, the expression of its partner protein OsNAR2.1 was significantly up-regulated in 0.5 mM NO3- (Figure 2). The OsNRT2.1 lines could still promote NO3- uptake (Figure 3) into the different tissues compared to WT plants (Supplementary Figure S10D). Thus, the up-regulation of OsNAR2.1 expression in NO3- condition (Figure 2B) promotes NO3- uptake in transgenic plants compared with WT (Supplementary Figure S10D). The observation that the upregulation of OsNRT2.1/OsNAR2.1 is favorable to the transport of NO3- in plants and improves rice yield, is consistent with our previous findings (Chen et al., 2016b; Chen J.G. et al., 2017). As we did not observe enhanced expression of either OsNRT2.3a or OsNRT2.4 lines in NO3- condition, we speculate that the regulation of OsNAR2.1 differs from other OsNRT2 genes according to the plant NO3- content (Supplementary Figure S2, Yan et al., 2011; Wei et al., 2018).
AWD vs. WL Conditions in WT vs. Transgenic Lines
In field experiments, the grain weight of WT lines under AWD condition decreased by 31% compared to WL condition. The NO3- concentrations in OsNRT2.1 transgenic lines also differed across plant areas in AWD condition and which were higher than WT lines (Supplementary Figure S10D). However, the total N concentration did not significantly differ across the lines (Supplementary Figure S10C). The total N of seeds in transgenic lines increased compared to WT (Figure 5). Thus, the overexpression of OsNRT2.1 improves NO3- uptake and assimilation efficiency to increase N accumulation in grain, leading to enhanced grain yields. In hydroponic experiments, the overexpression of OsNRT2.1 also enhanced NO3- uptake (Figure 3B) and N accumulation (Figure 5D), maintaining plant grain yields in WL condition. This is because in AWD condition, a high concentration of dissolved oxygen is present, which can influence nitrification by nitrifying bacterial, or chemical oxidation for the conversion of NH4+ to NO3- at the root surface (Li et al., 2008; Steffens et al., 2011). Thus, under AWD condition, NO3- plays an important role in N accumulation and contributes to enhanced grain yields. However, for WT plants, the capacity to uptake NO3- is limited; and thus, grain yields are dramatically reduced. This observed loss of grain in WT type rice can likely be explained by a multitude of mechanisms.
Surprisingly, we found that under AWD, Mn in the grain of transgenic plants was greatly increased compared to WL condition, but in WT rice, no changes were evident (Figure 5E). In addition, in WL condition, Mn levels also increased in the transgenic lines compared to WT (Figure 5E). We observed no differences in Mn concentrations in other parts of the plant under AWD condition (Supplementary Figure S10). Thus, higher levels of Mn were transported to grain and accumulated (Figure 5). This explains the improvement in seed quality, emergence, and seeding growth observed, as the positive effects of Mn on these processes is well documented (Dimkpa and Bindraban, 2016). The seeds of OsNRT2.1 overexpression lines not only increased in their total N accumulation, but enhanced Mn content was also observed (Figure 5). The length/width of these seeds were also better than WT (Figure 5A and Supplementary Figure S6), demonstrating that Mn plays an important role in increasing crop nutritional quality, crop yield and biomass production. Other metal elements such as Mg and Fe were not influenced by OsNRT2.1 overexpression (Supplementary Figures S8, S11).
Enhanced Expression of Mn Transporters Explains Enhanced Mn Uptake in Transgenic Lines
We verified gene expression profiles in the organs responsible for grain filling and discovered that the expression of OsNRT2.1 and OsNAR2.1 were enhanced in the panicle-neck, flag leaves and sheaths (Figure 6A). In the same plant areas, the expression of OsNRAMP5 and OsNRAMP6 increased in the OsNRT2.1 lines. Interestingly, OsNRAMP3/6 expression was enhanced in node I (Figure 6B). The expression of related genes involved in Mg and Fe uptake were also altered by OsNRT2.1 overexpression (Figure 7). It is understood that node I is a junction of vasculatures that link leaves, stems and panicles and so is important for the transport of nutrient elements into grain (Yamaji and Ma, 2009, 2014; Yamaji et al., 2013a). Transporters responsible for the delivery of minerals into seeds have been reported, including OsYSL16 for Cu (Zheng et al., 2012), OsHMA2 for Zn and Cd (Yamaji et al., 2013b) and AtNIP6;1 that is expressed in the node region for B distribution (Tanaka et al., 2008). Accordingly, the majority of these genes are also strongly expressed in node I (Tanaka et al., 2008; Yamaji and Ma, 2009, 2014; Zheng et al., 2012; Yamaji et al., 2013a).
Conclusion
Taken together, we show that AWD treatment can induce the expression of NO3- and Mn transporters in grain filling organs which increases the accumulation of N and Mn in grain. NO3- uptake in OsNRT2.1 transgenic lines can improve Mn accumulation, however, the Mn concentration does not increase in the seeds and husk of OsNRT2.3a/b overexpression lines, which also display increased NO3- uptake compared to WT lines (Fan et al., 2016). Thus, the mechanism(s) linking NO3- and Mn in OsNRT2.1 overexpressing plants differ from other OsNRT2 overexpression lines and is worthy of further investigation. From our findings, we propose a new application to improve both N and water efficiency in agricultural systems and demonstrate how high OsNRT2.1 expression improves Mn content in rice grain.
Author Contributions
BiL, JC, and XF conceived the study, analyzed the data, and drafted the manuscript. BiL, LZ, and SL cultivated the rice materials and collected the rice samples. LZ, BL, and HL extracted RNA and performed the qRT-PCR experiments. BL and LZ participated in field and material management. BiL and JC conducted the statistical analysis of raw data. XF, GX, and GY revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are also grateful for the Anhui Provincial Natural Science Foundation of China Science Foundation of China (No. 1608085MC59) and Major Special Science and Technology Project of Anhui Province (No. 16030701102).
Footnotes
Funding. This study was financially supported by China National Key Program for Research and Development (2016YFD0100700), National Natural Science Foundation (Grant No. 31372122), Jiangsu Science Fund for Distinguished Young Scholars (Grant No. BK20160030), the Transgenic Project (Grant 2016ZX08001003-008).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01192/full#supplementary-material
Assessment of the total N-content under different N treatments. (A) total N concentration, (B) total N content of roots and shoots from different N treatments, (C) total N content of whole plants. 0.5/2.5A: 0.5 mM/2.5 mM NH4+ as an N source; 0.5/2.5 N: 0.5/2.5 mM NO3- as an N source. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Expression pattern of NO3- transporters and Mn transporters under different N treatments; total RNA was isolated from WT rice supplied with 0.5/2.5N: 0.5/2.5 mM NO3- and 0.5/2.5 A:0.5/2.5 mM NH4+ as an N source for 2 weeks. (A) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (D) OsNRAMP3/OsNRAMP5/OsNRAMP6 in leaves; (B) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (E) OsNRAMP3/OsNRAMP5/OsNRAMP6 in sheath; (C) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (F) OsNRAMP3/OsNRAMP5/OsNRAMP6 in roots. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between different N treatments (P < 0.05, one-way ANOVA).
Mn concentration in seeds and husk of OsNRT2.1/OsNRT2.3b overexpression lines. Mn concentrations in seeds of (A) OsNRT2.1 overexpression lines, (B) OsNRT2.3b overexpression lines and (C) OsNRT2.3a overexpression lines. Mn concentration in husk of (D) OsNRT2.1 overexpression lines, (E) OsNRT2.3b overexpression lines and (F) OsNRT2.3a overexpression lines. b-O1/2/8: three OsNRT2.3b overexpression lines; a-O1/2: two OsNRT2.3a overexpression lines. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Identification of transgenic lines. (A) Southern blot of genomic DNA isolated from WT and transgenic plants. Hybridization was performed using a hygromycin gene probe. P, positive control; M, marker. Extraction of total RNA from roots and shoots of WT and transgenic lines and qRT-PCR results under. (B) M: DNA molecular-weight marker II, DIG – labeled; P: positive controls. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Comparison of total N/Mn concentrations and content of transgenic plants at different nitrogen supply levels. (A–C) Under 0.5 mM NH4+ treatments, (A) total N concentration, (B) total N content and (C) Mn content of roots and shoots. (D–F) Under 0.5 mM NO3- treatments, (D) total N concentration, (E) total N content and (F) Mn content of roots and shoots. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Assessment of the length and width of seeds in different lines under WL and AWD treatments. (A) Seeds lengths (mm), (B) seed widths (mm). Error bars: standard error (n = 4 plants), 15 repeats. Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Effects of different irrigation conditions on Mn concentrations in rice husk. Under WL and AWD, (A) total N concentration and (B) Mn concentration of rice husk were assessed. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between the irrigation conditions of all lines (P < 0.05, one-way ANOVA).
Effects of different irrigation conditions on other elements in rice seeds. Under WL and AWD, Fe and Mg concentrations of husk (A,B) and seeds (C,D). Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines in different irrigation conditions (P < 0.05, one-way ANOVA).
Diagram of RNA sampling in WT and transgenic plants.
Effect of transgenic lines on total N/Mn content in vegetative organs under AWD conditions. (A) Dry weight of different parts in all lines, (B) Total N concentration, (C) Total N content, (D) NO3- concentration, (E) Manganese concentration, and (F) Manganese content from different parts of all lines. Error bars: standard error (n = 4 plants). Other leaves: second and third leaves. Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Concentration of other elements in different parts of transgenic lines under AWD conditions. (A) Mg concentration, (C) Mg content, (B) Fe concentration, (D) Fe content. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Correlation analysis between expression of OsNRT2.1 and total nitrogen/nitrate concentration in flag leaves of wt and transgenic lines. (A) Linear Analysis of relative expression of OsNRT2.1 and total N concentration. (B) Linear Analysis of relative expression of OsNRT2.1 and nitrate concentration.
Primers used to amplify the OsNRT2.1 open reading frame.
Primers used for quantitative real-time polymerase chain reaction.
References
- Ai P. H., Sun S. B., Zhao J. N., Fan X. R., Xin W. J., Guo Q., et al. (2009). Two rice phosphate transporters, OsPht1;2 and OsPht1;6 have different functions and kinetic properties in uptake and translocation. Plant J. 57 798–809. 10.1111/j.1365-313X.2008.03726.x [DOI] [PubMed] [Google Scholar]
- Borlotti A., Vigani G., Zocchi G. (2012). Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biol. 12:189. 10.1186/1471-2229-12-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botrill D. E., Possingham J. V., Kriedmmann P. E. (1970). The effect of nutrient deficiencies on photosynthesis and respiration in spinach. Plant Soil 32 424–438. 10.1007/BF01372881 [DOI] [Google Scholar]
- Cailliatte R., Schikora A., Briat J. F., Mari S., Curie C. (2010). High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22 904–917. 10.1105/tpc.109.073023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. F., Zhang Q., Cai H. M., Zhou W., Xu F. S. (2018). H2O2 mediates nitrate-induced iron chlorosis by regulating iron homeostasis in rice. Plant Cell Environ. 41 767–781. 10.111/pce.13145 [DOI] [PubMed] [Google Scholar]
- Chen J. G., Zhang Y., Tan Y. W., Xu G. H., Fan X. R. (2016a). The effects of OsNRT2.1 over-expression on plant growth and nitrogen use efficiency in rice nipponbare (Oryza sativa L. ssp. japoncia). Mol. Plant Breed. 14 1–9. [Google Scholar]
- Chen J. G., Zhang Y., Tan Y. W., Zhang M., Zhu L. L., Xu G. H., et al. (2016b). Agronomic nitrogen-use efficiency of rice can be increased by driving OsNRT2.1 expression with the OsNAR2.1 promoter. Plant Biotechnol. J. 14 1705–1715. 10.1111/pbi.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. G., Fan X. R., Qian K. Y., Zhang Y., Song M. Q., Liu Y., et al. (2017). pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants. Plant Biotechnol. J. 15 1273–1283. 10.1111/pbi.12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. C., Yamaji N., Horie T., Chen J., Li J., An G. H., et al. (2017). A magnesium transporter OsMGT1 plays a critica role in salt tolerance in rice. Plant Physiol. 174 1837–1849. 10.1104/pp.17.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton J. M., Webster R. E., Cheng N., Pittman J. K. (2012). Knockout of multiple Arabidopsis cation/H(+) exchangers suggests isoform-specific roles in metal stress response, germination and seed mineral nutrition. PLoS One 7:e47455. 10.1371/journal.pone.0047455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Glass A. D. M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3 389–395. 10.1016/s1360-1385(98)01311-9 [DOI] [Google Scholar]
- Dimkpa C. O., Bindraban P. S. (2016). Fortification of micronutrients for efficient agronomic production: a review. Agron. Sustain. Dev. 36:7 10.1007/s13593-015-0346-6 [DOI] [Google Scholar]
- Duan Y. H., Zhang Y. L., Ye L. T., Fan X. R., Xu G. H., Shen Q. R. (2007). Response of rice cultivars with different nitrogen use efficiency to partial nitrate nutrition. Ann. Bot. 99 1153–1160. 10.1093/aob/mcm051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziwornu K. A., Shrestha A., Matthus E., Ali B., Wu L. B., Frei M. (2018). Responses of contrasting rice genotypes to excess manganese and their implications for lignin synthesis. Physiol. Biochem. Plant 123 252–259. 10.1016/j.plaphy.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Emery L., Whelan S., Hirschi K. D., Pittman J. K. (2012). Protein phylogenetic analysis of Ca2+/cation Antiporters and insights into their evolution in plants. Front. Plant Sci. 3:1. 10.3389/fpls.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Tang Z., Tan Y., Zhang Y., Luo B., Yang M., et al. (2016). Over expression of a pH sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. U.S.A. 113 7118–7123. 10.1073/pnas [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H. M., Yan M., Fan X. R., Li B. Z., Shen Q. R., Miller A. J., et al. (2011). Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 62 2319–2332. 10.1093/jxb/erq403 [DOI] [PubMed] [Google Scholar]
- Gong X. L., Qu C. X., Liu C., Hong M. M., Wang L., Hong F. S. (2011). Effects of manganese deficiency and added cerium on nitrogen metabolism of maize. Biol. Trace Elem. Res. 144 1240–1250. 10.1007/s12011-011-9105-y [DOI] [PubMed] [Google Scholar]
- Hebbern C. A., Pedas P., Schjoerring J. K., Knudsen L., Husted S. (2005). Genotypic differences in manganese efficiency: field experiments with winter barley (Hordeum vulgare L.). Plant and Soil. 272 233–244. 10.1007/s11104-004-5048-9 [DOI] [Google Scholar]
- Ishimaru Y., Masuda H., Bashir K., Inoue H., Tsukamoto T., Takahashi M., et al. (2010). Rice metal-nicotianamine transporter, OsYSL 2, is required for the long distance transport of iron and manganese. Plant J. 62 379–390. 10.1111/j.1365-313X.2010.04158.x [DOI] [PubMed] [Google Scholar]
- Jiang W. Z. (2006). Mn use efficiency in different wheat cultivars. Environ. Exp. Bot. 57 41–50. 10.1016/j.envexpbot.2005.04.008 [DOI] [Google Scholar]
- Jin Z., Zhu Y., Li X., Dong Y., An Z. (2015). Soil N retention and nitrate leaching in three types of dunes in the Mu Us desert of China. Sci. Rep. 5:14222. 10.1038/srep14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T., Krapp A. (2016). Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol. 57 707–717. 10.1093/pcp/pcw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Inoue H., Mizuno D., Takahashi M., Nakanishi H., Mori S., et al. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 39 415–424. 10.1111/j.1365-313X.2004.02146.x [DOI] [PubMed] [Google Scholar]
- Lee S., An G. (2009). Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 32 408–416. 10.1111/j.1365-3040.2009.01935.x [DOI] [PubMed] [Google Scholar]
- Li B., Xin W., Sun S., Shen Q., Xu G. (2006). Physiological and molecular responses of nitrogen-starved rice plants to re-supply of different nitrogen sources. Plant Soil 287 145–159. 10.1007/s11104-006-9051-1 [DOI] [Google Scholar]
- Li X. L., Ziadi N., Bélanger G., Cai Z. C., Xu H. (2011). Cadmium accumulation in wheat grain as affected by mineral N fertilizer and soil characteristics. Can. J. Soil Sci. 91 521–531. 10.1139/CJSS10061 [DOI] [Google Scholar]
- Li Y. L., Fan X. R., Shen Q. R. (2008). The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ. 31 73–85. 10.1111/j.1365-3040.2007.01737.x [DOI] [PubMed] [Google Scholar]
- Lux A., Martinka M., Vaculík M., White P. J. (2011). Root responses to cadmium in the rhizosphere: a review. J. Exp. Bot. 62 21–37. 10.1093/jxb/erq281 [DOI] [PubMed] [Google Scholar]
- Marschner H. (1995). Mineral Nutrition of Higher Plants. London: Academic Press. [Google Scholar]
- Muños S., Cazettes C., Fizames C., Gaymard F., Tillard P., Lepetit M., et al. (2004). Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter. NRT2.1. Plant Cell. 16 2433–2447. 10.1105/tpc.104.024380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Koopmans G. F., Bonten L. T., Song J., Luo Y., Temminghoff E. J., et al. (2016). Temporal variability in trace metal solubility in a paddy soil not reflected in uptake by rice (Oryza sativa L.). Environ. Geochem. Health 38 1355–1372. 10.1007/s10653-016-9803-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Peris C., Serra-Cardona A., Sánchez-Sanuy F., Campo S., Ariño J., Segundo B. S. (2017). Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Mol. Plant Microbe Interact. 30 385–398. 10.1094/MPMI-01-17-0005-R [DOI] [PubMed] [Google Scholar]
- Sasaki A., Yamaji N., Xia J., Ma J. F. (2011). OsYSL6 is involved in thedetoxification of excess manganese in rice. Plant Physiol. 157 1832–1840. 10.1104/pp.111.186031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. R. (2015). The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 66 23–48. 10.1146/annurev-arplant-050312-120129 [DOI] [PubMed] [Google Scholar]
- Socha A. L., Guerinot M. L. (2014). Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 5:106. 10.3389/fpls.2014.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B., Geske T., Sauter M. (2011). Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 190 369–378. 10.1111/j.1469-8137.2010.03496.x [DOI] [PubMed] [Google Scholar]
- Stitt M. (1999). Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 2 178–186. 10.1016/S1369-5266(99)80033-8 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Wallace I. S., Takano J., Roberts D. M., Fujiwara T. (2008). NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissue in Arabidopsis. Plant Cell 20 2860–2875. 10.1105/tpc.108.058628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Fan X., Li Q., Feng H., Miller A. J., Shen Q., et al. (2012). Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol. 160 2052–2063. 10.1104/pp.112.204461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhao X. Q., Hu Z. M., Shao J. F., Che J., Chen R. F., et al. (2015). Aluminium alleviates manganese toxicity to rice by decreasing root symplastic Mn uptake and reducing availability to shoots of Mn stored in roots. Ann. Bot. 116 237–246. 10.1093/aob/mcv090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Zheng Y., Feng H., Qu H., Fan X., Yamaji N. (2018). OsNRT2.4 encodes a dual-affinity nitrate transporter and functions in nitrate-regulated root growth and nitrate distribution in rice. J. Exp. Bot. 69 1095–1107. 10.1093/jxb/erx486 [DOI] [PubMed] [Google Scholar]
- Xie H. L., Jiang R. F., Zhang F. S., McGrath S. P., Zhao F. J. (2009). Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens. Plant Soil. 318 205–215. 10.1007/s11104-008-9830-y [DOI] [Google Scholar]
- Xu G., Fan X., Miller A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63 153–182. 10.1146/annurev-arplant-042811-105532 [DOI] [PubMed] [Google Scholar]
- Xu X., Chen X., Shi J., Chen Y., Wu W., Perera A. (2007). Effects of manganese on uptake and translocation of nutrients in a hyperaccumulator. J. Plant Nutr. 30 1737–1751. 10.1080/01904160701615582 [DOI] [Google Scholar]
- Yamaji N., Ma J. F. (2009). A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21 2878–2883. 10.1105/tpc.109.069831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N., Ma J. F. (2014). The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 19 556–563. 10.1016/j.tplants.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Yamaji N., Sasaki A., Xia J. X., Yokosho K., Ma J. F. (2013a). A node based switch for preferential distribution of manganese in rice. Nat. Commun. 4:2442. 10.1038/ncomms3442 [DOI] [PubMed] [Google Scholar]
- Yamaji N., Xia J., Mitani-Ueno N., Yokosho K., Ma J. F. (2013b). Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 162 927–939. 10.1104/pp.113.216564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Fan X. R., Feng H. M., Miller A. J., Shen Q. R., Xu G. H. (2011). Rice OsNAR2.1 interacts with OsNRT2. 1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 34 1360–1372. 10.1111/j.1365-3040.2011.02335.x [DOI] [PubMed] [Google Scholar]
- Yan X. L., Wu P., Ling H. Q., Xu G. H., Xu F. S., Zhang Q. F. (2006). Plant Nutriomics in China: an overview. Ann. Bot. 98 473–482. 10.1093/aob/mcl116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Zhang Y. Y., Zhang L. J., Hu J. T., Zhang X., Lu K., et al. (2014). OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 17 4849–4861. 10.1093/jxb/eru259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Yamaji N., Yokosho K., Ma J. F. (2012). YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 24 3767–3782. 10.1105/tpc.112.103820 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of the total N-content under different N treatments. (A) total N concentration, (B) total N content of roots and shoots from different N treatments, (C) total N content of whole plants. 0.5/2.5A: 0.5 mM/2.5 mM NH4+ as an N source; 0.5/2.5 N: 0.5/2.5 mM NO3- as an N source. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Expression pattern of NO3- transporters and Mn transporters under different N treatments; total RNA was isolated from WT rice supplied with 0.5/2.5N: 0.5/2.5 mM NO3- and 0.5/2.5 A:0.5/2.5 mM NH4+ as an N source for 2 weeks. (A) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (D) OsNRAMP3/OsNRAMP5/OsNRAMP6 in leaves; (B) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (E) OsNRAMP3/OsNRAMP5/OsNRAMP6 in sheath; (C) relative expression of OsNRT2.1/OsNRT2.3/OsNAR2.1 and (F) OsNRAMP3/OsNRAMP5/OsNRAMP6 in roots. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between different N treatments (P < 0.05, one-way ANOVA).
Mn concentration in seeds and husk of OsNRT2.1/OsNRT2.3b overexpression lines. Mn concentrations in seeds of (A) OsNRT2.1 overexpression lines, (B) OsNRT2.3b overexpression lines and (C) OsNRT2.3a overexpression lines. Mn concentration in husk of (D) OsNRT2.1 overexpression lines, (E) OsNRT2.3b overexpression lines and (F) OsNRT2.3a overexpression lines. b-O1/2/8: three OsNRT2.3b overexpression lines; a-O1/2: two OsNRT2.3a overexpression lines. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Identification of transgenic lines. (A) Southern blot of genomic DNA isolated from WT and transgenic plants. Hybridization was performed using a hygromycin gene probe. P, positive control; M, marker. Extraction of total RNA from roots and shoots of WT and transgenic lines and qRT-PCR results under. (B) M: DNA molecular-weight marker II, DIG – labeled; P: positive controls. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between N treatments (P < 0.05, one-way ANOVA).
Comparison of total N/Mn concentrations and content of transgenic plants at different nitrogen supply levels. (A–C) Under 0.5 mM NH4+ treatments, (A) total N concentration, (B) total N content and (C) Mn content of roots and shoots. (D–F) Under 0.5 mM NO3- treatments, (D) total N concentration, (E) total N content and (F) Mn content of roots and shoots. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Assessment of the length and width of seeds in different lines under WL and AWD treatments. (A) Seeds lengths (mm), (B) seed widths (mm). Error bars: standard error (n = 4 plants), 15 repeats. Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Effects of different irrigation conditions on Mn concentrations in rice husk. Under WL and AWD, (A) total N concentration and (B) Mn concentration of rice husk were assessed. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between the irrigation conditions of all lines (P < 0.05, one-way ANOVA).
Effects of different irrigation conditions on other elements in rice seeds. Under WL and AWD, Fe and Mg concentrations of husk (A,B) and seeds (C,D). Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines in different irrigation conditions (P < 0.05, one-way ANOVA).
Diagram of RNA sampling in WT and transgenic plants.
Effect of transgenic lines on total N/Mn content in vegetative organs under AWD conditions. (A) Dry weight of different parts in all lines, (B) Total N concentration, (C) Total N content, (D) NO3- concentration, (E) Manganese concentration, and (F) Manganese content from different parts of all lines. Error bars: standard error (n = 4 plants). Other leaves: second and third leaves. Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Concentration of other elements in different parts of transgenic lines under AWD conditions. (A) Mg concentration, (C) Mg content, (B) Fe concentration, (D) Fe content. Error bars: standard error (n = 4 plants). Different letters indicate a significant difference between WT and overexpression lines (P < 0.05, one-way ANOVA).
Correlation analysis between expression of OsNRT2.1 and total nitrogen/nitrate concentration in flag leaves of wt and transgenic lines. (A) Linear Analysis of relative expression of OsNRT2.1 and total N concentration. (B) Linear Analysis of relative expression of OsNRT2.1 and nitrate concentration.
Primers used to amplify the OsNRT2.1 open reading frame.
Primers used for quantitative real-time polymerase chain reaction.