Abstract
BACKGROUND:
High-intensity interval training (HIIT) has been shown to improve cardiometabolic health during supervised lab-based studies but adherence, enjoyment and health benefits of HIIT performed independently are yet to be understood. We compared adherence, enjoyment and cardiometabolic outcomes after 8-weeks of HIIT or moderate-intensity continuous training (MICT), matched for energy expenditure, in overweight and obese young adults.
METHODS:
17 adults were randomized to HIIT or MICT. After completing 12 sessions of supervised training over 3 weeks, participants were asked to independently perform HIIT or MICT for 30 minutes, 4 times/week for 5 weeks. Cardiometabolic outcomes included cardiorespiratory fitness (VO2peak), lipids, and inflammatory markers. Exercise enjoyment was measured by the validated Physical Activity Enjoyment Scale. RESULTS: Exercise adherence (93.4±3.1% vs 93.1±3.7%, respectively) and mean enjoyment across the intervention (100.1±4.3 vs 100.3±4.4, respectively) were high, with no differences between HIIT and MICT (p>0.05). Similarly, enjoyment levels did not change over time in either group (p>0.05). After training, HIIT exhibited a greater decrease in low-density lipoprotein cholesterol than MICT (−0.66 mmol·L−1 vs. −0.03 mmol·L−1, respectively) and a greater increase in VO2peak than MICT (p<0.05, +2.6 ml·kg.min−1 vs. +0.4 ml·kg.min−1, respectively). Interleukin-6 and C-reactive protein increased in HIIT (+0.5 pg·mL−1 and +31.4 nmol·L−1, respectively) and decreased in MICT (−0.6 pg·mL−1 and −6.7 nmol·L−1, respectively, p<0.05).
CONCLUSIONS:
Our novel findings suggest that HIIT is enjoyable and has high unsupervised adherence rates in overweight and obese adults. However, HIIT may be associated with an increase in inflammation with short-term exercise in this population.
Keywords: C-reactive protein, cardiorespiratory fitness, interleukin-6, cardiovascular disease, obesity, Physical activity, inflammation
Introduction
It is well established that physical activity is associated with decreased morbidity and mortality from cardiometabolic disease. Despite this evidence, only 20% of US adults meet current physical activity guidelines and 25% report no leisure-time physical activity (Centres for Disease Control and Prevention, 2014). One of the most common barriers to engaging in regular exercise is a lack of time (Burgess, Hassmen, & Pumpa, 2017; Trost, Owen, Bauman, Sallis, & Brown, 2002), with current research focusing on time-efficient strategies to promote exercise adherence and cardiometabolic health. Recently, high intensity interval training (HIIT) has gained popularity as a novel, time-efficient exercise strategy that has been shown to improve cardiometabolic disease risk factors in a variety of populations.
The majority of studies on HIIT have been supervised, laboratory-based interventions with only one mode of exercise (Elmer, Laird, Barberio, & Pascoe, 2015; Fex, Leduc-Gaudet, Filion, Karelis, & Aubertin-Leheudre, 2015; Fisher et al., 2015; Higgins, Baker, Evans, Adams, & Cobbold, 2015; Saanijoki et al., 2015), which limits the generalizability of the findings to free-living conditions. While these studies provide important information on the effects of HIIT on specific cardiometabolic outcomes in the short term, they do not address enjoyment of and adherence to HIIT under unsupervised conditions. Enjoyment is an important motive for engaging in and adhering to exercise (Aaltonen et al., 2012) yet there is a paucity of data on this topic related to HIIT. Most studies on enjoyment of HIIT have measured enjoyment after acute or short-term exercise in a laboratory environment, with conflicting findings (Bartlett et al., 2011; Jung, Bourne, & Little, 2014; Martinez, Kilpatrick, Salomon, Jung, & Little, 2015; Saanijoki et al., 2015). Research has yet to adequately evaluate HIIT as an exercise strategy that can be sustained independently, over a meaningful period of time. To our knowledge only one study to date has investigated the adherence to HIIT using multiple modes of exercise and during unsupervised conditions (Jung, Bourne, Beauchamp, Robinson, & Little, 2015). This intervention study was six weeks in duration, with two weeks of supervised conditions, in participants with prediabetes. Further studies are needed to determine adherence to longer term HIIT and enjoyment of this type of exercise in previously sedentary adults. The purpose of this study was to compare adherence to and enjoyment of unsupervised HIIT and moderate-intensity continuous training (MICT) in sedentary, overweight and obese young adults. A secondary purpose of this study was to compare cardiometabolic outcomes post intervention between HIIT and MICT.
Methods
Seventeen sedentary, overweight or obese adults aged 18–44 years (7 males, 10 females) participated in the study. Participants were recruited from a university and its surrounding community in the Pacific Northwest via flyers with a quick response code to the study website, word-of-mouth, university email advertisements and local newspaper advertisements. Interested participants were screened for inclusion and exclusion criteria via phone and participants were provided a gift card to the university bookstore after completing the study. Exclusion criteria included diagnosed cardiovascular, metabolic, or pulmonary disease; currently using antihypertensive or lipid-lowering medications; currently pregnant or breast feeding; irregular menstrual cycles; current smoker or smoked within past 6 months; or unable to perform exercise. The study protocols were submitted to and approved by the University’s Institutional Review Board for ethical testing of human subjects.
After consent, all participants completed baseline testing at week 0 and post testing at week 9 of the study. Testing at all time points was similar and described below. Participants were instructed to abstain from exercise for at least 12h, caffeine for at least 4h, and food for at least 2h, prior to each visit for testing. Participants were asked to fast for 12h prior to blood draws.
Following baseline testing (including fasting blood draw, anthropometrics, blood pressure, dietary recall, and cardiorespiratory fitness), participants were randomized to HIIT or MICT for 4 days per week for 8 weeks. The first 3 weeks of the 8-week intervention included one-on-one supervised training, specific to the group assignment, on 3 sessions per week. The fourth session was unsupervised and designed to increase participant autonomy. The last 5 weeks of the 8-week intervention was unsupervised.
The exercise modes included treadmill, cycle ergometer, and elliptical in a university gym-based setting where all participants had free access. Participants were instructed to use one mode for each exercise session and rotate modes such that all modes were used each week. For the fourth exercise session each week, participants self-selected their preferred exercise modality from the three they had been prescribed. Participants were also given the opportunity to jog or cycle outdoors on the days that the treadmill and cycle ergometer were prescribed, as long as heart rate was monitored and the prescribed intensities were achievable. Only one participant engaged in exercise outdoors during the study. The exercise prescription was progressive over the first 3 to 4 weeks of the intervention and groups were matched for energy expenditure.
Exercise training
Training intensities were derived from a VO2peak test at week 0 and 4, allowing for a progressive workload adjustment, and were prescribed as heart rate reserve (HRR), as recommended by the American College of Sports Medicine (ACSM, 2017). Participants randomized to MICT performed 20-min of continuous exercise at 55–59% HRR, with a 5-minute warm-up and cool down at 35–40% HRR for a 30-minute exercise session. Participants randomized to HIIT performed ten 1-minute bouts of high intensity exercise at 75–80% HRR, separated by ten 1-minute recovery bouts at 35–40% HRR. The high-intensity intervals were equivalent to 84 to 87% HR max in this sample, consistent with the intensities used in the HIIT literature. Warm-up and cool down periods were matched to MICT.
Participants wore a heart rate monitor (Polar FT7, Polar Electro Inc, New York, USA) during each exercise session to monitor exercise intensity. Participants also completed a log book for each training session, logging exercise heart rate data and session summary data (heart rate and energy expenditure) from the heart rate monitor. The heart rate monitors stored all exercise session data and were used to confirm exercise adherence, target heart rates and energy expenditure against the log books.
Anthropometric, blood pressure, and body composition measurements
Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively. Waist circumference was measured to the nearest 0.1 cm, in duplicate, at the level of the iliac crest. Overweight and obese were defined as body mass index of 25.0–29.9 and ≥30.0 kg·m−2, respectively (National Heart Lung and Blood Institute, 1998). After five minutes of seated rest two readings of blood pressure, within 5 mmHg, were averaged (Omron HEM-907XL; Kyoto, Japan). Body composition was estimated via air displacement plethysmography with measured thoracic gas volume (BOD POD®; COSMED, Rome, Italy) using standard procedures from the manufacturer.
Fasting blood samples
Fasting blood draws (12h fast) were performed at least 48h after the final exercise session for the determination of glucose, insulin, lipids, and inflammatory markers (C-reactive protein [CRP], interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor alpha (TNF-α), leptin, and adiponectin). Blood samples for glucose and lipids were processed in duplicate by a local CLIA certified laboratory. Glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, and triglycerides were measured using a Dimension RxL Max Integrated Chemistry System (Siemens; Erlangen, Germany). Glucose was measured using the hexokinase method with a minimum sensitivity of 0.0 mmol·L−1 and an intra-assay coefficient of variation (CV) of 0.6%. HDL cholesterol was assessed using the polyethylene glycol direct method with a minimum sensitivity of 0.3 mmol·L−1 and an intra-assay CV of 0.9%. LDL cholesterol was measured using the direct method with a minimum sensitivity of 0.13 mmol·L−1 and an intra-assay CV of 1.4%. Total cholesterol was measured via cholesterol oxidase, esterase, and peroxidase, and had a minimum sensitivity of 0.39 mmol·L−1 and an intra-assay CV of 1.1%. Triglycerides were measured using the enzymatic endpoint method and had a minimum sensitivity of 0.6 mmol·L−1 and an intra-assay CV of 1.2%.
Serum samples for insulin and inflammatory markers were sent to the University of Alabama Centre for Clinical and Translational Sciences Core Laboratory for analysis. Insulin was measured using an immunoenzymatic method (TOSOH AIA-600 II; TOSOH Bioscience, South San Francisco, CA, USA). This assay had a minimum sensitivity of 0.5 uU·mL−1 and intra-assay CV of 1.49%. Adiponectin and leptin were measured by radioimmunoassay (Millipore RIA kit, EMD Millipore, Billerica, MA) with a minimum sensitivity (90% bound) of 1.07 ug·mL−1 and 0.88 ng·mL−1, respectively and intra-assay CV of 6.79% and 5.70%, respectively. High sensitivity CRP was measured using an immunoturbidimetric assay (Pointe Scientific, Canton, MI, USA). This assay had a minimum sensitivity of 0.5 mg·L−1 and intra-assay CV of 7.49%. TNF-a, IL-6 and IL-8 were measured using 7-plex pro-inflammatory kits (Meso Scale Diagnostics, Rockville, MD) with a minimum sensitivity of 0.055 pg·mL−1, 0.137 pg·mL−1, and 0.06 pg·mL−1, respectively and intra-assay CV of 6.28%, 4.75%, and 1.97%, respectively.
Cardiorespiratory fitness measurements
VO2peak and maximal heart rate were measured using a continuous incremental exercise test to exhaustion on a treadmill and computerized metabolic system (TrueOne 2400; ParvoMedics, Salt Lake City, UT, USA). Resting expired gases were measured for 2 minutes followed by a 2 minute warm up performed at 2.5 to 3.5 mph, 0% grade. The treadmill speed was then increased to a comfortable but challenging walking or jogging pace (3–5 mph) for 2 minutes with the grade remaining at 0%. The treadmill grade was then increased by 1% each minute, with participants encouraged to continue until volitional fatigue. Standard criteria of respiratory exchange ratio greater than or equal to 1.10 and failure of heart rate to increase with increases in workload were used to confirm that a maximal effort was reached (ACSM, 2017). For all analyses of oxygen consumption, data were smoothed with a 15-breath moving average and the highest value obtained during the last minute of exercise was recorded (Robergs, Dwyer, & Astorino, 2010).
Exercise enjoyment
To assess exercise enjoyment, participants were asked to complete the 18-item Physical Activity Enjoyment Scale (Kendzierski & DeCarlo, 1991) during the first, fourth, and final week of exercise training. The questionnaires were completed immediately after the third exercise session of the week. This instrument consists of questions relating to enjoyment immediately after exercise with the instruction “Please rate how you feel at the moment about the physical activity you have been doing”. This 18-item survey is scored on a 7-point bipolar scale. Example items include “I enjoy it/I hate it”, “I find it energizing/I find it tiring”, “It gives me a strong sense of accomplishment/It does not give me any sense of accomplishment at all”. Scores range from 18–126 with higher scores indicating higher levels of enjoyment. Previous research has shown good internal consistency of this instrument (Cronbach’s alpha=0.96; Kendzierski & DeCarlo, 1991).
Dietary Intake
Daily intakes of energy and macronutrients were analysed using 24-h dietary recalls (2 week days and 1 weekend day) over a 1-week period at pre and post testing (Nutrition Data System for Research software, University of Minnesota, Minneapolis, MN; Version 2013). Participants were asked to maintain their current diet throughout the duration of the study.
Statistics
Sample size estimates were generated with an ANOVA repeated measures within-between interaction design using GPower version 3.1.9.2 (Universitat Kiel, Germany). Our primary outcome variable was enjoyment of exercise, with a minimum important difference of 18 between MICT (48±14) and HIIT (66±10; Kong et al., 2016) and a correlation of 0.6 between repeated measures (Kendzierski and DeCarlo, 1991). Based on data from Kong et al. (2016) we calculated the pooled variance (12.1), effect size f (0.75), Cohen’s d (1.5) and eta squared (0.36) to use for the sample size estimation. Using an ANOVA, repeated measures within-between interaction design, a sample size of 8 participants per group was required for an alpha of 0.05 and 95% power.
Data were analysed using SPSS Statistical Software (IBM SPSS Statistics for Windows 22.0, Armonk, NY, USA). The Shapiro–Wilk test was used to check the assumption of normality, and non-normally distributed variables were log transformed. Multivariate analysis of variance (ANOVA) was used to compare mean characteristics and exercise variables (e.g., enjoyment) between groups at baseline. Repeated measures ANOVA was used to evaluate changes in exercise enjoyment and dietary intake from pre to post intervention.
Analysis of covariance (ANCOVA) was used to test differences between the groups while controlling for baseline values (Rausch, Maxwell, & Kelley, 2003). This statistical approach tests whether groups are different on the post-test while controlling for pretest values and whether groups change differently from pre to post test. We used this approach because it is generally more powerful than a repeated-measures ANOVA (group main effect and time by group interaction) when interest lies in group differences in the change from pre-test to post-test within the context of a randomized pre-to-post design (Rausch et al 2003). Variables that were significantly associated with the outcome variable were used as covariates. Covariates for lipids and inflammatory markers included baseline value, age, and body fat. Covariates for VO2peak and blood pressure included baseline value and sex. An alpha value of p<0.05 was accepted as the minimal level of significance.
Results
Ten participants were randomized into MICT (6 men and 4 women) and 9 were randomized into HIIT (2 men and 7 women) exercise groups. Seventeen participants completed the 8-week intervention (Figure 1). There were no adverse events during testing or training in MICT or HIIT. Baseline characteristics by training group are shown in Tables 1 and 2. All participants were overweight or obese, with an average body mass index of 31.6±5.0 kg·m2 (range 25.6–43.5 kg·m2) and average body fat percentage of 35.2±6.8% (range 19.1–43.6%).
Figure 1.
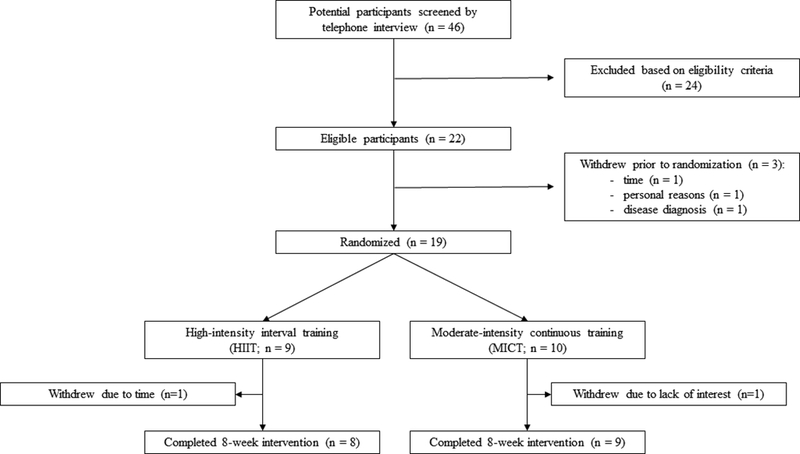
Flow of participants through the intervention.
Table 1.
Participant characteristics (mean±SD), p>0.05 for all.
| Variable | MICT (n=9) | HIIT (n=8) | ALL (N=17) |
|---|---|---|---|
| Age (years) | 28.9±8.1 | 23.1±6.6 | 26.2±7.8 |
| Sex (female [%]) | 4 [44%] | 6 [75%] | 10 [59%] |
| Body mass (kg) | 99.5±7.1 | 86.6±4.1 | 93.4±18.2 |
| Body mass index (kg·m−2) | 33.1±6.0 | 29.9±3.3 | 31.6±5.0 |
| Fat (%) | 35.3±7.2 | 35.2±6.8 | 35.2±6.8 |
| Fat mass (kg) | 35.7±13.1 | 30.4±9.0 | 33.2±11.3 |
| Fat free mass (kg) | 63.6±11.0 | 54.8±6.5 | 59.5±10.0 |
| Exercise Enjoyment (end of week 1) | 97.6±10.7 | 99.9±15.7 | 98.7±12.9 |
| Exercise adherence (%) | 93.1±10.6 | 93.4±8.3 | 93.2±9.3 |
| Average daily exercise energy expenditure (kcals) |
295.0±67.5 | 284.3±30.9 | 290.0±52.2 |
| Average weekly exercise energy expenditure (kcals) |
1086.1±242.1 | 1048.3±150.5 | 1068.3±199.0 |
p>0.05 for all
Table 2.
Cardiometabolic and fitness outcomes in MICT and HIIT at baseline and post-intervention (mean±SE).
| MICT (n=9) | HIIT (n=8) | ||||
|---|---|---|---|---|---|
| Variable | Baseline | Post- intervention† (95% CI) |
Baseline | Post- intervention† (95% CI) |
Effect size (power) |
| VO2peak (mL/kg·min−1) a | 34.5±2.1 | 34.9±0.8 (33.3, 36.6) |
34.8±2.9 | 37.4±0.8* (35.7, 39.2) |
0.27 (52%) |
| SBP (mmHg) a | 117.0±3.9 | 116.2±2.2 (111.4, 121.0) |
114.0±1.7 | 118.3±2.3 (113.2, 123.3) |
0.03 (9%) |
| DBP (mmHg) a | 74.0±3.5 | 71.5±2.0 (67.3, 75.7) |
72.0±2.9 | 71.1±2.1 (66.6, 75.5) |
0.01 (5%) |
| Waist (cm) b | 108.1±4.9 | 106.1±1.4 (103.1, 109.0) |
101.3±3.2 | 104.5±1.5 (101.2, 107.6) |
0.04 (10%) |
| Glucose (mmol·L−1) b | 5.2±0.1 | 5.1±0.1 (5.0, 5.3) |
4.9±0.2 | 5.3±0.1 (5.1, 5.5) |
0.13 (23%) |
| Insulin (μU·mL−1) b | 21.5±5.0 | 16.6±3.0 (10.4, 22.8) |
12.5±3.1 | 17.1±3.0 (10.5, 23.7) |
0.01 (5%) |
| Lipids | |||||
| HDL (mmol·L−1) b | 1.0±0.1 | 1.2±0.1 (1.1, 1.3) |
1.4±0.1* | 1.1±0.1 (1.0, 1.2) |
0.05 (11%) |
| LDL (mmol·L−1) b | 2.9±0.3 | 2.9±0.1 (2.7, 3.2) |
3.0±0.4 | 2.4±0.1* (2.1, 2.6) |
0.41 (76%) |
| Triglycerides (mmol·L−1) b | 1.1±0.1 | 1.2±0.2 (0.8, 1.6) |
1.0±0.2 | 1.1±0.2 (0.7, 1.5) |
0.01 (6%) |
| Total chol (mmol·L−1) b | 4.2±0.3 | 4.1±0.2 (3.7, 4.5) |
4.5±0.4 | 3.8±0.2 (3.4, 4.2) |
0.09 (17%) |
| Inflammatory Markers | |||||
| CRP (nmol·L−1) b | 21.9±5.7 | 14.9±5.4 (2.9, 26.7) |
14.3±3.8 | 37.6±5.4* (25.7, 49.5) |
0.41 (71%) |
| IL-6 (pg·mL−1) b | 1.0±0.3 | 0.4±0.2 (<0.01, 0.7) |
0.5±0.1 | 1.0±0.2* (0.6, 1.3) |
0.30 (55%) |
| IL-8 (pg·mL−1) b | 8.2±1.0 | 11.7±0.9 (9.8, 13.6) |
10.7±1.0 | 8.6±1.0 (6.5, 10.6) |
0.28 (51%) |
| TNFα (pg·mL−1) b | 2.0±0.1 | 2.1±0.1 (2.0, 2.3) |
2.1±0.2 | 2.1±0.1 (1.9, 2.2) |
0.01 (7%) |
| Leptin (ng·mL−1) b | 26.7±4.2 | 27.8±1.7 (24.1, 31.4) |
26.3±4.5 | 30.3±1.8 (26.3, 34.9) |
0.07 (14%) |
| Adiponectin (μg·mL−1) b | 6.7±1.5 | 6.4±0.3 (5.7, 7.1) |
7.9±1.3 | 6.9±0.3 (6.2, 7.7) |
0.07 (14%) |
CI, confidence interval; VO2peak, peak oxygen consumption; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; Total chol, total cholesterol; CRP, C-reactive protein; IL, interleukin; TNFα, tumour necrosis factor alpha
marginal means from ANCOVA
p≤0.05 compared to MICT
Covariates included baseline value and sex
Covariates included baseline value, age, and body fat mass
Macronutrient and energy intake (p>0.05) were similar across the 8-week study, indicating diet did not change with adoption of the exercise program. Additionally, there were no changes in body composition, body weight, or body mass index across the 8-week study (p>0.05).
Exercise adherence to the 8-week program was similar between MICT and HIIT (p>0.05, Table 1). Both exercise deliveries were enjoyable with enjoyment scores ranging from 74 to 125 and no differences in enjoyment between groups or over time (p>0.05, Figure 2).
Figure 2.
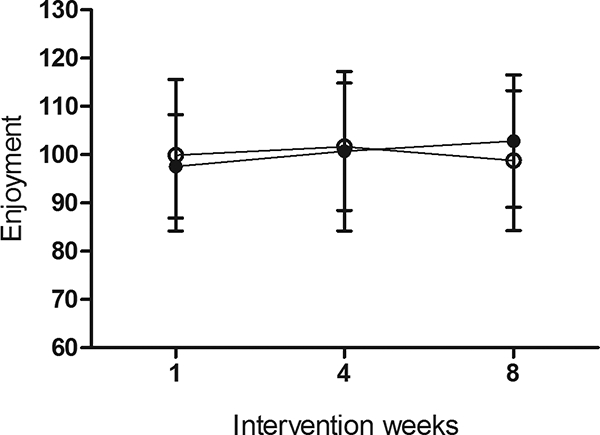
Physical activity enjoyment scale during the intervention (mean±SD). Closed circles MICT, open circles HIIT, p>0.05.
Average daily exercise energy expenditure was similar between MICT and HIIT groups (295.0±67.5 vs. 284.3±30.9 kcals, respectively) indicating both groups were able to perform the prescribed exercise protocol and maintain heart rate targets when exercising on their own.
Table 2 displays cardiometabolic baseline and post-intervention values for MICT and HIIT groups. There was a significant difference in LDL cholesterol post-intervention between groups with HIIT exhibiting a significantly greater decrease from pre to post intervention than MICT, when controlling for baseline values, age and body fat (p<0.05). There were no group differences in HDL cholesterol, triglycerides, total cholesterol, glucose, insulin, blood pressure, or waist circumference following the 8-week study. There was a significant difference in VO2peak post-intervention between groups with HIIT showing a significantly greater increase in relative VO2peak than MICT (p=0.04), when controlling for covariates. Results were similar when absolute VO2peak was used in the model.
Mean values of IL-6 and CRP were significantly different at post intervention between groups when controlling for baseline values, age, and body fat (p<0.05, Table 2). Over the 8-week intervention, levels of IL-6 and CRP decreased in MICT and increased in HIIT (p<0.05). There were no group differences in IL-8, TNF-α, leptin, or adiponectin following the 8-week intervention.
Discussion
Our novel findings provide preliminary evidence that adherence to and enjoyment of HIIT, carried out in a free-living environment, in overweight and obese adults is high and similar to that of MICT matched for time and energy expenditure. These findings suggest that overweight and obese participants can adhere to a HIIT program independently and that enjoyment of exercise was high throughout the intervention. Our results also suggest that 8 weeks of HIIT improves low-density lipoprotein and VO2peak levels in previously sedentary overweight and obese adults when compared to MICT, despite a similar energy expenditure. Further, these positive health changes were evident despite the exercise program not meeting current guidelines for physical activity and no change in body mass or body fat percentage.
A unique finding in the study was that adherence to and enjoyment of HIIT was similar to that of MICT. Enjoyment is important for long term adherence to exercise (Aaltonen et al., 2012). Yet few studies investigate enjoyment of HIIT after an intervention lasting more than 2 weeks (Foster et al., 2015). Moreover, there is debate over the value and practicality of HIIT relative to MICT and whether adherence to HIIT would be high enough to promote positive health outcomes and be used as a public health strategy (Biddle & Batterham, 2015). We show that enjoyment was high and stayed high throughout the 8-week intervention. This is in contrast to Foster et al. (2015) who showed enjoyment of HIIT decreased over 8 weeks in untrained college-aged subjects. The study by Foster et al. (2015) employed intervals that were at a higher intensity and shorter duration than the current study and participants performed exercise on a cycle ergometer in a laboratory, which may have contributed to the decreased enjoyment over the course of the study. Our study had participants exercise using 3 modes of exercise in a gym setting or outside, which may have increased enjoyment levels. Our adherence levels of 93.4% and 93.1% for HIIT and MICT, respectively was similar to that reported from laboratory-based studies (Keating et al., 2014).
Recent studies have reported mixed results regarding the effects of HIIT on lipid levels. Elmer et al. (2015) reported that 8 weeks of laboratory-based HIIT, similar to the protocol in the current study but in men only, showed a significant decrease in triglycerides in HIIT when compared to moderate-intensity endurance training. Fisher et al. (2015) reported that 6 weeks of laboratory based HIIT significantly reduced total cholesterol and triglycerides in overweight and obese young men but there was no difference between HIIT and moderate-intensity exercise training. Others have shown little impact of HIIT on lipid levels (Ciolac et al., 2010; Higgins et al., 2014; Kessler, Sisson, & Short, 2012). Our results extend these findings by demonstrating greater improvements in LDL cholesterol following unsupervised HIIT in overweight and obese men and women when compared to MICT of similar energy expenditure. Our participants maintained the same diet throughout the 8-week intervention and did not lose body mass or fat mass, suggesting that the changes were due to the exercise program. We did not see significant changes in other lipids with either exercise program, which is consistent with previous studies.
Numerous studies have established a strong association between low cardiorespiratory fitness and cardiovascular disease morbidity and mortality. We found that an 8-week HIIT program significantly increased VO2peak by 7.5% in young overweight and obese adults, but this change was not evident in the MICT program despite a similar energy expenditure. This improvement in VO2peak may be clinically significant, translating to about a 14% reduction in cardiovascular disease mortality (Lee et al., 2011). Our findings are consistent with the majority of the literature showing improvements in VO2peak in the ranges of 7–24% after 4–16 weeks of HIIT in a variety of healthy and at-risk populations. A limitation in the literature includes the inability to directly compare the results of HIIT interventions because the interventions are vastly different. HIIT protocols can vary in interval intensity, length, duration, and number of intervals as well as the ratio of intervals to recovery periods and type of recovery. Moreover, some of these protocols may not be achievable or sustainable in an overweight or obese population, particularly the supramaximal protocols. Our study adds to the literature by demonstrating this change in cardiorespiratory fitness in overweight and obese adults engaging in the intervention under free-living conditions.
Our results suggest that HIIT and MICT may elicit different effects on markers of inflammation in overweight and obese adults. Levels of CRP and IL-6 increased in HIIT and decreased in MICT. The majority of studies on exercise and inflammation have investigated the effects of moderate-intensity aerobic training with equivocal results. Some studies show decreases in CRP and IL-6 with 6 to 12 months of aerobic training in healthy and diseased samples, whereas others show no change in inflammation with training (Beavers, Brinkley, & Nicklas, 2010). To our knowledge, only four studies have examined the effects of HIIT on inflammation with conflicting findings (Elmer et al., 2015; Gerosa-Neto et al., 2016; Keating et al., 2014; Robinson, Durrer, Simtchouk, Jung, Bourne, Voth, & Little 2015). Three of the four studies report no changes in CRP, IL-6, IL-8, or TNF-α, whereas Gerosa-Neto et al. (2016) report favourable changes in IL-6 and unfavourable changes in TNF-α and adiponectin with HIIT. It is important to note that although IL-6 levels were increased in HIIT in the present study, IL-6 can be anti-inflammatory by acting to stimulate production of anti-inflammatory cytokines IL-1 receptor antagonist and IL-10, and supressing TNF-α through IL-6 dependent and independent pathways (Pederson and Pederson, 2005). No changes in adiponectin levels were observed in the present study, supporting the findings of previous research (Arikawa, Thomas, Schmitz, & Kurzer, 2011; Marcell, McAuley, Traustadottir, & Reaven, 2005). A review on exercise and adiponectin levels indicated that moderate-to-vigorous exercise lasting at least 90 minutes may be necessary to increase adiponectin levels in adults (Simpson & Singh, 2008). Our findings should be confirmed in larger samples to determine whether HIIT may be more likely to produce an inflammatory response than MICT in certain samples, particularly obese individuals, or whether longer term exercise (i.e., greater than 8 weeks) is needed to show decreases in inflammation.
This study has several strengths including investigating enjoyment of exercise under free-living conditions, using accurate and reliable measures of cardiorespiratory fitness and body composition, having participants maintain a similar diet throughout the study, matching energy expenditure between groups, and employing a randomized study design that included unsupervised exercise in a free-living environment. Limitations of the study included lack of a no-exercise control group and determination of the minimum important difference for enjoyment of exercise with the Physical Activity Enjoyment Scale. Currently there is no consensus in the literature on the minimum important difference in enjoyment of exercise, which may have implications for sample size calculations. Thus, estimated sample size for this study was based on group differences reported in the literature (Kong et al., 2016).
In summary, our findings suggest that HIIT is enjoyable and has high unsupervised adherence rates in a free-living environment in overweight and obese adults. Our results also suggest that 8 weeks of HIIT improves LDL cholesterol and VO2peak levels in previously sedentary overweight and obese adults when compared to MICT, despite a similar energy expenditure. However, HIIT may be associated with an increase in inflammation with short-term exercise in this population.
Acknowledgments
This research was funded by CTR-IN NIH NIGMS #1U54GM104944–01A1. This publication was made possible by an Institutional Development Award from NIH NIGMS under grant P20GM103408.
Footnotes
Disclosure of Interests
The authors declare that they have no conflicts of interest.
All procedures performed were in accordance with the ethical standards of the institutional ethics review board and conformed to the policies established by the U.S. Department of Health, Education, and Welfare and the American Physiological Society. The study had prior approval by the University ethics review board and all participants provided informed consent.
References
- Aaltonen S, Leskinen T, Morris T, Alen M, Kaprio J, Liukkonen J, & Kujala U (2012). Motives for and barriers to physical activity in twin pairs discordant for leisure time physical activity for 30 years. International Journal of Sports Medicine, 33, 157–163. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine (2017). ACSM’s guidelines for exercise testing and prescription (10th ed.). Philadelphia, PA: Wolters Kluwer. [Google Scholar]
- Arikawa AY, Thomas W, Schmitz KH, & Kurzer MS (2011). Sixteen weeks of exercise reduces C-reactive protein levels in young women. Medicine and Science in Sports and Exercise, 43, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, & Morton JP (2011). High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. Journal of Sports Science, 29, 547–553. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Brinkley TE, & Nicklas BJ (2010). Effect of exercise training on chronic inflammation. Clinica Chimica Acta, 411, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle SJH, & Batterham AM (2015). High-intensity interval exercise training for public health: A big HIT or shall we HIT it on the head? International Journal of Behavioral Nutrition and Physical Activity, 12(1), 95 10.1186/s12966-015-0254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess E, Hassmen P, Pumpa KL (2017). Determinants of adherence to lifestyle intervention in adults with obesity: a systemic review. Clinical Obesity, March 5 e-pub ahead of print Doi: 10.1111/cob.12183. [DOI] [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention. (2014). State Indicator Report on Physical Activity, 2014. Atlanta, GA: U.S. Department of Health and Human Services. [Google Scholar]
- Ciolac EG, Bocchi EA, Bortolotto LA, Carvalho VO, Greve JMD, & Guimaraes GV (2010). Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertension Research, 33, 836–843. [DOI] [PubMed] [Google Scholar]
- Elmer DJ, Laird RH, Barberio MD, & Pascoe DD (2015). Inflammatory, lipid, and body composition responses to interval training or moderate aerobic training. European Journal of Applied Physiology, 16, 601–609. 10.1007/s00421-015-3308-4 [DOI] [PubMed] [Google Scholar]
- Fex A, Leduc-Gaudet JP, Filion ME, Karelis AD, & Aubertin-Leheudre M (2015). Effect of elliptical high intensity interval training on metabolic risk factor in pre- and type 2 diabetes patients: A pilot study. Journal of Physical Activity and Health, 12, 942–946. [DOI] [PubMed] [Google Scholar]
- Fisher G, Brown AW, Bohan Brown MMB, Alcorn A, Noles C, Winwood L, … Allison DB (2015). High intensity interval versus moderate intensity training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. Plos One, 10(10), 1–15. 10.1371/journal.pone.0138853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C, Farland CV, Guidotti F, Harbin M, Roberts B, Schuette J, … Porcari JP (2015). The effects of high intensity interval training versus steady state training on aerobic and anaerobic capacity. Journal of Sports Science and Medicine, 14, 747–755. [PMC free article] [PubMed] [Google Scholar]
- Gerosa-Neto J, Antunes BMM, Campos EZ, Rodrigues J, Ferrari GD, Rosa Neto J…Lira FS (2016). Impact of long-term high-intensity interval and moderate-intensity continuous training on subclinical inflammation in overweight and obese adults. Journal of Exercise Rehabilitation, 12, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins TP, Baker MD, Evans SA, Adams RA, & Cobbold C (2015). Heterogeneous responses of personalized high intensity interval training on type 2 diabetes mellitus and cardiovascular disease risk in young healthy adults. Clinical Hemorheology and Microcirculation, 59, 365–377. 10.3233/CH-141857 [DOI] [PubMed] [Google Scholar]
- Jung ME, Bourne JE, & Little JP (2014). Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate and continuous vigorous intensity exercise in the exercise intensity affect continuum. Plos One, 9(12), e114541 10.1371/journal.pone.0114541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Bourne JE, Beauchamp MR, Robinson E, & Little JP (2015). High-intensity interval training as an efficacious alternative to moderate-intensity continuous training for adults with prediabetes. Journal of Diabetes Research, 10.1155/2015/191595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SE, Machan EA, O’Connor HT, Gerofi JA, Sainsbury A, Caterson ID, & Johnson NA (2014). Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. Journal of Obesity, 2014, 834865 10.1155/2014/834865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzierski D, & DeCarlo KJ (1991). Physical activity enjoyment scale: Two validation studies. Journal of Sport and Exercise Psychology, 13(1), 50–64. [Google Scholar]
- Kessler HS, Sisson SB, & Short KR (2012). The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Medicine, 42, 489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kong Z, Fan X, Shengyan S, Song L, Shi Q, & Nie J (2016). Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: A randomized controlled trial. PLOS One, 11(7), e0158589 10.1371/journal.pone.0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Xui X, Artero EG, Lee IM, Church TS, McAuley PA, … Blair SN (2011). Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men. Circulation, 124, 2483–2490. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcell TJ, McAuley KA, Traustadottir T, & Reaven PD (2005) Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism, 54, 533–541. [DOI] [PubMed] [Google Scholar]
- Martinez N, Kilpatrick MW, Salomon K, Jung ME, & Little JP (2015). Affective enjoyment responses to high intensity interval training in overweight to obese and insufficiently active adults. Journal of Sport and Exercise Psychology, 37, 138–149. doi: 10.1123/jsep.2014-0212. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute, Obesity education initiative expert panel on the identification, evaluation, and treatment of obesity in adults (1998). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Heart Lung and Blood Institute. Report, 98–4083. [Google Scholar]
- Petersen AMW & Pedersen BK (2005). The anti-inflammatory effect of exercise. Journal of Applied Physiology, 98,1154–1162. [DOI] [PubMed] [Google Scholar]
- Rausch JR, Maxwell SE, & Kelley K (2003). Analytic methods for questions pertaining to a randomized pretest, posttest, follow-up design. Journal of Clinical Child and Adolescent Psychology, 32, 467–486. doi: 10.1207/S15374424JCCP3203_15. [DOI] [PubMed] [Google Scholar]
- Ridker PM (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107, 363–369. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Dwyer D, & Astorino T (2010). Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Medicine, 40, 95–111. doi: 10.2165/11319670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Robinson E, Durrer C, Simtchouk S Jung ME, Bourne JE, Voth E, & Little JP (2015). Short-term high-intensity interval and moderate intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. Journal of Applied Physiology, 119, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saanijoki T, Nummenmaa L, Eskelinen JJ, Savolainen AM, Vahlberg T, Kalliokoski KK, & Hannukainen JC (2015). Affective responses to repeated sessions of high intensity interval training. Medicine and Science in Sports and Exercise, 47, 2604–2611. doi: 10.1249/MSS.0000000000000721. [DOI] [PubMed] [Google Scholar]
- Simpson KA, & Singh MA (2008). Effects of exercise on adiponectin: A systematic review. Obesity (Silver Spring), 16, 241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- Thum JS, Parsons G, Whittle T, Astorino TA (2017). High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLOS One, doi: 10.1371/journal.pone.0166299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, & Brown W (2002). Correlates of adults’ participation in physical activity: Review and update. Medicine and Science in Sports and Exercise, 34, 1996–2001. doi: 10.1249/01.MSS.0000038974.76900.92. [DOI] [PubMed] [Google Scholar]


