Abstract
Hematopoietic stem cells (HSCs) can self renew and differentiate into all cell types of the blood. This is therapeutically important as HSC transplants can provide a curative effect for blood cancers and disorders. The process by which HSCs develop has been the subject of extensive research in a variety of model organisms, however, efforts to produce bona-fide HSC from pluripotent precursors capable of long term multi-lineage reconstitution have fallen short. Studies in zebrafish, chicken and mice have been instrumental in guiding efforts to derive HSCs from human pluripotent stem cells and have identified a complex set of molecular signals and cellular interactions mediated by such developmental regulators as FGF, Notch, TGFβ and Wnt, which collectively promote the stepwise developmental progression towards mature HSCs. Tight temporal and spatial control of these signals is critical to generate the appropriate numbers of HSCs needed for the life of the organism. The role of the Wnt family of signaling proteins in hematopoietic development has been the subject of many studies owing in part to the complex nature of its signaling mechanisms. By integrating cell fate specification with cell polarity establishment, Wnt is uniquely capable of controlling complex biological processes, including at multiple stages of embryonic HSC development, from HSC specification to emergence from the hemogenic epithelium to subsequent expansion. This review highlights key signaling events where specific Wnt signals instruct and guide hematopoietic development in both zebrafish and mice and extends these findings to current efforts of generating HSCs in vitro.
Keywords: Hematopoietic stem cells, HSCs, hematopoiesis, Wnt signaling, development, blood
Introduction
Hematopoietic stem cells (HSCs) are adult stem cells that are capable of self-renewal and giving rise to all terminally differentiated cells of the blood. This property has allowed HSCs to be used as a therapeutic for various blood disorders and cancers by repopulating the patient’s deficient blood system with a full complement of healthy blood cells. Currently, HSCs are harvested from healthy donor bone marrow, peripheral blood, or umbilical cord blood, and most procedures require the donor cells and the patient recipient to be human leukocyte antigen (HLA)-matched. In some cases, the patient’s own HSCs may be harvested and banked for later therapeutic use. Though HSCs have been a viable therapeutic for decades, many patients lack a HLA-matched donor (Hatzimichael and Tuthill 2010, Peters et al. 2010).
The advent of induced pluripotent stem cell (iPSC) technology has made possible facile derivation of pluripotent stem cells from patients, thus creating a possible source of autologous HSCs for each patient in need of a transplant (Takahashi et al. 2007). Pluripotent stem cells are, in theory, capable of differentiating into all cells that make up an organism, including HSCs. However, it is currently not possible to generate therapeutically viable HSCs for human patients (reviewed in Slukvin 2013, Vo and Daley 2015). A more thorough understanding of the molecular cues that instruct the native development of HSCs will contribute to improving protocols to generate these cells in vitro. This review focuses on the role of the Wnt signaling pathway during HSC development.
Hematopoietic stem cell development in vivo
Hematopoietic development is separated into two phases. The first phase, termed primitive, produces mostly erythrocytes and macrophages that transiently sustain the organism during early development. These cell types arise in the yolk sac in mammals and in the intermediate cell mass/cephalic mesoderm in the zebrafish (reviewed in Davidson and Zon 2004, and Batta et al. 2016). In the zebrafish, these waves are temporally and spatially distinct from the definitive waves of hematopoiesis, which give rise first to committed erythromyeloid precursors (EMPs) in the posterior blood island then to HSCs that appear along the floor of the dorsal aorta. [Figure 1] HSCs are derived from the mesodermal lineage, the generation of which is dependent on the coordinate regulation of multiple signaling pathways, including Nodal, bone morphogenic protein (BMP), fibroblast growth factor (FGF), and Wnt (reviewed in Clements and Traver 2013). A subset of mesodermal cells, specifically lateral plate mesoderm, migrates laterally past the somites, which provide critical signaling and guidance cues, to the midline of the organism, eventually forming the vasculature (reviewed in Medvinsky et al. 2011). Cooperation between the Vegf, Hedgehog and Notch signaling pathways further specify these cells to become either arterial or venous endothelium (Rowlinson and Gering 2010). Specific cells within the floor of the aorta termed hemogenic endothelium undergo an endothelial to hematopoietic transition (EHT) to become HSCs. These cells undergo a change in morphology, transitioning from a flattened endothelial cell to a round hematopoietic cell, and bud from the wall of the aorta (Kissa et al. 2008, Eilken et al. 2009, Bertrand et al. 2010, Kissa and Herbomel 2010, Mizuochi et al. 2012). These nascent HSCs enter circulation and home to the placenta and fetal liver (mice) or the caudal hematopoietic tissue (zebrafish), where HSCs proliferate before transitioning to the adult niche that maintains the HSC population for the remainder of the lifetime of the animal; the bone marrow in the mouse and the kidney marrow in the zebrafish (Murayama et al. 2006, reviewed in Medvinsky et al. 2011). The journey of a developing HSC in the model organisms focused on here (mouse and zebrafish) proceeds through similar stages of development: specification, emergence, and expansion before moving to the adult maintenance niche (Figure 1). The specific anatomical regions for these events vary between organisms, but the niche functions appear conserved.
Figure 1.
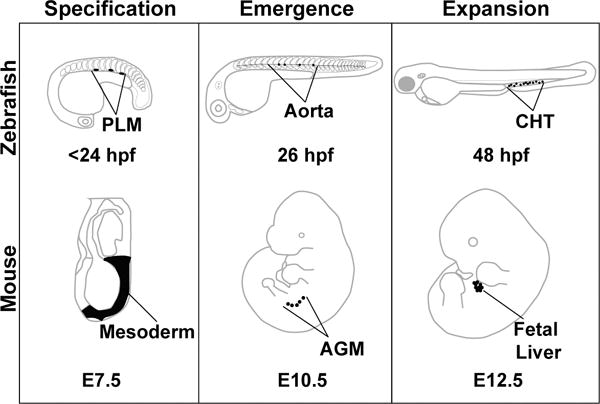
Model organisms used to study embryonic hematopoiesis. Early HSC development can be divided into three phases: specification, emergence, and expansion. These stages are conserved among vertebrates, but the precise anatomical locations where these events take place vary slightly between model organisms. This review focuses on three model systems: zebrafish, mouse, and the human embryonic stem cell in vitro differentiation system. Specification is the process by which developing HSCs receive molecular cues that inform their fate before they emerge. In the zebrafish, these cells arise from the posterior lateral mesoderm (PLM), which migrate beneath the somites to the midline of the embryo to form the vasculature. This process is similar in the mouse embryo, with HSCs deriving from cells of the mesoderm. Emergence in both the zebrafish and the mouse occurs in the aorta (fish), or the aorta – gonad – mesonephros (AGM) region (mouse). HSCs that are embedded within the aortic endothelium emerge from the aorta in a process called the endothelial to hematopoietic transition, and enter circulation into the vein (fish) or the aorta (mouse). Relatively few HSCs emerge from the aorta, so their numbers are expanded in a niche that supports proliferation. In fish, this is the caudal hematopoietic tissue (CHT), and in mouse this is the fetal liver. Eventually, the HSCs seed the adult hematopoietic organs where they will be maintained for the lifetime of the animal (fish: kidney marrow, mouse: bone marrow).
The Wnt signaling pathway
Wnt signaling is an evolutionarily highly conserved pathway critical for the generation of cell diversity and polarity amongst all metazoan species. Although much of the published literature distinguishes Wnt signaling into two broad types, the canonical and non-canonical pathways, recent studies suggest a more integrated view where Wnt proteins through their short range signaling nature simultaneously activate “cell fate” (= canonical) and “cell polarity” (= non-canonical) cascades (reviewed in Loh et al. 2016). Broadly speaking, both pathways employ the Wnt signaling molecules, their cognate receptors encoded by the Frizzled (Fzd) gene family and the intracellular signaling molecule Dishevelled (Dvl/Dsh). Downstream of these shared signaling units, the two pathways are quite distinct, with the cell fate cascade defined by the signaling components Glycogen Synthase Kinase 3 (GSK3), Axin and Adenomatous Polyposis Coli (APC), and β-catenin. In contrast, the cell polarity cascades acts through proteins like Vangl, Celsr and Prickle to regulate cellular orientation within a group of cells. Over the years, the cell fate pathway has garnered most attention, yielding important insights into its mode of action and its roles in development and disease. [Figure 2]
Figure 2.
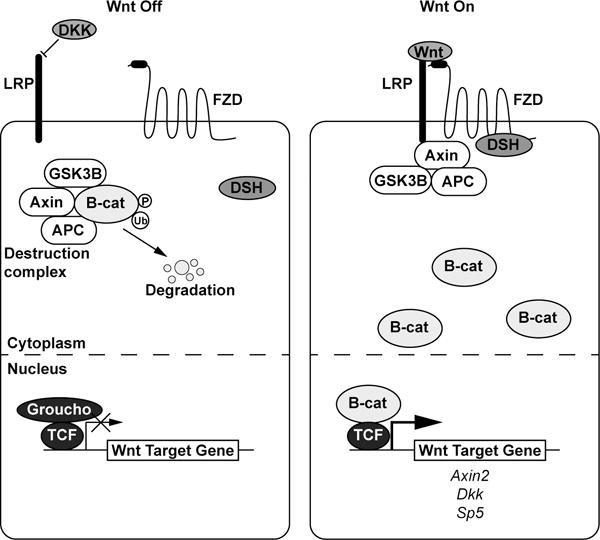
The (β–catenin mediated) Wnt signaling pathway. In the absence of a Wnt ligand, a destruction complex phosphorylates β–catenin and targets it for ubiquitination and degradation. The transcription factor TCF is bound to Wnt target genes and acts as a co-repressor with Groucho. When a Wnt ligand is present, it binds a Frizzled receptor and LRP co-receptor which causes the destruction complex to associate away from β–catenin, which builds up in the cytoplasm and translocates to the nucleus to act as a transcriptional co-activator with TCF to initiate transcription of Wnt target genes such as Axin2, Dkk, and Sp5.
The key mediator of the cell fate cascade is β-catenin: in the absence of Wnt signal, a destruction complex consisting of Axin, GSK3β, APC, and other proteins promotes phosphorylation of β-catenin, thereby targeting it for ubiquitination and degradation by the proteasome (Aberle et al. 1997). Transcription factors T-cell factor (TCF) and lymphoid enhancer binding factor (LEF) reside in the nucleus bound to regulatory regions of Wnt target genes and to co-repressors, such as Groucho to inhibit transcription (Cavallo et al. 1998). Upon transduction of a Wnt signal through a Fzd receptor complexed with an LRP5/6 co-receptor, Dvl/Dsh and components of the destruction complex are re-localized to the membrane (Bhanot et al. 1996, Yang-Snyder et al. 1996, Holmen et al. 2002), releasing β-catenin from constitutive degradation. Increased cytosolic β-catenin translocates to the nucleus, where it binds TCF and LEF to act as a co-activator to initiate transcription of Wnt target genes (Daniels and Weis 2005) (Figure 2).
Several features ensure tight control of this signaling pathway, which is critical for proper cell fate diversification and specification. First, the signaling range of Wnt proteins is highly restricted, a feature afforded by the covalent attachment of a lipid, thus rendering the protein highly hydrophobic and poorly soluble once secreted from a cell (Willert et al. 2003, Takada et al. 2006). Second, a host of negative regulators act at multiple levels of the signaling cascade, including on the Wnt proteins themselves (e.g. Sfrp, Notum), the Fzd/LRP receptor complexes (e.g. Rnf43, Dkk), the intracellular signaling cascade (e.g. Axin2, Nkd), and on the transcriptional response (e.g. ICAT, Sp5). Several of these negative regulators are target genes of Wnt/β-catenin signaling, thus establishing negative feedback loops that restrict the spatial and temporal response to Wnt signals.
In contrast to this cell fate cascade, which exerts much of its effects through changes in gene expression, the Wnt cell polarity pathway acts independently of β-catenin and regulates complex biological processes, such as planar cell polarity, convergent extension and cell migration. The study of the Wnt polarity pathway has proven more difficult than the study of the Wnt cell fate pathway, owing in large part to the scarcity of in vitro assays and the need for complex biological systems, such as imaging of explants or whole animals. In addition to this pathway utilizing distinct intracellular effectors, such as Vangl, Celsr and Prickle, the interactions of which remain poorly understood, this pathway in certain contexts employs non-Fzd receptors, such as Ror1/2 and Ryk. While this review will focus primarily on the cell fate pathway, we will also highlight notable roles of the Wnt cell polarity pathway. It should be stressed that development of HSCs, like many complex biological processes, requires input from both Wnt signaling pathways.
Zebrafish hematopoietic development
As zebrafish HSC precursors develop from cells of the posterior lateral mesoderm, they receive a complement of molecular cues that informs their identity as HSCs; this process is termed specification. Eventually, specialized cells of the endothelium will undergo an endothelial to hematopoietic transition and bud off from the endothelium in a process termed emergence, which initiates at 26 hours post fertilization (hpf). Next, nascent HSCs move to the caudal hematopoietic tissue (CHT) where they proliferate during the expansion phase of HSC development. We will discuss the requirement for Wnt signaling during each of these three phases: specification, emergence and expansion (Figure 1).
The Wnt cell polarity pathway is required for zebrafish HSC specification
To date, a requirement for the Wnt cell fate pathway (= Wnt/β-catenin) in HSC specification in zabrafish has not been demonstrated. However, Wnt16 acting in a β-catenin-independent manner is required in the somites for HSC specification in a non – cell autonomous manner (Clements et al. 2011). Knockdown of wnt16 via injection of an antisense morpholino oligonucleotide decreases HSC marker gene expression at 24 hpf, before HSCs have begun to emerge, indicating a defect in HSC specification. This decrease in HSC number is sustained into later stages of hematopoietic development. Knockdown of wnt16 causes a loss of Notch ligand deltaC and deltaD expression in the somites, and overexpression of deltaC and deltaD in the context of the wnt16 morpholino is sufficient to rescue the loss of HSC phenotype. This Wnt16 signal sets up a Notch3 cue that is not received directly by the HSCs, but must be received by somite – adjacent cells between 15 – 17 hpf (Clements et al. 2011, Kim et al. 2014). Knockdown of wnt16 led to a reduction in expression of markers of the sclerotome, a compartment of the somite that has been shown to be important for HSC development, although the exact mechanisms by which it is required is not known (reviewed in Butko et al. 2016). Rspondin1, an activator of the Wnt pathway, regulates this requirement for Wnt16. Loss of Rspondin1 results in decreased wnt16 expression, and subsequent loss of HSC specification (Genthe and Clements 2017).
Wnt is required for zebrafish HSC emergence
Many lines of evidence support the conclusion that zebrafish HSC emergence is dependent upon Wnt cell fate signaling. Our recent work showed that loss of Wnt9a in the somites caused a decrease in HSC number after initiation of emergence with no discernible negative impact on HSC specification (Grainger and Richter et al. 2016). This Wnt signal is unrelated to the somitic requirement for wnt16, as it is temporally distinct and does not impact specification. Interestingly, Wnt9a is required pre –20 hpf, but the HSC phenotype does not occur until much later, around 32 hpf, indicating that Wnt9a establishes a permissive environment for later HSC amplification. Similarly, overexpression of the Wnt antagonist dkk1 causes a decrease in HSCs and progenitors at 36 hpf (as detected by expression of cmyb), which is during the emergence window (Goessling et al. 2009). Overexpression of wnt8, which in this context potently activates the cell fate pathway, caused an increase in HSCs during the emergence window (Goessling et al. 2009). Inhibiting the secretion of all Wnt ligands using a chemical inhibitor of Porcupine (Porcn) (Chen et al. 2009), an enzyme which is required for the lipid modification and subsequent secretion of Wnts, also caused a loss of HSCs during the emergence window (Biechele et al. 2011, Grainger and Richter et al. 2016).
Results from manipulation of cytoplasmic components of the Wnt pathway also support the conclusion that Wnt cell fate is required for HSC emergence. Overexpressing axin1 or stabilizing Axin1 protein by chemically inhibiting Tankyrases, which promote degradation of Axin, inhibited the Wnt pathway and resulted in a decrease of cmyb+ cells during HSC emergence (Goessling et al. 2009, Wang et al. 2013). Conversely, using a small molecule to increase the association between Axin and LRP6 to stimulate the Wnt pathway caused an increase in cmyb+ cells during HSC emergence (Wang et al. 2013). Activating the pathway by inhibiting GSK3β with lithium increased the number of flk1/cmyb – double positive HSCs emerging from the aortic floor (Grainger and Richter et al. 2016). Overexpression of a constitutively active β–catenin also increased the number of cmyb+ cells within the HSC emergence window (Grainger and Richter et al. 2016). Altogether, these experiments provide strong evidence that cytoplasmic components of the Wnt cell fate pathway are necessary for HSC emergence.
Inhibition of Wnt signaling at the level of target gene activation also demonstrated that Wnt is required for HSC emergence. Expression of a dominant-negative Tcf transgene (dntcf) that lacks the β-catenin binding domain results in cells unable to respond to an extracellular Wnt signal. Downregulation of the Wnt pathway via expression of dntcf resulted in decreased numbers of cmyb+ hematopoietic cells during HSC emergence in multiple studies (Goessling et al. 2009, Grainger and Richter et al. 2016). Interestingly, expression of dntcf also caused a decrease in gata1+ primitive blood cells, suggesting Wnt may also play a role in earlier waves of hematopoietic development (Lengerke et al. 2008). Experiments utilizing the dntcf transgene have provided insight into the critical tissue that must receive this canonical Wnt cue. Tissue specific expression of dntcf in fli1a+ endothelium and, more specifically, gata2b+ hemogenic endothelium was sufficient to recapitulate whole-embryo dntcf expression (Grainger and Richter et al. 2016). This suggests that cells of the hemogenic endothelium must receive a critical Wnt cell fate cue to successfully develop and emerge from the aortic endothelium.
The role of Wnt signaling in zebrafish HSC expansion
The caudal hematopoietic tissue has long been considered the main site of HSC proliferation in the zebrafish embryo, analogous to the mouse placenta or fetal liver. Recent reports have provided evidence for HSCs undergoing expansion within the aorta prior to emergence and migration to the caudal hematopoietic tissue (Goessling et al. 2009, Grainger and Richter et al. 2016). It is not clear whether Wnt signaling plays a role in HSC expansion within the caudal hematopoietic tissue, but the Wnt pathway does have a critical function in the more recently described intra-aortic expansion of cells fated to become HSCs. It is not yet clear whether this proliferation occurs within hemogenic endothelial cells or in nascent HSCs due to a lack of marker genes that differentiate these cell types. Proliferation of intra-aortic hematopoietic cells has been described in mouse development; providing evidence that this aortic expansion event is conserved between species (Boisset et al. 2015). Inhibition of Wnt via overexpression of axin1, dkk1, or dntcf decreased proliferative cells within the aorta as measured by the incorporation of BrdU into dividing cells. Conversely, overexpression of wnt8 to stimulate the Wnt pathway resulted in an increase in proliferation (Goessling et al. 2009). Proliferative cells within the aorta have been shown to be positive for the HSC marker gata2b (Grainger and Richter et al. 2016). Upon morpholino-mediated knockdown of wnt9a, gata2b+ HSCs are arrested in the G1 phase of the cell cycle and failed to undergo intra-aortic proliferation. This phenotype is likely due to a reduction in expression of the cell cycle regulator and Wnt target gene myca (the zebrafish homolog of Myc) that occurs when the Wnt pathway is inhibited. The importance of myca is further evidenced by its ability to rescue the HSC defect in wnt9a morphants (Grainger and Richter et al. 2016). Interestingly, Goessling et al. (2009) reported that overexpression of negative regulators of Wnt signaling caused both an increase in apoptosis and a decrease in proliferation, while Grainger and Richter et al. 2016 did not observe an increase in apoptosis with the overexpression of dntcf, but saw a lack of proliferation consistent with previously published results. These studies provide strong evidence that Wnt cell fate signaling is required for proliferation of developing HSCs within the aorta.
Mouse hematopoietic development
The majority of the research on the role of Wnt signaling in mouse hematopoietic development has focused on the emergence and expansion of HSCs. There has been no direct evidence that Wnt signaling plays a role in mouse HSC specification, though Wnt has been implicated in the development of primitive blood and erythromyeloid progenitors in the mouse (Nostro et al. 2008, Frame et al. 2016). We will focus on the role of Wnt in the emergence and expansion of HSCs in the mouse embryo (Figure 1).
Mouse HSC emergence is regulated by Wnt signaling
Mouse HSCs emerge directly from aortic endothelium that undergoes an endothelial to hematopoietic transition within the aorta–gonad–mesonephros (AGM) region. Wnt pathway components such as Dishevelled, TCF, and β–catenin are expressed in the AGM around the time of HSC emergence (E10-E12), and nuclear β–catenin is restricted to distinct endothelial cells at the base of intra-aortic hematopoietic clusters, which hints at a possible role for Wnt during HSC emergence (Orelio and Dzierzak 2003, Ruiz-Herguido et al. 2012). This was further investigated using explant culture experiments where the AGM region was dissected from mouse embryos and cultured in vitro. Treating E10.5 AGM explants with a GSK3 inhibitor (SB216763) to activate the Wnt pathway increased HSC emergence as measured by a colony forming cell (CFC) assay and by hematopoietic reconstitution of irradiated recipients. Conversely, inhibiting Wnt with a small molecule that interferes with the β–catenin/TCF complex (PKF-115) caused a decrease in HSCs (Ruiz-Herguido et al. 2012). Tissue specific loss of Wnt using a conditionally inactivatable β–catenin allele in VE-Cadherin-positive endothelial cells caused a significant decrease in HSC emergence, as measured by a CFC assay. Interestingly, loss of Wnt after hematopoietic fate acquisition by inactivating β–catenin in Vav1+ hematopoietic cells had no affect on HSC function (Zhao et al. 2007, Ruiz-Herguido et al. 2012). Together, these data suggest that Wnt is required in endothelial cells during HSC emergence from the aorta, but is dispensable after HSCs have emerged and begun to express mature hematopoietic markers.
Wnt is required in the fetal liver for HSC function
After HSCs emerge from the aorta in the mouse embryo they migrate to the placenta and fetal liver, both niches that promote HSC proliferation. Wnt pathway components such as β–catenin and Wnt3a are expressed in the fetal liver at E12.5, a time period when HSC numbers expand (Orelio and Dzierzak 2003, Luis et al. 2010). Loss of Wnt3a by genetic knockout caused early lethality at E12.5 due to many severe developmental phenotypes, but analysis of hematopoiesis in the fetal liver was still possible. Wnt3a−/− embryos displayed a severe reduction in HSC numbers (as defined by the HSC signature LSK+(Lineage-, Sca1+, c-kit-) Flt3-) in the fetal liver, with the remaining HSCs exhibiting defects in self-renewal and poor long-term reconstitution capacity in wild-type hosts (Luis et al. 2009). This loss of Wnt3a was not compensated by any other Wnt genes expressed in the fetal liver, suggesting that Wnt3a is the primary Wnt regulating fetal liver HSC function (Luis et al. 2010). It is not clear whether the HSC defects seen in the fetal liver of Wnt3a−/− embryos are a result of earlier hematopoietic events gone wrong, such as decreased HSC emergence due to lack of Wnt signaling, or if these experiments represent yet another requirement for canonical Wnt during the developmental journey of an HSC. Ex vivo experiments in which fetal liver cells were co-cultured on the bone marrow stromal cell line OP9 showed that exposure to exogenous Wnt3a affects HSC differentiation into downstream lineages; high Wnt3a arrested T-cell development in vitro and in vivo, and increased differentiation into B-cells in vivo (Famili et al. 2015). Together, these data suggest that Wnt3a acting through the cell fate pathway is required in the fetal liver for proper HSC function, including self-renewal and differentiation into downstream blood lineages.
Mouse hematopoietic development in vitro
The importance of Wnt signaling in hematopoietic development has been investigated in mouse embryonic stem cells differentiating in vitro to hematopoietic lineages. Activation of the Wnt pathway with exogenous Wnt3a increased the number of hematopoietic cells either by CFC assay or by expression of hematopoietic markers by qPCR (Naito et al. 2006, Goessling et al. 2009). Inhibition of Wnt via the addition of DKK1 decreased hematopoietic output as measured by the expression of hemoglobin y (Hbb-y) (Rai et al. 2012). These results are consistent with the general positive correlation between Wnt signaling and hematopoietic development. However, this does not seem to be true for all Wnts: Wnt2−/− embryonic stem cells gave rise to an increased number of blast colony-forming cells (BL-CFCs), suggesting that some Wnts, like Wnt2, have a repressive affect on hematopoietic differentiation (Wang et al. 2007). This provides support for the theory that Wnts can have unique functions and may be required in a non-redundant manner for various hematopoietic processes, such as Wnt9a in the zebrafish hemogenic endothelium and Wnt3a in the mouse fetal liver.
Human hematopoietic development in vitro
Although derivation of HSCs capable of multilineage engraftment from human pluripotent stem cells (hPSCs) has not been achieved, significant insights have been made on the role of Wnt signaling during hematopoietic development in vitro, which largely mimics development in an organism: cells are first committed to the mesodermal lineage and are further specified towards a specialized type of hemogenic endothelium, which then gives rise to hematopoietic stem and progenitor cells (HSPCs (Figure 3)) (reviewed in Ditadi et al. 2017). The spatial compartmentalization of in vivo HSC development is nonexistent in this in vitro system, and differences among individual hPSC lines and between differentiation protocols confounds comparison of multiple studies, as timing of developmental stages may vary. However, the requirement for Wnt signaling in the in vitro differentiation system largely mirrors the requirements for Wnt in hematopoietic development in model organisms. [Figure 3]
Figure 3.
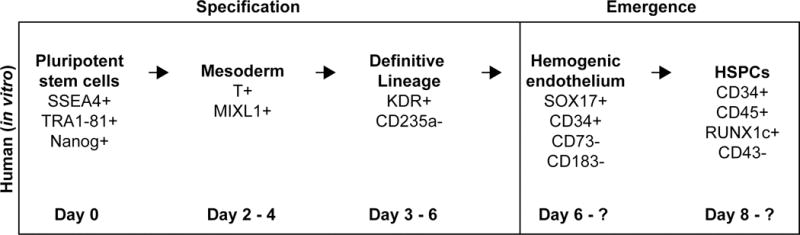
Hematopoietic development during in vitro differentiation. The development process is similar between human cells in vitro and cells in vivo; albeit with a lack of spatial separation in vitro. Hematopoietic stem and progenitor cells (HSPCs) are derived from the mesodermal lineage that is further specified to become cells that will contribute to definitive hematopoiesis. These cells are pushed towards an endothelial fate by a growth factor cocktail usually containing VEGF, and eventually become hemogenic endothelium. The emergence process yields HSPCs expressing various hematopoietic marker genes.
Multiple studies provide evidence that Wnt is required for specification of HSPCs in vitro. Stimulating the pathway using a GSK3β inhibitor (CHIR99021) early in the differentiation protocol promoted the specification of posterior mesoderm that gave rise to hemogenic endothelium (Kitajima et al. 2015). These results are consistent with previously described roles for Wnt during the specification of mesoderm in vivo (reviewed in Clements and Traver 2013). Wnt also is required for the specification of definitive hematopoiesis at the expense of primitive hematopoiesis as inhibition of Wnt secretion (with the Porcn inhibitor IWP-2) during a mid-early stage of differentiation abrogated T-cell differentiation potential of hematopoietic progenitors. Stimulating the pathway with a GSK3β inhibitor during the same time frame inhibited primitive hematopoiesis and enhanced definitive hematopoiesis, as measured by T-cell potential (Sturgeon et al. 2014). These findings are contradictory to others suggesting that Wnt is required for development of primitive blood in the mouse (Nostro et al. 2008). However, these distinctions are consistent with differences in the ways that mesoderm is patterned in the human and mouse embryo (reviewed in Ditadi et al. 2017). Activating the pathway via addition of Wnt3a or Wnt1 protein throughout the course of differentiation resulted in an increase in HSPCs, and the addition of DKK1 inhibited HSPC production (Woll et al. 2008, Wang and Nakayama 2009, Gertow et al. 2013). These results support the model that Wnt signaling is required for the development of HSPCs, mirroring the requirements identified in vivo.
Conclusions
HSCs are capable of giving rise to all cells of the blood. The ability to derive patient-specific HSCs in vitro is of great interest to the scientific and medical communities, as these cells have high therapeutic potential. However, it is still not possible to generate therapy-grade HSCs from pluripotent precursors. A better understanding of signals, including Wnt, that promote the differentiation to HSCs is critical in achieving this goal.
As documented in this review, Wnt signaling influences HSCs at multiple stages and in many systems, at times with varying conclusions as to the role that Wnt plays in the context of HSC biology (Table 1). Depending on the age of the animal or the system used to analyze HSCs, Wnt has been shown to promote the development, expansion, and maintenance of HSCs (reviewed in Lento et al. 2013). In other contexts, Wnt has been shown to inhibit self-renewal and eliminate the HSC pool (Kirstetter et al. 2006, Scheller et al. 2006). In the adult system, the dosage of Wnt dictates its effect on the maintenance of HSCs and differentiation into downstream lineages (Luis et al. 2011). [Table 1]
Table 1.
Summary of the effects of Wnt pathway perturbations on hematopoietic development.
| Gene (perturbed) | Wnt up/down-regulated? | Model System | Phenotype | Tissue | Time | Notes | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Human | Extracellular | Exogenous DKK1 | Down | Human (in vitro ESC differentiation) | Decrease in HSPCs | N/A | d0 - endpoint | Decrease in CD34/CD45+ cells, decrease in CFCs | Woll et al. Blood 2008 |
| Exogenous Wnt1 | Up | Human (in vitro ESC differentiation) | Increase in HSPCs | N/A | d0 - endpoint | Increase in CD34/CD45+ cells, decrease in CFCs | Woll et al. Blood 2008 | ||
| Exogenous WNT3A | Up | Human (in vitro ESC differentiation) | Increase in HSPCs | N/A | d0 - 4? | Increase in CFCs | Gertow et al. Stem Cell Reports 2013 | ||
| Exogenous Wnt3a | Up | Human (in vitro iPSC differentiation) | Increase in HSPCs | N/A | d0 - endpoint | Wnt3a protein; Increase in HSC markers, EM-CFC colonies in CFC assay | Wang and Nakayama, Stem Cell Research 2009 | ||
| Exogenous Wnt3a | Up | Human (in vitro ESC differentiation) | Increase in HSPCs | N/A | d0 | Increased HSPCs as measured by CFC assay | Wang and Nakayama, Stem Cell Research 2009 | ||
| Inhibition of Wnt secretion | Down | Human (in vitro ESC differentiation) | Decrease in HSPCs | N/A | d2-3 | IWP2; Decreased HSCs as measured by lack of T cell potential | Sturgeon et al. Nature Biotechnology 2014 | ||
| Cytoplasmic | GSK3B inhibition | Up | Human (in vitro) | Increase in HSPCs, lack of primitive blood | N/A | d2-3 | CHIR99021; Increased HSCs as measured by T cell potential | Sturgeon et al. Nature Biotechnology 2014 | |
| GSK3B inhibition | Up | Human (in vitro) | Increased HSPCs | N/A | d0-1 | CHIR99021; increased CFU-mix colonies, increased %CD34+/CD43- | Kitajima et al. Experimental Hematology 2016 | ||
| Mouse | Extracellular | Wnt2 | Down | Mouse (in vitro ESC differentiation) | Increased HSPCs | N/A | N/A | Wnt2 KO, increased BL-CFC colonies in EB differentiation | Wang et al. Journal of Biological Chemistry 2007 |
| Wnt3a | Down | Mouse (in vivo) | Decrease in LSK cells | Fetal liver | E12.5 | Wnt3a KO, Decreased long term reconstitution capacity - secondary recpients had poor engraftment; decreased self-renewal; reduction of myeloid progenitors | Luis et al. Blood 2009 | ||
| Exogenous Wnt3a | Up | Mouse (ex vivo) | Increase in EMPs | Yolk sac explants | N/A | CFC assay | Frame et al. Stem Cells 2016 | ||
| Increased Wnt3a (high, exogenous) | Up | Mouse (ex vivo) | Decrease in T cells, increase in B cells | Fetal liver on OP9 stroma | N/A | Coculture assay, and fetal liver LSK+ cells transplanted into adult mice | Famili et al. Cell Death and Disease 2015 | ||
| Increased Wnt3a (physiological) | Up | Mouse (ex vivo) | Acceleration of T cell development | Fetal liver on OP9 stroma | N/A | Coculture assay, and fetal liver LSK+ cells transplanted into adult mice | Famili et al. Cell Death and Disease 2015 | ||
| Exogenous Wnt3a | Up | Mouse (in vitro ESC differentiation) | Increase in HSPCs | N/A | Tx: d4 | CFC assay | Goessling et al. Cell 2009 | ||
| Exogenous Wnt3a | Up | Mouse (in vitro ESC differentiation) | Increase in HSPCs | N/A | d5-10 | Increase in blood markers by qPCR (CD31, CD45, VE-Cad, Bh1-globin) | Naito et al. PNAS 2006 | ||
| Exogenous Dkk1 | Down | Mouse (in vitro ESC differentiation) | Decrease in primitive blood | N/A | d4 - 6 | Decrease in primitive erythrocyte colonies | Nostro et al. Cell Stem Cell 2008 | ||
| Exogenous DKK1 | Down | Mouse (in vitro ESC differentiation) | Decrease in hematopoietic cells | Ubiquitous | d2-4 | Decrease in Hbb-y expression by qPCR | Rai et al. Stem Cells and Development 2012 | ||
| Cytoplasmic | GSK3B inhibition | Up | Mouse (AGM explants, E10.5) | Increase in HSCs | AGM | E10.5 | SB216763, CFC assay, hematopoietic reconstitution assay | Ruiz-Herguido et al. JEM 2012 | |
| B-catenin inhibition | Down | Mouse (AGM explants, E10.5) | Decrease in HSCs | AGM | E10.5 | PKF-115, CFC assay, hematopoietic reconstitution assay | Ruiz-Herguido et al. JEM 2012 | ||
| Stabilized B-catenin overexpression | Up | Mouse (in vitro ESC differentiation) | Increase in primitive blood | N/A | d4 - 6 | Increase in primitive erythrocyte colonies | Nostro et al. Cell Stem Cell 2008 | ||
| B-catenin | Down | Mouse (in vivo) | Decrease in EMPs | Cdh5:Cre; conditional B-cat | E9.5 - 10.5 | CFC assay, focus on yolk sac | Frame et al. Stem Cells 2016 | ||
| B-catenin | Down | Mouse (in vivo) | Decrease in HSCs | AGM (VECad-Cre) | E10.5-11.5 | CFC assay; mutants had hematopoietic and vascular defects | Ruiz-Herguido et al. JEM 2012 | ||
| B-catenin | Down | Mouse (in vivo) | No effect on HSCs | HSCs (Vav-Cre) | E12.5 onward | Adult analysis; Wnt is required in the endothelium | Ruiz-Herguido et al. JEM 2012 | ||
| B-catenin | Down | Mouse (in vivo) | No effect on HSC number | HSCs (Vav-Cre) | E12.5 onward | Decreased HSC function - poor reconstitution capacity | Zhao et al. Cancer Cell 2007 | ||
| Zebrafish | Extracellular | wnt8 overexpression | Up | Zebrafish (Tg) | Increase in HSCs | Ubiquitous | 10 somite stage | hsp:wnt8 (8a); increase in cmyb+ cells at 36hpf; Wnt regulated by PGE2 | Goessling et al. Cell 2009 |
| wnt9a | Down | Zebrafish (MO and Tg) | Decrease in HSC emergence, specification OK | Somite | pre-20 hpf | Decrease in an aortic expansion of HSCs | Grainger and Richter et al. Cell Reports 2016 | ||
| wnt16 | Down | Zebrafish (MO) | Lack of HSC specification | Somite | pre-15 hpf | Sets up Notch cue needed for specification | Clements et al., Nature 2011 | ||
| dkk1 overexpression | Down | Zebrafish (Tg) | Decrease in HSCs | Ubiquitous | 12 somite stage | hsp:dkk1; decrease in cmyb+ cells at 36 hpf; Wnt regulated by PGE2 | Goessling et al. Cell 2009 | ||
| Porcupine inhibition (no Wnt secretion) | Down | Zebrafish (drug) | Decrease in HSCs | Ubiquitous | 1ss - 40 hpf | IWP-2, decrease in flk1/cmyb+ cells | Grainger and Richter et al. Cell Reports 2016 | ||
| Cytoplasmic | axin1 overexpression | Down | Zebrafish (Tg) | Decrease in HSCs | Ubiquitous | 11 somite stage | hsp:axin1; decrease in cmyb+ cells at 36 hpf; Wnt regulated by PGE2 | Goessling et al. Cell 2009 | |
| increased Axin - Lrp6 interaction | Up | Zebrafish (drug) | Increased HSCs | Ubiquitous | 3ss - 36 hpf | HLY78, decreased cmyb+ cells | Wang et al. Nature Chemical Biology 2013 | ||
| Tankyrase inhibition | Down | Zebrafish (drug) | Decreased HSCs | Ubiquitous | 3ss - 36 hpf | XAV929, decreased cmyb+ cells | Wang et al. Nature Chemical Biology 2013 | ||
| Constitutively active B-catenin | Up | Zebrafish (Tg) | Increased HSCs | Endothelium | 10 - 40 hpf | Increase in cmyb+ cells | Grainger and Richter et al. Cell Reports 2016 | ||
| GSK3B inhibition | Up | Zebrafish (drug) | Increase in HSCs | Ubiquitous | 1ss - 40 hpf | LiCl, increased flk1/cmyb+ cells | Grainger and Richter et al. Cell Reports 2016 | ||
| Nuclear | dntcf overexpression | Down | Zebrafish (Tg) | Lack of primitive blood gata1 expression in PLM | Ubiquitous | 8h | Signals coordinately with BMP to turn on Cdx and Hox genes | Lengerke et al., Cell Stem Cell 2008 | |
| dntcf overexpression | Down | Zebrafish (Tg) | Increase in HSCs | Ubiquitous | 13 somite stage | hsp:dntcf; increase in cmyb+ cells at 36 hpf; Wnt regulated by PGE2 | Goessling et al. Cell 2009 | ||
| dntcf overexpression | Down | Zebrafish (Tg) | Decrease in HSC emergence, specification OK | Hemogenic endothelium (gata2b:Gal4) | pre-20 hpf | Decrease in an aortic expansion of HSCs | Grainger and Richter et al. Cell Reports 2016 | ||
Evidence from the mouse and zebrafish systems indicates that Wnt is required in the endothelium for HSCs to emerge from the aorta, but is dispensable after HSCs have already emerged (Zhao et al. 2007, Ruiz-Herguido et al. 2012, Grainger and Richter et al. 2016). Wnt may also be necessary for the embryonic expansion of HSCs (Luis et al. 2009, Grainger et al. 2016). Wnt has also been shown to promote the expansion of adult HSCs in vitro (Reya et al. 2003, Willert et al. 2003). Wnt is required for HSC specification in the human embryonic stem cell differentiation system, suggesting that this requirement for Wnt during HSC development is highly conserved amongst different organisms (Sturgeon et al. 2014).
Although there is clear evidence supporting a role for Wnt during HSC emergence and expansion, we do not yet have a clear understanding of the mechanism by which a Wnt signal acts on HSCs. In the zebrafish, Wnt acts as a proliferative cue for HSCs in the aorta by signaling through myca, a previously described Wnt target (Grainger and Richter et al. 2016). However, we do not know whether the downstream response to Wnt is similar in other systems, like in the mouse endothelium. It may also be important to understand the specific ligands that mediate the Wnt signal, as multiple studies have hinted that other Wnts cannot compensate for the loss of critical ligands, and various Wnts affect hematopoietic development in different ways (Wang et al. 2007, Luis et al. 2010, Grainger and Richter et al. 2016). This is likely due to a combination of receptor-ligand specificity and differences in spatio-temporal expression of Wnts and Frizzleds. Most differentiation protocols utilize small molecule activators or inhibitors of the Wnt pathway, many of which have off-target effects. Stimulation of the Wnt pathway using the specific molecules that direct HSC development in vivo may improve differentiation protocols to generate HSCs in vitro.
Acknowledgments
Funding
This work was supported in part by the NIH/NHLBI under Grant R01HL135205 and was made possible in part by the CIRM Major Facilities grant (FA1-00607) to the Sanford Consortium for Regenerative Medicine. JR was partially supported by the American Heart Association Predoctoral Fellowship 16PRE27340012.
Biographies
Jenna Richter received her BS degree from the University of Minnesota in 2012. She entered the Biomedical Sciences PhD program at UC San Diego in 2012, and joined the Willert and Traver laboratories where she is currently working towards her PhD thesis.
Karl Willert received his PhD from UC San Francisco where he studied the role of Wnt signalling in the laboratory of Harold Varmus. As a postdoctoral researcher at Stanford University with Roel Nusse, he was first to purify a biologically active Wnt protein and show that these signalling molecules are lipid-modified. He joined UC San Diego as Assistant Professor in 2008.
David Traver performed his graduate work in the laboratory of Irving Weissman at Stanford University where he developed mouse models of myeloid leukemia and identified myeloid-restricted progenitor subsets. He then did his postdoctoral research at Harvard University where he characterized the cellular biology of the zebrafish hematopoietic system. He started his own laboratory at UC San Diego in 2004.
Footnotes
Jenna Richter, ORCiD: 0000-0002-4486-9926
Disclosure statement
The authors declare no conflict of interest.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin–proteasome pathway. The EMBO Journal. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batta K, Menegatti S, Garcia-Alegria E, Florkowska M, Lacaud G, Kouskoff V. Concise Review: Recent Advances in the In Vitro Derivation of Blood Cell Populations. STEM CELLS Translational Medicine. 2016;5(10):1330–1337. doi: 10.5966/sctm.2016-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382(6588):225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Developmental Biology. 2011;355(2):275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Boisset JC, Clapes T, Klaus A, Papazian N, Onderwater J, Mommaas-Kienhuis M, Cupedo T, Robin C. Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood. 2015;125(3):465–469. doi: 10.1182/blood-2014-07-588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Developmental Biology. 2016;409(1):129–138. doi: 10.1016/j.ydbio.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395(6702):604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chemical Biology. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474(7350):220–224. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nature Reviews Immunology. 2013;13(5):336–348. doi: 10.1038/nri3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nature Structural & Molecular Biology. 2005;12(4):364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23(43):7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- Ditadi A, Sturgeon CM, Keller G. A view of human haematopoietic development from the Petri dish. Nature Reviews Molecular Cell Biology. 2017;18(1):56–67. doi: 10.1038/nrm.2016.127. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa SI, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Famili F, Naber BAE, Vloemans S, de Haas EFE, Tiemessen MM, Staal FJT. Discrete roles of canonical and non-canonical Wnt signaling in hematopoiesis and lymphopoiesis. Cell Death & Disease. 2015;6(11):e1981. doi: 10.1038/cddis.2015.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame JM, Fegan KH, Conway SJ, McGrath KE, Palis J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. STEM CELLS. 2016;34(2):431–444. doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthe JR, Clements WK. R-spondin-1 is required for specification of hematopoietic stem cells through Wnt16 and Vegfa signaling pathways. Development. 2017 doi: 10.1242/dev.139956. dev.139956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K, Hirst CE, Yu QC, Ng ES, Pereira LA, Davis RP, Stanley EG, Elefanty AG. WNT3A Promotes Hematopoietic or Mesenchymal Differentiation from hESCs Depending on the Time of Exposure. Stem Cell Reports. 2013;1(1):53–65. doi: 10.1016/j.stemcr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic Interaction of PGE2 and Wnt Signaling Regulates Developmental Specification of Stem Cells and Regeneration. Cell. 2009;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger S, Richter J, Palazón RE, Pouget C, Lonquich B, Wirth S, Grassme KS, Herzog W, Swift MR, Weinstein BM, Traver D, Willert K. Wnt9a Is Required for the Aortic Amplification of Nascent Hematopoietic Stem Cells. Cell Reports. 2016;17(6):1595–1606. doi: 10.1016/j.celrep.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells and Cloning : Advances and Applications. 2010;3:105–117. doi: 10.2147/SCCAA.S6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Salic A, Zylstra CR, Kirschner MW, Williams BO. A Novel Set of Wnt-Frizzled Fusion Proteins Identifies Receptor Components That Activate β-Catenin-dependent Signaling. Journal of Biological Chemistry. 2002;277(38):34727–34735. doi: 10.1074/jbc.M204989200. [DOI] [PubMed] [Google Scholar]
- Kim AD, Melick CH, Clements WK, Stachura DL, Distel M, Panáková D, MacRae C, Mork LA, Crump JG, Traver D. Discrete Notch signaling requirements in the specification of hematopoietic stem cells. The EMBO journal. 2014;33(20):2363–2373. doi: 10.15252/embj.201488784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SEW, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nature Immunology. 2006;7(10):1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kissa K, Murayama E, Zapata A, Cortés A, Perret E, Machu C, Herbomel P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111(3):1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Nakajima M, Kanokoda M, Kyba M, Dandapat A, Tolar J, Saito MK, Toyoda M, Umezawa A, Hara T. GSK3β inhibition activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells. Experimental Hematology. 2015 doi: 10.1016/j.exphem.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JBA, Zon LI, Daley GQ. BMP and Wnt Specify Hematopoietic Fate by Activation of the Cdx-Hox Pathway. Cell Stem Cell. 2008;2(1):72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt Signaling in Normal and Malignant Hematopoiesis. Cold Spring Harbor Perspectives in Biology. 2013;5(2):a008011. doi: 10.1101/cshperspect.a008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, van Amerongen R, Nusse R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Developmental Cell. 2016;38(6):643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Luis TC, Naber BAE, Fibbe WE, van Dongen JJM, Staal FJT. Wnt3a nonredundantly controls hematopoietic stem cell function and its deficiency results in complete absence of canonical Wnt signaling. Blood. 2010;116(3):496–497. doi: 10.1182/blood-2010-04-282624. [DOI] [PubMed] [Google Scholar]
- Luis TC, Naber BAE, Roozen PPC, Brugman MH, de Haas EFE, Ghazvini M, Fibbe WE, van Dongen JJM, Fodde R, Staal FJT. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell stem cell. 2011;9(4):345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Luis TC, Weerkamp F, Naber BAE, Baert MRM, de Haas EFE, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJM, Staal FJT. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113(3):546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138(6):1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Mizuochi C, Fraser ST, Biasch K, Horio Y, Kikushige Y, Tani K, Akashi K, Tavian M, Sugiyama D. Intra-Aortic Clusters Undergo Endothelial to Hematopoietic Phenotypic Transition during Early Embryogenesis. PLoS ONE. 2012;7(4):e35763. doi: 10.1371/journal.pone.0035763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing Hematopoietic Precursor Migration to Successive Hematopoietic Organs during Zebrafish Development. Immunity. 2006;25(6):963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proceedings of the National Academy of Sciences. 2006;103(52):19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, Activin, and BMP Signaling Regulate Distinct Stages in the Developmental Pathway from Embryonic Stem Cells to Blood. Cell Stem Cell. 2008;2(1):60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelio C, Dzierzak E. Identification of 2 novel genes developmentally regulated in the mouse aorta-gonad-mesonephros region. Blood. 2003;101(6):2246–2249. doi: 10.1182/blood-2002-07-2260. [DOI] [PubMed] [Google Scholar]
- Peters A, Burridge PW, Pryzhkova MV, Levine MA, Park TS, Roxbury C, Yuan X, Peault B, Zambidis ET. Challenges and strategies for generating therapeutic patient-specific hemangioblasts and hematopoietic stem cells from human pluripotent stem cells. The International Journal of Developmental Biology. 2010;54(6-7):965–990. doi: 10.1387/ijdb.093043ap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Walthall JM, Hu J, Hatzopoulos AK. Continuous antagonism by Dkk1 counter activates canonical Wnt signaling and promotes cardiomyocyte differentiation of embryonic stem cells. Stem Cells and Development. 2012;21(1):54–66. doi: 10.1089/scd.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Rowlinson JM, Gering M. Hey2 acts upstream of Notch in hematopoietic stem cell specification in zebrafish embryos. Blood. 2010;116(12):2046–2056. doi: 10.1182/blood-2009-11-252635. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herguido C, Guiu J, D’Altri T, Inglés-Esteve J, Dzierzak E, Espinosa L, Bigas A. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. The Journal of Experimental Medicine. 2012;209(8):1457–1468. doi: 10.1084/jem.20120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nature Immunology. 2006;7(10):1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122(25):4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nature Biotechnology. 2014;32(6):554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Developmental Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Vo LT, Daley GQ. De novo generation of HSCs from somatic and pluripotent stem cell sources. Blood. 2015;125(17):2641–2648. doi: 10.1182/blood-2014-10-570234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gilner JB, Bautch VL, Wang DZ, Wainwright BJ, Kirby SL, Patterson C. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. The Journal of Biological Chemistry. 2007;282(1):782–791. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- Wang S, Yin J, Chen D, Nie F, Song X, Fei C, Miao H, Jing C, Ma W, Wang L, Xie S, Li C, Zeng R, Pan W, Hao X, Li L. Small-molecule modulation of Wnt signaling via modulating the Axin-LRP5/6 interaction. Nature Chemical Biology. 2013;9(9):579–585. doi: 10.1038/nchembio.1309. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama N. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Research. 2009;3(2–3):113–125. doi: 10.1016/j.scr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, Moon RT, Kaufman DS. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111(1):122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Current biology: CB. 1996;6(10):1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of β-Catenin Impairs the Renewal of Normal and CML Stem Cells In Vivo. Cancer Cell. 2007;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


