Abstract
The intergenerational impact of engineered nanomaterials in plants is a key knowledge gap in the literature. A soil microcosm study was performed to assess the effects of multi-generational exposure of wheat (Triticum aestivum L.) to cerium oxide nanoparticles (CeO2-NPs). Seeds from plants that were exposed to 0, 125, and 500 mg CeO2-NPs/kg soil (Ce-0, Ce-125 or Ce-500, respectively) in first generation (S1) were cultivated in factorial combinations of Ce-0, Ce-125 or Ce-500 to produce second generation (S2) plants. The factorial combinations for first/second generation treatments in Ce-125 were S1-Ce-0/S2-Ce-0, S1-Ce-0/S2-Ce-125, S1-Ce-125/S2-Ce-0 and S1-Ce-125/S2-Ce-125, and in Ce-500 were S1-Ce-0/S2-Ce-0, S1-Ce-0/S2-Ce-500, S1-Ce-500/S2-Ce-0 and S1-Ce-500/S2-Ce-500. Agronomic, elemental, isotopic, and synchrotron X-ray fluorescence (XRF) and X-ray absorption near-edge spectroscopy (XANES) data were collected on second generation plants. Results showed that plants treated during the first generation only with either Ce-125 or Ce-500 (e.g. S1-Ce-125/S2-Ce-0 or S1-Ce-500/S2-Ce-0) had reduced accumulation of Ce (61 or 50%), Fe (49 or 58%) and Mn (34 or 41%) in roots, and δ15N (11 or 8%) in grains compared to the plants not treated in both generations (i.e. S1-Ce-0/S2-Ce-0). Plants treated in both generations with Ce-125 (i.e. S1-Ce-125/S2-Ce-125) produced grains that had lower Mn, Ca, K, Mg and P relative to plants treated in the second generation only (i.e. S1-Ce-0/S2-Ce-125). In addition, synchrotron XRF elemental chemistry maps of soil/plant thin-sections revealed limited transformation of CeO2-NPs with no evidence of plant uptake or accumulation. The findings demonstrated that first generation exposure of wheat to CeO2-NPs affects the physiology and nutrient profile of the second generation plants. However, the lack of concentration-dependent responses indicate that complex physiological processes are involved which alter uptake and metabolism of CeO2-NPs in wheat.
Keywords: Carbon, First generation, Isotope, Nitrogen, δ15N, Second generation, XRF
Introduction
Cerium oxide nanoparticles (CeO2-NPs) possess unique surface redox chemistry that gives them biological-like activity.1 This peculiar property of CeO2-NPs stimulates physiological and biochemical changes in seeds harvested from plants that have been exposed to CeO2-NPs. Studies have revealed that CeO2-NPs induced significant changes on the quality of S1 seeds in wheat (Triticum aestivum L.),2,3 barley (Hordeum vulgare L.),4,5 kidney beans (Phaseolus vulgaris),6 and soybean (Glycine max L.).7 Findings have invariably established alterations in nutrient profile, grain/embryo physiology, and macromolecular composition in seeds. Interestingly, such changes often occur regardless of Ce/CeO2-NPs accumulation in seeds, and also without other responses in plants. However, the effects of those changes on succeeding generations remain unexplored.
Studies using uncoated CeO2-NPs revealed that these particles are highly stable, showing negligible dissolution in various environmental media and persisting as intact nanoparticulate CeO2 in soil, sewage sludge, and plant tissues.8,9 CeO2-NPs are widely used in industry and predicted to accumulate in sewage sludge, landfills, and biosolids.10 Frequent application of biosolids will result in accumulation of CeO2-NPs in agricultural soil,10,11 and consequently repeated multigenerational exposure of plants to these particles is highly possible. Therefore, impacts on plant from one generation to the next in response to CeO2-NPs exposure should be examined.
Environmental health and safety issues associated with engineered nanomaterial (ENM) exposure across multiple generations in plants is a key knowledge gap.12 A few published articles have examined the effects of previous exposure on plant performance in the succeeding generation. Wang et al.13 observed that offspring of CeO2-NPs treated tomato displayed growth retardation and tendency to accumulate Ce. A recent report on multigenerational exposure to CeO2-NPs showed diminished growth and productivity in the succeeding generations of Brassica rapa.14 In another study, first generation exposure of Arabidopsis thaliana to CuO-NPs induced changes in gene expression and stimulated uptake of nanosized CuO in second generation plants.15 Nevertheless, current data is insufficient to assess intergenerational impacts of CeO2-NPs in food crops.
The effects of exposure to CeO2-NPs during the first, second, or both generations on physiology, phenology, yield and nutrient imbalances in wheat were assessed. It was hypothesized that first generation exposure to CeO2-NPs would alter physiological and agronomic performance, increase cerium accumulation in plant tissues, and modify nutrient uptake in second generation wheat. This was hypothesized because first generation plants and seeds showed changes in nutrient profile and macromolecular contents. Seeds from wheat exposed to two levels of CeO2-NPs during the first generation (S1) were cultivated, with and without additional exposure during the second generation, to assess responses in second generation (S2) plants and seeds. Synchrotron X-ray spectroscopy was also employed to investigate the transformation and movement of CeO2-NPs in the rhizosphere. This study provides new insights on the longer-term impacts of ENMs following soil exposure to CeO2-NPs.
Materials and Method
Experimental design
This study is a continuation of research on long-term impacts of CeO2-NPs in wheat. In the first generation study, wheat (Jubilee) seeds were cultivated to full maturity in 0, 125, and 500 mg CeO2-NPs/kg soil (Ce-0, Ce-125 and Ce-500, respectively) to obtain first generation seeds.3 In this study, the S1 seeds produced in the first generation study were cultivated in factorial combinations of Ce-0, Ce-125 or Ce-500 to produce second generation (S2) plants and seeds. The experimental set-up and the factorial treatments were presented in SI Figure 1. Each treatment was replicated six times except for the control which was replicated five times due to lack of seeds. Analyses were performed in second generation plants, i.e. offspring of S1-untreated plants (those untreated in first generation and exposed or unexposed in the second generation, e.g. S1-Ce-0/S2-Ce-0, S1-Ce-0/S2-Ce-500) or S1-treated plants (those treated in the first generation and exposed or unexposed in the second generation, e.g. S1-Ce-0/S2-Ce-500, S1-Ce-500/S2-Ce-500) (SI Figure 1).
Pot preparation and CeO2-NPs addition in soil
The CeO2-NPs (Meliorum Technologies, Rochester, NY) were obtained from the University of California Center for Environmental Implications of Nanotechnology. The particles are rods with primary size of 8 ± 1 nm, particle size of 231 ± 16 nm in DI water, surface area of 93.8 m2/g, and 95.14% purity.16 We have included the XRD analysis and properties of pure suspension of CeO2-NPs in the Supplemental Information (SI Figure 2, SI Table 1). The pots were prepared by filling each plastic pot (15 cm diameter × 30 cm high) with 1.45 kg wet potting soil (MiracleGro® potting soil) to get an equivalent of 800 g dry weight soil. The amount of CeO2-NPs necessary to prepare Ce-125 and Ce-500 treatments (100 and 400 mg/800 g dry weight soil, respectively) were weighed and sonicated in 125 mL Millipore water (MW) at 25°C for 30 min in a water bath (Branson Ultrasonics, Danbury, CT). The suspension was poured evenly into the soil. The pots were placed in the growth chamber for three days before use.
Plant cultivation
Wheat seedlings were prepared and grown to full maturity as described.3 Briefly, nine-day-old seedlings were transplanted totaling eight seedlings/pot or one seedling/100 g dry weight soil. The pots were placed on a saucer to prevent loss of water from leaching and any leachate was rinsed back into the soil. Equal amount of distilled water was added to the pots regularly to keep the root zone moist. The plants were cultivated in the growth chamber (Environmental Growth Chamber, Chagrin Falls, OH) with these conditions: 16-h photoperiod, 20/10°C, 70% humidity, 300 µmol/m2-s for the first 40 days, after which the conditions were kept at 16-h photoperiod, 25/15°C, 70% humidity, 600 µmol/m2-s until harvest. Yoshida nutrient solution17 (100 mL) was added to the pots at 0, 1, 5, 25, 39 and 45 days after exposure (DAE; counted from the day the seedlings were transplanted) to fertilize the plant. Biological controls like Stratiolaelaps scimitus and Ablysius (Neoseiulus) cucumeris (BeneficialInsectary, Inc.) were applied at 7 and 69 DAE to control thrips population.
Determinations of plant growth and yield
Plant growth and yield parameters (plant height, biomass, number of open and dry spikes, spike length, grain yield/spike, and 100-grain weight) were measured according to Rico et al.3 as described in details in SI. Leaf gas exchange was measured using a Li-Cor Portable Photosynthesis System as described in Li-Cor Biosciences manual (Using the LI-6400/LI-6400XT Version 6). Fully expanded leaves with the area of 2-cm2 were placed in the chamber and readings were recorded after five minutes. The chamber condition was kept at 300 µmol/m2-s, 20°C and 65–67% humidity. At harvest, roots, shoots, and spikes were harvested, cleaned, and stored as previously described.3
Analytical methods
Plant tissues for multi-elemental assay were digested according to EPA method 3050A described in Gavlak et al.18 Samples (250 mg) were digested sequentially in 6 mL concentrated nitric acid and 8 mL ammonium sulfate/sulfuric acid (1:2.5 w:v) solution, and digestate was diluted to 50 mL using MW. Blanks, duplicates and reference standards (i.e. BCR-670 aquatic plant duck weed and NIST-1547 peach leaves) were used to validate the digestion and analytical method obtaining percent recoveries between 90% and 115%. Analyses were performed at W. M. Keck Collaboratory for Plasma Spectrometry at Oregon State University. Elemental analysis was performed using Inductively Coupled Plasma - Optical Emission Spectroscopy (ICP-OES) (Prodigy7, Teledyne Leeman Labs) while trace analysis of Ce was accomplished using ICP - Mass Spectroscopy (ICP-MS) (Thermo X-Series II CCT, ThermoFisher Scientific). For quality control, blank and spiked samples analyses were repeated every 12 samples.
For changes in 13C/12C and 15N/14N ratios, samples were analyzed using an Elementar Vario Isotope Cube (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to a Isoprime 100 isotope ratio mass spectrometer (Isoprime Ltd, Stockport UK). Three laboratory isotope standards were also analyzed to assess quality assurance or check calibration. The final values were expressed relative to internal standards V-PDB (Vienna PeeDee Belemite) and Air for carbon and nitrogen, respectively.
Synchrotron µXRF and µXANES studies
At harvest, a soil core (column) was collected from each treatment in a 5 cm × 12 cm (diameter × height) PVC cylinder to contain the plant roots, crown and remaining shoot material. The cores ends were wrapped in plastic wrap and frozen (−80 C°) until used. The frozen soil/plant cores were thawed, dried and embedded with epoxy resin. They were then cut in half along the long axis and 3–5 cm by 7 cm glass slides were glued to cover the entire cut surface on one half of each core. Further processing (i.e., cutting and polishing) produced soil/plant thin-sections with a thickness of ~100 µm.19,20 Micro X-ray fluorescence (µXRF) mapping of Ce and other elements in the wheat soil/plant thin-sections was performed at the 10.3.2 X-ray Microprobe Beamline at the Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory. To acquire accurate elemental maps, ‘multiple-energy’ maps of Ce were collected at both 5710 eV (below the Ce L3 edge) and 5729 eV (the Ce L3 edge). The maps were collected by scanning each line of pixels twice, once at 5710eV and once at 5729eV, an energy chosen to give equal signals from Ce3+ and CeO2. Energy calibration was such that the first peak for CeO2 was at 5730.33 eV. Data processing was done using ALS BL10.3.2 software ‘process multi-E chem maps’ and ‘Difference maps’ programs, by which the 5710eV map was subtracted from the 5729eV map, and the Ce channel data from that difference combined with the other detector data channels to form a composite map in which Ce and Ti (whose K-edge is below the Ce L3 edge and whose fluorescence energy is close to that of Ce) are accurately separated from each other. The resulting elemental maps were examined to locate areas and points of interest that contain Ce.
Cerium L-edge μ-X-ray absorption near edge structure (XANES) spectra were collected from points (spots) of interest in the thin section, based on the apparent presence of Ce at these locations as determined by the µXRF chemistry maps collected near the soil-root interface and on soil particles. Normalization and least squares combination fitting (LCF) were performed with ALS BL10.3.2 software. Reference component XANES spectra for the LCF were obtained from Ce(III) sorbed on ferrihydrite for Ce(III), Ce(IV) sorbed on MnO2 (samples provided by Y. Takahashi) and Ce(IV) oxide for Ce(IV). The proportion of Ce(III) and Ce(IV) at each spot was determined by LCF using ALS BL 10.3.2 software and the normalized intensities of the Ce(III) and Ce(IV) standards. Note that while Ce in CeO2 is tetravalent, its XANES spectrum is different from that of most Ce(IV) compounds or Ce sorbed on MnO2. Although the data on most spots was not good enough to rule out the presence of non-CeO2 Ce(IV), there were no spectra in which the addition of such species yielded a significant improvement in fit quality.
Data analysis
Data collected from roots, shoots and grains of second generation plants were subjected to a two-way ANOVA using General Linear Model in SAS statistical package (SAS Institute, Cary, NC, USA) to determine the statistical significance of first generation (S1), second generation (S2), and the interaction between first and second generations (S1×S2). In all cases, mean and standard error (SE) were calculated.
Results and Discussion
Agronomic and yield parameters
Agronomic and yield performance of second generation wheat is presented in Figure 1, and Tables 3–5 and Figure 3 of the Supplementary Information (SI). Two-way ANOVA demonstrated that first generation (S1) exposure to Ce-500 altered plant height, shoot biomass and spike characters, but first generation exposure to Ce-125 only affected plant height (SI Table 2). However, CeO2-NPs did not affect leaf gas exchange, spike length, grain weight, or grain yield (SI Table 3). The statistically significant effect of S1 CeO2-NPs treatment (SI Tables 2, 4) highly suggests that the modifications in plant growth and development were due to changes in first generation seeds induced by S1 CeO2-NPs treatment.
Figure 1.
Plant growth (A,B), grain formation (C,D), and grain maturity (E,F) responses of second generation wheat plants due to exposure to 0, 125 or 500 mg CeO2-NPs/kg during the first (S1) generations. Values are means ± SE. *, **, *** indicate significance at p ≤ 0.1, 0.05, 0.01, respectively.
Plant height of second generation plants was significantly altered by Ce exposure (Figure 1A,B). For example, at 10, 20, and 31 DAE, growth was enhanced by 52, 19, and 5%, respectively, in S1-Ce-500 (i.e. mean of S1-Ce-500/S2-Ce-0 and S1-Ce-500/S2-Ce-500) compared to S1-Ce-0 (i.e. mean of S1-Ce-0/S2-Ce-0 and S1-Ce-0/S2-Ce-500) plants (Figure 1B). These data suggest that CeO2-NP exposure in the first generation stimulates growth in second generation plants. For the spike formation, S1-Ce-500 produced more spikes in the second generation plants than S1-Ce-0 from 53 to 84 DAE (Fig. 1D). Consequently, S1-Ce-500 produced more dry spikes in second generation plants than S1-Ce-0 from 81 to 88 DAE (Figure 1F). However, S1-Ce-125 treatment in general did not affect spike production or maturity (Figure 1C,E). These results suggest that first generation exposure to CeO2-NPs may affect the productivity of succeeding generations in wheat.
In addition, S1-Ce-500 improved shoot biomass and number of spikes by 3 and 10%, respectively, and decreased number of grains per spike by 6%, compared to S1-Ce-0 plants (SI Table 4). Despite these changes, the total grain yield was not affected (SI Table 3). These results also indicate that first generation exposure to CeO2-NPs modifies the agronomic and yield properties of succeeding generations.
The modifications observed in plant performance of second generation wheat suggest that CeO2-NPs treatment induced biochemical and physiological changes in seeds that were produced during the first generation. Previous studies showed that CeO2-NPs promoted down-regulation of proteins in kidney beans S1 seeds, and altered starch and protein contents in wheat seeds.2,6 It is difficult to determine the cause of accelerated growth and grain formation observed in the present study; however, analysis of first generation wheat seeds appeared to have improved grain biomass and amino and fatty acid concentrations.3 Such alterations in macromolecular contents could have triggered the enhanced growth and early onset of grain production/maturity in the offspring of plants whose parents were exposed to Ce-500 during the first generation.
The current study also demonstrated one peculiar observation: exposure to Ce-500 in first generation only (S1-Ce-500/S2-Ce-0) enhanced growth and grain formation compared to exposure in both the first and second generations (S1-Ce-500/S2-Ce-500) (SI Figures 3A,B; SI Tables 2, 4). Related study on CeO2-NPs exposed Brassica napus indicated a similar pattern of response wherein Ma et al.15 reported a reduction in seed production in Brassica napus exposed to CeO2-NPs (10–1000 mg/L) in the first, second and third generations. On the contrary, Wang et al.14 found that first generation exposure of tomato to CeO2-NPs resulted in poor growth performance of plants untreated in the next generation.
Cerium accumulation
The data also suggest that exposure to CeO2-NPs during first generation caused physiological or biochemical changes that altered the uptake of Ce in the second generation (Table 1, SI Table 5). Exposure to CeO2-NPs significantly altered Ce concentrations in roots and shoots, but not grains (SI Table 5). Notably, Ce accumulation in roots and shoots of second generation plant was dependent on CeO2-NPs exposure at second generation.
Table 1.
Isotope analysis of wheat cultivated to seed production in second generation plants exposed to CeO2-NPs during the first (S1), second (S2), or both generations. Values are means ± SE (n = 6 except for S1-Ce-0/Ce-0 wherein n = 5).
| Roots | ||||
|
| ||||
| δ15N (‰) | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | 1.11 ± 0.10 | 1.06 ± 0.14 | 1.08 ± 0.08 | |
| S1-Ce-500 | 0.91 ± 0.22 | 0.57 ± 0.12 | 0.74 ± 0.13* | |
| Mean | 1.00 ± 0.13 | 0.82 ± 0.11 | ||
|
| ||||
| Shoots | ||||
|
| ||||
| C (%) | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | 36.58 ± 0.29 | 36.51 ± 0.20 | 36.54 ± 0.16 | |
| S1-Ce-500 | 37.24 ± 0.22 | 37.12 ± 0.17 | 37.18 ± 0.14** | |
| Mean | 36.94 ± 0.20 | 36.82 ± 0.16 | ||
|
| ||||
| δ13C (‰) | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | −29.67 ± 0.25 | −29.50 ± 0.13 | −29.58 ± 0.13 | |
| S1-Ce-500 | −29.14 ± 0.16 | −29.17 ± 0.19 | −29.16 ± 0.11* | |
| Mean | −29.38 ± 0.16 | −29.34 ± 0.12 | ||
|
| ||||
| N (%) | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | 2.05 ± 0.10 | 2.10 ± 0.08 | 2.07 ± 0.06 | |
| S1-Ce-500 | 1.80 ± 0.08 | 1.84 ± 0.09 | 1.82 ± 0.05** | |
| Mean | 1.91 ± 0.07 | 1.97 ± 0.07 | ||
|
| ||||
| C:N | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | 18.07 ± 1.03 | 17.56 ± 0.72 | 17.79 ± 0.58 | |
| S1-Ce-500 | 20.92 ± 0.94 | 20.43 ± 1.04 | 20.67 ± 0.67** | |
| Mean | 19.62 ± 0.80 | 19.00 ± 0.74 | ||
|
| ||||
| Grains | ||||
|
| ||||
| δ15N (‰) | S2-Ce-0 | S2-Ce-500 | Mean | |
| S1-Ce-0 | 1.66 ± 0.06 | 1.67 ± 0.04 | 1.67 ± 0.03 | |
| S1-Ce-500 | 1.55 ± 0.05 | 1.53 ± 0.04 | 1.54 ± 0.03* | |
| Mean | 1.60 ± 0.04 | 1.60 ± 0.04 | ||
|
| ||||
| δ15N (‰) | S2-Ce-0 | S2-Ce-125 | Mean | |
| S1-Ce-0 | 1.66 ± 0.06 | 1.74 ± 0.10 | 1.71 ± 0.06 | |
| S1-Ce-125 | 1.46 ± 0.08 | 1.57 ± 0.07 | 1.52 ± 0.05* | |
| Mean | 1.55 ± 0.6 | 1.66 ± 0.06 | ||
indicate significance at p ≤ 0.05, 0.01, respectively.
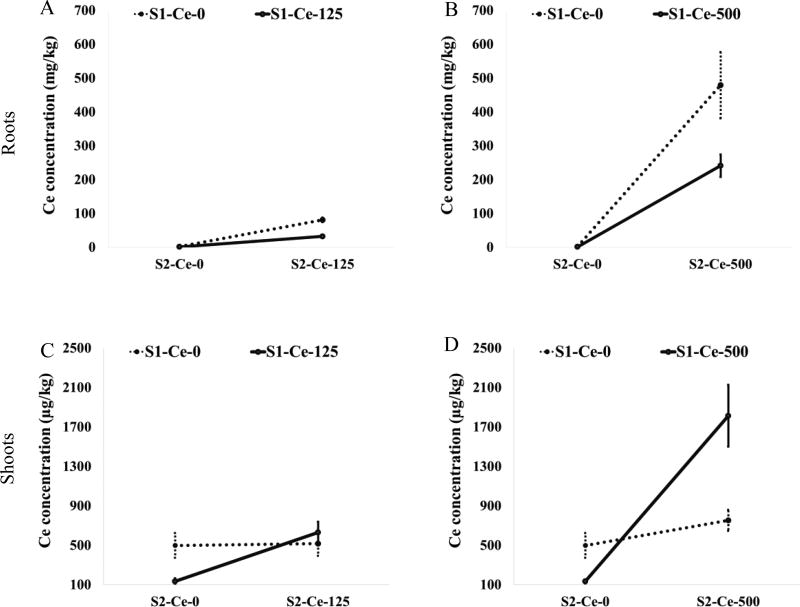
In roots, exposure at both first and second generations to CeO2-NPs (S1-Ce-125/S2-Ce-125, S1-Ce-500/S2-Ce-500) dramatically decreased Ce accumulation by 61% and 50%, respectively, compared to plants that were exposed in second generation only (S1-Ce-0/S2-Ce-125, S1-Ce-0/S2-Ce-500) (Figure 2A–B). These findings suggest that exposure to CeO2-NPs for two consecutive generations decreases Ce uptake in roots.
Figure 2.
Changes in cerium accumulation in roots (A,B) and shoots (C,D) of second generation wheat plants exposed to 0, 125 or 500 mg CeO2-NPs/kg due to interaction effects between first (S1) and second (S2) generation exposures to CeO2-NPs. Values are means ± SE (n = 6 except for S1-Ce-0/Ce-0 wherein n = 5).
In the case of shoots, only Ce-500 treatment at first and second generations (S1-Ce-500/S2-Ce-500) resulted in higher Ce concentration compared to second generation exposure only (S1-Ce-0/S2-Ce-500) (Figure 2D). Interestingly, Ce accumulation in S1-treated plants not exposed in the second generation (S1-Ce-125/S2-Ce-0, S1-Ce-500/S2-Ce-0) was significantly reduced by 73% compared to plants not treated in both generations (S1-Ce-0/S2-Ce-0) (Figure 2C,D). What triggered this response is not clear, but evidently parental exposure to CeO2-NPs results in decreased Ce accumulation in second generation plants. Contrary to this finding, the second generation plants from CeO2-NPs exposed tomato and CuO-NPs treated Arabidopsis exhibited enhanced accumulation of Ce and CuO-NPs, respectively.14,15
The results further indicated that despite high concentration of Ce in the shoots when both the first and second generations were exposed to Ce-500 (Figure 2D), Ce was not translocated to the grains (data not shown). Prior reports have shown that Ce did not translocate to grains in CeO2-NPs treated wheat.2,3
Nutrient accumulation
Exposure to CeO2-NPs significantly altered the concentrations of Al, Fe and Mn in wheat roots (Figure 3, SI Table 5). Second generation plants exposed to CeO2-NPs in the first generation only (e.g. S1-Ce-500/S2-Ce-0) had reduced Al, Fe, and Mn accumulations by 56–70%, 49–58%, and 34–41%, respectively, relative to control plants unexposed in both first and second generations (S1-Ce-0/S2-Ce-0) (Figure 3A–F). The influence of CeO2-NPs on nutrient concentrations in plants has been abundantly discussed in the literature, but studies show varying and non-uniform responses in uptake in response to ENM exposure. In the present study it is not clear how first generation exposure to CeO2-NPs decreased the uptake of Al, Fe and Mn in the roots of second generation plants. Nevertheless, the similar responses in Al, Fe and Mn accumulations to CeO2-NPs exposure highly suggest that similar mechanisms may be involved in the uptake of these elements in second generation wheat plants. The decrease in Fe and Mn is probably beneficial to plants since increased concentrations of these elements in roots or shoots usually results in decreased yield.21 The reduced Al uptake in roots is also beneficial since Al is toxic to plants.22
Figure 3.
Elemental uptake in roots of wheat cultivated to grain production in second generation wheat plants exposed to 0, 125 or 500 mg CeO2-NPs/kg due to interaction effects between first (S1) and second (S2) generation exposures to CeO2-NPs. Values are means ± SE (n = 6 except for S1-Ce-0/Ce-0 wherein n = 5).
In contrast to the large reduction of Al, Fe and Mn observed in roots, exposure to CeO2-NPs had little influence on element concentrations in wheat shoots. P uptake in S1-Ce-125 was 9% higher whereas K storage in S1-Ce-500 was 7% lower compared to those in unexposed control (data no shown). The large reduction of Fe and Mn concentrations in roots and the slight changes in P and K concentrations in the shoots did not affect agronomic and yield attributes (i.e. gas exchange and grain yield) in this study.
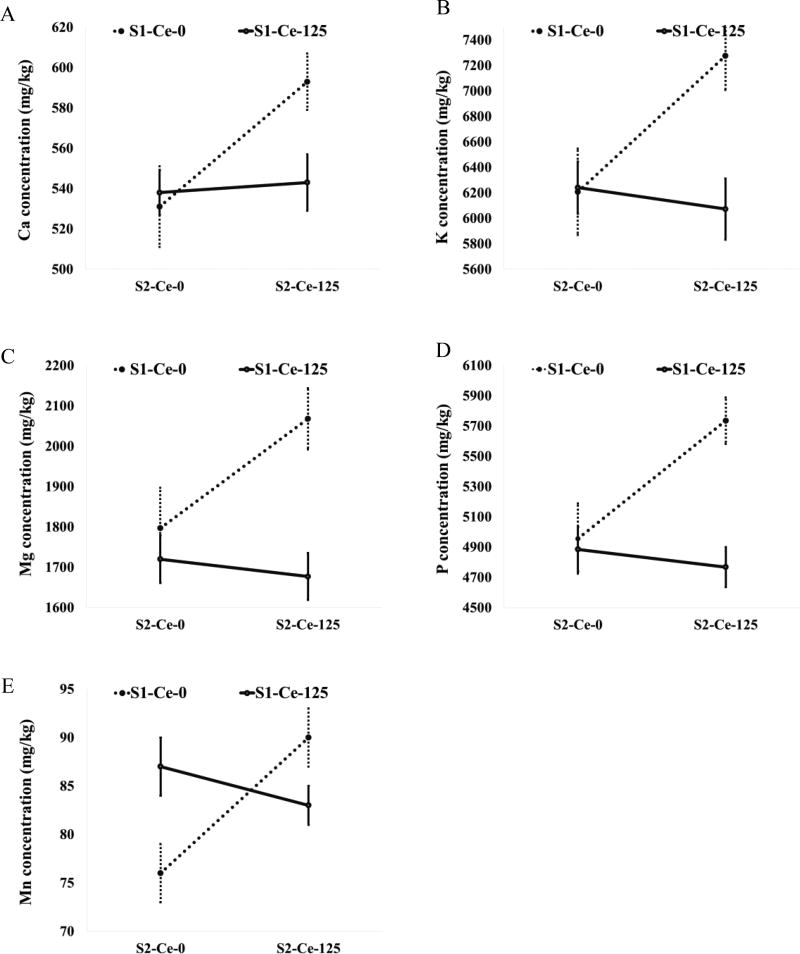
There was some indication that nutrients in grains were affected by CeO2-NPs, providing a possible mechanism for intergenerational effects through lowered seed quality. Significant effects on Mn, Ca, K, Mg and P in grains were found only in the Ce-125 treated plants, especially when treated in both the first and second generations (Figure 4, SI Table 5). Data showed that S1-Ce-125/S2-Ce-125 caused a reduction in the concentrations of Mn, Ca, K, Mg and P by 8, 8, 17, 20, and 19%, respectively, relative to S1-Ce-0/S2-Ce-125 (Figure 4). The decreased uptake in S1-Ce-125/S1-Ce-125 with respect to S1-Ce-0/S2-Ce-125 signifies that exposure to Ce-125 in both the first and second generations negatively affected the accumulation of these nutrients in the grains.
Figure 4.
Changes in elemental content of grains harvested from second generation wheat plants exposed to 125 mg CeO2-NPs/kg due to interaction effects between first (S1) and second (S2) generation exposures to CeO2-NPs. Values are means ± SE (n = 6 except for S1-Ce-0/Ce-0 wherein n = 5).
The results reported here for grains suggest that exposure to different concentrations of CeO2-NPs may influence different physiological mechanisms, and demonstrate the lack of concentration-dependent responses in wheat. Exposure to CeO2-NPs did not alter the Ce concentration but exposure to Ce-125 in the first and second generations reduced Mn, Ca, K, Mg and P accumulation in grains. Ce-125 did not affect the yield parameters but modified nutrient content in grains, whereas Ce-500 treatment affected agronomic and yield parameters but did not alter elemental content in grains. These findings suggest that in second generation plants, Ce-125 affects grain quality more than Ce-500, but Ce-500 influences growth and productivity more than Ce-125. This lack of concentration-dependent trend or inconsistent effects on the endpoints have been reported in the literature as a major challenge in assessing environmental effects of engineered nanomaterials.
C and N stable isotope analysis
Since CeO2-NPs altered physiological performance and mineral uptake, C and N stable isotope discrimination was evaluated to assess subtle effects of CeO2-NPs in wheat. Studies on C N dynamics in ENM-treated plants are yet to be reported in the literature. Nevertheless, there are some reports of isotope discrimination in metal-exposed plants: dimethylarsinate decreased δ15N in nasturtium (Tropaelum majus) while salinity increased δ13C in beans.23,24 The stable isotope ratio reflects the amount in which heavier isotopes are discriminated with respect to lighter isotopes, and changes in isotope composition is a sensitive measure of the physiological and biochemical responses of plants to different environmental conditions.25,26 For example, C and N isotope discrimination in metal-exposed plants (i.e. Na, Cd, Pb, Zn) has been attributed to physiological changes associated with photosynthesis (i.e. leaf gas exchange, photosynthetic electron transport), inhibition of nitrate assimilation, and/or decrease in nitrogen uptake or protein biosynthesis.25,27
In the current study, analyses were performed on seeds produced following the first generation of exposure (S1 seeds), and plant tissues and seeds following the second generation of exposure (Table 1, SI Tables 6–8). Results demonstrated that percent C and N and δ13C and δ15N ratios in wheat S1 seeds were not different between Ce-0, Ce-125, and Ce-500 (data not shown). However, ANOVA showed that when S1 seeds were grown to the S2 generation with or without receiving additional CeO2-NPs, significant changes in isotope discrimination in offspring plants were observed (SI Tables 6–7).
The results indicate that first generation exposure to Ce-500 induced biochemical changes in wheat S1 seeds that resulted in changes in C, N and their stable isotopes, but these changes were only observed in second generation plants. At harvest, only the Ce-500 treatment modified δ15N ratio in roots, and C or N values in shoots. The shoots yielded higher C, δ13C, and C:N ratio but lower N content (2, 1, 16, 12%, respectively), while roots had lower (32%) δ15N in S1-Ce-500 compared to S1-Ce-0 (Table 1). Analysis of shoots at mid-life growth stage (41 DAE) revealed a similar trend for C, N and C:N ratio (SI Tables 6, 8). The consistent trend for C, N, C:N ratio in shoots at mid-life and end-of-life growth stages suggest that these changes occurred at an early stage in plant development, and persisted until the end-of-life cycle. In addition, the high C:N ratio reflects the increase in C accompanied by large decrease in N. The nearly 12% drop in shoot N and 32% decrease in root δ15N in S1-Ce-500 signify possible consequential effects on plant metabolism and nitrogen cycling. It is interesting to note that these alterations were due to first generation exposure to Ce-500 because the shoots of second generation plants in S1-Ce-500 regardless of exposure to S2-Ce-0 or S2-Ce-500 had altered C or N values relative to those in S1-Ce-0 (Table 1).
Although δ15N values of seeds produced during the first generation exposure to CeO2-NPs (S1 seeds) were unchanged, seeds produced during the second generation did exhibit significant changes in δ15N (Table 1). The wheat S1 seeds did not exhibit measurable changes in C or N isotope ratios, but cultivating them to the second generation produced statistically significant changes in S2 seeds. Both Ce-125 and Ce-500 exhibited similar effects on δ15N in grains: S1-Ce-125 and S1-Ce-500 decreased δ15N ratios compared to their respective Ce-0 treatments (11 and 8% lower, respectively). The data indicated that first generation exposure to CeO2-NPs caused a decrease in δ15N; this means that wheat S1 seeds exposed or not to CeO2-NPs in the second generation yielded lower δ15N in S2 grains. However, the mechanisms involved in the intergenerational effects of ENMs on nutrient dynamics and stable isotope ratios in wheat are unknown. Additional research is needed to better understand how CeO2-NPs alter nutrient dynamics in plants, especially in agronomic species such as wheat since exposure may result in altered food quality.
It should be noted that alterations in C and N stable isotopes were due to S1 CeO2-NPs treatment (SI Tables 6–8). This observation is consistent with those recorded in plant growth and nutrient profile; which indicates that when wheat is exposed to CeO2-NPs for two generations, the effects of S1 CeO2-NPs treatment is more dominant than S2 CeO2-NPs treatment.
Localization and in situ speciation of Ce in soil columns
Data collected at the ALS revealed the spatial distribution and chemistry of Ce and CeO2-NPs in soil, plant tissues, and at the root-soil interface in wheat (Figure 5, SI Figure 4). Micro X-ray fluorescence (µXRF) elemental maps of the soil column from the S1-Ce-0/S1-Ce-500 sample showed that Ce(IV) adhered to root surfaces or was aggregated in the soil outside the roots, but was not detected inside the roots, root crown, or shoots. Furthermore, we were expecting the µXRF data to provide evidence for Ce inside the roots since our ICP-MS results indicated a relatively high concentration of Ce (479 ± 98 mg/kg) in these roots (Figure 2B). Similarly, our µXRF data is in contrast with the results from other studies on the same CeO2-NPs and treatment (500 mg/kg soil) used in this experiment which showed CeO2-NPs taken up in soy and kidney beans.28,29 However, the absence of Ce particles/aggregates inside the wheat roots, crowns and shoots at high CeO2-NPs concentration (500 mg/kg) in the treatment and long exposure time (90 days of full life cycle) in our study strongly suggests that wheat does not take up or accumulate Ce from CeO2-NPs. Previous reports have also indicated root adsorption of CeO2-NPs and a lack of root-to-shoot translocation of Ce in wheat.2,3,30,31
Figure 5.
Images of thin sections of soil profile collected at harvest from wheat treated with 500 mg CeO2-NPs/kg soil. (A) Image showing the shoots, crown, roots, and rhizosphere. (B) Tricolor µXRF map of shoot (red = Ce3+, green = Ti, blue = Ce4+). (C) Tricolor µXRF map of crown (red = Ce, green = Ti, blue = Ca). (D,E) Tricolor µXRF maps of root 1 (red = Ce, green = Ti, blue = Ca) and root 2 (red = Ce3+, green = Ce4+, blue = Ca), respectively. (C1, C2, D1, D2, E1, E2) Ce µXANES spectra from representative spots on crown, root, and soil. Spectra in red line represents linear combination (LC) fits, and white solid line spectra represents µXANES from the sample. Shoot 1 spots 0 and 1, crown spots 2 and 4, and root 1 spot 4 did not show spectra for cerium. Spectra of the other spots are shown in SI Figure 4.
Micro X-ray absorption near edge spectroscopy (µXANES) analysis of Ce hotspots revealed that Ce was bound as CeO2 maintaining the original CeO2-NP coordination (IV). Ce adsorbed on wheat root surfaces was 92–98% CeO2-NPs with small amount of Ce3+ species (3–7%), whereas, Ce aggregated on soil particles was estimated to be 86–94% CeO2-NPs with small fraction in ionic Ce3+ form (4–12%). These results demonstrate that CeO2-NPs undergo very limited transformation in the wheat/soil system as consistently noted in previous synchrotron studies (SI Table 9).29–32 However, this is the first study to exhibit synchrotron localization and speciation of CeO2-NPs in the rhizosphere (i.e. soil-root interface and areas in root vicinity).
Taken together these findings show that CeO2-NPs are not transformed or accumulated in wheat, which suggests that the modifications in plant development, nitrogen uptake, and isotope discrimination were probably due to either CeO2-NPs affecting root processes and interfering with nutrient uptake or ion transport. Alternatively, CeO2-NPs affected microbial activity and functions in the rhizosphere that altered nutrient cycling in soil-wheat system.
Conclusion
The study revealed that first generation exposure to CeO2-NPs has implications on the physiology, phenology and nutrient composition of second generation wheat plants. With the exception of cerium accumulation in shoots and roots, alterations in all other parameters (growth and productivity, nutrient profile, C and N stable isotope) were due to first generation CeO2-NPs treatment as demonstrated by strong statistical significance of this treatment. Exposure during the first generation only shortened the vegetative period and physiological maturity, without harmful effects on plant productivity. First generation exposure to CeO2-NPs also resulted in concomitant decreases in Ce, Al, Fe and Mn accumulations in roots, and δ15N in grains of second generation plants. Nutrient content in grains was sensitive to multigenerational exposure to Ce-125, while growth and development and C and N concentrations in shoots were altered following repeated Ce-500 treatment. Finally, synchrotron imaging revealed exterior root surface adsorption and soil agglomeration of CeO2-NPs with very limited transformation (i.e., Ce4+ → Ce3+). These findings illustrate important but complex changes induced following CeO2-NPs exposure, and identify possible new research approaches and endpoints for assessing effects of engineered nanomaterials in plants.
Supplementary Material
Acknowledgments
The first author acknowledges funding for first generation seed production from The University of Texas at El Paso, and University of California Center for Environmental Implications of Nanotechnology supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI-0830117. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has been subjected to EPA review and no official endorsement should be inferred. The synchrotron work was supported by a grant from the Advanced Light Source at Lawrence Berkeley National Laboratory. The operations at the Advanced Light Source are supported by the Director, Office of Science, Office of Basic Energy Sciences, US Department of Energy under Contract No. DE-AC02-05CH11231.
Footnotes
Electronic Supplementary Information
Data on elemental, isotopic, and synchrotron analyses, plant growth, and two-way ANOVA. This information is available at http://rsc.org.
References
- 1.Heckert EG, Karakoti AS, Seal S, Self WT. Biomaterials. 2008;29:2705. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du W, Gardea-Torresdey JL, Ji R, Yin Y, Zhu J, Peralta-Videa JR, Guo H. Environ. Sci. Technol. 2015;49:11884. doi: 10.1021/acs.est.5b03055. [DOI] [PubMed] [Google Scholar]
- 3.Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, Gardea-Torresdey JL. J. Agric. Food Chem. 2014;62:9669. doi: 10.1021/jf503526r. [DOI] [PubMed] [Google Scholar]
- 4.Rico CM, Barrios AC, Tan W, Rubenecia R, Lee SC, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-015-4243-y. [DOI] [PubMed] [Google Scholar]
- 5.Marchiol L, Mattiello A, Poscic F, Fellet G, Zavalloni C, Carlino E, Musetti R. Int. J. Environ. Res. Public Health. 2016;13:332. doi: 10.3390/ijerph13030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumdar S, Almeida IC, Arigi EA, Choi H, VerBerkmoes NC, Trujillo-Reyes J, Flores-Margez JP, White JC, Peralta-Videa JR, Gardea-Torresdey JL. Environ. Sci. Technol. 2015;49:13283. doi: 10.1021/acs.est.5b03452. [DOI] [PubMed] [Google Scholar]
- 7.Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Corral Diaz B, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. Plant Physiol. Bioch. 2014;80:128. doi: 10.1016/j.plaphy.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis G, Ryan B, McLaughlin MJ, Kirby JK, Beak D, Chittleborough D. Environ. Sci. Technol. 2011;45:2777. doi: 10.1021/es103769k. [DOI] [PubMed] [Google Scholar]
- 9.Gardea-Torresdey JL, Rico CM, White JC. Environ. Sci. Technol. 2014;48:2526. doi: 10.1021/es4050665. [DOI] [PubMed] [Google Scholar]
- 10.Keller A, McFerran S, Lazareva A, Suh S. J. Nanopart. Res. 2013;15:1692. [Google Scholar]
- 11.Suppan S. Nanomaterials in Soil: Our Future Food Chain? Institute for Agriculture and Trade Policy; 2013. [Google Scholar]
- 12.Servin AD, White JC. NanoImpact. 2016;1:9. [Google Scholar]
- 13.Wang Q, Ebbs SD, Chen Y, Ma X. Metallomics. 2013;5:753. doi: 10.1039/c3mt00033h. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Wang Q, Rossi L, Ebbs SD, White JC. NanoImpact. 2016;1:46. [Google Scholar]
- 15.Wang Z, Xu L, Zhao J, Wang X, White JC, Xing B. Environ. Sci. Technol. 2016;50:6008. doi: 10.1021/acs.est.6b01017. [DOI] [PubMed] [Google Scholar]
- 16.Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z. Environ. Sci. Technol. 2010;44:1962. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory Manual for Physiological Studies of Rice. International Rice Research Institute; Los Banos, Laguna, Philippines: 1976. [Google Scholar]
- 18.Gavlak R, Horneck D, Miller RO. Soil, plant and water reference methods for the western region. 3. Vol. 125. WREP; 2005. [Google Scholar]
- 19.Langner P, Mikutta C, Suess E, Marcus MA, Kretzchmar R. Environ. Sci. Technol. 2013;47:9706. doi: 10.1021/es401315e. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi N, Ohkura T, Takahashi Y, Maegima Y, Arao T. Environ. Sci. Technol. 2014;48:1549. doi: 10.1021/es402739a. [DOI] [PubMed] [Google Scholar]
- 21.Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C. Integr. Comp. Biol. 2012;52:77. doi: 10.1093/icb/ics041. [DOI] [PubMed] [Google Scholar]
- 22.Rao IM, Miles JW, Beebe SE, Horst WJ. Ann. Bot. 2016 doi: 10.1093/aob/mcw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt AC, Mattusch J, Reisser W, Wennrich R. Appl. Organometal. Chem. 2005;19:590. [Google Scholar]
- 24.Brugnoli E, Lauteri M. Plant Physiol. 1991;95:628. doi: 10.1104/pp.95.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrio JP, Voltas J. Manage. Environ. Quality. 2003;14:82. [Google Scholar]
- 26.Ariz I, Cruz C, Neves T, Irigoyen JJ, Garcia-Olaverri C, Nogues S, Aparicio-Tejo PM, Aranjuelo I. Front. Plant Sci. 2015;6:1. doi: 10.3389/fpls.2015.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutter K, Jung K, Krauss GJ. Environ. Sci. Pollut. Res. 2002;9:417. doi: 10.1007/BF02987592. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL. ACS Nano. 2013;7:1415–1423. doi: 10.1021/nn305196q. [DOI] [PubMed] [Google Scholar]
- 29.Majumdar S, Peralta-Videa JR, Trujillo-Reyes J, Sun Y, Barrios AC, Niu G, Flores-Margez JP, Gardea-Torresdey JL. J. Hazard. Mater. 2014;278:279–287. [Google Scholar]
- 30.Schwabe F, Schulin R, Limbach LK, Stark W, Burge D, Nowack B. Chemosphere. 2013;9:512. doi: 10.1016/j.chemosphere.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Viezcas JA, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL. ACS Sustain Chem. Eng. 2016;4:1187. [Google Scholar]
- 32.Zhang P, Ma Y, Liu S, Wang G, Zhang J, He X, Zhang J, R Y, Zhang Z. Environ. Pollut. 2016 doi: 10.1016/j.envpol.2016.10.094. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.