Abstract
Objective
To compare the perioperative outcomes of hand-assisted laparoscopic donor nephrectomy (HALDN) and pure LDN, as HALDN and LDN are the two most widely used techniques of DN to treat end-stage renal disease.
Methods
In this systematic review and meta-analysis, we performed a literature search of PubMed, Embase, Web of Science, and Cochrane from 01/01/1995 to 31/12/2014. The primary outcome was conversion to an open procedure. Secondary outcomes were warm ischaemia time (WIT), operation time (OT), estimated blood loss (EBL), complications, and length of stay (LOS). Data analysed were presented as odds ratios (ORs) or weighted mean differences (WMDs) with 95% confidence intervals (CIs), I2, and P values. Subgroup analysis was performed.
Results
There were 24 studies included in the meta-analysis; three randomised controlled trials (RCTs), one randomised pilot study, two prospective, and 18 retrospective cohort studies. There were no differences in conversion to an open procedure between the two techniques for both RCTs (OR 0.42, 95% CI 0.06, 2.90; I2 = 0%, P < 0.001) and cohort studies (OR 1.06, 95% CI 0.63, 1.78; I2 = 0%, P = 0.84). WIT was shorter for the HALDN (−41.79 s, 95% CI −71.85, −11.74; I2 = 96%, P = 0.006), as was the OT (−26.32 min, 95% CI −40.67, −11.97; I2 = 95%, P < 0.001). There was no statistically significant difference in EBL, complications or LOS.
Conclusion
There is little statistical evidence to recommend one technique. HALDN is associated with a shorter WIT and OT. LDN has equal safety to HALDN. Further studies are required.
Abbreviations: BMI, body mass index; (L)DN, (laparoscopic) donor nephrectomy; EBL, estimated blood loss; FEM, fixed-effects model; HALDN, hand-assisted laparoscopic donor nephrectomy; HARPDN, hand-assisted retroperitoneal donor nephrectomy; LOS, length of stay; OR, odds ratio; OT, operation time; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RALDN, robot-assisted laparoscopic donor nephrectomy; RCT, randomised controlled trial; REM, random-effects model; WIT, warm ischaemia time; WMD, weighted mean difference
Keywords: Laparoscopic donor nephrectomy, Hand-assisted donor nephrectomy, Renal transplantation
Introduction
Renal transplantation improves both the quantity and quality of life for recipients [1]. Worldwide >40% of the ∼69 000 kidney transplants carried out in 2008 were from live donors [2]. Live-donor nephrectomies (DNs) are more cost effective [3] and offer superior graft survival particularly in the longer term [4], [5]. When carefully screened, healthy kidney donors have been shown to have no increased risk of developing end-stage renal disease than the average population [6].
Laparoscopic DN (LDN) was introduced in 1995 by Ratner et al. [7], with lower postoperative pain, quicker recovery time, shorter hospital stay, and better cosmesis [8]. LDN has become the reference standard for DN for these reasons and has been shown to increase recruitment of live donors [9], [10]. However, some of the earlier studies raised questions over the safety of the procedure due to intraoperative events, so hand-assisted LDN (HALDN) was introduced in 1998 as an alternative technique [11]. This enabled a combination of the minimally invasive approach with tactile feedback and immediate control of the hilum should intraoperative bleeding occur. The learning curve associated with HALDN was another advantage over LDN [12].
Both LDN and HALDN techniques have been shown to have advantages over the open procedure [13], [14], [15]. However, the superiority of one technique over another is still not entirely clear when it comes to technical variations in LDN. Similarly, for the hand-assisted technique there have been few studies directly comparing the transperitoneal and retroperitoneal approaches and no randomised controlled trial (RCT) in this area.
In 2007, a systematic review and meta-analysis of nine studies comparing 174 LDN and 202 HALDN procedures found that HALDN had a lower rate of conversion to an open procedure (2.97% vs 4.6%), a shorter warm ischaemia time (WIT) and length of procedure, as well as lower blood loss than LDN [16]. According to another review, HALDN trended towards a lower intraoperative complication rate and increased minor postoperative complications than LDN [17]. A qualitative review of evidence in 2010 found that most studies comparing different minimal invasive techniques were similar in terms of intra- and postoperative outcome for both the donor and the recipient [15]. Wadstrom et al. [18] carried out a systematic review and meta-analysis comparing traditional open DN to pure LDN and HALDN methods in 2011. This covered 30 original articles relating to DN but also included 21 articles concerning radical nephrectomy and 14 nephroureterectomy.
Since 2007 there has been at least nine further studies comparing LDN to HALDN or hand-assisted retroperitoneal DN (HARPDN), including three RCTs. Therefore, an updated analysis of the outcomes for these procedures is warranted.
Methods
Study design
A systematic review of RCTs, as well as prospective and retrospective cohort studies, was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist [19].
Search strategy
The keywords ‘donor nephrectomy’, ‘laparoscopic’, and ‘hand-assisted laparoscopic’ were entered into databases including PubMed, Embase, Web of Science and Cochrane. The dates covered were from 1 January 1995 to 31 December 2014. The search was limited to published studies on humans, with no limits on language. The lists of references of relevant primary research and review articles were searched for further studies. Authors were contacted to provide further information from their studies where necessary.
Eligibility criteria
RCTs, prospective and retrospective cohort studies comparing the purely LDN to either transperitoneoscopic or retroperitoneoscopic hand-assisted techniques reported in a single cohort were included. Non-comparative studies or those that compared to another technique were excluded, also where no full text was available. When more than one study containing the same population of patients was reported from the same institution the most recent publication was included. The primary outcome was conversion to an open procedure. Other secondary outcome measures included WIT (the time from cross clamping of the renal artery until cold perfusion), operation time (OT), estimated blood loss (EBL), complications (both intra- and postoperative), and length of stay (LOS).
Study selection
If the title of the article appeared to address the inclusion criteria the abstract was reviewed by two independent authors (M.P.B. and L.G.K.), then the full article if deemed eligible. Studies were included if they addressed any of the above-mentioned outcomes as either primary or secondary outcomes. If there was disagreement over the eligibility of a particular study, conclusion was reached by consensus agreement.
Data required
The data taken from each study included sample size, demographic data such as mean age, gender, mean body mass index (BMI), side of nephrectomy, as well as the outcomes mentioned above.
Quality assessment of methods
The methodological quality of RCTs was assessed using the Cochrane risk of bias tool [20]. For each category, a judgement of ‘yes’, ‘no’ or ‘unclear’ was given. The methodological quality of both retrospective and prospective cohort studies was assessed using the Newcastle-Ottawa Scale [21]. A score of 0–9 (allocated as stars) was allocated to each of the cohort studies. A score of 0–3 stars was deemed low quality, 4–6 stars medium quality, and 7–9 stars were considered to be of high quality. Quality assessments were made by two independent reviewers (M.P.B. and L.G.K.).
Data synthesis and statistics
‘Review Manager’ (Revman version 5.2 The Cochrane Collaboration, Oxford, UK) was used to perform meta-analysis of the data. Data were presented as odds ratios (ORs) for dichotomous and weighted mean differences (WMDs) for continuous variables with 95% CIs. For studies that presented continuous data as means and range values and where standard deviation (SD) was not available or not provided on contacting the authors, it was calculated using the range rule [22]. Analysis was carried out separately for RCTs and cohort studies due to their different study designs. Subgroup analysis was performed to assess the retroperitoneal HARPDN technique. A fixed-effects model (FEM) was used for analysis, except when I2 was >50% a random-effects model (REM) was used. Statistical heterogeneity was measured using chi-squared and I2 models [23]. Sensitivity analysis was performed for studies performed in 2010 or later.
Results
Included studies
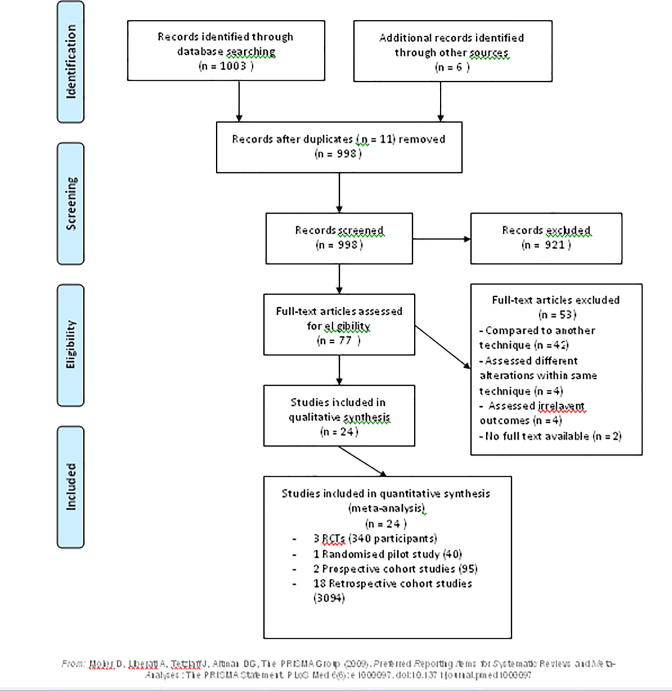
The literature search yielded 1003 citations. A further six were obtained from other sources, such as reference lists of other studies, and there were 11 duplicates. Overall, 77 full text articles were assessed for eligibility, with studies excluded for the reasons listed below (Fig. 1). In total, there were 24 included studies, three RCTs, one randomised pilot study, two prospective and 18 retrospective cohort studies.
Fig. 1.
PRISMA flow chart for study selection.
Included studies characteristics
The characteristics of the included studies are summarised in the table below (Table 1) [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]. Good geographic distribution of participants was seen, with studies mainly from Northern Europe/Scandinavia, South Asia, and USA. There was a wide range in the number of study participants. Only five studies had >200 participants, whilst 12 studies had <100 participants. All but one of the HARPDN studies, including both randomised studies, came from Northern Europe/Scandinavia illustrating the popularity of the technique in this region.
Table 1.
Characteristics of the 24 included studies.
| References | Year | Country | Type of study | Technique | No. of patients |
|---|---|---|---|---|---|
| Bargman et al. [24] | 2006 | USA | RCT | HALDN vs LDN | 20 vs 20 |
| Branco et al. [25] | 2008 | Brazil | Retrospective cohort | HALDN vs LDN | 67 vs 89 |
| Buell et al. [26] | 2004 | USA | Prospective cohort | HALDN vs RLDN | 31 vs 28 |
| Cho et al. [27] | 2013 | Korea | RCT | HALDN vs LDN | 50 vs 50 |
| Choi et al. [28] | 2014 | Korea | Retrospective cohort | HALDN vs LDN | 80 vs 80 |
| Dols et al. [29] | 2014 | Netherlands | RCT | HARP vs LDN | 95 vs 95 |
| El-Galley et al. [30] | 2004 | UK | Retrospective cohort | HALDN vs LDN vs ODN | 55 vs 17 vs 28 |
| Gershbein et al. [31] | 2002 | USA | Retrospective cohort | HALDN vs LDN | 15 vs 30 |
| Gjertsen et al. [32] | 2006 | USA | Retrospective cohort | HARP vs LDN vs ODN | 11 vs 15 vs 25 |
| Klop et al. [33] | 2014 | Netherlands | Randomised pilot study | HARP vs LDN | 20 vs 20 |
| Kocak et al. [34] | 2007 | USA | Retrospective cohort | HALDN vs LDN | 318 vs 482 |
| Lai et al. [35] | 2010 | Taiwan | Retrospective cohort | HALDN vs LDN | 52 vs 45 |
| Lucas et al. [36] | 2013 | USA | Retrospective cohort | HALDN vs LDN | 116 vs 152 |
| Mateo et al. [37] | 2003 | USA | Retrospective cohort | HALDN vs LDN | 18 vs 29 |
| Mjøen et al. [38] | 2010 | Norway | Retrospective cohort | HALDN vs LDN vs HARPDN | 177 vs 196 vs 26 |
| Percegona et al. [39] | 2008 | Brazil | Retrospective cohort | HALDN vs LDN | 34 vs 21 |
| Ruiz-Deya et al. [40] | 2001 | USA | Retrospective cohort | HALDN vs LDN vs ODN | 23 vs 11 vs 14 |
| Ruszat et al. [41] | 2006 | Switzerland | Retrospective cohort | HALDN vs LDN vs RLDN vs ODN | 34 vs 14 vs 65 vs 69 |
| Salazar et al. [42] | 2005 | Canada | Retrospective cohort | HALDN vs LDN vs ODN | 24 vs 11 vs 15 |
| Sundqvist et al. [43] | 2004 | Sweden | Prospective cohort | HARPDN vs LDN vs. ODN | 11 vs 14 vs 11 |
| Ungbhakorn et al. [44] | 2012 | Thailand | Retrospective cohort | HALDN vs LDN vs ODN | 23 vs 82 vs 95 |
| Velidedeoglu et al. [45] | 2002 | USA | Retrospective cohort | HALDN vs LDN vs ODN | 60 vs 40 vs 50 |
| Wadstrom et al. [46] | 2003 | Sweden | Retrospective cohort | HALDN vs LDN vs HARPDN | 14 vs 11 vs 18 |
| Yoo et al. [47] | 2006 | Korea | Retrospective cohort | HALDN vs LDN vs ODN | 177 vs 24 vs 42 |
HALDN: Hand-assisted laparoscopic donor nephrectomy.
LDN: Laparoscopic donor nephrectomy.
HARPDN: Hand-assisted retroperitoneoscopic donor nephrectomy.
RLDN: Retroperitoneal laparoscopic donor nephrectomy.
Quality of studies summary
The quality of the randomised studies was assessed using the Cochrane Risk of Bias Tool. A summary can be found in the table below (Table 2) [24], [27], [29], [33]. The two Dutch studies [29], [33] had a much lower risk of bias, particularly when it came to sequence generation, allocation concealment, and attempt at blinding. They also addressed incomplete outcomes more overtly. The other two studies Bargman et al. [24] and Cho et al. [27] both had a high risk of bias on overall assessment.
Table 2.
Risk of bias assessment of randomised studies.
| Randomised study | Adequate sequence generation? | Allocation concealment? | Adequate blinding? | Incomplete outcome data addressed? | Free of selective reporting? | Free of other bias? |
|---|---|---|---|---|---|---|
| Dols et al. 2014 [29] | Yes | Yes | No - single | Yes | Yes | Yes |
| Klop et al. 2014 [33] | Yes | Yes | No - single | Yes | Yes | Yes |
| Cho et al. 2013 [27] | Unclear | No | No | No | Unclear | Unclear |
| Bargman et al. 2006 [24] | Unclear | No | No | No | Unclear | Unclear |
An assessment of the cohort studies was made using the Newcastle-Ottawa Scale and is summarised in the table below (Table 3). The overall quality was medium (4–6 stars). Kocak et al. [34], Mjøen et al. [38] and Choi et al. [28] were the three studies that were given a higher quality score (7–9 stars). All cohorts scored 3 on selection criteria. The outcomes were already present when the study began for all but two prospective cohort studies [26], [43]. Most studies had groups that were comparable in terms of age, gender and BMI. Those that were matched for side of nephrectomy, multiple renal arteries, and previous abdominal surgery, as well were given a higher rating. Most of the studies outlined the perioperative protocol and stated that both groups were exposed to the same protocol. Whilst duration of follow-up was stated in most studies, there is little direct information on completeness of follow-up on participants. Overall there was heterogeneity between studies in terms of the characteristics and number of participants, study methodology and follow-up.
Table 3.
Quality assessment of cohort studies using the Newcastle-Ottawa Scale.
| References | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|
| Ruiz-Deya et al. [40] | *** | * | ** | 5 |
| Velidedeoglu et al. [45] | *** | * | * | 5 |
| Gershbein et al. [31] | *** | * | ** | 6 |
| Wadstrom et al. [46] | *** | * | * | 5 |
| Mateo et al. [37] | *** | ** | ** | 6 |
| Sundqvist et al. [43] | *** | * | ** | 6 |
| Buell et al. [26] | *** | * | ** | 6 |
| El-Galley et al. [30] | *** | * | * | 5 |
| Salazar et al. [42] | *** | * | * | 5 |
| Gjertsen et al. [32] | *** | * | * | 5 |
| Yoo et al. [47] | *** | * | * | 5 |
| Ruszat et al. [41] | *** | * | ** | 6 |
| Kocak et al. [34] | *** | ** | ** | 7 |
| Branco et al. [25] | *** | * | ** | 6 |
| Percegona et al. [39] | *** | * | 4 | |
| Lai et al. [35] | *** | * | ** | 6 |
| Mjøen et al. [38] | *** | ** | ** | 7 |
| Ungbhakorn et al. [44] | *** | * | * | 5 |
| Lucas et al. [36] | *** | * | ** | 6 |
| Choi et al. [28] | *** | ** | *** | 8 |
0–3 low quality; 4–6 medium quality; 7–9 high quality.
Meta-analysis
Firstly, an overall analysis was carried out comparing hand-assisted (both HALDN and HARPDN) vs pure LDN.
RCTs
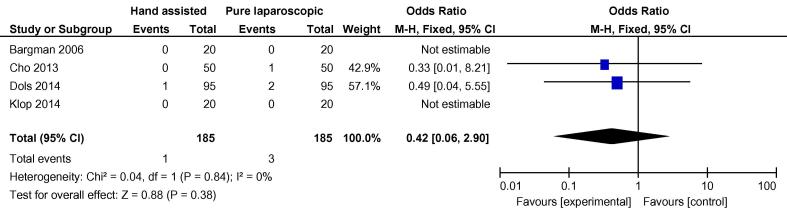
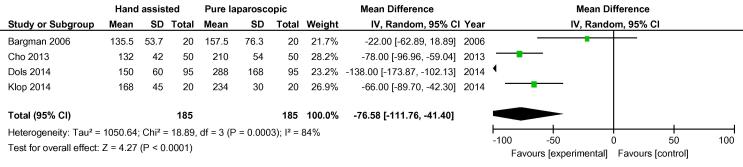
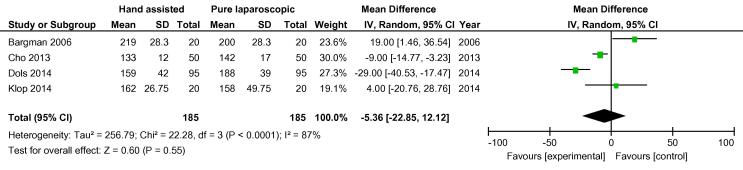
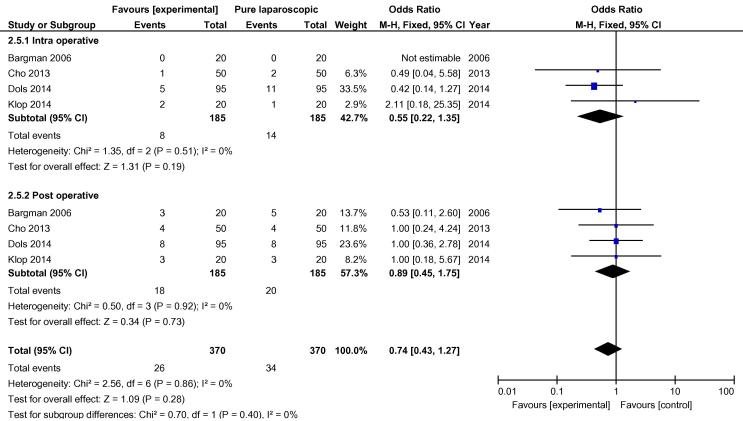
On analysis of the four randomised studies, there was a total of 370 patients; 185 in each group. There was one conversion in the hand-assisted group vs three in the laparoscopic group, OR 0.42 (FEM, 95% CI 0.06, 2.90) (Fig. 2). For secondary outcomes, WIT was significantly shorter in the hand-assisted group (76.58 s, 95% CI −111.76, −41.40) (Fig. 3). There was no statistically significant difference in OT (−5.36 min, 95% CI −22.85, 12.12), intraoperative complication rate (OR 0.55, 95% CI 0.22, 1.35,), or postoperative complication rate (OR 0.89, 95% CI 0.45, 1.75) (Fig. 4, Fig. 5). There was no statistically significant difference between the two groups in EBL at 31.13 mL (REM, 95% CI −45.84, 108.09; I2 = 85%, P = 0.43), or LOS postoperatively at 0.16 days (FEM, 95% CI −0.06, 0.38; I2 = 0%, P = 0.14).
Fig. 2.
Odds difference in open conversions between all HALDN (experimental) and pure LDN (control) procedures for RCTs.
Fig. 3.
Mean difference in WIT between all HALDN (experimental) and pure LDN (control) procedures for RCTs.
Fig. 4.
Mean difference in operation time between all HALDN (experimental) and pure LDN (control) procedures for RCTs.
Fig. 5.
Odds difference in complications between all HALDN (experimental) and pure LDN (control) procedures for RCTs.
All hand-assisted vs laparoscopic pooled cohorts
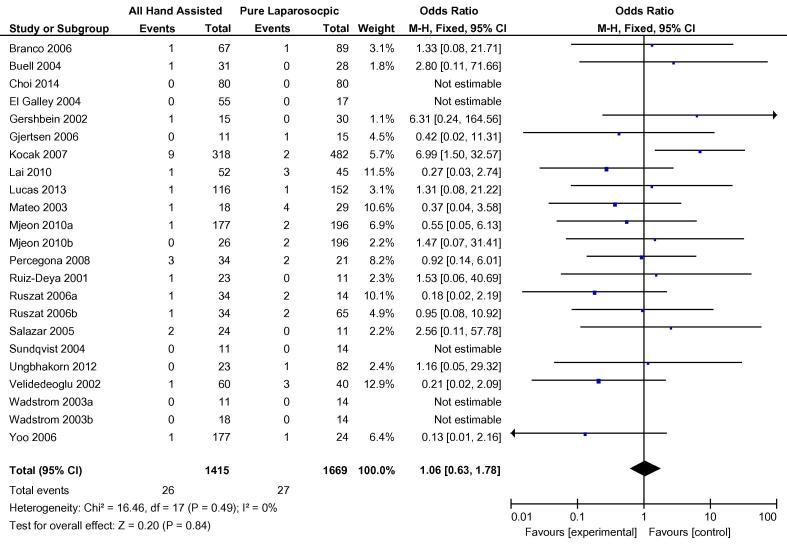
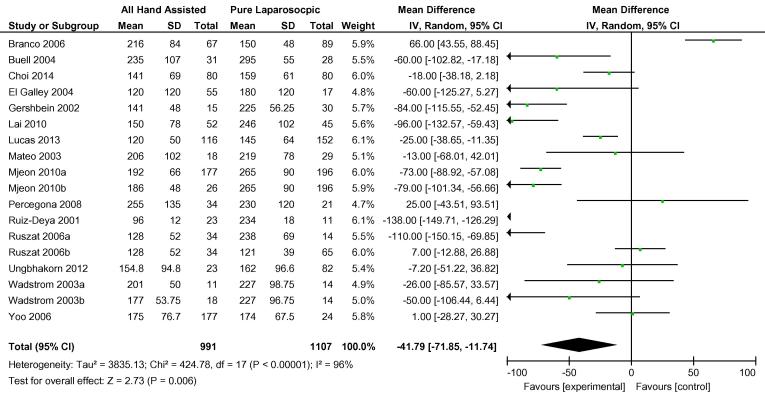
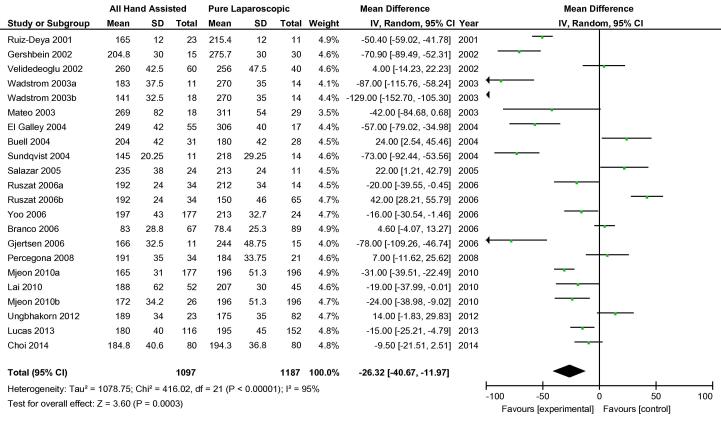
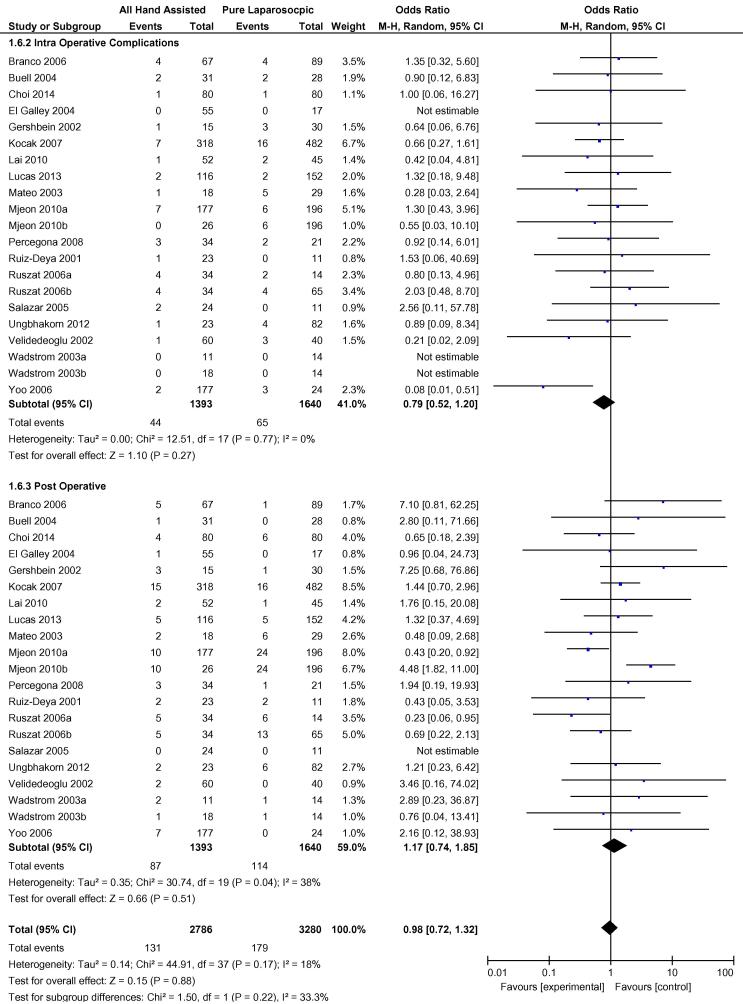
Overall, 20 cohort studies were analysed to compare hand-assisted techniques to pure LDN. The two hand-assisted techniques of HALDN and HARPDN were pooled for this analysis. Three cohort studies carried out more than one relevant comparison [38], [41], [46], so these were included separately e.g. Mjøen et al. 2010a and Mjøen et al. 2010b [38]. There was no statistical difference in conversions to open procedure between the two groups. In total, 26/1415 hand-assisted procedures were converted vs 27/1669 pure LDN procedures, OR 1.06 (95% CI 0.63, 1.78) (Fig. 6). Both WIT and OT were shorter in the hand-assisted group by −41.79 s (95% CI −71.85, −11.74) and −26.32 min (95% CI −40.67, −11.97), respectively (Fig. 7, Fig. 8). There was no difference in overall complications between the two groups OR 0.98 (95% CI 0.72, 1.32) (Fig. 9). Only 11 cohort studies assessed EBL. There was no statistically significant difference between the two groups in terms of EBL at −11.08 mL (REM, 95% CI −33.45, 11.28; I2 = 73%, P = 0.33), or LOS at 0.20 days (REM, 95% CI −0.11, 0.51; I2 = 95%, P = 0.2).
Fig. 6.
Odds difference in open conversions between all HALDN (experimental) and pure LDN (control) procedures for pooled cohort studies.
Fig. 7.
Mean difference in WIT between all HALDN (experimental) and pure LDN (control) procedures for pooled cohort studies.
Fig. 8.
Mean difference operation time between all HALDN (experimental) and pure LDN (control) procedures for pooled cohort studies.
Fig. 9.
Odds difference in complications between all HALDN (experimental) and pure LDN (control) procedures for pooled cohort studies.
Subgroup analysis -– HARPDN vs LDN cohorts
A total of six studies compared specifically the retroperitoneal hand-assisted approach with pure LDN. This included one RCT, one randomised pilot study, one prospective cohort and four retrospective cohort studies. Only the cohorts were analysed in this subgroup. Out of a total of 305 patients, there were no conversions in the HARPDN group vs three LDN patients, OR 0.76 (FEM, 95% CI 0.08, 7.33; I2 = 0%, P = 0.81). Three studies assessed WIT, with a total of 279 patients. WIT was shorter in the HARPDN group at −54.78 s (REM, 95% CI −90.93, −18.63; I2 = 66%, P = 0.003), as was OT at −75.43 min (REM, 95% CI −121.81, −29.06; I2 = 95%, P = 0.001). There was a lower intraoperative events risk in the HARPDN group, OR 0.49 (FEM, 95% CI 0.05, 4.44; I2 = 0%, P = 0.53). On the other hand, postoperative complications were significantly higher in this group, OR 3.54 (FEM, 95% CI 1.55, 8.09; I2 = 0%, P = 0.003).
Sensitivity analysis
There was statistical heterogeneity between the studies. A sensitivity analysis was performed for more recently published cohort studies. A cut-off of 2010 or later was used. For conversions to open procedure the OR reduced from 1.06 (FEM, 95% CI 0.63, 1.78; I2 = 0%, P = 0.84) to 0.65 (FEM, 95% CI 0.20, 2.14; P = 0.48), whilst I2 remained 0%, chi-squared reduced from 16.46 to 1.20. WIT increased from −41.79 s (REM, 95% CI −71.85, −11.74; I2 = 96%, P = 0.006) to −56.96 s (REM, 95% CI −87.09, −26.82; P < 0.001). The chi-squared score reduced substantially from 424.78 to 36.87 and I2 reduced slightly from 96% to 89%. There was no significant change in OT, EBL, complications, and LOS. Overall there was less heterogeneity for the studies after 2010 for most outcomes, which is likely due to the established nature of each technique.
Discussion
Statement of principal findings
The present meta-analysis had conversions to an open procedure as the primary outcome and none of the analyses showed a statistically significant difference in conversion rates. Whilst conversion is not a complication in itself, it implies issues regarding technical difficulty and intraoperative safety and will have an effect on postoperative recovery. It has previously been shown in a large series that the most common reason for conversion is vascular injury (38.5%) and open conversion is inversely related to case volume and accumulated experience [48].
There was a consistent difference in the secondary outcome of WIT, both overall and in the subgroup analysis. This is most likely due to the fact that the kidney is manually extracted through the handport quickly after the vessels are ligated in the hand-assisted approach, whilst the purely laparoscopic approach requires a retrieval bag and incision. There is limited long-term data relating to graft function in the setting of longer WITs. One study found similar survival rates but a trend towards reduced long-term function with longer WIT; however, this was in non-heart beating donors [49]. An RCT of 200 patients found no difference in long-term graft function associated with longer WIT when comparing LDN to open DN [50].
OT was shorter overall in the hand-assisted group, with over an hour reduction for the HARPDN subgroup. The duration of procedure is difficult to compare with certainty because the endpoint can vary amongst institutions and how it is measured is often not stated. Despite this possible confounder, we observed a wide range in OT for each technique (83–269 min for HALDN, 78.4–311 min for LDN). The variance in OT for HARPDN was much smaller 141–172 min. This suggests an effect of uniform recording, as well as certain centre-specific practices and operator experience.
There was little difference in the overall complication rate between the two groups. There were stricter rates of reporting of even very minor complications in certain centres [29], [33]. Whilst the intraoperative event rate was lower and the postoperative complications risk higher in the hand-assisted group, none of these were statistically significant. Based on the present meta-analysis LDN is as safe as HALDN and HARPDN. Finally, there was no statistically significant advantage in EBL or LOS between the techniques.
Results in the context of current literature
Kokkinos et al. [16] carried out a meta-analysis of nine cohort studies published in 2007. The present study has produced comparable results over 8 years later. Although not statistically significant, the conversion rate was lower in the Kokkinos et al. [16] meta-analysis, OR 0.58 (95% CI 0.18, 1.82). The conversion rate in our present study is almost the same in both groups, OR 1.06. The advantage of hand-assisted of shorter WIT and OT has been shown in both the Kokkinos et al. [16] and our present study. The present study reconfirms the evidence from Kokkinos et al. [16] that there is no statistical difference in the complication rate between HALDN and LDN, although intraoperative complications were lower for the HARPDN subgroup analysis.
Halgrimson et al. [17] published a highly-powered review of 37 articles with >9000 patients in 2009, although it was not limited to comparative studies like our present study. It found a statistically significant difference in our primary outcome, conversion to open procedure in favour of HALDN (0.4% vs 0.8%, P = 0.015). The pooled cohort in the present study showed no statistical difference in conversion rates (1.88% vs 1.85%, P = 0.88). They also found a statistically significant increase in intraoperative events rate for LDN (5.2% vs 2%, P < 0.001). This was not reproduced in our present study (4.54% LDN vs 3.23% HALDN, P = 0.27).
Strengths and weaknesses of the study
One of the strengths of the present meta-analysis is that it includes a substantial number of studies, with 24 in total. The study provides an updated and thorough analysis of some of the important perioperative outcomes associated with DN. The methodological quality was assessed by two independent reviewers using validated criteria. Broad search terms were used to identify the totality of evidence. A robust methodology was followed to screen and select studies. RCTs were analysed separately to cohort studies at all times. In addition to an overall assessment, subgroup analysis on HARPDN was also carried out.
There are also limitations to the present study. Firstly, there were only three RCTS and one randomised pilot study, which were lowly powered in all but one study. It was, therefore, not possible to perform a sensitivity analysis on RCTs. The main weight of the results has come from cohort studies. There was a high risk of bias for two of the RCTs [24], [27]. Whilst the risk of bias was lower for the other two randomised studies; there were still aspects that were judged unclear. Only three cohort studies were deemed high quality [28], [34], [38]. It would have been preferable to analyse graft survival, postoperative pain scores, and analgesia requirement, but these were poorly reported. Finally, there was significant characteristic and statistical heterogeneity between studies as evidenced by sensitivity analysis accounting for studies from 2010 or later.
Clinical/policy implications
Both HALDN and purely LDN techniques are generally safe and the differences between them are negligible. HALDN has consistently shown to have a shorter WIT than pure LDN. Whilst it is desirable to minimise WIT, previous studies including the Cochrane Review 2011 have shown that laparoscopic techniques having a longer WIT has not had a significant detrimental effect on graft survival [13]. The difference in OT ranged from being negligible in the analysis of RCTs to >1 h in the HARPDN subgroup. Experience, learning curve, and frequency of case load are obvious factors that may influence the duration of a procedure but other factors, such as coordination with the recipient transplant procedure are not explicitly addressed. Based on the findings of our present study, the risk of intraoperative events and requirement to convert to an open procedure for both techniques was lower than in previous studies. EBL and LOS postoperatively should not influence procedure choice. As one technique cannot be advocated over another it is left to personal preference for the operating surgeon. A retroperitoneoscopic approach may be advantageous in the setting of previous abdominal surgery with potential adhesions. Finally, as major complications in DN are relatively rare, it is important to set up an international register of conversions, intraoperative events, and major postoperative complications, as this is the only way to truly determine safety in minimally invasive DN.
Areas for further research
As DN should minimise the harm to the patient and maximise potential for a quick recovery, further research into other minimally invasive techniques is warranted and is already being carried out. Robot-assisted LDN (RALDN) was first described in 2001/2002 [51], [52]. It has reported good results with no difference in complications or early graft function [53], with the benefit proposed of even earlier discharge from hospital [54]. There have been no RCTs comparing RALDN to other techniques and these higher-level studies would be recommended. Further emerging techniques have shown promising results such as the laparoendoscopic single site (LESS LDN) technique [55] and natural orifice DN [56]. Other areas of research that would be of benefit relate to cost analysis and quality-of-life outcomes using uniform assessment tools.
Conclusion
The present systematic review with meta-analysis has found that there is no major difference between HALDN and pure LDN techniques in terms of perioperative outcomes. There is no statistical difference between the two groups in the rates of conversion to an open procedure and perioperative complications. The hand-assisted method has consistently been shown to have a shorter WIT and duration of procedure. Further studies of higher level of evidence are required to further establish any benefit of one technique over the other.
Author participation
Mark P Broe – Data collection or management, data analysis, manuscript writing/editing.
Rose Galvin – Protocol/project development, methodology supervision, manuscript editing.
Lorna G. Keenan – Data collection or management, data analysis, manuscript editing.
Richard E. Power – Protocol/project development, manuscript editing.
Conflict of interest
There was no conflict of interest regarding any author involved in the study. There was no funding for the study. There were no studies carried out on human participants or animals.
Upper Tract Surgery
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L., Ojo A.O., Ettenger R.E., Agodoa L.Y. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.United Network for Organ Sharing. Facts about organ transplantation. Available from: http://www.unos.org/. Accessed April 2015
- 3.Matas A.J., Schnitzler M. Payment for living donor (vendor) kidneys: a cost-effectiveness analysis. Am J Transplant. 2004;4:216–221. doi: 10.1046/j.1600-6143.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 4.Kanellis J. The CARI guidelines. Justification for living donor kidney transplantation. Nephrology. 2010;15:S72–S79. doi: 10.1111/j.1440-1797.2009.01212.x. [DOI] [PubMed] [Google Scholar]
- 5.Nemati E., Einollahi B., Lesan Pezeshki M., Porfarziani V., Fattahi M.R. Does kidney transplantation with deceased or living donor affect graft survival? Nephrourol Mon. 2014;6:e12182. doi: 10.5812/numonthly.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim H.N., Foley R., Tan L., Rogers T., Bailey R.F., Guo H. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratner L.E., Ciseck L.J., Moore R.G., Cigarroa F.G., Kaufman H.S., Kavoussi L.R. Laparoscopic live donor nephrectomy. Transplantation. 1995;60:1047–1049. [PubMed] [Google Scholar]
- 8.Merlin T.L., Scott D.F., Rao M.M., Wall D.R., Francis D.M., Bridgewater F.H. The safety and efficacy of laparoscopic live donor nephrectomy: a systematic review. Transplantation. 2000;70:1659–1666. doi: 10.1097/00007890-200012270-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ratner L.E., Hiller J., Sroka M., Weber R., Sikorsky I., Montgomery R.A. Laparoscopic live donor nephrectomy removes disincentives to live donation. Transplant Proc. 1997;29:3402–3403. doi: 10.1016/s0041-1345(97)00955-x. [DOI] [PubMed] [Google Scholar]
- 10.Schweitzer E.J., Wilson J., Jacobs S., Machan C.H., Philosophe B., Farney A. Increased rates of donation with laparoscopic donor nephrectomy. Ann Surg. 2000;232:392–400. doi: 10.1097/00000658-200009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf J.S., Jr, Tchetgen M.B., Merion R.M. Hand-assisted laparoscopic live donor nephrectomy. Urology. 1998;52:885–887. doi: 10.1016/s0090-4295(98)00389-6. [DOI] [PubMed] [Google Scholar]
- 12.Leventhal J.R., Kocak B., Salvalaggio P.R., Koffron A.J., Baker T.B., Kaufman D.B. Laparoscopic donor nephrectomy 1997 to 2003: lessons learned with 500 cases at a single institution. Surgery. 2004;136:881–890. doi: 10.1016/j.surg.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Wilson C.H., Sanni A., Rix D.A., Soomro N.A. Laparoscopic versus open nephrectomy for live kidney donors. Cochrane Database Syst Rev. 2011;11:CD006124. doi: 10.1002/14651858.CD006124.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta P., Challacombe B., Compton F., Khan S. A systematic review of hand-assisted laparoscopic live donor nephrectomy. Int J Clin Pract. 2004;58:474–478. doi: 10.1111/j.1368-5031.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Dols L.F., Kok N.F., Ijzermans J.N. Live donor nephrectomy: a review of evidence for surgical techniques. Transplant Int. 2010;23:121–130. doi: 10.1111/j.1432-2277.2009.01027.x. [DOI] [PubMed] [Google Scholar]
- 16.Kokkinos C., Nanidis T., Antcliffe D., Darzi A.W., Tekkis P., Papalois V. Comparison of laparoscopic versus hand-assisted live donor nephrectomy. Transplantation. 2007;83:41–47. doi: 10.1097/01.tp.0000248761.56724.9c. [DOI] [PubMed] [Google Scholar]
- 17.Halgrimson W.R., Campsen J., Mandell M.S., Kelly M.A., Kam I., Zimmerman M.A. Donor complications following laparoscopic compared to hand-assisted living donor nephrectomy: an analysis of the literature. J Transplant. 2010;2010:825689. doi: 10.1155/2010/825689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadstrom J., Martin A.L., Estok R., Mercaldi C.J., Stifelman M.D. Comparison of hand-assisted laparoscopy versus open and laparoscopic techniques in urology procedures: a systematic review and meta-analysis. J Endourol. 2011;25:1095–1104. doi: 10.1089/end.2010.0348. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed March 2015.
- 22.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autorino R., Kaouk J.H., Yakoubi R., Rha K.H., Stein R.J., White W.M. Urological laparoendoscopic single site surgery: multi-institutional analysis of risk factors for conversion and postoperative complications. J Urol. 2012;187:1989–1994. doi: 10.1016/j.juro.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 24.Bargman V., Sundaram C.P., Bernie J., Goggins W. Randomized trial of laparoscopic donor nephrectomy with and without hand assistance. J Endourol. 2006;20:717–722. doi: 10.1089/end.2006.20.717. [DOI] [PubMed] [Google Scholar]
- 25.Branco A.W., Kondo W., Branco Filho A.J., De George M.A., Rangel M., Stunitz L.C. A comparison of hand-assisted and pure laparoscopic techniques in live donor nephrectomy. Clinics (Sao Paulo) 2008;63:795–800. doi: 10.1590/S1807-59322008000600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buell J.F., Abreu S.C., Hanaway M.J., Ng C.S., Kaouk J.H., Clippard M. Right donor nephrectomy: a comparison of hand-assisted transperitoneal and retroperitoneal laparoscopic approaches. Transplantation. 2004;77:521–525. doi: 10.1097/01.tp.0000109689.55999.fa. [DOI] [PubMed] [Google Scholar]
- 27.Cho H.J., Choi Y.S., Bae W.J., Bae J.H., Hong S.H., Lee J.Y. Another option for laparoscopic living donor nephrectomy: a single center experience comparing two-port versus hand-assisted technique. J Endourol. 2013;27:587–591. doi: 10.1089/end.2012.0577. [DOI] [PubMed] [Google Scholar]
- 28.Choi S.W., Kim K.S., Kim S., Choi Y.S., Bae W.J., Hong S.H. Hand-assisted and pure laparoscopic living donor nephrectomy: A matched-cohort comparison over 10 yr at a single institute. Clin Transplant. 2014;28:1287–1293. doi: 10.1111/ctr.12462. [DOI] [PubMed] [Google Scholar]
- 29.Dols L.F., Kok N.F., d'Ancona F.C., Klop K.W., Tran T.C., Langenhuijsen J.F. Randomized controlled trial comparing hand-assisted retroperitoneoscopic versus standard laparoscopic donor nephrectomy. Transplantation. 2014;97:161–167. doi: 10.1097/TP.0b013e3182a902bd. [DOI] [PubMed] [Google Scholar]
- 30.El-Galley R., Hood N., Young C.J., Deierhoi M., Urban D.A. Donor nephrectomy: a comparison of techniques and results of open, hand-assisted and full laparoscopic nephrectomy. J Urol. 2004;171:40–43. doi: 10.1097/01.ju.0000100149.76079.89. [DOI] [PubMed] [Google Scholar]
- 31.Gershbein A.B., Fuchs G.J. Hand-assisted and conventional laparoscopic live donor nephrectomy: a comparison of two contemporary techniques. J Endourol. 2002;16:509–513. doi: 10.1089/089277902760367476. [DOI] [PubMed] [Google Scholar]
- 32.Gjertsen H., Sandberg A.K., Wadstrom J., Tyden G., Ericzon B.G. Introduction of hand-assisted retroperitoneoscopic living donor nephrectomy at Karolinska University Hospital Huddinge. Transplant Proc. 2006;38:2644–2645. doi: 10.1016/j.transproceed.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Klop K.W., Kok N.F., Dols L.F., Dor F.J., Tran K.T., Terkivatan T. Can right-sided hand-assisted retroperitoneoscopic donor nephrectomy be advocated above standard laparoscopic donor nephrectomy: a randomized pilot study. Transpl Int. 2014;27:162–169. doi: 10.1111/tri.12226. [DOI] [PubMed] [Google Scholar]
- 34.Kocak B., Baker T.B., Koffron A.J., Leventhal J.R. Laparoscopic living donor nephrectomy: a single-center sequential experience comparing hand-assisted versus standard technique. Urology. 2007;70:1060–1063. doi: 10.1016/j.urology.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Lai I.R., Yang C.Y., Yeh C.C., Tsai M.K., Lee P.H. Hand-assisted versus total laparoscopic live donor nephrectomy: comparison and technique evolution at a single center in Taiwan. Clin Transplant. 2010;24:E182–E187. doi: 10.1111/j.1399-0012.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 36.Lucas S.M., Liaw A., Mhapsekar R., Yelfimov D., Goggins W.C., Powelson J.A. Comparison of donor, and early and late recipient outcomes following hand-assisted and laparoscopic donor nephrectomy. J Urol. 2013;189:618–622. doi: 10.1016/j.juro.2012.07.142. [DOI] [PubMed] [Google Scholar]
- 37.Mateo R.B., Sher L., Jabbour N., Singh G., Chan L., Selby R.R. Comparison of outcomes in noncomplicated and in higher-risk donors after standard versus hand-assisted laparoscopic nephrectomy. Am Surg. 2003;69:771–778. [PubMed] [Google Scholar]
- 38.Mjøen G., Holdaas H., Pfeffer P., Line P.D., Øyen O. Minimally invasive living donor nephrectomy - introduction of hand-assistance. Transplant Int. 2010;23:1008–1014. doi: 10.1111/j.1432-2277.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 39.Percegona L.S., Bignelli A.T., Adamy A., Jr, Pilz F., Chin E.W., Meyer F. Hand-assisted laparoscopic donor nephrectomy: comparison to pure laparoscopic donor nephrectomy. Transplant Proc. 2008;40:687–688. doi: 10.1016/j.transproceed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Deya G., Cheng S., Palmer E., Thomas R., Slakey D., Jacobs S.C. Open donor, laparoscopic donor and hand-assisted laparoscopic donor nephrectomy: a comparison of outcomes. J Urol. 2001;166:1270–1273. [PubMed] [Google Scholar]
- 41.Ruszat R., Sulser T., Dickenmann M., Wolff T., Gurke L., Eugster T. Retroperitoneoscopic donor nephrectomy: donor outcome and complication rate in comparison with three different techniques. World J Urol. 2006;24:113–117. doi: 10.1007/s00345-006-0051-9. [DOI] [PubMed] [Google Scholar]
- 42.Salazar A., Pelletier R., Yilmaz S., Monroy-Cuadros M., Tibbles L.A., McLaughlin K. Use of a minimally invasive donor nephrectomy program to select technique for live donor nephrectomy. Am J Surg. 2005;189:558–563. doi: 10.1016/j.amjsurg.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Sundqvist P., Feuk U., Haggman M., Persson A.E., Stridsberg M., Wadstrom J. Hand-assisted retroperitoneoscopic live donor nephrectomy in comparison to open and laparoscopic procedures: a prospective study on donor morbidity and kidney function. Transplantation. 2004;78:147–153. doi: 10.1097/01.tp.0000133280.74695.34. [DOI] [PubMed] [Google Scholar]
- 44.Ungbhakorn P., Kongchareonsombat W., Leenanupan C., Kijvikai K., Wisetsingh W., Patcharatrakul S. Comparative outcomes of open nephrectomy, hand-assisted laparoscopic nephrectomy, and full laparoscopic nephrectomy for living donors. Transplant Proc. 2012;44:22–25. doi: 10.1016/j.transproceed.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Velidedeoglu E., Williams N., Brayman K.L., Desai N.M., Campos L., Palanjian M. Comparison of open, laparoscopic, and hand-assisted approaches to live-donor nephrectomy. Transplantation. 2002;74:169–172. doi: 10.1097/00007890-200207270-00005. [DOI] [PubMed] [Google Scholar]
- 46.Wadstrom J., Lindstrom P., Engstrom B.M. Hand-assisted retroperitoneoscopic living donor nephrectomy superior to laparoscopic nephrectomy. Transplant Proc. 2003;35:782–783. doi: 10.1016/s0041-1345(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 47.Yoo K.Y., Hong S.H., Hwang T.K. Donor nephrectomy: comparison of open, hand-assisted and laparoscopic donor nephrectomy. Korean J Urol. 2006;47:1309–1314. [Google Scholar]
- 48.Richstone L., Seideman C., Baldinger L., Permpongkosol S., Jarrett T.W., Su L.M. Conversion during laparoscopic surgery: frequency, indications and risk factors. J Urol. 2008;180:855–859. doi: 10.1016/j.juro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 49.Barlow A.D., Metcalfe M.S., Johari Y., Elwell R., Veitch P.S., Nicholson M.L. Case-matched comparison of long-term results of non-heart beating and heart-beating donor renal transplants. Br J Surg. 2009;96:685–691. doi: 10.1002/bjs.6607. [DOI] [PubMed] [Google Scholar]
- 50.Simforoosh N., Basiri A., Shakhssalim N., Gooran S., Tabibi A., Khoshdel A. Long-term graft function in a randomized clinical trial comparing laparoscopic versus open donor nephrectomy. Exp Clin Transplant. 2012;10:428–432. doi: 10.6002/ect.2012.0010. [DOI] [PubMed] [Google Scholar]
- 51.Horgan S., Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 2001;11:415–419. doi: 10.1089/10926420152761950. [DOI] [PubMed] [Google Scholar]
- 52.Horgan S., Vanuno D., Sileri P., Cicalese L., Benedetti E. Robotic-assisted laparoscopic donor nephrectomy for kidney transplantation. Transplantation. 2002;73:1474–1479. doi: 10.1097/00007890-200205150-00018. [DOI] [PubMed] [Google Scholar]
- 53.Renoult E., Hubert J., Ladriere M., Billaut N., Mourey E., Feuillu B. Robot-assisted laparoscopic and open live-donor nephrectomy: a comparison of donor morbidity and early renal allograft outcomes. Nephrol Dial Transplant. 2006;21:472–477. doi: 10.1093/ndt/gfi150. [DOI] [PubMed] [Google Scholar]
- 54.Cohen A.J., Williams D.S., Bohorquez H., Bruce D.S., Carmody I.C., Reichman T. Robotic-assisted laparoscopic donor nephrectomy: decreasing length of stay. Ochsner J. 2015;15:19–24. [PMC free article] [PubMed] [Google Scholar]
- 55.Autorino R., Brandao L.F., Sankari B., Zargar H., Laydner H., Akca O. Laparoendoscopic single-site (LESS) vs laparoscopic living-donor nephrectomy: a systematic review and meta-analysis. BJU Int. 2015;115:206–215. doi: 10.1111/bju.12724. [DOI] [PubMed] [Google Scholar]
- 56.Gurluler E., Berber I., Cakir U., Gurkan A. Transvaginal route for kidney extraction in laparoscopic donor nephrectomy. JSLS. 2014;18 doi: 10.4293/JSLS.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]