Abstract
The most recent revolution in our understanding and knowledge of the human body is the introduction of new technologies allowing direct magnified vision of internal organs, as in laparoscopy and robotics. The possibility of viewing an anatomical detail, until now not directly visible during open surgical operations and only partially during dissections of cadavers, has created a ‘new surgical anatomy’. Consequent refinements of operative techniques, combined with better views of the surgical field, have given rise to continual and significant decreases in complication rates and improved functional and oncological outcomes. The possibility of exploring new ways of approaching organs to be treated now allows us to reinforce our anatomical knowledge and plan novel surgical approaches. The present review aims to clarify some of these issues.
Abbreviations: BNP, bladder neck preservation; 3D, three-dimensional; DVC, deep venous complex; NVB, neurovascular bundle; RP, radical prostatectomy
Keywords: Robotic surgery, Anatomy, Laparoscopy, Prostatectomy
A new medicine
Until 1500, surgery and anatomy were considered of little importance in comparison with other branches of medicine. In fact, until that century, professors had taught Anatomy simply by reading Galen’s works ex cathedra. Although Galen was considered the standard authority on the topic, for religious reasons, he based most of his information on anatomy on what he saw when he dissected the bodies of animals, thus unintentionally making many gross errors.
Modern anatomy saw the light of day in Padua, thanks to Andreas Vesalius, Professor of Surgery and Anatomy at the University of Padua from 1537. Vesalius believed that surgery had to be grounded in anatomy, and performed some dissections of human bodies. Only after a Paduan judge decided to make the bodies of executed criminals available for dissection did Vesalius start systematically to dissect and compare human bodies. He always performed the dissections himself and produced anatomical charts, as reference aids for his students. In 1543 (the same year which saw the publication of Nicolaus Copernicus’ work on the revolutionary heliocentric theory), Vesalius published De Humani Corporis Fabrica, a revolutionary anatomical atlas based largely on human dissection, transforming anatomy into a sphere of knowledge which relied on observations taken from direct visualisation of the human body. Vesalius was the first to place particular importance on ‘ocular evidence’.
Vesalius’s disciples continued his work in Padua: his first student, Gabriele Falloppio, discovered the tubes, which are now named after him, the ‘fallopian tubes’; and Girolamo Fabrici d’Acquapendente was the first to describe valves in veins. William Harvey later completed studies on circulation. Further progress was also made on many fronts, mainly by practising surgeons.
New tools
Antonio Vallisneri (1661–1730) was an Italian medical scientist, physician and naturalist, who held the chairs of Practical Medicine first and Theoretical Medicine later at the University of Padua.
In performing his animal studies and medical research, he decided to use some ‘English microscopes with eight orders of lens’ because, in his opinion, ‘some objects are only visible with great patience and the eye armed with a very fine and perfect microscope’ (‘e solo visibili con gran pazienza coll’occhio armato d’un finissimo e perfettissimo microscopio’).
The microscope allowed Vallisneri to discover and be the first to describe new structures in human subjects, such as spermatozoa (‘vermicelli spermatici’) [1], In the same way, in Padua, during long cold nights spent observing the heavens, in January 1610 Galileo Galilei discovered the first four moons of Jupiter (‘cosmica sidera’) ‘armed with the first telescope’ (‘cannocchiale’). In both cases, a manufactured tool augmented the human power of vision, towards the two extremes of very small and very large.
In the field of surgery, the concept of an ‘armed eye’ augments our ability to see and identify much finer details in the surgical field, using optical magnification.
A new ‘vision’
The most recent revolution in our knowledge and understanding of the human body is represented by the new technologies that allow us direct magnified views of organs, as in laparoscopy. The possibility of examining anatomical details, until now not directly visible during open surgical operations and only partially during post-mortem dissections, has created a ‘new anatomy’. In fact, being able to see microscopic structures in vivo (rather than ex vivo) has greatly increased our knowledge of surgical anatomy.
The consequent refinements in operative techniques, as well as better views of the surgical field, have led to continual and significant decreases in complication rates and improvements in functional outcomes.
However, in some situations, improved views of laparoscopic surgery have not always been followed by better surgical work. This is the case, for example, of the real difficulty in placing a suture or performing a continuous suture using laparoscopic instruments. Only after a (very) long learning curve can an inexperienced surgeon place a single suture as well as an expert surgeon, who can quickly complete a long suture after only a few operations.
A new platform
Less than 20 years ago, another revolution profoundly changed our way of understanding, learning and consequently teaching anatomy: the introduction of robotic surgery with the da Vinci® Robot System (Intuitive Surgical, Sunnyvale, CA, USA). This platform is used in minimally invasive general surgery, paediatric surgery, gynaecology, otorhinolaryngology, and cardiothoracic surgery, although most robotic operations are planned for urological surgery.
One of the major advantages for surgeons using robots is the possibility of achieving sufficient skill in a (much) shorter time compared with laparoscopic surgery.
Until this revolution, surgeons in training always had to gain operative experience through ‘supervised trial and error’ on real patients, with consequent prolonged training (and sometimes compromising patients’ safety!). With robotic systems, surgeons can practise operations in three-dimensional (3D) visual simulations, using soft-tissue models that re-create the textures of human tissues.
The development of specific and increasingly technologically advanced simulators has certainly shortened the learning curve and increased surgeons’ skills, consequently improving results. In addition, special image-guided simulations allow naive surgeons to practise procedures on 3D reconstructions of the specific anatomical details of each patient.
For all these reasons, the great success and spread of this minimally invasive technology is clear-cut, combining as it does enhanced dexterity and greater precision with improved visualisation.
A new surgical anatomy allows new surgical techniques
This enhanced ability to view includes clarifying the mutual relationships of each structure in the operative field. Associated with the development and spread of robotic surgery, knowledge of new anatomy gives rise to some modifications (and improvements) in surgical techniques.
Radical prostatectomy (RP) as an example
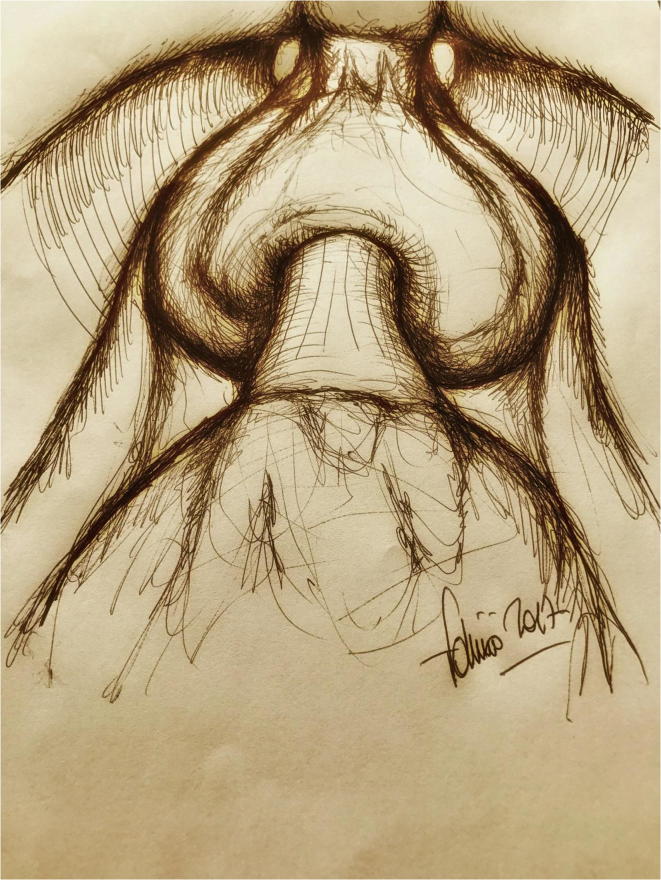
The most powerful example is probably RP (Fig. 1). After the initial description of the gross anatomical landmarks during removal of the prostate, thanks to the pioneering experience of Patrick Walsh in the 1980s, our knowledge of several structures surrounding the gland has progressively increased. However, it was only after the recent introduction of robotic surgery that magnified vision allowed detailed identification of microstructures, and ‘millimetric’ dissection of such anatomical components turned out to be useful in improving oncological and functional outcomes [2]. For all these reasons, the advent of robotic surgery heralded a new dawn in the treatment of prostate cancer, especially in some problematical cases.
Fig. 1.
A ‘new surgical anatomy’ came into being after the introduction of robotic surgery allowing a more precise dissection of surgical planes – artistic representation of the prostate during robotic prostatectomy.
Bladder neck
In order to undertake bladder neck preservation (BNP) in cases of open RP, only a few useful anatomical landmarks are present; this often means that it is simply transected.
Through magnified vision, we can clearly see the three separate muscle layers described by anatomists using microscopes after cadaver dissections: the inner longitudinal layer, middle circular layer, and outer longitudinal layer [3].
Clear-cut identification of these layers allows meticulous preservation of a long tract of the intraprostatic neck through sharp cold-scissor dissection of natural tissue planes [4], avoiding cautery to create the surgical plane and the consequent possible release of energy near the periprostatic neurovascular bundles (NVBs) [5].
More recent studies show that, during robotic RP, BNP hastens continence without compromising cancer control [6]. BNP has also shown several other advantages, including a lower risk of bladder neck contracture [7], lower rates of urethral injury [8], and reduced anastomotic urinary leakage.
Deep vein complex
Another challenging step during RP is how to manage the so-called ‘deep venous complex’ (DVC) because placing a selective suture, as initially described by Walsh [9], is not always possible during laparoscopic RP: surgeons are obliged to place the suture deeply to avoid bleeding, with the consequent risk of involving some fibres of the urethral sphincter in the suture. Magnified vision allows precise identification of the single layers and components of the DVC (e.g., the peculiar small arteries amongst veins). Only laparoscopically skilled surgeons can manage the DVC with selective ligation, avoiding damage to the sphincter and improving functional outcomes [10].
The precise movements of robotic instruments allow parsimonious apical dissection to preserve both the external sphincteric structures and the NVBs, avoiding or reducing the risk of positive surgical margins.
After selective ligation and section, the DVC can be suspended: this technique, initially described for open RP [9], can help to control venous bleeding and support the striated sphincter. With 3D magnified and close-up vision, and dexterity with the robotic system, this technique can be performed robotically and very precisely, thus enhancing early recovery of postoperative urinary continence [11].
NVBs
Although the definite advantage of the robotic approach in terms of preservation of erectile function after RP has not yet been demonstrated, there is little doubt about the possibility of performing lateral dissection layer-by-layer during robotic RP [12]. Cavernosal nerves are millimetric in size and run round the prostate inside a bundle of fatty tissue and vascular components.
Starting from this microscopic anatomical knowledge and with the aid of the improved magnification and visualisation provided by the robotic platform, some authors have proposed using the prostatic vasculature as a landmark to perform nerve-sparing dissection. This vascular network is not visible (or is extremely difficult to see) during open surgery, but it can easily be identified during minimally invasive approaches, allowing selective sparing of the surrounding tissues, and even choosing the specific percentage of layer to be preserved [13]. Membranes that were previously believed to be single or double-layered have even turned out to be multi-layered structures.
Posterior reconfiguration
The ability to insert several sutures accurately in a limited space also allows robotic surgeons to improve on previous techniques. This is the case, for example, of posterior reconstruction after removal of the prostate. The traditional technique described by Rocco et al. [14] for open RP can be refined robotically by means of a long suture, adding other passages and involving other anatomical structures [15]: the magnification, better visualisation, and wristed instrumentation of the robotic platform have helped to improve functional outcomes [16], starting from the same correct idea and philosophy.
New techniques
The possibility of exploring new ways to approach organs to be treated has allowed us to reinforce our anatomical knowledge and to initiate novel approaches. The most exemplary intervention is the Retzius-sparing approach [17]: the trans-Douglas route is generally poorly known to open surgeons, due to the difficulty of visualising the posterior plane. Using robotic technology, we have begun to see this other (dark) side of the prostate and to better identify some anatomical structures, such as the tips of seminal vesicles and the posterior wall of the bladder neck.
In this way, preservation of the periprostatic neural network and complete intrafascial dissection of the prostate can be achieved through the Douglas space, with excellent functional and oncological outcomes.
Accurate preoperative imaging can also allow partial robotic prostatectomy [18], with potential preservation of some portion(s) of the gland if the surrounding prostatic tissue is shown to be benign.
Conclusions
Today, the introduction of previously impossible techniques of imaging, such as MRI and 3D reconstructions, has dramatically changed our understanding of anatomy, progressively increasing our knowledge not only of the ultra-detailed structures of every single apparatus, but also their specific functions.
This ‘new surgical anatomy’ came into being and grew after the introduction of minimally invasive techniques, allowing better 3D-magnified visualisation of the surgical field and, with robotic systems, more accurate and precise dissection of surgical planes. Knowing this anatomy and, above all, respecting it, should result in better ‘surgical acts’, improving cancer control and postoperative functional outcomes.
Acknowledgments
Acknowledgements
None.
Conflict of interest
None.
Pelvic Surgery
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Vallisneri A. appresso Gio. Gabbriel Hertz; Venezia: 1721. Istoria della generazione dell'uomo, e degli animali, se sia da' vermicelli spermatici o dalle uova; con un trattato nel fine della sterilità e de' suoi rimedi. [Google Scholar]
- 2.Walz J., Epstein J.I., Ganzer R., Graefen M., Guazzoni G., Kaouk J. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70:301–311. doi: 10.1016/j.eururo.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Kingsnorth A.N., Skandalakis P.N., Colborn G.L., Weidman T.A., Skandalakis L.J., Skandalakis J.E. Embryology, anatomy, and surgical applications of the preperitoneal space. Surg Clin North Am. 2000;80:1–24. doi: 10.1016/s0039-6109(05)70394-7. [DOI] [PubMed] [Google Scholar]
- 4.Dal Moro F. A thermal bladder neck dissection during robot-assisted radical prostatectomy. Int Braz J Urol. 2014;40:433–434. doi: 10.1590/S1677-5538.IBJU.2014.03.22. [DOI] [PubMed] [Google Scholar]
- 5.Ong A.M., Su L.M., Varkarakis I., Inagaki T., Link R.E., Bhayani S.B. Nerve sparing radical prostatectomy: effects of hemostatic energy sources on the recovery of cavernous nerve function in a canine model. J Urol. 2004;172:1318–1322. doi: 10.1097/01.ju.0000139883.08934.86. [DOI] [PubMed] [Google Scholar]
- 6.Ma X., Tang K., Yang C., Wu G., Xu N., Wang M. Bladder neck preservation improves time to continence after radical prostatectomy: a systematic review and meta-analysis. Oncotarget. 2016;7:67463–67475. doi: 10.18632/oncotarget.11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licht M.R., Klein E.A., Tuason L., Levin H. Impact of bladder neck preservation during radical prostatectomy on continence and cancer control. Urology. 1994;44:883–887. doi: 10.1016/s0090-4295(94)80175-4. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins L.C., Nogueira M., Wilding G.E., Tan W., Kim H.L., Mohler J.L. Median lobe in robot-assisted radical prostatectomy: evaluation and management. Urology. 2008;71:810–813. doi: 10.1016/j.urology.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Walsh P.C. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160:2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 10.Porpiglia F., Fiori C., Grande S., Morra I., Scarpa R.M. Selective versus standard ligature of the deep venous complex during laparoscopic radical prostatectomy: effects on continence, blood loss, and margin status. Eur Urol. 2009;55:1377–1383. doi: 10.1016/j.eururo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M., Kakuma T., Suekane S., Nakashima O., Mohamed E.R., Matsuoka K. A randomized clinical trial of suspension technique for improving early recovery of urinary continence after radical retropubic prostatectomy. BJU Int. 2008;102:958–963. doi: 10.1111/j.1464-410X.2008.07759.x. [DOI] [PubMed] [Google Scholar]
- 12.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 13.Patel V.R., Schatloff O., Chauhan S., Sivaraman A., Valero R., Coelho R.F. The role of the prostatic vasculature as a landmark for nerve sparing during robot assisted radical prostatectomy. Eur Urol. 2012;61:571–576. doi: 10.1016/j.eururo.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Rocco F., Gadda F., Acquati P., Carmignani L., Favini P., Dell'Orto P. Arch Ital Urol Androl. 2001;73:127–137. [PubMed] [Google Scholar]
- 15.Porpiglia F., Bertolo R., Manfredi M., De Luca S., Checcucci E., Morra I. Total anatomical reconstruction during robot-assisted radical prostatectomy: implications on early recovery of urinary continence. Eur Urol. 2016;69:485–495. doi: 10.1016/j.eururo.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Dal Moro F., Crestani A., Valotto C., Zattoni F. CORPUS – novel complete reconstruction of the posterior urethral support after robotic radical prostatectomy: preliminary data of very early continence recovery. Urology. 2014;83:641–647. doi: 10.1016/j.urology.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Galfano A., Ascione A., Grimaldi S., Petralia G., Strada E., Bocciardi A.M. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. 2010;58:457–461. doi: 10.1016/j.eururo.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Villers A., Flamand V., Arquímedes R.C., Puech P., Haber G.P., Desai M.M. Robot-assisted partial prostatectomy for anterior prostate cancer: a step-by-step guide. BJU Int. 2017;119:968–974. doi: 10.1111/bju.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]