Abstract
We employ Monte Carlo simulation and sensitivity analysis techniques to describe the population dynamics of pesticide exposure to a honey bee colony using the VarroaPop+Pesticide model. Simulations are performed of hive population trajectories with and without pesticide exposure to determine the effects of weather, queen strength, foraging activity, colony resources, and Varroa populations on colony growth and survival. The daily resolution of the model allows us to conditionally identify sensitivity metrics. Simulations indicate queen strength and forager lifespan are consistent, critical inputs for colony dynamics in both the control and exposed conditions. Adult contact toxicity, application rate and nectar load become critical parameters for colony dynamics within exposed simulations. Daily sensitivity analysis also reveals that the relative importance of these parameters fluctuates throughout the simulation period according to the status of other inputs.
Keywords: Pollinator, Honey bee, Sensitivity analysis, Colony population model, Pesticides
Graphical abstract
1. Introduction
Insect pollinator species richness and diversity has been in decline for a half century (Vanbergen, 2013). Honey bee colonies have increased globally but have shown significant decline in Europe and North America (Spleen et al., 2013; Steinhauer et al., 2014; Lee et al., 2015). Honey bees and wild pollinators are important for increased crop yields through higher quality harvests (Garibaldi et al., 2013) and have been estimated worldwide to support nearly 10% of agricultural production (Gallai et al., 2009). Declines in honey bee populations could potentially lead to unstable yields over time of pollinator-dependent crops (Sinnathamby et al., 2013). Pollination services have been valued at over $29 billion/year for U.S. agriculture with over half the contribution by honey bees (Calderone, 2012). Multiple stressors have been identified that threaten honey bee health, these include parasites and pests, pathogens, poor nutrition, and pesticide exposure (Goulson et al., 2015; Pettis and Delaplane, 2010). Insecticide effects on honey bee colonies can be through direct mortality, but sublethal exposures leading to adverse outcomes at the hive level also occur (Johnson, 2015). Generally, from a pesticide regulatory perspective, short-term experiments are used to derive ecological exposure levels that are protective of direct mortality (Steeger et al., 2015). However, sublethal effects can have a lower threshold for significant colony level effects and regulatory processes are adapting to address these effects for pollinators (EPA, 2012). Regulatory agencies assess potential risks to honey bees from pesticides through a tiered process that includes individual-based effects data, colony-based assessments under controlled test conditions and less controlled, but more environmentally-relevant conditions where bees forage freely. Model implementation is also tiered. Since extrapolating short-term exposures to colony-level effects over multiple seasons is problematic (Becher et al., 2013), simulation models that estimate stage-based exposure and subsequent effects on colony population dynamics have been identified as an important component of pollinator risk management (Becher et al., 2013). There is a need for a more detailed colony model to inform the design and interpretation of higher-tier studies, interpret the relevance of sublethal and lethal effects and estimate the effect of pesticides in conjunction with other known honey bee stressors (e.g., Varroa mites).
Detailed honey bee simulations for pesticides must consider the structure of the colony, which includes different life stages and castes (i.e., eggs, larvae, pupae, drones, workers, foragers, queen), as well as environmentally relevant magnitudes of exposure through direct or indirect routes (stage-specific pollen, nectar, honey, bee bread, and royal jelly consumption). Pesticide exposures include non-target exposure from agricultural use (Johnson et al., 2010) but also intentional use to extirpate hive pests (e.g. miticides). Such a simulation model therefore makes predictions for exposure concentrations and effects for all these combinations and must be compared to available data for these same combinations for verification and validation purposes. There have been a number of studies of managed bee colonies that have demonstrated a range of pesticide residues detected in bees and hive matrices (e.g., honey, pollen, wax). Chauzat et al. (2011) found that within honey bee colony matrices, pollen loads and beeswax had the highest frequency of occurrence of multiple pesticides used either directly within a hive or for agricultural uses. Unintentional exposure to agriculturally applied pesticides was high with a detection rate of >40%. Honey bees can be lethally impacted by exposure to pesticides but are most likely exposed to concentrations lower than lethal limits. At sublethal levels, pesticide exposure has been associated with changes in individual bee behavior such as reduced foraging efficiency and decreases in colony queen production (Henry et al., 2012; Schneider et al., 2012; Whitehorn et al., 2012; DeGrandi-Hoffman et al., 2013). Overall, the most frequently detected pesticides and the two that occur in the highest quantity are those used by beekeepers to control Varroa mites (coumaphos and fluvalinate) (Mullin et al., 2010).
When submitted pesticides fail screening assessment, more realistic and taxa-specific lines of evidence can be requested and evaluated before making a final registration decision, for instance additional empirical exposure and effects data. Requirements for a higher-tier honey bee colony model were identified in Fischer and Moriarty (2014) with a goal of addressing questions that cannot be answered with individual-level tests, semi-field and field studies. In addition, Sponsler and Johnson (2016) identify key components of exposure modeling that are often lacking in population and colony-level models: environmental heterogeneity and in-hive pesticide distribution. The ability to model all possible exposure pathways, from foraging dynamics to intra-colony interactions, is another important requirement. Fischer and Moriarty (2014) included a formal evaluation of existing candidate models that assessed the risks to honey bees from pesticides. None of the existing honey bee models were determined to be currently suitable for regulatory usage because of a variety of issues that included lack of linkage between foragers and surrounding landscape, insufficient testing with empirical data, lack of sensitivity analysis to understand controlling factors, non-incorporation of multiple stressors and insufficient documentation for some of the models. For the USEPA, an existing USDA model is being evaluated (USEPA, 2012, 2014) to simulate honey bee colony dynamics and provide an additional line of evidence for the pesticide evaluation process. VarroaPop is a population model that predicts the population growth and behavior of a honey bee (Apis mellifera) colony infested by Varroamites (Varroa destructor). The model was developed as an extension of a honey bee population model, BEEPOP, that was created by DeGrandi-Hoffman et al. (1989) to simulate colony dynamics. The modified version of BEEPOP can then be used to translate miteeffects on individuals and predict outcomes at the colony level and parameterized for specific environments (Purucker et al., 2007). VarroaPop uses weather conditions, mite population dynamics, and age-structured honey bee colony input parameters to calculate honey bee and mite population growth.
We updated the existing VarroaPop model to predict population growth and behavior by leveraging existing cohort development dynamics. Existing features included daily tracking of colony population size and demographics in which weather conditions, mite population dynamics, and age-structured honey bee colony input parameters informed output. This version of VarroaPop+Pesticide (v3.2.6.11) introduces pesticide treatments to model simulations, in which individuals can be exposed to the active ingredient by physical contact (i.e., foraging) or ingestion. The model can be used to evaluate risks to honey bee colony survival from pesticide exposure at different times of year and with different weather and colony conditions. The complexities of Varroa parasitism and its effects on worker longevity and colony growth also can be included in simulations with pesticide-induced sublethal and lethal effects at each life stage. It also provides a platform for estimating the sublethal effects of pesticide exposure through future enhancements to the model.
We implement Monte Carlo simulations in order to create spatial heterogeneity and to vary in-hive pesticide distribution in exposure scenarios. We use sensitivity analyses to identify parameters that are the most influential (contributing most to output variability) as part of the continuing development of the model. This helps highlight important parameters which may require additional research, allow for the calibration of sensitive parameters to realistically simulate collected data, determine parameters which are less important in order to avoid overparameterization and to assess the relative importance of subroutines that model elements of hive population dynamics. Sensitivity analysis has been a useful tool which has furthered understanding of other honey bee colony models (Schmickl and Crailsheim, 2007; Becher et al., 2014; Torres et al., 2015). We use the modified version of Varroapop, Varroapop+Pesticide, to evaluate temporally a baseline scenario without pesticide exposure and three exposure scenarios representing different application types: foliar application, seed treatment and soil application. The sensitivity analyses are employed at different temporal scales within each application type to conditionally identify important parameters.
2. Methods
2.1. VarroaPop model
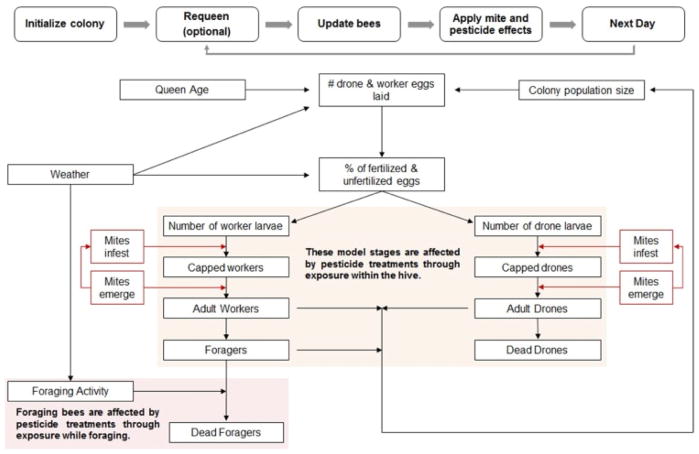
VarroaPop was developed as an extension of the BEEPOP colony population dynamicsmodel to determine the effects of Varroa mite parasitism on honey bee colony growth and survival. VarroaPop couples mite population growth from reproduction and immigration with colony growth based on queen egg laying rates and worker longevity (DeGrandi-Hoffman et al., 1989; DeGrandi-Hoffman and Curry, 2004; DeGrandi-Hoffman et al., 2016). Varroa mites affect colony dynamics by reducing the longevity of adult workers parasitized during development. An overview of the model routine is shown in Fig. 1. The flowchart at the top of the schematic diagram (Fig. 1) represents the overall daily model algorithm.
Fig. 1.
Main VarroaPop model routine. Primary components are estimated daily within the model code as a function of external driving variables and fluctuating conditions within the hive.
2.1.1. Queen fecundity and hive dynamics
The complete descriptions of BEEPOP and VarroaPop are available from DeGrandi-Hoffman et al. (1989), DeGrandi-Hoffman and Curry (2004) and DeGrandi-Hoffman et al. (2016). Briefly, colony growth was predicted based on the number of eggs laid per day. Egg laying was determined by the maximum number of eggs the queen can lay per day (a function of queen strength), maximum and minimum temperatures as expressed in heat units, photoperiod, and size of the adult worker population. Queen strength is initially parameterized as a continuous number between 1 and 5 which is used to linearly interpolate between values for the maximum daily number of eggs (1000–3000) and the initial sperm count (1.8–5.5 million). As a queen ages, the number of daily eggs laid by the queen declines as a quadratic function of the number of days the queen has been laying eggs. The proportion of eggs that develop into workers (i.e., fertilized eggs) was determined as a function of photoperiod, colony size, and the amount of sperm in the queen’s spermatheca. Workers are categorized as house bees (workers < 21 days old unless specified otherwise) and foragers (workers > 21 days old). Workers do not perform specific tasks in the simulations. Foragers return nectar and pollen to the colony. As the queen ages, the concentration of sperm in the spermatheca is reduced and the probabilities of producing unfertilized eggs that develop into drones increases. Even if the queen has sufficient amounts of sperm to fertilize the eggs she lays, colonies produce drones primarily in the late spring and summer. The proportion of unfertilized eggs produced by queens with sufficient sperm was estimated as a function of colony size and photoperiod. Requeening within VarroaPop can be enabled with “automatic” or “scheduled” settings. Automatic requeening can be triggered once the proportion of drone eggs fell below a specified proportion during the foraging season (April–September), however, we scheduled requeening to occur annually starting on 05/01/1989. In both requeening scenarios, an egg laying delay may be specified for the new queen. The number of eggs (fertilized and unfertilized) laid each day was treated as a cohort that were aged to emerged adult workers or drones. Adult workers remain in the hive for 21 days and then forage for a specified number of days. Foraging bees collect nectar and pollen that is either consumed or stored if more is collected in a day than required to feed the colony. Precocious foraging was simulated by reducing the number of days that worker bees remain in the hive after emergence. Resources are assumed to be unlimited thus simulating a managed colony where beekeepers feed sugar syrup and protein substitute during resource shortages. The total number of adult workers in all cohorts was reported as the colony population size and used to estimate egg laying.
2.1.2. Varroa mite infestation
External parasites are a common stressor in managed honey bee colonies. Adult Varroa destructor mites suck the hemolymph from adults and brood, transmitting diseases and viruses that negatively impact the chances of colony survival (Nazzi and Conte, 2016). Varroa populations are initialized in colonies by defining a percentage of adult workers and sealed brood cells with mites. Mites on adult workers infest worker and drone brood cells of appropriate age and reproduce. Mites that enter brood cells to reproduce are called ‘mother mites’. The proportion of infested worker and drone cells where mites successfully reproduce is an input. Literature values for the number of mated daughter mites produced in each worker or drone brood cell are used as default values. The daughter mites emerge from the brood cell with the adult bee. The daughter mites and a percentage of the mother mites can then infest and reproduce in brood cells, thus increasing the mite population. Infestation rates are predicted with a functional response equation based on the ratio of mites to brood cells. Colonies with high numbers of brood cells have higher brood cell infestation rates. If mite populations exceed the number of brood cells, the brood cells may be multiplicatively infested with mites. Mite reproduction per mother mite is reduced when cells are multiplicatively infested. Adult bees that were parasitized by mites during development have reduced longevity.
2.1.3. Accounting for pesticide exposures and effects to individual honey bees
Exposure routes for bees differ based on application type. In the model, bees foraging in a field treated with a pesticide through foliar spray could potentially be exposed to the pesticide through direct spray (contact exposure) as well through consuming contaminated food (oral exposure). Direct spray onto bees (contact exposure) is not expected for honey bees foraging in fields treated with a pesticide, through direct application to soil (e.g., drip irrigation), or through seed treatments. For these application methods, pesticide exposure through consumption of residues in nectar and pollen are expected to be the dominant routes. Foraging honey bees may also be exposed to pesticides via contact with dust from seed treatments or via consumption of water from surface water, puddles, dew droplet formation on leaves and guttation fluid; however, those routes will be implemented in a future version of the model. The exposure and effect algorithms and parameters used in this model are defined in DeGrandi-Hoffman et al. 2016 and Appendix ATable A1.
Estimating contact exposure to individual bees was assessed for forager bees following foliar spray applications (Koch and Weisser, 1997). Contact exposure was considered on the day of pesticide application. For in-hive bees, it was assumed that contact exposure was negligible relative to the oral exposure they would receive from consuming contaminated nectar and pollen collected from treated fields. For soil applications and seed treatments, contact exposure is not assessed in the version of VarroaPop used for this sensitivity analysis.
Estimating dietary exposure to individual bees was calculated by multiplying food intake rates by pesticide concentrations in pollen, nectar and jelly. Methods for estimating pesticide concentrations in pollen and nectar differ by application method, with different algorithms for foliar spray, soil applications and seed treatments (USEPA, 2014). Pesticide concentrations in royal and worker jelly are a factor of 100 lower than concentrations in pollen, based on the least conservative ratio of concentrations of a pesticide in nurse bee food when processed into royal jelly (USEPA, 2012).
Honey bee (Apis mellifera L.) adult workers (females), as well as drones (males), fulfill their nutritional requirements through consumption of a combination of nectar, honey, pollen and bee bread (stored pollen combined with nectar or honey). Food consumption rates are expressed as nectar and pollen equivalents for bees that consume honey and bee bread. Larval and adult queens require royal jelly, which is composed primarily of glandular secretions produced by nurse bees (Haydak, 1970); worker larvae and drones receive royal jelly during the first 3 days of development, and then receive brood food (jelly) for the remainder of their larval development period (Babendreier et al., 2004; Simpson, 1955). Over the course of the adult life stage, an adult worker bee requires different portions of nectar, honey and bee bread in order to meet the demands of the different tasks she must fulfill. For example, young bees that produce royal and brood jellies (i.e., nurse bees) require the most pollen (compared to other bees engaged in different tasks) because their glandular secretions provide protein and lipids to larvae and to the queen (Winston, 1987). Older forager bees require more nectar (or honey) to meet the energy demands of flying (Rortais et al., 2005), and generally do not consume much pollen (Crailsheim et al., 1992).
2.1.4. Pesticide exposure scenarios
Four different sets of Monte Carlo simulations were run in order to estimate the effects of pesticides on honey bee colony dynamics. The exposure scenarios included foliar spray, soil application and seed treatment. In addition, a set of control scenario simulations were implemented to serve as a baseline with which to contrast the application scenarios. All non-pesticide based life history and environmental variables were shared across the four sets of simulation inputs. The model has the ability to use empirical concentration data for pollen, nectar and bees. However, for the purposes of the sensitivity analysis, these components were calculated with algorithms based on the application methods being assessed.
Pesticide concentrations for foliar sprays on blooming plants (i.e., pollen and nectar contamination) was estimated by considering initial residue concentrations at the time of application (Fletcher et al., 2004) and the effect of the dissipation of the pesticide, as defined by a dissipation half-life (Willis and McDowell, 1987). For this scenario, required inputs that utilize input distributions include foliar dissipation half-life (in days), application rate (in lb a.i./A), number of applications and the application interval (in days). The initial residue value was multiplied by the application rate of the pesticide to derive starting pesticide concentrations in pollen and nectar. In modeling exposures over multiple days after application, the half-life accounts for the dissipation of pesticide residues.
Pesticide concentrations in pollen and nectar of crops growing in treated soil was estimated from a model published by Briggs et al. (1982, 1983) and modified by Ryan et al. (1988). The model depends upon the octanol-water and organic carbon partitioning of a chemical as well as basic soil properties. Required inputs include application rate, partitioning coefficients and the aerobic soil metabolism half-life. Soil properties include the fraction of organic carbon in soil, bulk density and soil water content. Since these properties were simulated from probability distributions, a correlation was induced between octanol-water and organic carbon partitioning to more accurately reflect their joint distributions. Pesticide concentration in soil at each daily time step can be estimated by dividing the application rate by the soil depth and incorporating loss processes. Degradation of the pesticide in soil was implemented with a chemical-specific aerobic soil metabolism half-life. The approach assumes no additional losses of the pesticide from soil via leaching, runoff or volatilization. A transpiration stream concentration factor is then calculated using the above inputs to estimate the overall movement with which a pesticide moves from the soil/root system and translocates to the aboveground portion of the plant, including pollen and nectar.
For seed treatments, the estimated pesticide concentration in pollen and nectar of treated crops is 1 μg a.i./g. This is based on EPPO’s (European Plant Protection Organization) screening value (EPPO, 2010). Since seed concentration is treated as a constant regardless of the application rate, this is an insensitive parameter within the sensitivity analysis.
2.1.5. Method for translating pesticide toxicity data into mortality of bees
To calculate the percent mortality to adults following field pollen, nectar and contact exposure as well as larvae to in-hive dietary exposure, the model uses three separate dose-response relationships that are based on toxicity inputs. The percent reduction due to mortality is applied to the proportion of groups of individuals that do not have a tolerance threshold greater than the calculated dose. Tolerance thresholds are pre-determined upon initializing the model or when births occur by dividing each cohort into equal numbers of bees and assigning each a set tolerance threshold. Note that the percent mortality predicted for different cohorts are different depending upon the age of exposure. For example, only foraging bees experience contact exposures. Also, hive bees and foraging bees receive different dietary exposures of pesticides. Therefore, toxicity parameters include adult oral LD50 and slope; adult contact LD50 and slope; and larval oral LD50 and slope.
The proportion of honey bees (p-hat) that do not survive a given exposure concentration was calculated using probit analysis, a specialized type of regression for binomial outcomes that accounts for the sigmoidal nature of the survival curve. In this case, for each combination of pesticide exposure and honey bee age class, the outcome is the survival of the honey bee. The algorithm translates environmental compartment exposure concentrations, dose and toxicity to estimate the percent mortality for each cohort. For larvae, dietary exposure concentrations were recalculated for each day of the simulation and used with larval toxicity parameters to assess mortality to each larval cohort. For all adults, dietary-based exposure concentrations were used in combination with the adult oral toxicity to estimate mortality to adult cohorts. Note that in-hive bees have different dietary exposures than forager bees due to differences in exposure concentrations and feeding rates (that can be attributed to different energetic requirements of their respective tasks). For forager bees, estimated exposures due to contact from foliar applications of pesticides were also used in conjunction with the contact-based toxicity parameters.
2.2. Programming for Monte Carlo simulations
VarroaPop was written in C++ using Microsoft Foundation Classes (MFC) as the interface to the Microsoft Windows environment. In the Doc/View architecture model in MFC, the document contains all the relevant data and model inputs and a set of views that express the output of the model in the user interface. In VarroaPop, the document is represented externally with a *.vrp file that contains all the input data accessed by the VarroaPop + Pesticide algorithms. The simulation was constructed around the model of a colony that experiences daily environmental updates. The model can be executed interactively where a user inputs information and graphical and tabular output is presented at the end of each simulation run or it can be executed non-interactively where the inputs are supplied with command line parameters and outputs are written to computer-readable text files.
In addition, the model can be executed via the command line with inputs passed in as text files. R (R Development Core Team, 2016) wrapper scripts were developed to leverage this feature and access the VarroaPop C++ binary and implement the Monte Carlo analyses for different exposure scenarios. The scripts are structured in a sequential way to setup a local execution environment, create the input distributions for the different exposure scenarios, write an input file for each Monte Carlo simulation and then execute all the Monte Carlo simulations in a parallel processing manner by passing the input files to the VarroaPop executable as an argument. The VarroaPop output files are written to disk as an external representation of the R objects for the inputs and outputs of each simulation. These objects can then be read back from these files as two-dimensional data frames for the sensitivity analysis post-processing in R.
2.2.1. Implementation of Monte Carlo simulations
Monte Carlo simulations were performed to assess how bee colony dynamics change with different methods of exposure with the VarroaPop + Pesticide model. The model presents 3 types of pesticide application methods: foliar spray, seed, and soil. In total, 20k simulations were run at 5000 runs per pesticide application method (i.e. foliar, soil, seed) and a “control” set in which pesticides were not applied. All four sets of 5000 simulations were run with identical colony input parameters in order to isolate the impact of pesticide exposure on colony dynamics. Inputs for each run were selected from random uniform distributions. The range limits of each distribution were defined according to previous studies, observational data, or expert opinion.
Table 1 displays parameter information for the input distributions used in the Monte Carlo simulations. Non-informative uniform distributions were used to capture a wide realistic range of parameter inputs that represent a wide range of possible colony settings and applied pesticides. Professional judgement was used to set the limits for the ranges since literature references only exist for specific settings. All simulations were run for a duration of three years starting on January 1, 1988 to December 31, 1990. Exposure simulations were run with a single pesticide application date on July 1, 1989 during the growing season with exposures occurring in the subsequent month. Weather data from Fresno, CA was used from January 1, 1988 to December 31, 1990. The weather file includes daily values for variables such as precipitation (cm/day), pan evaporation (cm/day), mean temperature (C), wind speed (cm/s) and solar radiation (Langleys/day) that are used as inputs for plant phenology and bee foraging behavior algorithms. All sets of simulations were initialized with 10,000 adult worker bees.
Table 1.
Input parameter distributions for Monte Carlo Simulations.
| Parameter | Units | Distribution Range | ||
|---|---|---|---|---|
| Bee Colony Base Parameters | ||||
| Queen Strength | Uniform (1–5) | |||
| Forager Lifespan | days | Uniform (4–16) | ||
| Requeening | Requeen Strength | Uniform (1–5) | ||
| Worker to Drone | Uniform (1–5) | |||
| Foraging Dynamics | ||||
| Adult Foragers | Pollen Load | mg/bee | Uniform (0–10) | |
| Nectar Load | mg/bee | Uniform (0–10) | ||
| Pollen Trips | Per day | Uniform (5–15) | ||
| Nectar Trips | Per day | Uniform (5–15) | ||
| Pesticide Toxicity Parameters | ||||
| Adult | Slope | p(s)/ug a.i./bee | Uniform (1–9) | |
| LD50 | ug a.i./bee | Uniform (0.001–100) | ||
| Slope Contact | p(s)/ug a.i./bee | Uniform (1–10) | ||
| LD50 Contact | ug a.i./bee | Uniform (0.001–100) | ||
| Larval | Slope | p(s)/ug a.i./bee | Uniform (1–9) | |
| LD50 | ug a.i./bee | Uniform (0.001–100) | ||
| Kow | unitless | Uniform (0.1–100,000) | ||
| Koc | unitless | Uniform (1–100,000) | ||
| Contact Dose Factor | ug/mL/bee | Uniform (0.26–2.4) | ||
| Foliar Treatment | Half-life | days | Uniform (0.1–35) | |
| Application Rate | lb/A | Uniform (0.001–10) | ||
| Soil Treatment | Soil P | g/cm3 | Uniform (1–2) | |
| Soil Foc | unitless | Uniform (0.001–0.02) | ||
| Soil Concentration | ppm | 1.0 | ||
| Seed Treatment | Seed Concentration | ppm | Uniform (1–10) | |
| Pollen and Nectar Consumption | ||||
| Life Stage | Caste (task in hive) | Average age (in days) | Daily intake rate (IR; mg/day) | |
| Nectarb | Pollen | |||
| Larval | Larval Worker | 4 | Uniform (0–100) | Uniform (0–4) |
| 5 | Uniform (0–150) | Uniform (0–6) | ||
| Larval Drone | 5 | Uniform (0–150)e | Uniform (0–6)e | |
| Adult | Worker (cell cleaning and capping) | 1–3 | Uniform (20–100)d | Uniform (2.2–8.2)c |
| Worker (brood and queen tending, nurse bees) | 4–10 | Uniform (20–200)d | Uniform (2.2–9.5)c | |
| Worker (comb building, cleaning and food handling) | 11–20 | Uniform (20–100)d | Uniform (0–3)c | |
| Drone | >10 | Uniform (133–337) | 0a | |
| Forager | >18 | Uniform (170–500) | 0a | |
Negligible amount consumed.
Consumption of honey was converted to nectar-equivalents using sugar contents of honey and nectar.
Pollen consumption rates for drone larvae are unknown. Pollen consumption rates for worker larvae are used as a surrogate.
2.3. Sensitivity analysis
Sensitivity analyses are used to identify parameters which are the most influential (contributing most to output variability) as part of the model calibration process, to highlight parameters which require additional research for strengthening the knowledge base, to determine parameters which are insignificant and can be eliminated from the final model to avoid overparameterization (Hamby, 1994; Iman and Helton, 1988; Saltelli et al., 2004, Saltelli et al., 2008). Sensitivity results are also used to assess the relative importance of subroutines that model elements of hive population dynamics.
Multiple techniques can be used to analyze the performance of a model. The partial correlation coefficient (PCC) function of the R package “sensitivity” (Pujol et al., 2015) was used as a primary sensitivity metric for analyzing model output. The PCC function is a bivariate correlation function based on the methods of Saltelli et al. (2000). This approach assumes a linear relationship between the input and output and provides measures of the relative contributions of various input parameters to the observed variations in output. This method of linear sensitivity analyses, PCC, can provide an effective measure of importance of model inputs. The Partial Correlation Coefficient (PCC) is a measure of interaction between two input variables when removed from any interdependencies or correlations of other variables in a linear system. PCC values range from −1 to +1, with positive and negative values indicating positive and negative correlations, respectively. The Partial Correlation Coefficient is given by Eq. (1):
| (1) |
where rABC is the PCC between variables A and B with variable C held constant.
Monte Carlo analyses were performed with the VarroaPop model to assess how bee colony dynamics change with exposure to pesticides. Inputs were parameterized with uniform distributions for relevant input variables in VarroaPop. Sensitivity analysis was performed by querying 5000 simulation results of a specific input and output variable within the 3D array for three pesticide-treated and one non-treated runs. A three year simulation period was used with a pesticide application in the middle of the second year to allow for burn-in and evaluation of sensitivity both before and after exposure/application. Simulations that included pesticide treatments were run with a single pesticide application date with a 30-day exposure period. All other colony input parameters were the same as in the non-treated simulations.
3. Results
3.1. Simulation time series and colony survival
Control simulations provide a statistical baseline with which to compare the partial correlation coefficients and model results of the exposed (i.e. soil, foliar, seed) simulations. We utilize partial correlation coefficients (PCC) for sensitivity analysis, addressed in Section 3.2, to assess model performance of VarroaPop for controlled versus three exposed application scenarios. Controlled and exposed sets of simulations were run with identical base parameters which includes the requeening ability and associated parameters (i.e. requeen strength). Colony size is a function of worker longevity, overwintering success, and spring time flight weather.
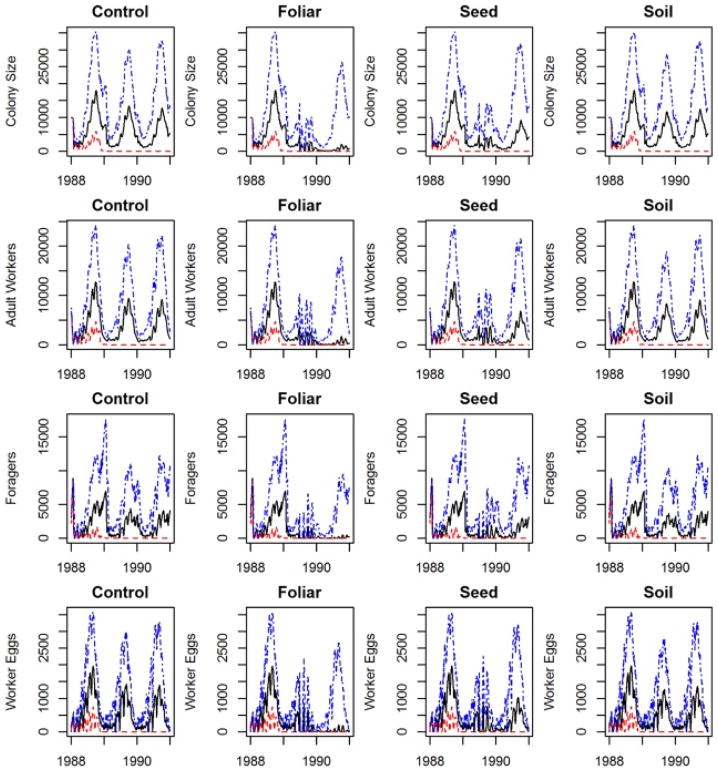
Simulation results of the controlled (i.e. no pesticide application) scenario exhibited rebounding colony survival during the foraging season (April–September) with die-offs occurring during the winter over the simulated time period (Fig. 2). Overwintering mortality leads to a sharp decrease in colony size, after which colony size continues to decline into the foraging season for 25% of the simulations across the controlled and exposed scenarios. Controlled and exposed simulations share identical colony input parameters, and thus colony size patterns, and diverge at the point of pesticide application. The lower quartile of colonies in the controlled and exposed scenarios continue to decline without response to the pesticide application, which is indicative of how parameters associated with intra-colony bee dynamics (i.e. queen strength and forager lifespan) may be more influential on colony size than pesticide parameters. The upper quartile of colonies in the exposed simulations display steeper rates of decline in colony size following the pesticide exposure compared to the controlled colonies.
Fig. 2.
Time series plots of the first (red), second (black), and third (blue) quartiles of colony size, adult workers, foragers, and worker eggs. Control vs. pesticide-applied simulations exhibit identical trends until the pesticide application on July 1, 1989. After which, the impact of the applied pesticide through direct and indirect exposure is evident by changes in bee colony dynamics. Both exposed sets exhibit suppressed recovery in which the pattern of recovery depends on the method of pesticide application.
The pattern of recovery of the foliar simulations displays a steady, prolonged decline in colony survival, whereas the soil simulations display rebounding colony dynamics following the exposure date (Fig. 2). Both methods of exposure (i.e. foliar and soil) struggle to recover, and the pattern of recovery depends on the method of pesticide application. Differences in these patterns are indicative of how each pesticide application method impacts colonies through alternate routes of exposure which translates to varying colony dynamics. Sensitivity analysis results allow us to determine specific critical parameters that influence colony dynamics once pesticides are applied to the model. Further time-conditional analysis reveals that the relative importance of these parameters fluctuates throughout the simulation period. These results are discussed in depth with sensitivity analysis in Section 3.2.
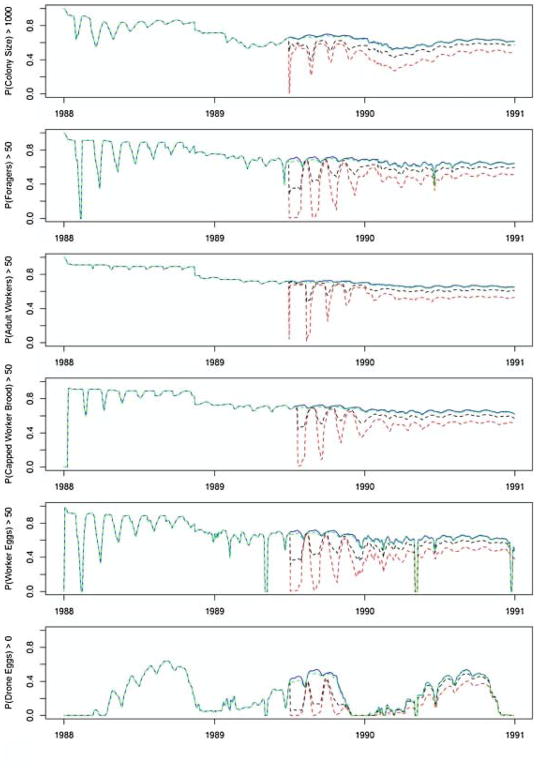
The output profile of the Monte Carlo simulations by proportion of surviving output variables (i.e. colony size, foragers, adult workers, and capped worker brood) shows the response behavior of colonies in different application scenarios (Fig. 3). Fig. 3 displays the proportion of colonies that rebound after a pesticide application and the pattern of recovery for the remainder of the simulation period. Foliar and soil application methods have the strongest impact on colony dynamics. Over half of the simulated bee colonies of the soil condition fail to recover within a week of exposure. Bee colonies with soil pesticide applications die off at a steady, linear rate. The foliar spray application condition has a substantial, but less severe impact on bee colonies than the soil condition when compared to the control scenario. Initially, almost 90% of the colonies rebound after the foliar application scenario. However, the impact of the foliar spray application intensifies within six months following the application date and simulated colonies experience rebounding recovery until equilibrium is reached after the month-long exposure period. Simulated honey bee colonies exposed to the seed condition maintain a steady rate of colony persistence, similar to the control scenario, throughout the exposure period and into the third year. The variation in response to pesticide exposure methods is attributed to different routes of exposure to the colonies. This is evident by breaking down the sensitivity analysis by day which is discussed in Section 3.2.
Fig. 3.
Proportion of control (blue) vs. exposed (foliar-red, seed-black, soil-green) simulations in which Capped Worker Brood, Adult Workers, Foragers, or Colony Size are greater than 50, 50, 50 or 5000, respectively. Foliar and soil application simulations exhibit steep declines in persisting adult workers, foragers, and colony size in response to pesticide application. This figure shows that foliar and seed pesticide conditions struggle to reach equilibrium in the aftermath of pesticide application. The soil application condition (black) exhibits a minor impact on colony dynamics following the pesticide application.
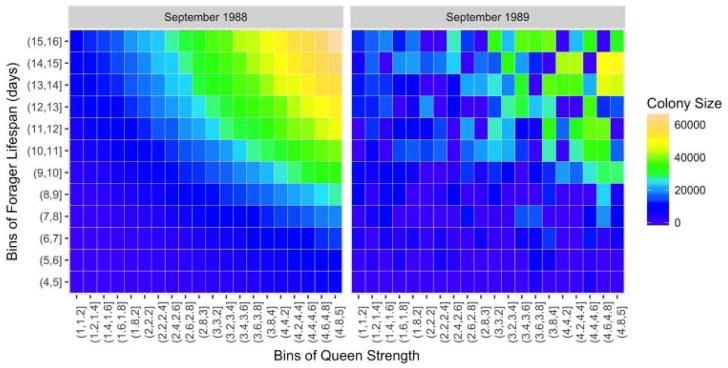
Colony persistence is a product of the interaction of queen strengths and forager lifespans. In Fig. 4, few colonies with weak queen strength (on a scale of 1–5) survive up to the pesticide application date at end of the second foraging season. The surviving colonies with weak queens are those with high average forager lifespans. Even so, smaller colonies with short forager lifespans survive to this point on account of strong queens. Queen strength is the primary determinant of colony size prior to pesticide application, and interactions between queen strengths of greater than 1 and high-average forager lifespans allow for stronger colonies. Simulated colonies of the control and exposed scenarios share identical input parameters up to the pesticide application date. We expect the relative importance of queen strength and forager lifespan to shift with time as colonies are exposed to pesticides. For these time-conditional results, the relative sensitivities are as expected with forager lifespan and queen strength being the most influential parameters in the spring, pesticide toxicity importance spiking in the summer during the pesticide application period, and queen strength being the most influential parameter the rest of the year.
Fig. 4.
Colony sizes of 5000 simulations at two different time points one year apart in the control scenario as a function of two primary sensitive parameters: queen strength and forager lifespan. Queen strength is the primary determinant of colony size, initial queen strengths of 1 or 2 rarely have viable colony sizes, but interactions between queen strengths of 3–5 and high average forager lifespans allow for stronger colonies.
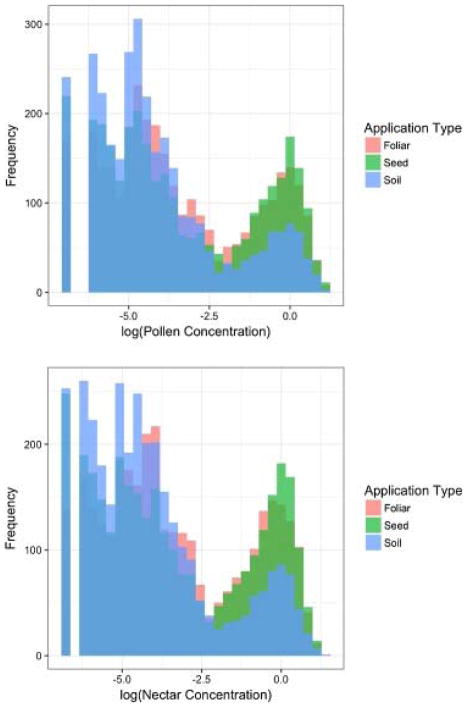
Pesticide residue concentration in pollen affects colony dynamics and colony survival in different modes depending on what pesticide application method is used for the simulated colonies. Fig. 5 summarizes each set of Monte Carlo simulations per application method and reveals how VarroaPop + Pesticide registers in-hive pollen concentrations of pesticide application method. Simulations employing the foliar spray method exhibit relatively moderate pollen concentrations in less than 5% of the Monte Carlo simulations during the month in which pesticide is applied. Seed applications show a high frequency of relatively low concentrations of pesticide within the in-hive pollen, and low pesticide concentration of in-hive pollen could explain the ability of simulated colonies to recover in the seed condition. Pollen concentrations resulting from the soil application method exhibit peculiar behavior as a result of internal model calculations for soil conditions.
Fig. 5.
In-hive pollen concentrations of pesticide residue. Concentrations are log-scaled to better differentiate the pesticide concentrations for the different application types. Soil applications had a significantly less frequency of high exposure concentrations compared to seed and foliar application types. This highlights differences in routes of exposure and how pesticide residue is modeled between each application type.
3.2. Sensitivity analysis and colony survival
Colony dynamics and the potential for colony survival is dependent upon different input parameters according to the pesticide application type. We utilize partial correlation coefficients (PCC) for sensitivity analysis to assess model performance of VarroaPop under the control scenario and three different pesticide application conditions. Initial sensitivity analysis of the control scenario demonstrates that colony size is primarily attributed to forager lifespan and queen strength across the entire simulation period regardless of the type of pesticide application (Fig. 6). We utilize colony size to examine model performance because it presents comprehensive simulation results for analysis. Further investigation reveals that the importance of forager lifespan and queen strength remain strong parameters for colony size over all the application scenarios, but their sensitivity fluctuates over time depending on pesticide application method and timing of exposure. A comparison of sensitivity analysis results before and after pesticide exposure exhibits a shift in influential parameters over colony size with each application method (Fig. 6). Colony size depends on forager lifespan and queen strength to propagate colonies through the simulation time period, but sensitivity results of each pesticide application method (i.e. foliar, seed, soil) reveals that toxicological parameters grow in influence with colony size. The influence of the parameters that contribute to pesticide exposure (slope and LD50 values for adults and larvae for the foliar scenario), when summed, have a similar level of influence as queen strength and forager lifespan.
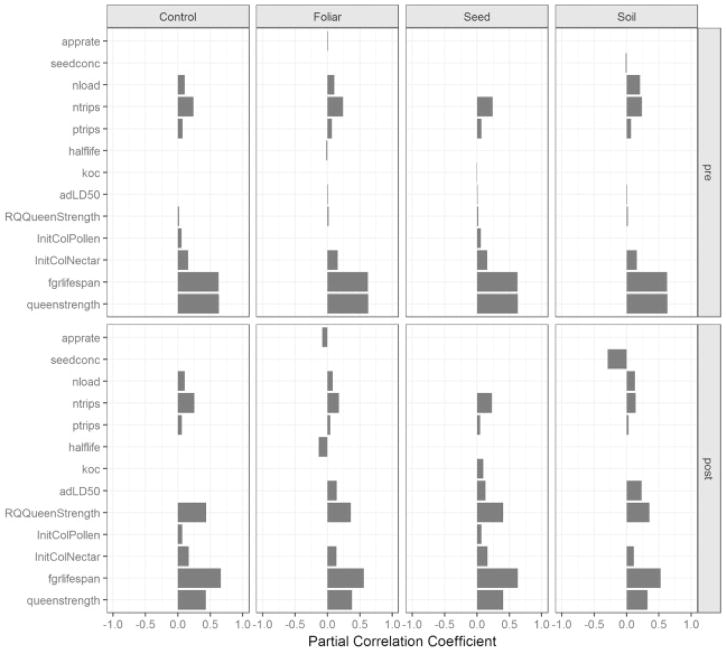
Fig. 6.
Before (top row) and after (bottom row) pesticide application daily sensitivity results. Each column presents PCC results of colony size in four different pesticide conditions (i.e. control, foliar spray, seed, soil). Foliar, seed, soil, and control share similar PCC rankings prior to the pesticide application due to identical base simulation parameters. PCC values and rankings shift with pesticide exposure and differ based on the pesticide application method, though control sensitivities show little change.
In order to further understand the relationships between input parameters on colony size, daily PCC values were calculated and examined across the simulated time period (Fig. 6). The time series of PCC values following the pesticide application date reveals which toxicological parameters influence colony size and give some insight into lag effects inherent in the model. Queen strength, requeen strength, and forager lifespan maintain significant, positive correlations on colony size amongst control and exposed scenarios. The reported PCC values in Table 2 represent the status colony dynamics 30 days after the pesticide application date. Daily breakout of these parameters for the foliar spray application simulations shows that the PCC value of adult contact LD50 falls below our minimum significance criterion over time while pesticide application rate remains a significant input parameter over the course of the simulation period. For the soil application method, sensitivity analysis shows that seed concentration significantly accounts for responses in colony size following pesticide application. The majority of other input parameters included in the sensitivity analysis did not exhibit significant impacts on colony size. However, daily time series of PCC values across the simulation period (Fig. 7) reveals influential input parameters that fall below the significance threshold of the sensitivity analysis.
Table 2.
Post-exposure (30 days) PCC (|r| > 0.062, n = 5000, p < 0.05) values of colony size.
| Input Parameter | Control | Seed | Soil | Foliar |
|---|---|---|---|---|
| Forager Lifespan (days) | 0.64 | 0.53 | 0.64 | 0.57 |
| Nectar Trips (per day) | 0.25 | 0.14 | 0.23 | 0.18 |
| Nectar Load (mg/Bee) | 0.10 | 0.04 | 0.09 | 0.08 |
| Queen Strength | −0.07 | 0.33 | 0.41 | 0.38 |
| Initial Colony Nectar Amount (g) | – | 0.11 | 0.17 | 0.14 |
| Adult LD50 | – | 0.23 | 0.13 | 0.14 |
| Requeen Strength | 0.29 | 0.35 | 0.41 | 0.36 |
| Seed Concentration | – | −0.29 | – | – |
| Koc | – | – | 0.10 | – |
| Pollen Trips (per day) | 0.05 | 0.036 | 0.05 | 0.04 |
| Application Rate (lb a.i./A) | – | – | – | −0.08 |
| Half-life (days) | – | – | – | −0.14 |
| Initial Colony Pollen Amount (g) | – | – | 0.07 | – |
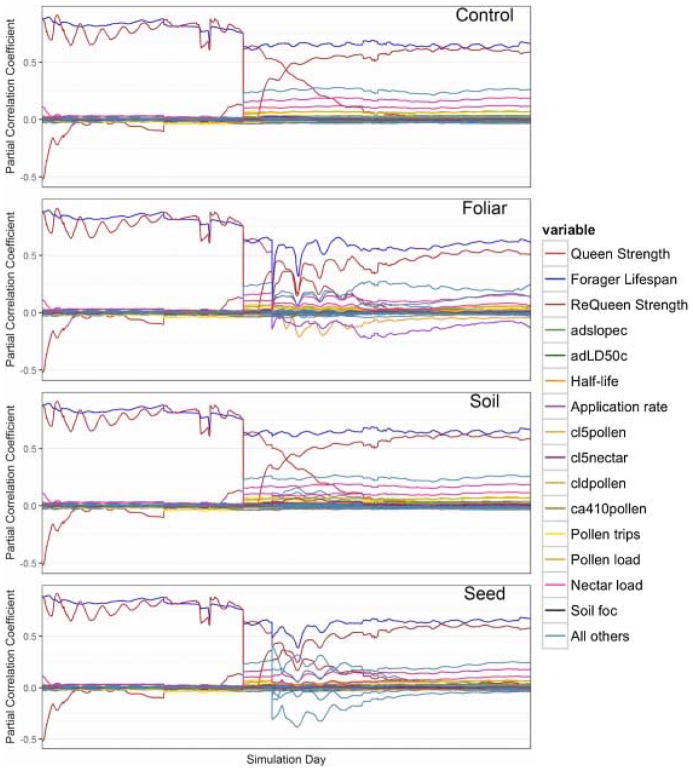
Fig. 7.
Daily simulation breakout of partial correlation coefficients (PCC) as time series. The control scenario exhibits the strength of forager lifespan and queen strength across the simulation period. Queen strength sensitivity diminishes after a requeening event and requeen strength grows over the remainder of the simulation time period. Furthermore, an analysis of daily PCC values over time reveals dynamic model behavior following pesticide applications and exhibits which toxicological input parameters emerge with each type of pesticide application method.
4. Discussion
4.1. Simulation results
Our simulation results show that honey bee colonies are less likely to recover from pesticide exposure relative to the control simulations. In particular, colonies with foliar spray and soil application methods exhibit the least recovery following pesticide application and exposure. Whereas, colonies exposed to pesticides via the seed application method showed little difference compared to the control scenario. We observe a minimum colony survival threshold in our Monte Carlo simulations. Queen strength and forager lifespan are strong factors for colony survival. Colonies with strong queens and workers with longer than average forager lifespan are more likely to survive than those with weak queens and short-lived foragers. This combined influence of queen strength and forager lifespan creates a minimum boundary condition for model parameterization and colony survival. This boundary can fluctuate according to other influential parameters that arise with each pesticide application method. Queen strength remains the dominant parameter for colony survival across controlled and exposed simulations and throughout the simulation period. 1.2% of simulated colonies with strong queen strengths do not survive through the first winter and up to the time when pesticide is applied in the second foraging season regardless of forager lifespan. Whereas, 10.3% of colonies with forager lifespans greater than 10 days did not survive up to the pesticide application date independent of queen strength. This highlights the influence of the queen strength parameter to direct colony dynamics independent of forager lifespan. Even so, the dynamic of the relationship between queen strength and forager lifespan is strongly perturbed by intra-colony events, such as re-queening.
Simulation and sensitivity analysis results of the exposed methods reveal two mechanisms by which colony survival is affected between foliar spray and soil applications. At face value, Monte Carlo simulations of the foliar and soil conditions exhibit very similar responses to pesticide application. However, further investigation with sensitivity analysis reveals different pathways of pesticide exposure within the VarroaPop + Pesticide model. Foliar spray applications are often applied in domestic settings for garden use and provides a topical defense against target pests. Seed and soil application techniques are generally employed with systemic pesticides in which chemicals are integrated into the plant tissues. Dively and Kamel (2012) reported higher insecticide residues in pollen and nectar of foliar applications than of soil or seed applications in their exposure study. Foliar spray application rate and adult contact LD50 become critical parameters in the foliar condition which indicates mortality of foraging individuals via direct exposure to spray applications. Our results, however, do not indicate if foraging bees that come into direct contact with foliar-applied pesticides die on contact, or lose cognitive function and expire before returning to the hive.
4.2. Sensitivity analysis
Sensitivity analysis indicates that colony survival is most influenced by routes of pesticide exposure outside of the hive via forager interaction with dosed pollen and nectar. Sensitivity analysis results of the foliar spray condition indicate application rate, nectar load, and adult contact LD50 as significant, critical parameters for colony persistence. In actual colonies, pesticides can enter the hive in pollen and nectar and be stored in comb cells. The model simulates this by determining the nectar and pollen requirements of a colony each day depending on population size. VarroaPop calculates pesticide residue in nectar and pollen from foliar spray as a function of application rate and dissipation with time. Therefore, the estimated field concentrations of topical pesticide residue in pollen and nectar are a direct factor of the application rate, however, pesticide residues inside the hive are a function of application rate and a number of other colony dynamic factors. Adult contact LD50 refers to the median lethal dose of adults via contact exposure to pesticide residue. In the VarroaPop + Pesticide model, foraging bees are the primary caste of workers that come into direct contact with dosed pollen and nectar. VarroaPop defines forager interaction with pollen and nectar as contact-based, or direct exposure. Queen strength and forager lifespan remain to be the most critical parameters for colony survival over the course of the simulation period. Other parameters associated with internal colony dynamics and individual exposure to pesticides react to pesticide application in the exposure scenarios and temporarily dictate colony dynamics, but these become less critical over time such that queen strength and forager lifespan become the main drivers of colony dynamics post-exposure. The importance of queen strength is associated with egg laying potential and how the colony perpetuates from the initial number of eggs laid and the worker to drone ratio. Forager lifespan determines the resource dynamics of the hive via foraging trips and collected nectar and pollen loads. Queen strength and forager lifespan, together, drive colony dynamics—including survival through the winter and in pesticide exposure conditions.
Sensitivity analysis of the VarroaPop + Pesticide model provides guidance for future model development. Linear sensitivity analysis reveals queen strength and forager lifespan as consistent, critical parameters for colony dynamics regardless of pesticide exposure. The relative importance of pesticide parameters is a function of toxicity and application rates, and can fluctuate over time as displayed in the time conditional sensitivity analysis. Adult contact LD50 and application rate became critical parameters of colony dynamics in the foliar application method simulations, whereas pollen load was the most critical toxicological parameter in the soil application method simulations. Sensitivity indices of the seed application method did not identify sensitive toxicological parameters since simulation results displayed colony dynamics that did not deviate significantly from the control conditions. Daily time series of sensitivity values allows for detailed observation of model behavior and interactions between critical parameters.
4.3. Model restrictions and potential modifications
With the recent sensitivity analysis results and conclusions in mind, opportunities exist to make additional model modifications to strengthen model behavior regarding colony dynamics and toxicological effects. Allowing for multiple pesticide applications at different times will warrant long-term simulations and strengthened toxicological modeling within VarroaPop + Pesticide. Further modifications may also be informed by evaluating simulation results against empirical data from field studies. The current model version includes resource storage, consumption, and gathering metrics which can serve as a foundation to add the impact of resource availability to the model (e.g., impacts to egg laying, brood cannibalism, seasonal effects). Implementing these details into colony dynamics could identify additional sensitive resource parameters.
Additionally, having these Monte Carlo capabilities provides opportunities for model calibration in specific exposure settings. For example, calibrating the model for specific data sets that contain application details and field-collected data (e.g., pollen concentrations, pollen loads, cohort survival rates) allows for more confidence in the realism of the model. This implementation also provides a basis for future incorporation of sublethal impacts of pesticide exposure and resulting effects on hive outcomes.
5. Conclusion
Sensitivity analyses with VarroaPop + Pesticide indicates queen strength and forager lifespan as consistent, critical parameters for colony dynamics regardless of pesticide exposure. The influence of the parameters that contribute to pesticide exposure and effects (e.g., application inputs, toxicity values for adults and larvae and foraging parameters) are partitioned amongst those parameters but have a similar level of influence as queen strength and forager lifespan when aggregated. The relative importance of pesticide parameters is a function of toxicity and application rate. Conditionally implementing the sensitivity analyses on a daily timescale demonstrates that the relative importance of these parameters fluctuates throughout the simulation period in response to external events. For these time-conditional results, the relative sensitivities are as expected, with forager lifespan and queen strength being most influential parameters in the spring, pesticide exposure and effects parameters spiking in importance in the aftermath of pesticide application, and queen strength being the most influential parameters the rest of the year. By using sensitivity analysis to assess model output and variability, the development, calibration and application of simulation models to environmental risk assessment can be better informed and potentially yield more accurate predictions and inference for complex ecological processes.
6. Software availability
The C binary for the latest release of the VarroaPop program and the R code that implements the sensitivity analysis and generates the manuscript figures are available from a GitHub repository located at https://github.com/puruckertom/beeRpop (Kuan et al., 2016).
Acknowledgments
Thanks to Mike Cyterski, Matthew Etterson, Anita Pease and Fran Rauschenberg for manuscript review and edits. This research was supported in part by an appointment to the Research Program at the at the National Exposure Research Laboratory in the Office of Research Development, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through Interagency Agreement No. DW8992298301 between the United States Department of Energy and the United States Environmental Protection Agency. This paper has been reviewed in accordance with Agency policy and approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the USEPA.
Appendix A
Table A1.
Parameter descriptions.
| Parameter symbol | Description | Units |
|---|---|---|
| AR | Single application rate. | lb a.i./A |
| Cgrass(t) | Pesticide concentration in grass at time t. Used as a surrogate to estimate pesticide concentrations in pollen and nectar when chemical-specific empirical data for pollen and nectar are not available. | μg a.i./g |
| Cjelly(t) | Pesticide concentration in jelly at time t. Assumed to be equal to Cpollen(t)/100. | μg a.i./g |
| Cnectar(t) | Pesticide concentration in nectar at time t. Dependent upon application rate and method. | μg a.i./g |
| Cpollen(t) | Pesticide concentration in pollen at time t. Dependent upon application rate and method. | μg a.i./g |
| Csoil | Pesticide concentration in soil. | μg a.i./g |
| d | Soil depth | cm |
| Dcontact(t) | Pesticide dose received at time t by forager bees through direct spray. | μg a.i./bee |
| Ddiet(t) | Pesticide dose received at time t through the diet. | μg a.i./bee |
| Dnectar(t) | Pesticide dose received at time t through consumption of contaminated nectar. | μg a.i./bee |
| Dpollen(t) | Pesticide dose received at time t through consumption of contaminated pollen. | μg a.i./bee |
| foc | fraction of organic carbon in soil | none |
| IRjelly | Daily intake rate of jelly by individual bee. | g/day |
| IRnectar | Daily intake rate of nectar by individual bee. Varies by type of bee. | g/day |
| IRpollen | Daily intake rate of pollen by individual bee. Varies by type of bee. | g/day |
| Koc | soil organic carbon-water partitioning coefficient | L/kg-oc |
| LD50 | Median lethal dose (i.e., pesticide dose resulting in mortality to 50% of exposed bees). | μg a.i./bee |
| Toral(larva) | Tolerance of individual honey bee larva receiving oral pesticide dose. | μg a.i./bee |
| Toral(adult) | Tolerance of individual honey bee adult receiving oral pesticide dose. | μg a.i./bee |
| Tcontact(adult) | Tolerance of individual honey bee adult receiving contact pesticide dose. | μg a.i./bee |
| TSCF | Transpiration stream concentration factor | none |
| t1/2(foliage) | Foliar dissipation half-life | days |
| t1/2(soil) | Aerobic soil metabolism half-life | days |
| θ | soil-water content by volume | cm3/cm3 |
| ρ | soil bulk density | g-dw/cm3 |
References
- Babendreier D, Kalberer N, Romeis J, Fluri P, Bigler F. Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie. 2004;35:293–300. [Google Scholar]
- Becher MA, Osborne JL, Thorbek P, Kennedy PJ, Grimm V. Review: towards a systems approach for understanding honey bee decline: a stocktaking and synthesis of existing models. J Appl Ecol. 2013;50(4):868–880. doi: 10.1111/1365-2664.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ, Osborne JL. BEEHAVE: a systems model of honey bee colony dynamics and foraging to explore multifactorial causes of colony failure. J Appl Ecol. 2014;51(2):470–482. doi: 10.1111/1365-2664.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs G, Bromilow R, Evans A, Williams M. Relationships between lipophilicity and the distribution of non-ionised chemicals in barley shoots following uptake by the roots. Pestic Sci. 1983;14:492–500. [Google Scholar]
- Briggs GG, Bromilow RH, Evans AA. Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pestic Sci. 1982;13:495–504. [Google Scholar]
- Calderone NW. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS One. 2012;7(5):e37235. doi: 10.1371/journal.pone.0037235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauzat MP, Martel AC, Cougoule N, Porta P, Lachaize J, Zeggane S, Aubert M, Carpentier P, Faucon JP. An assessment of honey bee colony matrices, Apismellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environ Toxicol Chem. 2011;30(1):103–111. doi: 10.1002/etc.361. [DOI] [PubMed] [Google Scholar]
- Crailsheim K, Hrassnigg N, Gmeinbauer R, Szolderits MJ, Schneider LHW, Brosch U. Pollen utilization in non-breeding honey bees in winter. J Insect Physiol. 1993;39(5):369–373. [Google Scholar]
- Crailsheim K, Schneider LHW, Hrassnigg N, Bühlmann G, Brosch U, Gmeinbauer R, Schöffmann B. Pollen consumption and utilization in worker honey bees (Apis mellifera carnica): dependence on individual age and function. J Insect Physiol. 1992;38(6):409–419. [Google Scholar]
- DeGrandi-Hoffman G, Roth SA, Loper GL, Erickson EH., Jr BeePop: a honey bee population dynamics simulation model. Ecol Model. 1989;45:133–150. [Google Scholar]
- DeGrandi-Hoffman G, Curry B. A mathematical model of Varroa mite (Varroa destructor Anderson and Trueman) and honey bee (Apis mellifera L.) population dynamics. Int J Acarol. 2004;30(3):259–274. [Google Scholar]
- DeGrandi-Hoffman G, Chen Y, Simonds R. The effects of pesticides on queen rearing and virus titers in honey bees (Apis mellifera L.) Insects. 2013;4(1):71–89. doi: 10.3390/insects4010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandi-Hoffman G, Ahumada F, Chambers M, Hidalgo G, de Jong E. Population growth of Varroa destructor (Acari: Varroidae) in honey bee colonies is affected by the number of foragers with mites. Exp Appl Acarol. 2016;69:21–34. doi: 10.1007/s10493-016-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dively GP, Kamel A. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J Agric Food Chem. 2012;60(18):4449–4456. doi: 10.1021/jf205393x. [DOI] [PubMed] [Google Scholar]
- EPA. White Paper in Support of the Proposed Risk Assessment Process for Bees. Office of Chemical Safety and Pollution Prevention; 2012. p. 275. [Google Scholar]
- EPPO. Environmental risk assessment scheme for plant protection products: chapter 10, honey bees. European and Mediterranean Plant Protection Organization (EPPO) Bull OEPP/EPPO Bull. 2010;40:323–331. [Google Scholar]
- Fischer D, Moriarty T. Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop. Society of Environmental Toxicology and Chemistry (SETAC); Pensacola: 2014. [Google Scholar]
- Fletcher JS, Nellesson JE, Pfleeger T. Literature review and evaluation of the EPA food-chain (Kenaga) nomogram, an instrument for estimating pesticide residues on plants. Environ Toxicol Chem. 2004;13(9):1383–1391. [Google Scholar]
- Gallai N, Salles JM, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ. 2009;68(3):810–821. [Google Scholar]
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O, Bartomeus I. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339(6127):1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347(6229):1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- Hamby DM. A review of techniques for parameter sensitivity analysis of environmental models. Environ Monit Assess. 1994;32(2):135–154. doi: 10.1007/BF00547132. [DOI] [PubMed] [Google Scholar]
- Haydak MH. Honey bee nutrition. Annu Rev Entomol. 1970;15:143–156. [Google Scholar]
- Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336(6079):348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Iman RL, Helton JC. An investigation of uncertainty and sensitivity analysis techniques for computer models. Risk Anal. 1988;8(1):71–90. [Google Scholar]
- Johnson RM, Ellis MD, Mullin CA, Frazier M. Pesticides and honey bee toxicity–USA. Apidologie. 2010;41(3):312–331. [Google Scholar]
- Johnson RM. Honey bee toxicology. Ann Rev Entomol. 2015;60(1):415. doi: 10.1146/annurev-ento-011613-162005. [DOI] [PubMed] [Google Scholar]
- Koch H, Weisser P. Exposure of honey bees during pesticide application under field conditions. Apidologie. 1997;28:439–447. [Google Scholar]
- Kuan AC, Purucker ST, Wolfe K, Kanarek A. R Wrapper Code for VarroaPop. Zenodo: 2016. [DOI] [Google Scholar]
- Lee KV, Steinhauer N, Rennich K, Wilson ME, Tarpy DR, Caron DM, Rose R, Delaplane KS, Baylis K, Lengerich EJ, Pettis J. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 2015;46(3):292–305. [Google Scholar]
- Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Pettis JS. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PloS one. 2010;5(3):e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi F, Conte YL. Ecology of Varroa destructor, the major ectoparasite of the Western honey bee, Apis mellifera. Annu Rev Entomol. 2016;61:417–432. doi: 10.1146/annurev-ento-010715-023731. [DOI] [PubMed] [Google Scholar]
- Pettis JS, Delaplane KS. Coordinated responses to honey bee decline in the USA. Apidologie. 2010;41:256–263. [Google Scholar]
- Pujol GB, Iooss Janon A. R Package Version 1.11.1. 2015. Sensitivity: Sensitivity Analysis. [Google Scholar]
- Purucker ST, Welsh CJE, Stewart RN, Starzec P. Use of habitat-contamination spatial correlation to determine when to perform a spatially explicit ecological risk assessment. Ecol Model. 2007;204(1):180–192. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. URL. http://www.R-project.org. [Google Scholar]
- Rortais A, Arnold G, Halm M, Touffet-Briens F. Modes of honey bees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie. 2005;36:71–83. [Google Scholar]
- Ryan JA, Bell RM, Davidson JM, O’Connor GA. Plant uptake of non-ionic organic chemicals from soils. Chemosphere. 1988;17:2299–2323. [Google Scholar]
- Saltelli A, Chan K, Scott EM. Wiley Series in Probability and Statistics. Wiley; 2000. [Google Scholar]
- Saltelli A, Tarantola S, Campolongo F, Ratto M. Sensitivity Analysis in Practice: A Guide to Assessing Scientific Models. Wiley; 2004. [Google Scholar]
- Saltelli A, Marco R, Terry A, Francesca C, Jessica C, Debora G, Michaela S, Stefano T. Global Sensitivity Analysis: The Primer. John Wiley & Sons; 2008. [Google Scholar]
- Schneider CW, Tautz J, Grünewald B, Fuchs S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One. 2012;7(1):e30023. doi: 10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmickl T, Crailsheim K. HoPoMo: a model of honey bee intracolonial population dynamics and resource management. Ecol Model. 2007;204(1):219–245. [Google Scholar]
- Simpson J. The significance of the presence of pollen in the food of worker larvae of the honey bee. Q J Microsc Sci. 1955;96(1):117–120. [Google Scholar]
- Sinnathamby S, Assefa Y, Granger AM, Tabor LK, Douglas-Mankin KR. Pollinator decline: US agro-socio-economic impacts and responses. J Nat Environ Sci. 2013;4(1):1–13. [Google Scholar]
- Spleen AM, Lengerich EJ, Rennich K, Caron D, Rose R, Pettis JS, Henson M, Wilkes JT, Wilson M, Stitzinger J, Lee K. A national survey of managed honey bee 2011–12 winter colony losses in the United States: results from the Bee Informed Partnership. J Apicult Res. 2013;52(2):44–53. [Google Scholar]
- Sponsler DB, Johnson RM. Mechanistic modeling of pesticide exposure: the missing keystone of honey bee toxicology. Environ Toxicol Chem. 2016;9999(9999):1–11. doi: 10.1002/etc.3661. [DOI] [PubMed] [Google Scholar]
- Steeger T, Baris R, Moriarty T, Hart C, Hou W. Assessing risks of pesticides to bees: putting the science into context to inform regulatory decision making. Julius-Kühn-Archiv. 2015;450:8. [Google Scholar]
- Steinhauer NA, Rennich K, Wilson ME, Caron DM, Lengerich EJ, Pettis JS, Rose R, Skinner JA, Tarpy DR, Wilkes JT, Vanengelsdorp D. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the Bee Informed Partnership. J Apicult Res. 2014;53(1):1–18. [Google Scholar]
- Torres DJ, Ricoy UM, Roybal S. Modeling honey bee populations. PLoS One. 2015;10(7):e0130966. doi: 10.1371/journal.pone.0130966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. Submitted to the FIFRA Scientific Advisory Panel for Review and Comment September 11–14, 2012. Office of Chemical Safety and Pollution Prevention Office of Pesticide Programs Environmental Fate and Effects Division, Environmental Protection Agency; Washington DC: Environmental Assessment Directorate, Pest Management Regulatory Agency, Health Canada; Ottawa, CN: California Department of Pesticide Regulation; 2012. White Paper in Support of the Proposed Risk Assessment Process for Bees. http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2012-0543-0004. [Google Scholar]
- USEPA. Guidance for Assessing Pesticide Risks to Bees. Office of Chemical Safety and Pollution Prevention Office of Pesticide Programs Environmental Fate and Effects Division, Environmental Protection Agency; Washington DC: Health Canada Pest Management Regulatory Agency; Ottawa, CN: California Department of Pesticide Regulation, Sacramento, CA; 2014. [Google Scholar]
- Vanbergen AJ. Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ. 2013;11(5):251–259. [Google Scholar]
- Whitehorn PR, O’Connor S, Wackers FL, Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336(6079):351–352. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- Willis GH, McDowell LL. Pesticide persistence on foliage. Environ Contam Toxicol. 1987;100:23–73. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge, MA: 1987. [Google Scholar]