Abstract
Excessive interferon (IFN) production and signaling can lead to immunological and developmental defects giving rise to autoimmune diseases referred to collectively as “type I interferonopathies.” A subset of these diseases is caused by monogenic mutations affecting proteins involved in nucleic acid sensing, homeostasis, and metabolism. Interferonopathic mutations in the cytosolic antiviral sensor MDA5 render it constitutively hyperactive, resulting in chronic IFN production and IFN-stimulated gene expression. Few therapeutic options are available for patients with interferonopathic diseases, but a large number of IFN evasion and antagonism strategies have evolved in viral pathogens that can counteract IFN production and signaling to enhance virus replication. To test the hypothesis that these natural IFN suppressors could be used to subdue the activity of interferonopathic signaling proteins, hyperactive MDA5 variants were assessed for susceptibility to a family of viral MDA5 inhibitors. In this study, Paramyxovirus V proteins were tested for their ability to counteract constitutively active MDA5 proteins. Results indicate that the V proteins are able to bind to and disrupt the signaling activity of these MDA5 proteins, irrespective of their specific mutations, reducing IFN production and IFN-stimulated gene expression to effectively suppress the hyperactive antiviral response.
Keywords: : interferon, interferonopathy, MDA5, paramyxovirus, V protein
Introduction
Type I interferon (IFN, including IFNβ and diverse IFNα subtypes) refers to a family of antiviral cytokines in mammalian cells. The production of and response to IFN is essential for establishing cellular protection against virus infections, and also influences the actions of downstream innate and adaptive immune responses (Stark and others 1998). IFN restricts virus replication by inducing expression of antiviral effectors that degrade host and viral RNAs, inhibit transcription and translation, induce growth arrest, activate apoptosis, and trigger autophagy pathways that together lead to virus suppression and infection clearance (Stark and others 1998; McNab and others 2015). IFN is also an immune modulator, regulating the activity of dendritic cells, macrophages, B cells, T cells, and natural killer cells to counteract infections and stimulate lasting immunity (Tough and others 1996; Trinchieri 2010; Kiefer and others 2012; Tough 2012; McNab and others 2015).
IFN production is stimulated by virus infection or accumulation of nonself nucleic acids from both extracellular and intracellular sources. Antiviral signaling is initiated when a pattern recognition receptor (PRR) engages a nonself nucleic acid. For viral RNAs, transmembrane PRRs include Toll-like receptors (TLRs), TLR3, TLR7, and TLR8 (Kawasaki and Kawai 2014), and intracellular PRRs include the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) family members RIG-I, MDA5, and LGP2 (Bruns and Horvath 2014). The PRRs activate downstream signaling that leads to activation and nuclear import of the master transcription regulators, IRF3 and NFκB, driving the production of diverse primary antiviral effectors, including IFNs (Freaney and others 2013).
IFNs are secreted from the cell and bind to a transmembrane receptor to induce tyrosine phosphorylation of STAT1 and STAT2, which form a heterotrimeric transcription factor with the DNA binding subunit IRF9 known as the interferon-stimulated gene factor 3 (ISGF3) (Fu and others 1990; Kessler and others 1990). ISGF3 translocates to the nucleus and binds to IFN-stimulated response element (ISRE) sequences present in hundreds of interferon-stimulated gene (ISG) promoters to induce their transcription (Reich and others 1987; Levy and others 1988). The ISG products combine to establish the cellular antiviral state that prevents virus replication and stimulates professional innate and adaptive immune cell activation.
The antiviral activities of the IFN response are usually rapid and transient, and unregulated IFN production can lead to cellular, immunological, and developmental defects (Gresser and others 1980). Increased type I IFN production and signaling and a signature of chronically upregulated ISG expression characterizes several diseases, including systemic lupus erythematosus (SLE) (Baechler and others 2003; Bennett and others 2003; Crow and Wohlgemuth 2003), rheumatoid arthritis (van der Pouw Kraan and others 2007), type 1 diabetes (Ferreira and others 2014), and Aicardi–Goutieres syndrome (AGS) (Rice and others 2013; Crow and others 2015). While many mechanisms have been proposed to account for the IFN signature, recent characterization of monogenic inborn diseases have elucidated several heritable mechanisms for enhanced IFN production and signaling (Crow 2011).
Defects in cellular machinery involved in nucleic acid metabolism and homeostasis have been identified in interferonopathic diseases. TREX1, a 3′-5′ DNA exonuclease, is mutated in SLE and AGS patients causing a loss of exonuclease activity and increase in cellular single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) (Crow and others 2006a; Fye and others 2011). The accumulation of DNA results in chronic activation of DNA damage checkpoint signaling (Crow and others 2006a; Yang and others 2007; Fye and others 2011). Mutations in the DNA endonucleases, DNase I (Martinez Valle and others 2008) and DNase II (Rodero and others 2017), have been identified in patients. Reduced activity prevents clearing of chromatin breakdown by-product and stimulates production of anti-DNA nucleoprotein antibodies.
SAMHD1, a triphosphohydrolase, normally degrades deoxyribonucleoside triphosphates (dNTPs) (Kretschmer and others 2015). Mutations in SAMHD1 in AGS patients result in elevated cellular dNTP concentrations that trigger cellular stress responses and senescence (Rice and others 2009; Kretschmer and others 2015). The RNA endonuclease, RNASE H2, is essential for removing ribonucleotides from the genome and degrading RNA:DNA hybrids (Crow and others 2006b; Hiller and others 2012). Loss of function mutations in RNASE H2 in patients with AGS and SLE (Crow and others 2006b; Gunther and others 2015) result in chronic DNA damage signaling and inflammatory signaling through the DNA sensor cyclic GMP-AMP synthase (cGAS) and its adaptor STING (Hiller and others 2012; Mackenzie and others 2016).
Mutations to the adenosine deaminase acting on RNA 1, ADAR1, are linked to AGS (Rice and others 2012). The mutant ADAR1 loses its ability to modify cellular double-stranded RNAs (dsRNAs), such as retroelements, which normally prevents immune recognition (Nishikura 2010; Liddicoat and others 2016). Instead, accumulated unmodified endogenous dsRNAs trigger antiviral signaling and IFN production through the RLR pathway (Zhao and others 2018). Increased expression of endogenous retroelements, such as LINE1, is also thought to stimulate IFN production (Mavragani and others 2016). LINE1 expression and activity are regulated by pathways involving ADAR1, TREX1, and SAMHD1 (Zhao and others 2013, 2018; Orecchini and others 2017; Choi and others 2018).
In addition to defects that contribute to imbalances in endogenous nucleic acid accumulation and processing, nucleic acid sensing PRRs themselves have also been implicated in type I interferonopathies (Kato and others 2017). Analysis of single nucleotide polymorphisms and genetic linkage have implicated TLRs (Laska and others 2014; Wang and others 2014; Wu and others 2015), and gain of function mutations were identified in RIG-I (Jang and others 2015). Mutations to MDA5 have been identified repeatedly in patients, and gain of MDA5 function is not only more prevalent, but has also been mechanistically characterized (Funabiki and others 2014; Oda and others 2014; Rice and others 2014; Rutsch and others 2015; Van Eyck and others 2015).
MDA5 is comprised of a central conserved DECH-box helicase region, an N-terminal caspase activation and recruitment domain (CARD) region, a pincer domain and a C-terminal domain (Fig. 1A) (Cordin and others 2006; Wu and others 2013). The helicase domain is divided into domain 1 (Hel1) containing the conserved motifs Q, I, II, and III, the insertion domain (Hel2i), and domain 2 (Hel2) containing the conserved motifs IV, V, and VI (Cordin and others 2006; Wu and others 2013). The helicase domain has intrinsic RNA-binding and adenosine triphosphate (ATP) hydrolysis activities, enabling MDA5 to recognize nonself dsRNAs and form oligomers in an ATP-dependent process (Peisley and others 2011, 2012; Wu and others 2013; Bruns and others 2014). Upon oligomerization, the CARD region contacts the downstream antiviral signaling protein, MAVS, to induce the activation of IRF3 and NFκB transcription factors that lead to the production of IFN.
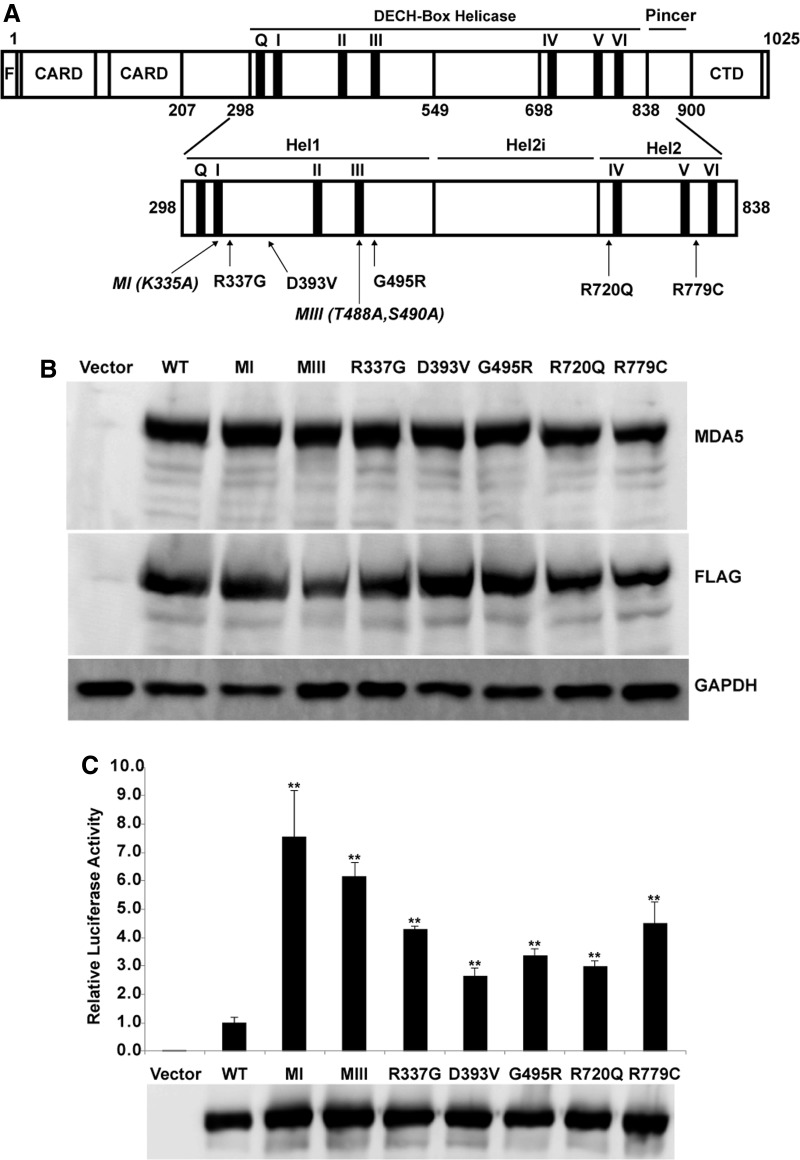
FIG. 1.
Construction and characterization of mutant MDA5 proteins. (A) A diagram illustrating MDA5 domain structure, including FLAG (F) epitope tag, CARDs, DECH-box helicase domain, pincer domain, and CTD. The MDA5 minimal V protein-binding region coincides with the Hel2 domain within the helicase domain. The expanded view of the helicase domain below shows the relative locations of the naturally arising activating mutations (R337G, D393V, G495R, R720Q, and R779C), as well as the laboratory-generated mutations [italicized, MI (K335A) and MIII (T488A, S490A)]. (B) HEK293T cells were transfected with empty vector, WT, or CA-MDA5 and assayed by immunoblotting with antibodies for MDA5, FLAG epitope tag, and GAPDH. (C) HEK293T cells were transfected with empty vector, WT, or CA-MDA5 as indicated along with an IFNβ promoter-driven luciferase reporter and a Renilla luciferase vector for normalization. After 24 h, cells were lysed and assayed for luciferase activity. Data are reported relative to WT MDA5 signal normalized to 1. Bars indicate average values (n = 3) with error bars representing standard deviation. Statistical analysis was done using a 2-tailed Student's t-test. (**P < 0.005) Immunoblot analysis indicates similar expression between WT MDA5 and CA-MDA5. CA-MDA5, constitutively active MDA5; CARD, caspase activation and recruitment domain; CTD, C-terminal domain; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN, interferon; MI, motif I; MIII, motif III; WT, wild type.
In type I interferonopathies, including SLE and AGS, several mutations have been identified within the helicase domain of MDA5 (Funabiki and others 2014; Oda and others 2014; Rice and others 2014; Rutsch and others 2015; Van Eyck and others 2015). These mutations result in more stable dsRNA binding and oligomerization that leads to both constitutive and hyperactive IFN production and signaling activity (Rice and others 2014). The interferonopathic MDA5 mutations may thereby result in a loss of tolerance to endogenous retroelements and stimulate constitutive IFN production (Ahmad and others 2018). Most patient-isolated MDA5 variants retain ATP hydrolysis activity, but at least 1 mutation, R337G, leads to a decrease in ATP hydrolysis (Rice and others 2014). These properties resemble those of constitutively active MDA5 (CA-MDA5) variants generated in the laboratory during systematic investigations of RNA binding, ATP hydrolysis, and IFN stimulation by all the RLRs (Bamming and Horvath 2009). Specific alterations to MDA5 helicase domain motif I (MI) and motif III (MIII) were found that led to constitutive and hyperactive IFN production despite the loss of ATP hydrolysis.
In the course of their evolution, RNA viruses have developed a number of strategies to escape, avoid, or antagonize host immune surveillance and antiviral response (Gotoh and others 2002; Gerlier and Lyles 2011). In many cases, viral strategies have been described that impinge on IFN production or responses. One large family of enveloped negative-strand RNA viruses, the Paramyxoviridae, is known to interfere with both IFN production and IFN signaling (Ramachandran and Horvath 2009; Versteeg and Garcia-Sastre 2010). For many viruses in this family, IFN evasion and antagonism is mediated in part or whole by nonstructural proteins known as V proteins.
The Paramyxovirus V proteins are recognizable by the presence of a highly conserved cysteine-rich C-terminal zinc-binding finger domain that forms a structural module that is linked to suppression of IFN production. V proteins use this conserved domain to directly interfere with cellular MDA5. Despite high amino acid sequence conservation of the RLRs within a defined minimal V protein-binding region (MVBR), V proteins show specificity in their ability to bind to and antagonize MDA5 and its associate, LGP2, but they do not bind to the analogous region of RIG-I (Parisien and others 2009; Rodriguez and Horvath 2013, 2014). Evidence regarding the mechanism of V protein-mediated MDA5 suppression suggests that V protein interferes with MDA5 dsRNA interaction (Childs and others 2009) and prevents MDA5 ATP hydrolysis (Parisien and others 2009). This specific MDA5 targeting capacity of Paramyxovirus V proteins suggested a hypothesis that they could be used to antagonize or suppress interferonopathic MDA5 signaling. To test this concept, both laboratory and patient-derived CA-MDA5 proteins were generated, characterized for IFN and ISG expression, and assessed for sensitivity to V proteins from PIV5, mumps virus, measles virus, Nipah virus, and Hendra virus. Results indicate that Paramyxovirus V proteins are effective inhibitors of CA-MDA5 proteins that can significantly suppress interferonopathic activity.
Materials and Methods
Cells and viruses
HEK293T and 2fTGH cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies, Thermo Fisher Scientific) supplemented with 10% Cosmic calf serum (HyClone, GE Healthcare Life Sciences) and 1% Penicillin–Streptomycin (Gibco, Thermo Fisher Scientific). Vesicular Stomatitis Virus (VSV, Indiana strain) was titered and propagated on U3A cells.
Plasmids
Wild-type (WT) MDA5 was cloned into the p3xFLAG-CMV-10 vector as previously described to provide an N-terminal FLAG epitope tag (Rodriguez and Horvath 2014). Point mutations in this plasmid were created using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). Paramyxovirus V proteins from PIV5, mumps virus, measles virus, Nipah virus, and Hendra virus were cloned into the pEF-HA vector as previously described to provide an N-terminal HA epitope tag (Rodriguez and others 2002, 2003; Ulane and others 2005; Ramachandran and others 2008).
Immunoblotting
Cell extracts for immunoblotting were prepared by lysing samples in whole cell extract buffer (WCEB; 50 mM Tris, pH 8.0, 280 mM NaCl, 0.5% IGEPAL, 0.2 mM EDTA, 2 mM EGTA, 10% glycerol, 1 mM DTT, and 0.1 mM NaVO4) supplemented with protease inhibitor cocktail set III (MilliporeSigma). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, probed with antibodies and visualized by chemiluminescence (PerkinElmer, Inc.). The antibodies used were anti-FLAG (Cat. No. F3165; MilliporeSigma), anti-HA (Cat. No. H3663; MilliporeSigma), anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH; Cat. No. sc-47724; Santa Cruz Biotechnology), and anti-MDA5 (monoclonal antibody generated at Northwestern University) (Bamming and Horvath 2009).
Luciferase reporter gene assay
HEK293T cells were seeded in 24-well tissue culture plates and transfected using the Lipofectamine 2000 reagent (Invitrogen, Thermo Fisher Scientific). The −110 IFNβ promoter-driven firefly luciferase reporter was cotransfected with a Renilla luciferase plasmid for signal normalization. The MDA5 plasmids were transfected at 25 ng/well and the V protein plasmids at 75 ng/well. Luciferase activity was measured 24 h after transfection with the Dual Luciferase Reporter Assay (Promega Corporation). Data are plotted as average values (n ≥ 3) with error bars representing the standard deviation. Statistical analysis was done using a 2-tailed Student's t-test.
Coimmunoprecipitation
HEK293T cells were seeded in 10-cm tissue culture plates and transfected using standard calcium phosphate procedures. Each plate was transfected with 5 μg of the MDA5 plasmids along with 5 μg of each V protein. In some coprecipitation experiments, V proteins that could readily be distinguished by their SDS-PAGE electrophoretic mobilities were multiplexed, that is, measles virus V (33 kDa) and PIV5 virus V (25 kDa) or Hendra virus V (51 kDa) and mumps virus V (25 kDa). Lysates for immunoprecipitation were prepared in WCEB. After preclearing with Sepharose beads, 10% of the clarified lysate was analyzed directly and the rest was subjected to immunoprecipitation. Protein complexes were purified by overnight incubation with the FLAG M2 affinity resin (MilliporeSigma) and washed with WCEB. After elution with SDS, proteins were analyzed by immunoblotting.
RNA isolation and gene expression analysis
Gene expression analysis was done by reverse transcription–quantitative polymerase chain reaction (RT-qPCR). Total RNA was extracted using the TRIzol Reagent (Invitrogen, Thermo Fisher Scientific). Samples were treated with DNAse I (Invitrogen, Thermo Fisher Scientific) and 1 μg of total RNA was random primed and reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen, Thermo Fisher Scientific). Gene expression was measured by quantitative real-time PCR and normalized to GAPDH. Data are representative of ≥3 replicate experiments and plotted as average value of technical replicates (n = 3) with error bars representing standard deviation in technical replicates. Statistical analysis was done using a 2-tailed Student's t-test. Primers used for this were as follows: CCL5 (F: 5′-CTGCTTTGCCTACATTGCCC-3′, R: 5′-TCGGGTGACAAAGACGACTG-3′); GAPDH (F: 5′-ACAGTCAGCCGCATCTTCTT-3′, R: 5′-ACGACCAAATCCGTTGACTC-3′); IFI6 (F: 5′-CTGTGCCCATCTATCAGCAG-3′, R: 5′-GCAGGTAGCACAAGAAAAGC-3′); IFIT1 (F: 5′-CAGAACGGCTGCCTAATTT-3′, R: 5′-GGCCTTTCAGGTGTTTCAC-3′); IFNβ (F: 5′-ACGCCGCATTGACCATCTAT-3′, R: 5′-AGCCAGGAGGTTCTCAACAA-3′); ISG15 (F: 5′-GACCTGACGGTGAAGATGCT-3′, R: 5′-CGATCTTCTGGGTGATCTGC-3′); and RSAD2 (F: 5′-CCTGTCCGCTGGAAAGTGTT-3′, R: 5′-GACACTTCTTTGTGGCGCTC-3′).
Antiviral cytopathic effect assay
Cytopathic effect (CPE) assays were carried out essentially as described (Bamming and Horvath 2009). HEK293T cells were seeded in 10-cm tissue culture plates and transfected using standard calcium phosphate procedures. Supernatants from HEK293T cells transfected with various MDA5 proteins alone and along with PIV5 V protein were harvested and used to treat 2fTGH cells. As a control, cells were also directly treated with recombinant IFNα (Roche). Following an 8-h incubation, 2fTGH cells were infected with VSV at 6 × 103 plaque-forming units/well in serum-free medium for 1 h. Medium was changed and cells incubated for 17 h before fixing with 4% formaldehyde and staining with 0.3% Crystal Violet in 50% ethanol.
Results
Construction and characterization of CA-MDA5 proteins
MDA5 mutations that confer hyperactive signaling profiles have been identified in patients with interferonopathic diseases, as well as in laboratory experiments designed to characterize the MDA5 catalytic domains. Seven CA-MDA5 mutations were generated using a well-characterized N-terminal FLAG epitope-tagged WT human MDA5 complementary DNA (cDNA) as the template for site-directed mutagenesis (Fig. 1A) (Bamming and Horvath 2009; Rodriguez and Horvath 2013; Bruns and others 2014).
Specifically, 5 of these CA-MDA5 mutants (R337G, D393V, G495R, R720Q, and R779C), were identified in patients characterized with an IFN signature (Oda and others 2014; Rice and others 2014). All 5 of the patient-derived CA-MDA5 proteins can induce the IFNβ promoter reporter gene in the absence of virus infection or dsRNA stimulation, and each bind RNA, but one of the mutations, R337G, is defective for ATP hydrolysis activity. The other 2 CA-MDA5 mutations, a point mutation to helicase domain MI (K335A) or a double mutation to helicase domain MIII (T488A/S490A) are defective in ATP hydrolysis activity, yet are able to strongly induce IFN gene expression in human and murine cell lines in the absence of virus infection or dsRNA stimulation (Bamming and Horvath 2009).
To establish a baseline to compare the activities of the mutant MDA5 proteins, their expression and activity were compared with WT MDA5 following transfection into HEK293T cells. All of the mutant proteins accumulated to similar levels as the expressed WT MDA5, as demonstrated in immunoblotting experiments with antibodies for MDA5 as well as FLAG epitope tag (Fig. 1B).
The signaling capacity of the MDA5 proteins was evaluated using an IFNβ promoter-driven reporter gene assay. Protein expression vectors were cotransfected into cells along with the IFNβ firefly luciferase reporter gene and a normalization control Renilla luciferase. Expression of WT MDA5 stimulated reporter gene expression, giving rise to a 77-fold activation. Strikingly, all of the MDA5 mutations conferred hyperactive IFNβ promoter activity, ranging from 7.5-fold increase over WT MDA5 for the most active mutant (MI) to 2.6-fold increase over WT MDA5 for the least active mutant (D393V) (Fig. 1C). The relative hyperactivity of these MDA5 proteins is highly reproducible and establishes a rank order of constitutive activity: MI ≅ MIII >> R337G ≅ R779C > D393V ≅ G495R ≅ R720Q >> WT.
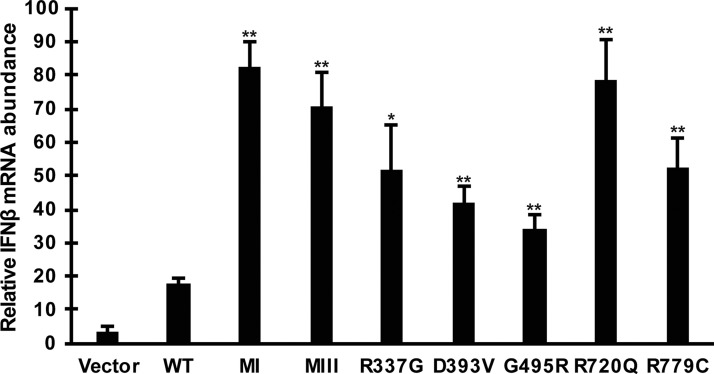
Additional characterization of these mutant MDA5 proteins was obtained from analysis of endogenous IFNβ gene expression. Total RNA was prepared from WT and CA-MDA5-expressing cells and IFNβ messenger RNA (mRNA) was measured by RT-qPCR (Fig. 2). Transfection efficiency in these samples was assessed to be >90% by GFP expression in parallel samples. MDA5 expression activates the expression of IFNβ, leading to a 5-fold stimulation over cells without MDA5 expression. All of the CA-MDA5 proteins exhibited hyperactivity in endogenous gene induction, ranging from 4.6-fold over WT MDA5 for the MI mutant to 1.9-fold for the G495R mutant, which was equal to the D393V mutant in exhibiting the lowest level of hyperactivity in IFNβ mRNA stimulation. Unexpectedly, the differences among the CA-MDA5 proteins in endogenous gene regulation were not as dramatic or variable compared with the reporter gene assay. This was most dramatically for R720Q, possibly indicating the influence of endogenous cellular mechanisms that function to attenuate or suppress the IFNβ response.
FIG. 2.
CA-MDA5 mutants increase endogenous IFNβ mRNA expression. Control empty vector, WT, or CA-MDA5 were expressed in HEK293T cells. Total RNA was harvested and analyzed by RT-qPCR. Data are representative of ≥3 replicate experiments and are shown normalized to GAPDH expression. Bars indicate average values of technical replicates (n = 3) with error bars representing standard deviation. Statistical analysis was done using a 2-tailed Student's t-test (*P < 0.05, **P < 0.005). mRNA, messenger RNA; RT-qPCR, reverse transcription–quantitative polymerase chain reaction.
V proteins bind to CA-MDA5 proteins
Paramyxovirus V protein suppression of MDA5 relies on direct protein–protein interaction through contact with the helicase domain C-terminal lobe (Andrejeva and others 2004; Parisien and others 2009). To test the ability of V proteins to associate with CA-MDA5 proteins, V proteins from diverse Paramyxoviruses were used in coimmunoprecipitation assays. Specifically, 5 V proteins from the Rubulaviruses, PIV5 and mumps virus; the Morbilliviruses, measles virus; and the Henipaviruses, Nipah virus and Hendra virus, were expressed in cells from vectors containing in-frame N-terminal HA epitope tags along with each of the FLAG epitope-tagged MDA5 proteins (Rodriguez and others 2002, 2003; Ulane and others 2005; Ramachandran and others 2008). Whole cell lysates were prepared and subjected to FLAG/M2 antibody immunoaffinity purification followed by immunoblotting with HA antibody to detect coprecipitation. All 5 of the tested V proteins were found to coprecipitate with all of MDA5 proteins irrespective of their mutations (Fig. 3).
FIG. 3.
V proteins bind CA-MDA5 mutants. HA-epitope-tagged V protein from PIV5, Nipah virus, measles virus, Hendra virus, or mumps virus was coexpressed with FLAG epitope-tagged WT or CA-MDA5 or the empty vector as indicated. Cell lysates were subjected to FLAG immunoprecipitation and assayed by immunoblot with antibody for the HA epitope tag to determine if the V protein coimmunoprecipitated with MDA5.
V proteins suppress CA-MDA5 signal transduction
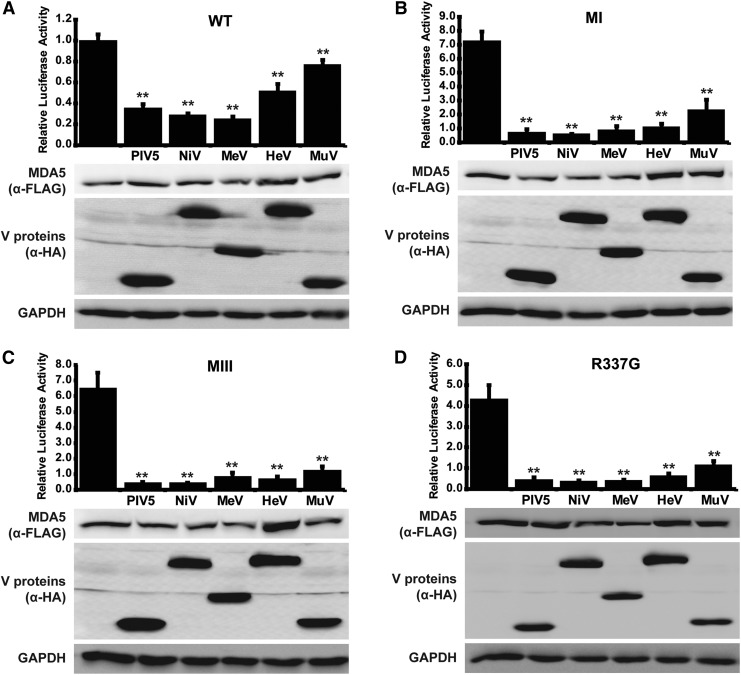
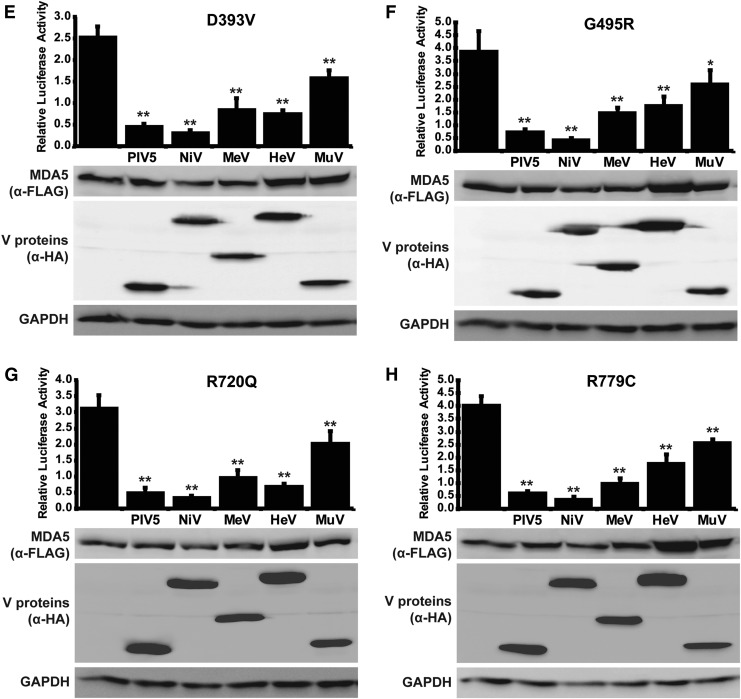
To confirm that the coprecipitation results in MDA5 suppression, signaling activity was measured with the IFNβ promoter luciferase reporter gene assay. WT or CA-MDA5 proteins were coexpressed with the Paramyxovirus V proteins and luciferase activity determined. In all cases, MDA5 expression was able to activate the reporter gene, with similar relative potency as noted in Fig. 1C, and this MDA5-mediated transcriptional activity was decreased by the presence of V proteins (Fig. 4). A specific order of inhibitory impact was found, with the PIV5 and Nipah virus V proteins most effective at inhibiting MDA5, irrespective of the activating mutation. Measles virus V protein was equally effective as PIV5 and Nipah virus V protein in suppressing WT MDA5 (Fig. 4A), as well as CA-MDA5 proteins MI, MIII, and R337G (Fig. 4B–D), but was less effective against D393V, G495R, R720Q, and R779C (Fig. 4E–H). Hendra virus and Mumps virus V proteins were the poorest inhibitors in this assay, but still mediated significant degree of interference with all the CA-MDA5 proteins.
FIG. 4.
V proteins suppress CA-MDA5 mutant signaling. (A) WT, or (B) CA-MDA5 were cotransfected with an IFNβ promoter-driven luciferase reporter and a control Renilla luciferase in the presence and absence of PIV5, Nipah virus (NiV), measles virus (MeV), Hendra virus (HeV), or mumps virus (MuV) V protein. After 24 h, cells were lysed and assayed for luciferase activity. Data are reported relative to WT MDA5 signal in the absence of V protein normalized to 1. Bars indicate average values (n ≥ 3) with error bars representing standard deviation. Statistical analysis was done using a 2-tailed Student's t-test. (*P < 0.05, **P < 0.005) Corresponding immunoblots of cell lysates assayed with anti-FLAG antibody for MDA5 expression and anti-HA antibody for V protein expression show similar protein expression for all samples.
PIV5 V protein suppresses CA-MDA5-mediated IFNβ gene expression
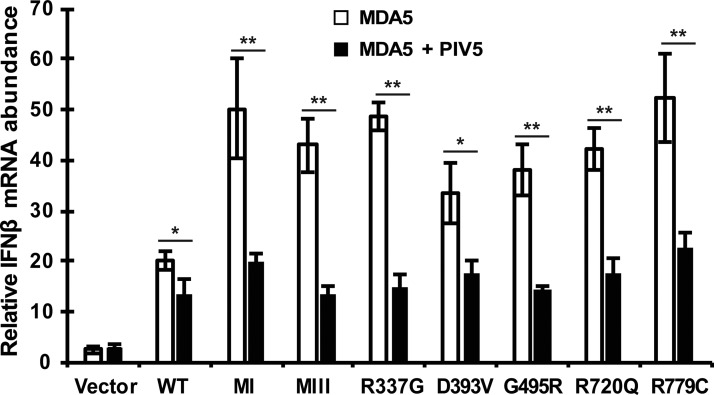
Of the V proteins tested, the PIV5 V protein was found to be uniformly effective at suppressing CA-MDA5 proteins in the reporter assay. To verify the observed suppression of CA-MDA5 protein activity, the ability of PIV5 V protein to interfere with MDA5 induction of endogenous IFNβ gene expression was tested. PIV5 V protein was coexpressed along with WT or CA-MDA5, and total RNA was subjected to RT-qPCR analysis of IFNβ mRNA. The PIV5 V protein interfered with the activity of all the CA-MDA5 proteins as indicated by greatly reduced IFNβ gene expression (Fig. 5).
FIG. 5.
PIV5 V protein suppresses CA-MDA5-induced IFNβ expression. Empty vector, WT, or CA-MDA5 were expressed in HEK293T cells in the presence and absence of PIV5 V protein. Total RNA was collected and analyzed for IFNβ mRNA expression by RT-qPCR. Data are representative of ≥3 replicate experiments and are shown normalized to GAPDH expression. Bars indicate average values of technical replicates (n = 3) with error bars representing standard deviation. Statistical analysis was done using a 2-tailed Student's t-test (*P < 0.05, **P < 0.005).
PIV5 V protein suppresses interferonopathic gene expression
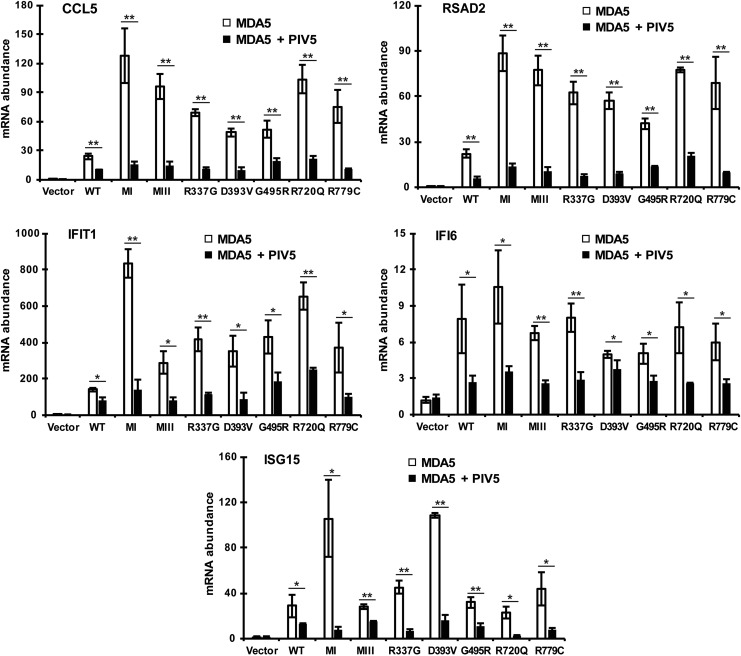
Patients with type I interferonopathies are characterized by elevated antiviral gene expression signatures even in the absence of infection, as diagnosed by small panels of ISG expression (Baechler and others 2003; Bennett and others 2003). To test the impact of V proteins on ISG responses, a panel of ISGs was measured in cells expressing WT or CA-MDA5 proteins alone and in combination with PIV5 V protein. Following protein expression, RNA was prepared for RT-qPCR using primers for the well-known ISGs CCL5, RSAD2, IFIT1, IFI6, and ISG15 (Schoggins and others 2011). All ISGs tested were activated by WT or CA-MDA5 expression, with similar extent of hyperactivity as observed for IFNβ mRNA induction. The ISG activation induced by all MDA5 proteins was suppressed robustly by coexpressed PIV5 V protein (Fig. 6).
FIG. 6.
PIV5 V protein suppresses CA-MDA5-induced ISG expression. Empty vector, WT, or CA-MDA5 were expressed in HEK293T cells in the presence and absence of PIV5 V protein. Total RNA was collected and analyzed for CCL5, RSAD2, IFIT1, IFI6, and ISG15 mRNA expression by RT-qPCR. Data are representative of ≥3 replicate experiments and are shown normalized to GAPDH expression. Bars indicate average values of technical replicates (n = 3) with error bars representing standard deviation. Statistical analysis was done using a 2-tailed Student's t-test (*P < 0.05, **P < 0.005). ISG, interferon-stimulated gene.
PIV5 V protein suppresses hyperactive antiviral response
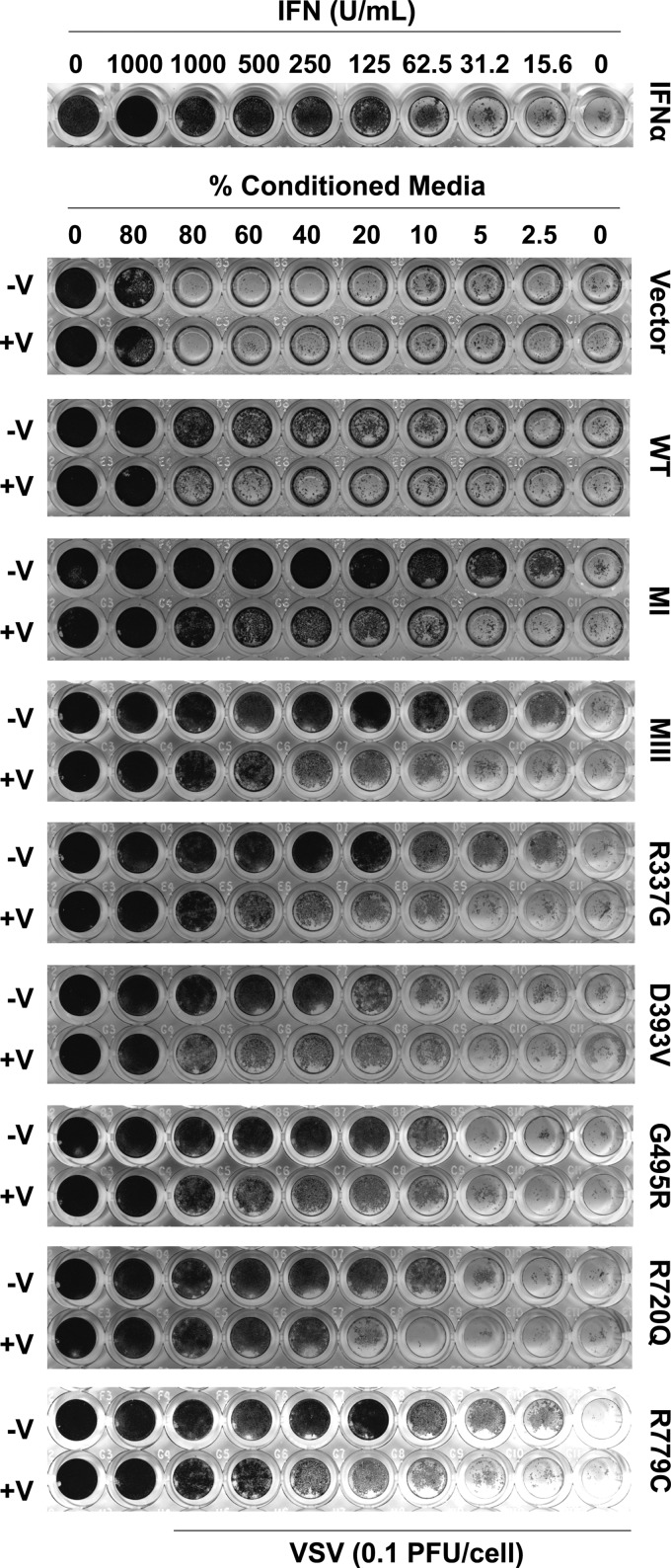
To further validate the suppression of CA-MDA5, a biological endpoint assay was tested. MDA5-mediated activation of IFN production is a first step in establishing the cellular antiviral state, and this can be evaluated using an antiviral CPE assay (Bamming and Horvath 2009). Conditioned medium was harvested from cells expressing WT or CA-MDA5 proteins both in the presence and in the absence of PIV5 V protein, and then applied to freshly plated 2fTGH cells for 8 h. These cells were then inoculated with VSV for 18 h. Medium from the MDA5 expressing cells, containing secreted IFN, protected the 2fTGH cells from VSV-induced cell death, and the CA-MDA5 mutants induced greater antiviral protection than WT MDA5 with similar rank order efficacy as observed in prior assays (Fig. 7). Comparison to a standard titration of IFNα-mediated CPE protection under these conditions shows that WT MDA5 produced an equivalent of 156.25 U/mL activity, whereas the most active CA-MDA5, MI, produced an equivalent of 2,500 U/mL of activity. The least protective CA-MDA5 D393V still produced 312.5 U/mL of activity. Coexpression of PIV5 V protein decreased protection from VSV-induced cytopathicity irrespective of the specific MDA5 mutation, consistent with the ability to interfere with MDA5 signal transduction.
FIG. 7.
PIV5 V protein suppresses antiviral response of CA-MDA5 mutants. Conditioned medium from HEK293T cells expressing empty vector, WT, or CA-MDA5 in the presence and absence of PIV5 V was collected and used to treat 2fTGH cells for 8 h. Cells were infected with VSV for 18 h, fixed, and stained to assay protection from virus-induced cytopathicity. As a control, 2fTGH cells were directly treated with IFNα for 8 h before virus infection. VSV, Vesicular Stomatitis Virus.
Discussion
Hosts and viruses have coevolved, with hosts developing innate immune strategies to combat viruses and viruses developing mechanisms to evade and antagonize the host innate immune system. As we look to develop treatments for human immune diseases, we should exploit these natural antagonists to benefit human health. One particularly interesting viral protein is the Paramyxovirus V protein that has evolved to prevent IFN antiviral responses (Ramachandran and Horvath 2009; Versteeg and Garcia-Sastre 2010). The V proteins share a common target to inhibit IFN production, the antiviral sensor MDA5 (Childs and others 2007; Ramachandran and Horvath 2009). In this study, the ability of Paramyxovirus V proteins to antagonize CA-MDA5 variants was tested as a potential means to control chronic IFN production. Results indicate that diverse Paramyxovirus V proteins were able to interact with both patient-derived and laboratory-generated CA-MDA5 proteins and suppress their chronic signaling and antiviral activities. The V proteins not only reduced IFN and ISG expression levels, but they also suppressed heightened antiviral responses observed in cells harboring CA-MDA5.
These results indicate that V proteins are an attractive system for developing new means to suppress MDA5-induced interferonopathic syndromes. As V proteins not only target MDA5-mediated IFN production, but also IFN-JAK-STAT signaling, they have the ability to counteract toxic effects of excessive IFN production and chronic IFN-stimulated gene expression (Ramachandran and Horvath 2009). This feature of V proteins can be seen in the increased suppression of ISG expression by PIV5 V protein (Fig. 6) compared with the suppression of IFNβ expression (Fig. 5). PIV5 V protein not only targets MDA5 but it also induces degradation of STAT1, likely leading to increased ISG suppression (Didcock and others 1999).
Interestingly, the diverse V proteins exhibited differential inhibition of MDA5 signaling (Fig. 4). Although the V protein C-terminal domains are highly conserved, previous work has determined that there are universal and virus-specific requirements for residues in MDA5 engagement (Ramachandran and Horvath 2010). PIV5 and Nipah virus V proteins do not require the first conserved histidine or the first conserved cysteine for MDA5 interference, whereas the mumps virus and measles virus V proteins require both of these residues (Ramachandran and Horvath 2010). These virus-specific requirements could indicate a difference in affinity for MDA5 and explain why PIV5 and Nipah virus V proteins are more potent inhibitors of MDA5 signaling than the V proteins of measles virus, Hendra virus, and mumps virus.
In this study, we used full-length V proteins to analyze their potential in antagonizing interferonopathic MDA5. Previous research has shown that the conserved V protein C-terminal domain is sufficient to interact with MDA5 and inhibit signaling (Andrejeva and others 2004; Childs and others 2007, 2009; Parisien and others 2009).
All the V proteins require the first conserved arginine in the C-terminal domain for MDA5 interaction and interference (Ramachandran and Horvath 2010), suggesting that peptides surrounding this region may retain MDA5 suppression activity. Dissection of minimal inhibitory regions is expected to define short peptides for inhibition of CA-MDA5 to be used as a starting point for rational design of compounds and assays that could yield small molecule inhibitors of MDA5 signaling.
In addition to providing proof of concept for V protein interference with CA-MDA5, this study refines our understanding of the mechanisms underlying V protein inhibition of MDA5. It was previously shown that V protein can interfere with MDA5 dsRNA binding (Childs and others 2009) as well as ATP hydrolysis (Parisien and others 2009). Three CA-MDA5 proteins analyzed (MI, MIII, and R337G) are defective for ATP hydrolysis, yet they are still suppressed by V proteins, suggesting that the V protein interference with ATP hydrolysis is a consequence of interaction that may not be directly linked to signaling inhibition. Further work is required to address both the mechanistic basis for hyperactivity in CA-MDA5 proteins, and the roles that ATP hydrolysis plays in MDA5 signaling.
This study provides an initial proof of the concept that viral IFN evasion proteins could be harnessed as effective antagonists of type I interferonopathies. Moreover, such a therapeutic strategy would not be limited for use only in patients with CA-MDA5 mutations. Interferonopathies caused by ADAR1 mutations develop due to increased recognition of unmodified endogenous retroelements by WT MDA5 (Ahmad and others 2018), and mutations in TREX1, SAMHD1, and RNase H2 lead to overexpression of endogenous LINE1 RNA that triggers signaling through WT MDA5 (Zhao and others 2013, 2018; Li and others 2017; Choi and others 2018). Even in these cases, targeting of MDA5 by V proteins would counteract overactive IFN and ISG expression.
Acknowledgments
The authors are grateful to members of the Horvath Laboratory for critical comments and helpful suggestions. This study was supported by NIH grants, AI073919, AI50707, and GM111652, to C.M.H. R.M. was supported by a predoctoral fellowship from the NIH Training Program in Viral Replication T32AI60523.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmad S, Mu X, Yang F, Greenwald E, Park JW, Jacob E, Zhang CZ, Hur S. 2018. Breaching self-tolerance to Alu duplex RNA underlies MDA5-mediated inflammation. Cell 172(4):797.e13–810.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101(49):17264–17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100(5):2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamming D, Horvath CM. 2009. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem 284(15):9700–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197(6):711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns AM, Horvath CM. 2014. Antiviral RNA recognition and assembly by RLR family innate immune sensors. Cytokine Growth Factor Rev 25(5):507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns AM, Leser GP, Lamb RA, Horvath CM. 2014. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol Cell 55(5):771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs K, Stock N, Ross C, Andrejeva J, Hilton L, Skinner M, Randall R, Goodbourn S. 2007. mda-5, But not RIG-I, is a common target for paramyxovirus V proteins. Virology 359(1):190–200 [DOI] [PubMed] [Google Scholar]
- Childs KS, Andrejeva J, Randall RE, Goodbourn S. 2009. Mechanism of mda-5 Inhibition by paramyxovirus V proteins. J Virol 83(3):1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Hwang SY, Ahn K. 2018. Interplay between RNASEH2 and MOV10 controls LINE-1 retrotransposition. Nucleic Acids Res 46(4):1912–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordin O, Banroques J, Tanner NK, Linder P. 2006. The DEAD-box protein family of RNA helicases. Gene 367:17–37 [DOI] [PubMed] [Google Scholar]
- Crow MK, Wohlgemuth J. 2003. Microarray analysis of gene expression in lupus. Arthritis Res Ther 5(6):279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ. 2011. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 1238:91–98 [DOI] [PubMed] [Google Scholar]
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GM, Gornall HL, Oojageer A, Anderson B, Pizzino A, Helman G, Abdel-Hamid MS, Abdel-Salam GM, Ackroyd S, Aeby A, Agosta G, Albin C, Allon-Shalev S, Arellano M, Ariaudo G, Aswani V, Babul-Hirji R, Baildam EM, Bahi-Buisson N, Bailey KM, Barnerias C, Barth M, Battini R, Beresford MW, Bernard G, Bianchi M, Billette de Villemeur T, Blair EM, Bloom M, Burlina AB, Carpanelli ML, Carvalho DR, Castro-Gago M, Cavallini A, Cereda C, Chandler KE, Chitayat DA, Collins AE, Sierra Corcoles C, Cordeiro NJ, Crichiutti G, Dabydeen L, Dale RC, D'Arrigo S, De Goede CG, De Laet C, De Waele LM, Denzler I, Desguerre I, Devriendt K, Di Rocco M, Fahey MC, Fazzi E, Ferrie CD, Figueiredo A, Gener B, Goizet C, Gowrinathan NR, Gowrishankar K, Hanrahan D, Isidor B, Kara B, Khan N, King MD, Kirk EP, Kumar R, Lagae L, Landrieu P, Lauffer H, Laugel V, La Piana R, Lim MJ, Lin JP, Linnankivi T, Mackay MT, Marom DR, Marques Lourenco C, McKee SA, Moroni I, Morton JE, Moutard ML, Murray K, Nabbout R, Nampoothiri S, Nunez-Enamorado N, Oades PJ, Olivieri I, Ostergaard JR, Perez-Duenas B, Prendiville JS, Ramesh V, Rasmussen M, Regal L, Ricci F, Rio M, Rodriguez D, Roubertie A, Salvatici E, Segers KA, Sinha GP, Soler D, Spiegel R, Stodberg TI, Straussberg R, Swoboda KJ, Suri M, Tacke U, Tan TY, te Water Naude J, Wee Teik K, Thomas MM, Till M, Tonduti D, Valente EM, Van Coster RN, van der Knaap MS, Vassallo G, Vijzelaar R, Vogt J, Wallace GB, Wassmer E, Webb HJ, Whitehouse WP, Whitney RN, Zaki MS, Zuberi SM, Livingston JH, Rozenberg F, Lebon P, Vanderver A, Orcesi S, Rice GI. 2015. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 167A(2):296–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. 2006a. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 38(8):917–920 [DOI] [PubMed] [Google Scholar]
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, Baumann C, Baxter P, Bertini E, Chandler KE, Chitayat D, Cau D, Dery C, Fazzi E, Goizet C, King MD, Klepper J, Lacombe D, Lanzi G, Lyall H, Martinez-Frias ML, Mathieu M, McKeown C, Monier A, Oade Y, Quarrell OW, Rittey CD, Rogers RC, Sanchis A, Stephenson JB, Tacke U, Till M, Tolmie JL, Tomlin P, Voit T, Weschke B, Woods CG, Lebon P, Bonthron DT, Ponting CP, Jackson AP. 2006b. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet 38(8):910–916 [DOI] [PubMed] [Google Scholar]
- Didcock L, Young DF, Goodbourn S, Randall RE. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J Virol 73(12):9928–9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, Cutler AJ, Doecke JD, Flint S, McKinney EF, Lyons PA, Smith KG, Achenbach P, Beyerlein A, Dunger DB, Clayton DG, Wicker LS, Todd JA, Bonifacio E, Wallace C, Ziegler AG. 2014. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 63(7):2538–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freaney JE, Kim R, Mandhana R, Horvath CM. 2013. Extensive cooperation of immune master regulators IRF3 and NFkappaB in RNA Pol II recruitment and pause release in human innate antiviral transcription. Cell Rep 4(5):959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XY, Kessler DS, Veals SA, Levy DE, Darnell JE., Jr. 1990. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci U S A 87(21):8555–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki M, Kato H, Miyachi Y, Toki H, Motegi H, Inoue M, Minowa O, Yoshida A, Deguchi K, Sato H, Ito S, Shiroishi T, Takeyasu K, Noda T, Fujita T. 2014. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40(2):199–212 [DOI] [PubMed] [Google Scholar]
- Fye JM, Orebaugh CD, Coffin SR, Hollis T, Perrino FW. 2011. Dominant mutation of the TREX1 exonuclease gene in lupus and Aicardi-Goutieres syndrome. J Biol Chem 286(37):32373–32382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlier D, Lyles DS. 2011. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol Mol Biol Rev 75(3):468–490, second page of table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B, Komatsu T, Takeuchi K, Yokoo J. 2002. Paramyxovirus strategies for evading the interferon response. Rev Med Virol 12(6):337–357 [DOI] [PubMed] [Google Scholar]
- Gresser I, Morel-Maroger L, Riviere Y, Guillon JC, Tovey MG, Woodrow D, Sloper JC, Moss J. 1980. Interferon-induced disease in mice and rats. Ann N Y Acad Sci 350:12–20 [DOI] [PubMed] [Google Scholar]
- Gunther C, Kind B, Reijns MA, Berndt N, Martinez-Bueno M, Wolf C, Tungler V, Chara O, Lee YA, Hubner N, Bicknell L, Blum S, Krug C, Schmidt F, Kretschmer S, Koss S, Astell KR, Ramantani G, Bauerfeind A, Morris DL, Cunninghame Graham DS, Bubeck D, Leitch A, Ralston SH, Blackburn EA, Gahr M, Witte T, Vyse TJ, Melchers I, Mangold E, Nothen MM, Aringer M, Kuhn A, Luthke K, Unger L, Bley A, Lorenzi A, Isaacs JD, Alexopoulou D, Conrad K, Dahl A, Roers A, Alarcon-Riquelme ME, Jackson AP, Lee-Kirsch MA. 2015. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest 125(1):413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller B, Achleitner M, Glage S, Naumann R, Behrendt R, Roers A. 2012. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J Exp Med 209(8):1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MA, Kim EK, Now H, Nguyen NT, Kim WJ, Yoo JY, Lee J, Jeong YM, Kim CH, Kim OH, Sohn S, Nam SH, Hong Y, Lee YS, Chang SA, Jang SY, Kim JW, Lee MS, Lim SY, Sung KS, Park KT, Kim BJ, Lee JH, Kim DK, Kee C, Ki CS. 2015. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 96(2):266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Oh SW, Fujita T. 2017. RIG-I-like receptors and type I interferonopathies. J Interferon Cytokine Res 37(5):207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS, Veals SA, Fu XY, Levy DE. 1990. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev 4(10):1753–1765 [DOI] [PubMed] [Google Scholar]
- Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. 2012. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 90(5):498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer S, Wolf C, Konig N, Staroske W, Guck J, Hausler M, Luksch H, Nguyen LA, Kim B, Alexopoulou D, Dahl A, Rapp A, Cardoso MC, Shevchenko A, Lee-Kirsch MA. 2015. SAMHD1 prevents autoimmunity by maintaining genome stability. Ann Rheum Dis 74(3):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska MJ, Troldborg A, Hansen B, Stengaard-Pedersen K, Junker P, Nexo BA, Voss A. 2014. Polymorphisms within Toll-like receptors are associated with systemic lupus erythematosus in a cohort of Danish females. Rheumatology (Oxford) 53(1):48–55 [DOI] [PubMed] [Google Scholar]
- Levy DE, Kessler DS, Pine R, Reich N, Darnell JE., Jr. 1988. Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev 2(4):383–393 [DOI] [PubMed] [Google Scholar]
- Li P, Du J, Goodier JL, Hou J, Kang J, Kazazian HH, Jr., Zhao K, Yu XF. 2017. Aicardi-Goutieres syndrome protein TREX1 suppresses L1 and maintains genome integrity through exonuclease-independent ORF1p depletion. Nucleic Acids Res 45(8):4619–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddicoat BJ, Chalk AM, Walkley CR. 2016. ADAR1, inosine and the immune sensing system: distinguishing self from non-self. Wiley Interdiscip Rev RNA 7(2):157–172 [DOI] [PubMed] [Google Scholar]
- Mackenzie KJ, Carroll P, Lettice L, Tarnauskaite Z, Reddy K, Dix F, Revuelta A, Abbondati E, Rigby RE, Rabe B, Kilanowski F, Grimes G, Fluteau A, Devenney PS, Hill RE, Reijns MA, Jackson AP. 2016. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J 35(8):831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Valle F, Balada E, Ordi-Ros J, Vilardell-Tarres M. 2008. DNase 1 and systemic lupus erythematosus. Autoimmun Rev 7(5):359–363 [DOI] [PubMed] [Google Scholar]
- Mavragani CP, Sagalovskiy I, Guo Q, Nezos A, Kapsogeorgou EK, Lu P, Liang Zhou J, Kirou KA, Seshan SV, Moutsopoulos HM, Crow MK. 2016. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol 68(11):2686–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. 2015. Type I interferons in infectious disease. Nat Rev Immunol 15(2):87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. 2010. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem 79:321–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Nakagawa K, Abe J, Awaya T, Funabiki M, Hijikata A, Nishikomori R, Funatsuka M, Ohshima Y, Sugawara Y, Yasumi T, Kato H, Shirai T, Ohara O, Fujita T, Heike T. 2014. Aicardi-Goutieres syndrome is caused by IFIH1 mutations. Am J Hum Genet 95(1):121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchini E, Doria M, Antonioni A, Galardi S, Ciafre SA, Frassinelli L, Mancone C, Montaldo C, Tripodi M, Michienzi A. 2017. ADAR1 restricts LINE-1 retrotransposition. Nucleic Acids Res 45(1):155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisien JP, Bamming D, Komuro A, Ramachandran A, Rodriguez JJ, Barber G, Wojahn RD, Horvath CM. 2009. A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2. J Virol 83(14):7252–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Jo MH, Lin C, Wu B, Orme-Johnson M, Walz T, Hohng S, Hur S. 2012. Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments. Proc Natl Acad Sci U S A 109(49):E3340–E3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Lin C, Wu B, Orme-Johnson M, Liu M, Walz T, Hur S. 2011. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc Natl Acad Sci U S A 108(52):21010–21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Horvath CM. 2009. Paramyxovirus disruption of interferon signal transduction: STATus report. J Interferon Cytokine Res 29(9):531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Horvath CM. 2010. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J Virol 84(21):11152–11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Parisien JP, Horvath CM. 2008. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J Virol 82(17):8330–8338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N, Evans B, Levy D, Fahey D, Knight E, Jr., Darnell JE., Jr. 1987. Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A 84(18):6394–6398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, Ali M, Gornall H, Couthard LR, Aeby A, Attard-Montalto SP, Bertini E, Bodemer C, Brockmann K, Brueton LA, Corry PC, Desguerre I, Fazzi E, Cazorla AG, Gener B, Hamel BC, Heiberg A, Hunter M, van der Knaap MS, Kumar R, Lagae L, Landrieu PG, Lourenco CM, Marom D, McDermott MF, van der Merwe W, Orcesi S, Prendiville JS, Rasmussen M, Shalev SA, Soler DM, Shinawi M, Spiegel R, Tan TY, Vanderver A, Wakeling EL, Wassmer E, Whittaker E, Lebon P, Stetson DB, Bonthron DT, Crow YJ. 2009. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet 41(7):829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, Bader-Meunier B, Baildam EM, Battini R, Beresford MW, Casarano M, Chouchane M, Cimaz R, Collins AE, Cordeiro NJ, Dale RC, Davidson JE, De Waele L, Desguerre I, Faivre L, Fazzi E, Isidor B, Lagae L, Latchman AR, Lebon P, Li C, Livingston JH, Lourenco CM, Mancardi MM, Masurel-Paulet A, McInnes IB, Menezes MP, Mignot C, O'Sullivan J, Orcesi S, Picco PP, Riva E, Robinson RA, Rodriguez D, Salvatici E, Scott C, Szybowska M, Tolmie JL, Vanderver A, Vanhulle C, Vieira JP, Webb K, Whitney RN, Williams SG, Wolfe LA, Zuberi SM, Hur S, Crow YJ. 2014. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46(5):503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Forte GM, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, Ackroyd S, Allcock R, Bailey KM, Balottin U, Barnerias C, Bernard G, Bodemer C, Botella MP, Cereda C, Chandler KE, Dabydeen L, Dale RC, De Laet C, De Goede CG, Del Toro M, Effat L, Enamorado NN, Fazzi E, Gener B, Haldre M, Lin JP, Livingston JH, Lourenco CM, Marques W, Jr., Oades P, Peterson P, Rasmussen M, Roubertie A, Schmidt JL, Shalev SA, Simon R, Spiegel R, Swoboda KJ, Temtamy SA, Vassallo G, Vilain CN, Vogt J, Wermenbol V, Whitehouse WP, Soler D, Olivieri I, Orcesi S, Aglan MS, Zaki MS, Abdel-Salam GM, Vanderver A, Kisand K, Rozenberg F, Lebon P, Crow YJ. 2013. Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol 12(12):1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, Jenkinson EM, Bacino CA, Battini R, Bertini E, Brogan PA, Brueton LA, Carpanelli M, De Laet C, de Lonlay P, del Toro M, Desguerre I, Fazzi E, Garcia-Cazorla A, Heiberg A, Kawaguchi M, Kumar R, Lin JP, Lourenco CM, Male AM, Marques W, Jr., Mignot C, Olivieri I, Orcesi S, Prabhakar P, Rasmussen M, Robinson RA, Rozenberg F, Schmidt JL, Steindl K, Tan TY, van der Merwe WG, Vanderver A, Vassallo G, Wakeling EL, Wassmer E, Whittaker E, Livingston JH, Lebon P, Suzuki T, McLaughlin PJ, Keegan LP, O'Connell MA, Lovell SC, Crow YJ. 2012. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet 44(11):1243–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero MP, Tesser A, Bartok E, Rice GI, Della Mina E, Depp M, Beitz B, Bondet V, Cagnard N, Duffy D, Dussiot M, Fremond ML, Gattorno M, Guillem F, Kitabayashi N, Porcheray F, Rieux-Laucat F, Seabra L, Uggenti C, Volpi S, Zeef LAH, Alyanakian MA, Beltrand J, Bianco AM, Boddaert N, Brouzes C, Candon S, Caorsi R, Charbit M, Fabre M, Faletra F, Girard M, Harroche A, Hartmann E, Lasne D, Marcuzzi A, Neven B, Nitschke P, Pascreau T, Pastore S, Picard C, Picco P, Piscianz E, Polak M, Quartier P, Rabant M, Stocco G, Taddio A, Uettwiller F, Valencic E, Vozzi D, Hartmann G, Barchet W, Hermine O, Bader-Meunier B, Tommasini A, Crow YJ. 2017. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nat Commun 8(1):2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Parisien JP, Horvath CM. 2002. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J Virol 76(22):11476–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Wang LF, Horvath CM. 2003. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J Virol 77(21):11842–11845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KR, Horvath CM. 2013. Amino acid requirements for MDA5 and LGP2 recognition by paramyxovirus V proteins: a single arginine distinguishes MDA5 from RIG-I. J Virol 87(5):2974–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KR, Horvath CM. 2014. Paramyxovirus V protein interaction with the antiviral sensor LGP2 disrupts MDA5 signaling enhancement but is not relevant to LGP2-mediated RLR signaling inhibition. J Virol 88(14):8180–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, MacDougall M, Lu C, Buers I, Mamaeva O, Nitschke Y, Rice GI, Erlandsen H, Kehl HG, Thiele H, Nurnberg P, Hohne W, Crow YJ, Feigenbaum A, Hennekam RC. 2015. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 96(2):275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472(7344):481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. 1998. How cells respond to interferons. Annu Rev Biochem 67:227–264 [DOI] [PubMed] [Google Scholar]
- Tough DF. 2012. Modulation of T-cell function by type I interferon. Immunol Cell Biol 90(5):492–497 [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272(5270):1947–1950 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. 2010. Type I interferon: friend or foe? J Exp Med 207(10):2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulane CM, Kentsis A, Cruz CD, Parisien JP, Schneider KL, Horvath CM. 2005. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J Virol 79(16):10180–10189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Voskuyl AE, Rustenburg F, Baggen JM, Ibrahim SM, Fero M, Dijkmans BA, Tak PP, Verweij CL. 2007. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis 66(8):1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyck L, De Somer L, Pombal D, Bornschein S, Frans G, Humblet-Baron S, Moens L, de Zegher F, Bossuyt X, Wouters C, Liston A. 2015. Brief report: IFIH1 mutation causes systemic lupus erythematosus with selective IgA deficiency. Arthritis Rheumatol 67(6):1592–1597 [DOI] [PubMed] [Google Scholar]
- Versteeg GA, Garcia-Sastre A. 2010. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol 13(4):508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Chang SW, Wu YJ, Lin JC, Ho HH, Chou TC, Yang B, Wu J, Chen JY. 2014. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci Rep 4:3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. 2013. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152(1–2):276–289 [DOI] [PubMed] [Google Scholar]
- Wu YW, Tang W, Zuo JP. 2015. Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol Sin 36(12):1395–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YG, Lindahl T, Barnes DE. 2007. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131(5):873–886 [DOI] [PubMed] [Google Scholar]
- Zhao K, Du J, Han X, Goodier JL, Li P, Zhou X, Wei W, Evans SL, Li L, Zhang W, Cheung LE, Wang G, Kazazian HH, Jr., Yu XF. 2013. Modulation of LINE-1 and Alu/SVA retrotransposition by Aicardi-Goutieres syndrome-related SAMHD1. Cell Rep 4(6):1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Du J, Peng Y, Li P, Wang S, Wang Y, Hou J, Kang J, Zheng W, Hua S, Yu XF. 2018. LINE1 contributes to autoimmunity through both RIG-I- and MDA5-mediated RNA sensing pathways. J Autoimmun 90:105–115 [DOI] [PubMed] [Google Scholar]