Abstract
Introduction
Individual patients with rheumatoid arthritis (RA) show divergent specific anti-citrullinated protein/peptide antibodies (ACPA) patterns, but hitherto no individual ACPA specificity has consistently been linked to RA pathogenesis. ACPA are also implicated in immune complexes (IC)-associated joint pathology, but until now, there has been no method to investigate the role of individual ACPA in RA IC formation and IC-associated pathogenesis.
Methods
We have developed a new technique based on IC binding to C1q-coated magnetic beads to purify and solubilise circulating IC in sera and synovial fluids (SF) from 77 patients with RA. This was combined with measurement of 19 individual ACPA in serum, SF and in the IC fractions from serum and SF. We investigated whether occurrence of individual ACPA as well as number of ACPA in these compartments was related to clinical and laboratory measures of disease activity and inflammation.
Results
The majority of individual ACPA reactivities were enriched in SF as compared with in serum, and levels of ACPA in IC were regulated independently of levels in serum and SF. No individual ACPA reactivity in any compartment showed a dominating association to clinical and laboratory measures of disease activity and severity. Instead, the number of individual ACPA reactivities in the IC fraction from SF associated with a number of markers of joint destruction and inflammation.
Conclusions
Our data highlight the polyclonality of ACPA in joint IC and the possibility that a broad ACPA repertoire in synovial fluid IC might drive the local inflammatory and matrix-degrading processes in joints, in analogy with antibody-induced rodent arthritis models.
Keywords: ant-ccp, autoantibodies, rheumatoid arthritis, synovial fluid
Introduction
Antibodies directed against citrullinated peptides (anti-citrullinated protein/peptide antibodies, ACPA) are found in sera of 60%–80% of patients with rheumatoid arthritis (RA).1 2 Numerous studies have confirmed that presence of ACPA in serum is associated with a more severe disease course.3–5
Total ACPA responses are commonly measured in serum with tests using proprietary cyclic citrullinated peptides (CCP) or other optimised mixtures of peptides.6–8 Already the first publications showed a considerable ACPA epitope variation between patients with RA.1 9 Commonly studied specificities include the originally described filaggrin molecule, as well as proteins found to be citrullinated in RA joints: α-enolase, vimentin, collagen type II, fibrinogen and histones.10–13 A number of microarray techniques have been developed to study the pattern of ACPA responses in a simplified way, using proteome microarrays, surface plasmon resonance imaging or addressable laser bead immunoassay.2 11 14 We have recently described a planar microarray where multiple ACPA are investigated in parallel to their native arginine-containing counterparts.10 In an extended follow-up study using 2825 patients with RA, we found that individual subtraction of arginine reactivity was beneficial, as it both increased diagnostic specificity and association to HLA-DRB1* shared epitope, while diagnostic specificity was unchanged.15
No individual ACPA specificity or group of ACPA specificities has hitherto showed any unique and consistent association to clinical phenotype.2 16 Such epitope studies have only been performed on serum samples. Two groups have reported divergent results concerning whether ACPA are enriched in synovial fluid after correction for IgG levels.8 17 18 Enrichment would imply that ACPA might be preferably produced in the joints of patients with RA. Thus, if ACPA have any direct pathogenetic association to the inflammatory process in RA, determinations of total ACPA and individual ACPA reactivities in synovial fluid (SF) might show stronger associations to clinical phenotype than corresponding serum levels.
Already 50 years ago, immune complexes (IC) were found in RA, especially in SF but also in the circulation of patients with extra-articular disease.19–23 However, results diverged considerably between studies and methods used.24 More recent studies have focused on biological IC function in RA. We have shown that levels of polyethylene glycol-precipitated IC from RA SF relate to in vitro-induced tumour necrosis factor alpha (TNF-α) production and rheumatoid factor (RF) levels, supporting the hypothesis that IC are directly linked to cytokine-dependent inflammation in RA.25 Using another in vitro model, we have produced surface-bound IC containing collagen type II (CII) and anti-CII, and related anti-CII IC-induced responses in vitro and anti-CII levels in vivo to an acute-onset RA phenotype.26–29 This IC-dependent RA phenotype shows resemblance to collagen antibody-induced arthritis (CAIA), an antibody-mediated arthritis model dependent on neutrophil granulocytes.28 30 Two groups have shown that ACPA-containing IC stimulate cytokine production via Fc receptors.31 32 Notably, except for some early studies, none of these studies have actually determined autoantibody levels in RA IC obtained in vivo.33 34 As previous studies have shown accumulation both of IC and of ACPA in SF as compared with in the circulation, ACPA levels in SF IC would then be an especially interesting target for such studies.
We have developed a bead-based assay for the quantification of multiple autoantibodies in soluble IC. Here, we have combined this assay with the ACPA multiplex array to investigate whether individual ACPA reactivities in sera and SF, and in IC from these compartments, can provide more or different prognostic information than conventional measurement of anti-CCP in serum.
Patients and methods
Subjects
The complete cohort study consisting of 121 patients with RA has been described previously, and of these, 77 patients had paired sera and knee SF available for the present study.35 Patients fulfilling the 1987 American College of Rheumatology classification criteria for RA and with knee synovitis were included at the rheumatology departments in Gävle, Falun and Uppsala, Sweden.36 Patients with function class 4 according to Steinbrocker, those receiving ≥10 mg oral prednisolone daily and those who had obtained glucocorticoid injection <3 months prior to the investigation were excluded.37 SF were aspirated from the knees whereafter 20 mg triamcinolone hexacetonide was injected. Sera and SF were collected in parallel, centrifuged for 20 min at 1800×g within 1 hour and stored at −70°C until analysis. Levels of mononuclear (MN) and polymorphonuclear (PMN) cells in SF were recorded. Patients were followed for 6 months, or until relapse if clinical synovitis re-appeared earlier. Information on patient characteristics (age, sex, disease duration and smoking habits) was recorded at the study inclusion. Disability was evaluated using the Swedish version of the Health Assessment Questionnaire.38 Disease Activity Score (DAS28)39 was assessed from the number of tender and swollen joints, erythrocyte sedimentation rate and Visual Analogue Scale for global health. Radiographic examination of knees was performed and joint destruction recorded according to Larsen et al.40 The study was approved by the regional ethical review board in Uppsala, and all patients had given informed consent in accordance with the Declaration of Helsinki.
Laboratory investigation of autoantibodies and measures of systemic and local inflammation was performed in parallel in serum and SF as described previously.35 41 42 Measurements of C1q-binding IC and IgG levels in sera and SF are described in the online supplementary file.
annrheumdis-2017-212627supp001.docx (10.1MB, docx)
Capturing and elution of circulating IC (CIC)
IC were purified from sera and SF by applying a technique we have established in our laboratory. Purified human C1q (Quidel, San Diego, California, USA; 1.2 mg/mL) was immobilised on magnetic tosylactivated microparticles (Dynabeads M-280; Life Technologies, Carlsbad, California, USA) according to the manufacturer’s recommendations for activation of amine groups.
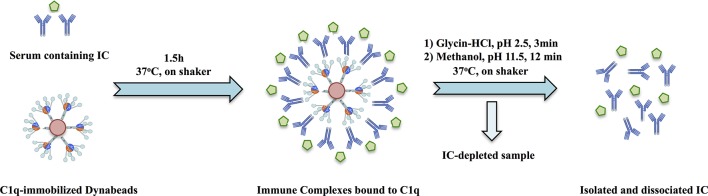
Ten-microlitre C1q beads were washed once with PBS–0.05% Tween–1% BSA (PBST-B) and incubated with 10 µL serum or SF and 30 µL PBST-B in a Bio-Plex flat-bottom 96-well plate for 1.5 hours on a microplate shaker (600 rpm) at 37°C. Antibodies from the C1q-bound IC were recovered in a two-step procedure. After washing with PBS–0.05% Tween, the beads were resuspended with a Bio-Plex hand-held magnetic washer in 50 µL 0.1 M glycine–HCl, pH 2.5 and incubated for 3 min on shaker at 37°C. IC eluates were collected and immediately neutralised with 4 µL 0.5 M NaOH on ice. In the second elution step, the beads were resuspended with 100 µL freshly prepared 25% methanol, pH 11.5, previously shown to dissociate antibodies without destroying antigen-binding capacity and incubated for additional 12 min on shaker at 37°C.43 44 The latter fraction was mixed with the glycine eluates. IC eluates that were not assayed the same day were stored at −70°C until testing; freezing/thawing did not obviously change autoantibody results. The full procedure of IC purification is graphically illustrated in figure 1, and validation data are shown in online supplementary figure 1 for artificial IC and in online supplementary figures 2 and 3 for IC-rich SLE sera.
Figure 1.
Schematic figure showing the process of immune complex purification with following solubilisation and measurement of individual anti-citrullinated protein/peptide antibody reactivities. Details of the procedure are described in the Patients and methods section and online supplementary file. IC, immune complexes.
Microarray-based analysis for detection of ACPA fine specificities
ACPA in serum and SF and in solubilised IC from serum and SF were investigated on a custom-made microarray based on the ImmunoCAP ISAC system (Phadia AB, Uppsala, Sweden).10 15 Each reaction site of the microarray slides contained 19 different citrullinated peptides and their native arginine-containing counterparts (table 1). Sera and SF were diluted 1:40 and IC eluates were, due to practical reasons, diluted twice with ImmunoCAP-specific IgA/IgG sample diluent (Phadia AB) corresponding to a 1:30 total dilution of the IC fraction as compared with serum and SF. For each individual ACPA reactivity, we subtracted the corresponding arginine peptide reactivity.15 Cut-offs for individual ACPA in serum and SF were the 98th percentile among 944 healthy control serum samples. Measurement of ACPA in IC failed in one patient and in SF of one other patient for technical reasons. As there are no data on distribution of individual ACPA in IC, cut-offs were set as median for the n=76 group for serum and SF IC, respectively. In alternative parallel evaluations, cut-offs were estimated from mean +2 SD for levels in IC prepared from 20 healthy donor sera. Three SF IC were investigated on two occasions concerning occurrence of 17 individual ACPA reactivities, with total kappa coefficient 0.92. The strengths of individual ACPA reactivities with and without arginine correction are shown in online supplementary table 1.
Table 1.
Relative concentration of specific ACPA, anti-CCP2 and circulating immune complexes (CIC) in sera and synovial fluids
| Sequence | Number of patients reacting in serum (%) | Serum median (mean) | SF median (mean) | P values (Wilcoxon) |
Compartment with highest level | Serum/ IgG median (mean) |
SF/ IgG median (mean) |
P values (Wilcoxon) |
Compartment with highest level | |
| Filaggrin 307–324 (CCP1) | HQCHQEST(cit)GRSRGRCGRGS (cyclic) | 55/77 (71) | 38.89 (233.82) | 17.07 (185.86) | 0.0005 | Serum | 3.16 (22.69) | 4.19 (36.59) | 0.0282 | SF |
| Vimentin 2–17 | ST(cit)SVSSSSY(cit)(cit)MFGG | 40/77 (52) | 2.7 (14.78) | −0.43 (19.40) | 0.9179 | 0.14 (1.27) | −0.11 (3.79) | 0.561 | ||
| Vimentin 60–75 | VYAT(cit)SSAV(cit)L(cit)SSVP | 48/77 (62) | 36.76 (195.93) | 11.5 (168.09) | 0.0575 | 2.63 (18.14) | 4.00 (36.36) | 0.0009 | SF | |
| Fibrinogen α36–50 | GP(cit)VVE(cit)HQSACKDS | 22/77 (29) | 0 (61.38) | 0 (53.56) | 0.1822 | 0 (4.67) | 0 (9.67) | 0.0355 | SF | |
| Fibrinogen α563–583 | HHPGIAEFPS(cit)GKSSSYSKQF | 47/77 (61) | 10.05 (107.30) | 6.48 (82.61) | 0.0378 | Serum | 1.02 (10.51) | 1.08 (18.02) | 0.0216 | SF |
| Fibrinogen α580–600 | SKQFTSSTSYN(cit)GDSTFESKS | 25/77 (32) | 0 (43.90) | 0 (27.70) | 0.1398 | 0 (4.65) | 0 (7.61) | 0.5338 | ||
| Fibrinogen α621–635 | (cit)GHAKS(cit)PV(cit)GIHTS | 36/77 (47) | 0 (121.15) | 0 (91.35) | 0.3756 | 0 (13.33) | 0 (20.48) | 0.0987 | ||
| Fibrinogen β36–52 | NEEGFFSA(cit)GHRPLDKK | 47/77 (61) | 14.38 (156.83) | 7.44 (132.73) | 0.039 | Serum | 1.33 (13.84) | 2.32 (27.04) | 0.0087 | SF |
| Fibrinogen β60–74 | (cit)PAPPPISGGGY(cit)A(cit) | 52/77 (68) | 17.7 (170.93) | 7.49 (156.08) | 0.0292 | Serum | 1.46 (14.62) | 2.40 (30.47) | 0.0005 | SF |
| Fibrinogen β62-81(Fib72) | APPPISGGGY(cit)ARPAKAAAT | 25/77 (32) | 0 (11.19) | 0 (7.09) | 0.9311 | 0 (1.16) | 0 (1.60) | 0.936 | ||
| Fibrinogen β62-81(Fib74) | APPPISGGGYRA(cit)PAKAAAT | 22/77 (29) | 0.55 (35.02) | 0.73 (32.12) | 0.2692 | 0.06 (2.76) | 0.11 (6.08) | 0.0305 | SF | |
| α-Enolase 5-21(CEP-1) | CKIHA(cit)EIFDS(cit)GNPTVEC (cyclic) | 49/77 (64) | 72.82 (263.44) | 32.4 (235.90) | 0.0183 | Serum | 7.85 (22.65) | 6.79 (55.09) | <0.0001 | SF |
| hnRNP 1 | (proprietary)* | 28/77 (36) | 9.79 (70.52) | 6.44 (53.49) | 0.0003 | Serum | 0.89 (6.06) | 1.72 (10.64) | <0.0001 | SF |
| hnRNP 5 | (proprietary)* | 49/77 (64) | 19.8 (134.97) | 9.14 (120.27) | 0.1191 | 1.67 (12.52) | 2.36 (26.61) | 0.0005 | SF | |
| hnRNP Z1 | (proprietary)* | 35/77 (45) | 0 (65.53) | 0.83 (56.09) | 0.4464 | 0 (6.47) | 0.25 (13.54) | 0.0283 | SF | |
| hnRNP Z2 | (proprietary)* | 46/77 (60) | 4.96 (128.27) | 6.21 (105.07) | 0.1726 | 0.43 (13.44) | 2.75 (24.81) | 0.0066 | SF | |
| hnRNP Bla26 | (proprietary)* | 41/77 (53) | 4.57 (45.95) | 0.81 (45.32) | 0.2491 | 0.43 (4.13) | 0.46 (8.74) | 0.005 | SF | |
| Histone4 14–34 | GAK(cit)H(cit)KVL(cit)DNIQGITKPAI | 32/77 (42) | 18.77 (109.76) | 11.64 (81.80) | <0.0001 | Serum | 1.66 (9.79) | 2.14 (15.51) | 0.0053 | SF |
| Histone4 31–50 | KPAI(cit)(cit)LA(cit)(cit)GGVK(cit)ISGLI | 47/77 (61) | 11.04 (101.92) | 4.79 (67.97) | 0.029 | Serum | 0.75 (10.42) | 1.36 (11.51) | 0.3402 | |
| Anti-CCP2 | – | 55/77 (71) | 132 (317.25) | 55 (153.99) | <0.0001 | Serum | 11.60 (29.44) | 10.48 (39.95) | 0.0053 | SF |
| CIC µg Eq/mL | – | – | 0.53 (12.35) | 0.27 (5.87) | 0.0252 | Serum | 0.042 (1.112) | 0.054 (0.914) | 0.5018 |
Values are shown both as arbitrary units in sera and SF, as well as after correction for total IgG levels in the corresponding serum or SF samples. Comparisons were performed with the Mann-Whitney U test; significant differences are depicted in bold. The second column details the sequences of the citrullinated peptides in the multiplex assay, using the single-letter amino acid code and ‘(cit)’ for citrulline. The not listed arginine-containing control peptides have identical sequences except that they contain arginine residues instead of citrulline.
*Peptides are derived from hnRNP A3. Available on request from KS; see authors’ details.
ACPA, anti-citrullinated protein/peptide antibodies; SF, synovial fluid.
Statistical analysis
Enrichment of individual ACPA reactivities in SF was defined as the ratio between ACPA levels in SF and serum, after normalisation for individual IgG levels. Groups were compared with the Mann-Whitney U test for unpaired comparisons, Wilcoxon signed-rank test for paired comparisons and χ2 test for ratios. Correlation between variables was assessed by Spearman’s rank correlation test. Multiple regression analyses were performed to determine which autoantibody measures predicted clinical and laboratory measures of disease activity and inflammation. Independent variables were in the first four regression analyses occurrence of 19 individual ACPA in serum, in SF and in the IC fractions obtained from serum and SF, respectively. Occurrence of ACPA was used as nominal variables, as ACPA distribution often is bimodal, grossly corresponding to anti-CCP2-positive and anti-CCP2-negative patients. In a second set of multiple regression analyses, the independent variables were anti-CCP2 levels in serum and in synovial fluid as well as the number of individual ACPA in serum, in SF and in the IC fractions obtained from serum and SF, respectively. Two independent ways to calculate cut-offs for individual ACPA reactivities in IC fractions were used, as described above.
The statistical calculations were performed with JMP V.11 (SAS Institute, Cary, North Carolina, USA). P values less than 0.05 were considered significant. No corrections for mass significance were performed.
Results
Distribution of individual ACPA reactivities in serum, SF and corresponding IC fractions
Individual ACPA reactivities were found in serum from 29% to 71% of the patients (table 1). Levels in corresponding synovial fluids were significantly lower for 8/19 ACPA. This was, however, also the case for total IgG levels measured with identical technique in both body fluids (median (mean)) levels in serum and SF (11.0 (12.6) vs 4.3 (5.2) g/L; p<0.0001). When individual ACPA reactivities were corrected for total IgG, the majority (14/19) of ACPA reactivities instead showed an increase in the synovial compartment as compared with serum. A corresponding relation was also seen for anti-CCP2 levels. Levels of CIC were lower in SF than in serum, after IgG correction this difference disappeared (table 1). Total IgG levels in sera and SF did not show any correlation to levels of anti-CCP2, IgM RF or IgA RF in any compartment (data not shown).
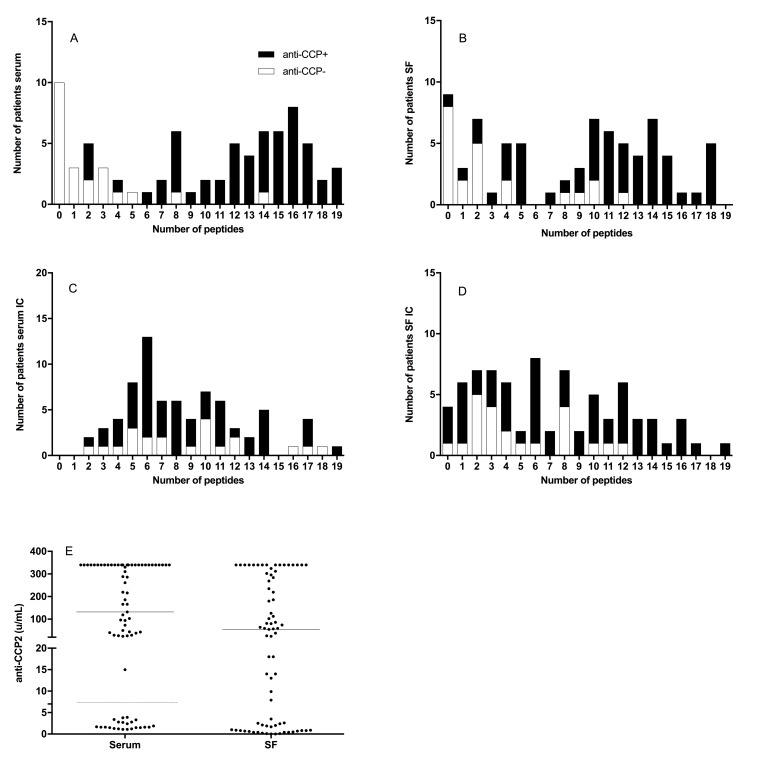
The number of individual ACPA reactivities in serum showed a bimodal distribution corresponding to low numbers in anti-CCP2-negative patients and higher numbers in anti-CCP2-positive patients (figure 2A). This was not as obvious for the distribution in SF (figure 2B). Number of individual ACPA reactivities in the IC fractions in serum and SF instead showed only one modal value, especially prominent for serum IC (figure 2C, D for cut-offs based on medians). Corresponding figures using the alternate cut-offs are shown in online supplementary figure 4.
Figure 2.
Distribution of number of antibodies against 19 citrullinated peptides in sera and synovial fluid (SF) as well as in immune complexes (IC) from sera and SF. Cut-offs for sera and SF (A, B) were determined as the 98th percentile for net anti-citrullinated protein/peptide antibodies (ACPA) reactivity among 944 healthy control samples, whereas cut-offs in the IC fractions from sera and SF (C, D) were determined as the median values for the respective compartment. For each compartment, empty bars represent the anti-CCP2-negative and filled bars represent the anti-CCP2-positive patients. In (E), the corresponding levels of anti-CCP2 in the investigated sera and SF are shown; horizontal bars representing medians. Corresponding figures using the alternate cut-offs for IC fractions from sera and SF are shown in online supplementary figure 4.
Relation between the appearance of individual ACPA reactivities in serum, SF and IC fractions
Levels of individual ACPA correlated strongly between sera and SF (Spearman’s ρ between 0.56 and 0.93, mean 0.78) as was also seen for anti-CCP2 (ρ=0.82; table 2). A much lower degree of correlation was seen between levels in serum and in the corresponding IC fraction (Spearman’s ρ 0.01–0.41, mean 0.20), whereas a somewhat larger degree of correlation was seen between levels in SF and in the corresponding IC fraction (ρ=0.06–0.61, mean 0.38; table 2).
Table 2.
Correlation between levels of specific ACPA in serum, synovial fluid and the immune complex fractions in serum and synovial fluid (correlation values are Spearman’s ρ values)
| ACPA specificity | Serum vs SF | Serum vs serum IC | SF vs SF IC |
| Filaggrin 307-324 (CCP1) | 0.85 | 0.31 | 0.61 |
| Vimentin 2–17 | 0.87 | 0.13 | 0.19 |
| Vimentin 60–75 | 0.93 | 0.15 | 0.38 |
| Fibrinogen α36–50 | 0.73 | 0.10 | 0.34 |
| Fibrinogen α563–583 | 0.85 | 0.24 | 0.49 |
| Fibrinogen α580–600 | 0.70 | 0.18 | 0.22 |
| Fibrinogen α621–635 | 0.87 | 0.33 | 0.55 |
| Fibrinogen β36–52 | 0.83 | 0.27 | 0.42 |
| Fibrinogen β60–74 | 0.82 | 0.28 | 0.53 |
| Fibrinogen β62–81(Fib72) | 0.56 | 0.01 | 0.17 |
| Fibrinogen β62–81(Fib74) | 0.63 | 0.04 | 0.43 |
| α-Enolase 5–21(CEP-1) | 0.88 | 0.41 | 0.58 |
| hnRNP 1 | 0.76 | 0.10 | 0.31 |
| hnRNP 5 | 0.78 | 0.36 | 0.34 |
| hnRNP Z1 | 0.67 | 0.14 | 0.22 |
| hnRNP Z2 | 0.82 | 0.32 | 0.55 |
| hnRNP Bla26 | 0.58 | 0.11 | 0.06 |
| Histone4 14–34 | 0.88 | 0.14 | 0.43 |
| Histone4 31–50 | 0.78 | 0.21 | 0.45 |
| Mean for 19 ACPA | 0.78 | 0.20 | 0.38 |
| Median for 19 ACPA | 0.82 | 0.18 | 0.42 |
| Anti-CCP2 | 0.82 |
ACPA, anti-citrullinated protein/peptide antibodies; IC, immune complexes; SF, synovial fluid.
Association between clinical and laboratory parameters and occurrence of individual ACPA reactivities in serum, SF and IC fractions
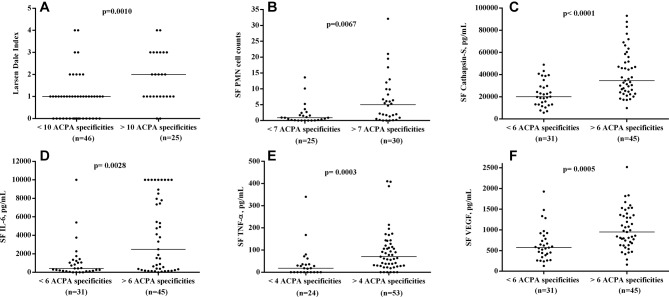
Analysis of the association between clinical and laboratory measures and the occurrence of individual ACPA in sera and SF yielded many parallel associations without any obvious dominance for certain ACPA (data not shown). When instead multiple regression with the occurrence of 19 individual ACPA reactivities in serum, SF and the corresponding IC fractions were used as independent variables, most of these associations disappeared, but again, no individual ACPA showed a general positive association to clinical and laboratory variables of disease activity and inflammation. For those ACPA showing many associations, there was often a mixture of positive and negative regression coefficients (online supplementary tables 2–5). Comparable data were found with the alternative approach of cut-off setting for ACPA in IC (data not shown). When the number of individual ACPA reactivities in serum, SF and in the corresponding IC fractions were employed as independent variables in parallel to anti-CCP2 levels in serum and SF, another picture emerged. The number of ACPA in the IC fraction in SF turned out to be the sole factor positively associating with Larsen-Dale score, and SF levels of PMN, IL-6, TNF-α, cathepsin S and VEGF, but without any association to the corresponding serum levels (table 3). This is graphically shown in figure 3, where Larsen-Dale index and SF levels of IL-6, cathepsin S and VEGF are shown dichotomised according to number of ACPA in the IC fraction in SF (optimal cut-offs for each variable were chosen according to calculations in online supplementary table 6). Also, when using the alternative cut-offs for the occurrence of individual ACPA in IC fractions from serum and SF, number of ACPA reactivities in SF IC was the determining factor for swollen joint count, and SF levels of PMN, IL-6, cathepsin S and VEGF, but also for serum IL-6 (data not shown).
Table 3.
Multiple regression with levels of anti-CCP2 in serum and synovial fluid together with number of ACPA reactivities in serum, synovial fluid, immune complex fraction in serum and in synovial fluid were used as independent variables and compared with clinical and laboratory measures (only significant p values (<0.05) are shown, and underlined if the regression coefficient and T statistics were negative)
| Age | Disease duration | DAS28 | Swollen joint count | Tender joint count | Global VAS | ESR | CRP | HAQ | Larsen-Dale | Time to relapse | SF PMN | SF MNC | Serum IL-6 | SF IL-6 | Serum TNF | SF TNF | Serum MMP3 | SF MMP3 | Serum PTX3 | SF PTX3 | Serum cathepsin L | SF cathepsin L | Serum cathepsin S | SF cathepsin S | Serum VEGF | SF VEGF | |
| Serum anti-CCP2 | 0.0182 | 0.0034 | |||||||||||||||||||||||||
| SF anti-CCP2 | 0.0157 | 0.0438 | 0.0026 | 0.0109 | |||||||||||||||||||||||
| No of ACPA in serum |
0.0385 | 0.0373 | |||||||||||||||||||||||||
| No of ACPA in SF |
|||||||||||||||||||||||||||
| No of ACPA in s-IC |
0.0149 | ||||||||||||||||||||||||||
| No of ACPA in SF IC | 0.0166 | 0.0459 | 0.0007 | 0.048 | 0.0441 | <0.0001 | 0.0377 |
ACPA, anti-citrullinated protein/peptide antibodies; CRP, C reactive protein; DAS, Disease Activity Score; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; MNC, mononuclear cell; MMP3, matrix metalloproteinase 3; PMN, polymorphonuclear; PTX3, pentraxin 3; SF, synovial fluid; TNF, tumour necrosis factor; VAS, visual analogue scale; VEGF, vascular endothelial growth factor.
Figure 3.
Difference between patients with high and low number of individual ACPA reactivities in SF IC concerning (A) Larsen-Dale index, (B) polymorphonuclear (PMN) cell counts, synovial fluid (SF) levels of (C) cathepsin S, (D) IL-6, (E) tumour necrosis factor alpha (TNF-α) and (F) vasclular endothelial growth factor (VEGF). For each measure, the optimal cut-off was chosen according to online supplementary table 6. Horizontal solid line represents the median levels in each group. For TNF-α, two measurements are outside of the axis limit but have been included in the statistics.
Discussion
In this study, we have shown that the majority of individual ACPA reactivities are enriched in SF as compared with in serum, and that levels of ACPA in IC are regulated independently of levels in serum and SF. No individual ACPA reactivity in serum or SF shows a dominating association with clinical and laboratory measures of disease activity and severity. Instead, the number of individual ACPA reactivities in the IC fraction from SF associates to a number of markers of joint destruction and inflammation.
We focused on determination of individual ACPA reactivities in sera and synovial fluid, and in IC fractions purified from sera and SF, in order to investigate if measurement of individual ACPA may provide more prognostic information than conventional measurement of CCP2 in serum and SF. We could not determine that any individual ACPA reactivity was dominant in the association with clinical disease activity or inflammation.
Anti-CCP2 and the majority (14/19) of individual ACPA reactivities were enriched in SF compared with in serum. When measuring crude ACPA levels, the majority of ACPA reactivities were lower in SF than in serum; enrichment in SF only appeared when antibody concentrations were corrected for total IgG, and also then, median values differed only modestly. No individual ACPA was considerably more enriched in SF than other ACPA. These data argue that most ACPA are locally produced in the joints. Snir and colleagues demonstrated that ACPA levels were increased in synovial fluid compared with in serum using two different techniques for IgG measurement.8 18 We used identical ELISA tests for serum and SF with γ-specific F(ab´)2 fragments of both the capture and detection antibodies to reduce the risk of non-specific binding of RF to Fc parts of assay antibodies. Consequently, we found no correlation between total IgG levels and anti-CCP2 or RF in serum or SF.
We found a strong correlation between serum and SF levels of individual ACPA, while associations were much lower between sera/SF and the corresponding IC fractions (table 2). This suggests that antibody levels in serum and SF are regulated by the same mechanisms, but that levels in IC are regulated differently. However, the correlations differed between peptides: anti-filaggrin 307–324 and anti-α-enolase 3–17 displayed strong correlation between serum and SF and the corresponding IC fractions, whereas the opposite was found for anti-fibrinogen β62–81 and anti-hnRNP Bla26 (table 2). This might implicate that certain individual ACPA reactivities have higher propensity to enrich in IC as compared with other ACPA, irrespective of body compartment. This first study using our new technique is however small, and such hypotheses should be confirmed in larger cohorts. We are aware that our results imply that some anti-CCP2-negative patients have ACPA in their IC, not found in the corresponding body fluids. Tentatively, occurrence of ACPA in IC might be a new measure of ACPA positivity with its own individual clinical associations, but there are also methodological concerns. Degree of ACPA positivity in IC varies with how cut-offs for ACPA in IC are determined (compare figure 2C and 2D with online supplementary figure 4) and might also be affected by method robustness. This will be subject to future studies.
The main finding of this study was that polyclonality in SF IC was the dominating factor that appeared in a number of independent estimates (8/27), always in positive association to clinical and laboratory measures of inflammation and joint destruction. Apart from serum IL-6 that associated with many ACPA measures, the only other ACPA measure that appeared frequently in the regression analyses was anti-CCP2 levels in SF, however usually with negative association. Regardless of which cut-off setting algorithm was used to determine occurrence of individual ACPA in IC fractions, the number of individual ACPA reactivities in SF IC appeared to be the most strongly associated factor.
Our finding indicates a key role for ACPA polyclonality in IC in the target organ. Thus, the breadth of the ACPA response, but no particular individual ACPA specificity, may be the determining factor. Our findings also put focus on the tentative role of ACPA in IC-driven joint pathology in RA. Our technique for the determination and quantification of individual autoantibodies in IC obtained in vivo was developed to target this kind of scientific questions, and we aim to pursue such studies in larger RA cohorts, including whether ACPA in general or with predilection for certain individual ACPA specificities are enriched in IC. We also plan to investigate IC heterogeneity concerning other antibody specificities, like RF and anti-type II collagen.
The tentative role for autoantibody polyclonality in RA IC makes it tempting to compare the present findings with two autoantibody-driven arthritis models in rodents. Both the CAIA as well as the K/BxN models depend solely on antibodies against type II collagen and glucose 6-phosphate isomerase, respectively. Injection of individual monoclonal IgG antibodies specific against major epitopes of the antigens into healthy recipients will only induce mild arthritis. However, when multiple antibodies directed against nearby epitopes on the same protein are injected simultaneously as a cocktail, they will induce severe arthritis, suggesting that pathogenicity is associated not to recognition of a specific epitope on the antigen but the ability to form polyclonal IC that may activate FcγR on the target cell.45 46
Although our results hitherto have not implicated any individual ACPA, it is of course tempting to speculate that our method might define a core group of individual ACPA reactivities in SF IC, which in vivo might target multiple epitopes on a pathogenetically central citrullinated antigen, and thereafter activate and perpetuate arthritis in RA as we have described for anti-type II collagen antibodies.26–29 47–49 It is possible that individual ACPA reactivities and also individual citrullinated antigen(s) can be recovered in isolated SF IC obtained with our new technique, and we intend to investigate this possibility.
The present version of our technique is a prototype method. If further studies will prove our findings to be of tentative interest in patient care by adding clinically useful information not provided by conventional autoantibody measurement, the laboratory procedures have to be further validated, for example, concerning robustness and variability.
Our data highlight the polyclonality of the ACPA in joint IC, and the possibility that a broad ACPA repertoire might drive the local inflammatory and matrix degrading process in the joint. Thus, our findings argue that ACPA are locally produced and participate in RA pathogenesis via formation of IC locally at the site of inflammation.
Acknowledgments
We thank Karin Fromell PhD for help with the QCM-D measurements.
Footnotes
TW and JR contributed equally.
Handling editor: Josef S Smolen
Contributors: AS and JR conceived the study. AS developed the IC purification technique described in this paper and performed most of the laboratory work. MH and LM-A developed the multiplex microarray. MC, KS and GS provided peptides for the analyses, including validation of their performance. AK, JL and TW included and performed clinical characterisation of the investigated patients. AL investigated inflammatory markers. AS and JR drafted the manuscript. All authors read, commented on and approved the final manuscript.
Funding: This study was funded by grants from the Swedish Research Council, the Swedish Rheumatism Association, King Gustav V 80-year foundation, ALF grants provided by the Uppsala County Council, the Rudberg Foundation and the Brunnberg Foundation.
Competing interests: LM-A is employed by Thermo Fisher Scientific. KS is co-inventor of the patents US 13/141,960, EP 09799354.7 describing the diagnostic use of the hnRNP-A3 peptide epitopes. GS is co-inventor of several international patents about ACPA antigens held by BioMérieux Cy and licensed to Eurodiagnostica Cy, and Axis-Shield Cy for commercialisation of the CCP2 assays; according to French laws, he receives a part of the royalties paid to the Toulouse III University and the University Hospital of Toulouse.
Patient consent: Not required.
Ethics approval: The ethics board in Uppsala.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Most data are shown in the manuscript and supplementary files. However, calculations using the alternative cut-offs (see Results section) are not included but can be obtained from JR.
References
- 1. Schellekens GA, de Jong BA, van den Hoogen FH, et al. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 1998;101:273–81. 10.1172/JCI1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Beers JJ, Willemze A, Jansen JJ, et al. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res Ther 2013;15:R140 10.1186/ar4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kastbom A, Strandberg G, Lindroos A, et al. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004;63:1085–9. 10.1136/ard.2003.016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2000;43:1831–5. [DOI] [PubMed] [Google Scholar]
- 5. Rönnelid J, Wick MC, Lampa J, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005;64:1744–9. 10.1136/ard.2004.033571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verpoort KN, Cheung K, Ioan-Facsinay A, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum 2007;56:3949–52. 10.1002/art.23127 [DOI] [PubMed] [Google Scholar]
- 7. Kakumanu P, Yamagata H, Sobel ES, et al. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies, but their sera also react with unmodified arginine-containing peptide. Arthritis Rheum 2008;58:1576–81. 10.1002/art.23514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snir O, Widhe M, Hermansson M, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum 2010;62:44–52. 10.1002/art.25036 [DOI] [PubMed] [Google Scholar]
- 9. Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol 1999;162:585–94. [PubMed] [Google Scholar]
- 10. Hansson M, Mathsson L, Schlederer T, et al. Validation of a multiplex chip-based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res Ther 2012;14:R201 10.1186/ar4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hueber W, Kidd BA, Tomooka BH, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum 2005;52:2645–55. 10.1002/art.21269 [DOI] [PubMed] [Google Scholar]
- 12. Nogueira L, Cornillet M, Singwe-Ngandeu M, et al. In Black Africans with rheumatoid arthritis, ACPA recognize citrullinated fibrinogen and the derived peptides α36-50Cit38,42 and β60-74Cit60,72,74, like in Caucasians. Clin Immunol 2014;152:58–64. 10.1016/j.clim.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 13. Pratesi F, Dioni I, Tommasi C, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis 2014;73:1414–22. 10.1136/annrheumdis-2012-202765 [DOI] [PubMed] [Google Scholar]
- 14. Wagner CA, Sokolove J, Lahey LJ, et al. Identification of anticitrullinated protein antibody reactivities in a subset of anti-CCP-negative rheumatoid arthritis: association with cigarette smoking and HLA-DRB1 ‘shared epitope’ alleles. Ann Rheum Dis 2015;74:579–86. 10.1136/annrheumdis-2013-203915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rönnelid J, Hansson M, Mathsson-Alm L, et al. Anticitrullinated protein/peptide antibody multiplexing defines an extended group of ACPA-positive rheumatoid arthritis patients with distinct genetic and environmental determinants. Ann Rheum Dis 2018;77:203–11. 10.1136/annrheumdis-2017-211782 [DOI] [PubMed] [Google Scholar]
- 16. Fisher BA, Plant D, Brode M, et al. Antibodies to citrullinated α-enolase peptide 1 and clinical and radiological outcomes in rheumatoid arthritis. Ann Rheum Dis 2011;70:1095–8. 10.1136/ard.2010.138909 [DOI] [PubMed] [Google Scholar]
- 17. Masson-Bessière C, Sebbag M, Durieux JJ, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol 2000;119:544–52. 10.1046/j.1365-2249.2000.01171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mannik M. Are antibodies to citrullinated antigens enriched in synovial fluids of patients with rheumatoid arthritis?: comment on the article by Snir et al. Arthritis Rheum 2010;62:3515–6. 10.1002/art.27669 [DOI] [PubMed] [Google Scholar]
- 19. Hannestad K. Monoclonal and polyclonal gamma M rheumatoid factors with anti-di- and anti-trinitrophenyl activity. Clin Exp Immunol 1969;4:555–78. [PMC free article] [PubMed] [Google Scholar]
- 20. Gordon DA, Bell DA, Baumal R, et al. Studies into the occurrence of soluble antigen-antibody complexes in disease. IV. Correlation between the rheumatoid biologically active factor and the clinical features of rheumatoid arthritis. Clin Exp Immunol 1969;5:57–66. [PMC free article] [PubMed] [Google Scholar]
- 21. Winchester RJ, Agnello V, Kunkel HG. The joint-fluid gammaG-globulin complexes and their relationship to intraarticular complement dimunition. Ann N Y Acad Sci 1969;168:195–203. 10.1111/j.1749-6632.1969.tb43108.x [DOI] [PubMed] [Google Scholar]
- 22. Cohen S, Ward PA. In vitro and in vivo activity of a lymphocyte and immune complex-dependent chemotactic factor for eosinophils. J Exp Med 1971;133:133–46. 10.1084/jem.133.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward PA, Zvaifler NJ. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest 1971;50:606–16. 10.1172/JCI106531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDougal JS, Hubbard M, Strobel PL, et al. Comparison of five assays for immune complexes in the rheumatic diseases: performance characteristics of the assays. J Lab Clin Med 1982;100:705–19. [PubMed] [Google Scholar]
- 25. Mathsson L, Lampa J, Mullazehi M, et al. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther 2006;8:R64 10.1186/ar1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullazehi M, Mathsson L, Lampa J, et al. Surface-bound anti-type II collagen-containing immune complexes induce production of tumor necrosis factor alpha, interleukin-1beta, and interleukin-8 from peripheral blood monocytes via Fc gamma receptor IIA: a potential pathophysiologic mechanism for humoral anti-type II collagen immunity in arthritis. Arthritis Rheum 2006;54:1759–71. 10.1002/art.21892 [DOI] [PubMed] [Google Scholar]
- 27. Mullazehi M, Mathsson L, Lampa J, et al. High anti-collagen type-II antibody levels and induction of proinflammatory cytokines by anti-collagen antibody-containing immune complexes in vitro characterise a distinct rheumatoid arthritis phenotype associated with acute inflammation at the time of disease onset. Ann Rheum Dis 2007;66:537–41. 10.1136/ard.2006.064782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manivel VA, Sohrabian A, Rönnelid J. Granulocyte-augmented chemokine production induced by type II collagen containing immune complexes is mediated via TLR4 in rheumatoid arthritis patients. Eur J Immunol 2016;46:2822–34. 10.1002/eji.201646496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manivel VA, Mullazehi M, Padyukov L, et al. Anticollagen type II antibodies are associated with an acute onset rheumatoid arthritis phenotype and prognosticate lower degree of inflammation during 5 years follow-up. Ann Rheum Dis 2017;76:1529–36. 10.1136/annrheumdis-2016-210873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol 2003;163:1827–37. 10.1016/S0002-9440(10)63542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clavel C, Nogueira L, Laurent L, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum 2008;58:678–88. 10.1002/art.23284 [DOI] [PubMed] [Google Scholar]
- 32. Sokolove J, Zhao X, Chandra PE, et al. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum 2011;63:53–62. 10.1002/art.30081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menzel J, Steffen C, Kolarz G, et al. Demonstration of antibodies to collagen and of collagen-anticollagen immune complexes in rheumatoid arthritis synovial fluids. Ann Rheum Dis 1975;35:446–50. 10.1136/ard.35.5.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steffen C, Dichtl M, Knapp W, et al. Immunogenicity and specificity of collagen. XII. Demonstration by immunofluorescence and haemagglutination of antibodies with different specificity to human collagen. Immunology 1971;21:649–57. [PMC free article] [PubMed] [Google Scholar]
- 35. Weitoft T, Rönnelid J, Knight A, et al. Outcome predictors of intra-articular glucocorticoid treatment for knee synovitis in patients with rheumatoid arthritis—a prospective cohort study. Arthritis Res Ther 2014;16:R129 10.1186/ar4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 37. Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc 1949;140:659–62. 10.1001/jama.1949.02900430001001 [DOI] [PubMed] [Google Scholar]
- 38. Ekdahl C, Eberhardt K, Andersson SI, et al. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the stanford health assessment questionnaire. Scand J Rheumatol 1988;17:263–71. [DOI] [PubMed] [Google Scholar]
- 39. Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 40. Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 1977;18:481–91. 10.1177/028418517701800415 [DOI] [PubMed] [Google Scholar]
- 41. Weitoft T, Larsson A, Saxne T, et al. Pentraxin 3 in serum and synovial fluid of patients with rheumatoid arthritis with and without autoantibodies. Scand J Rheumatol 2017;46 1 7. 10.1080/03009742.2016.1244288 [DOI] [PubMed] [Google Scholar]
- 42. Weitoft T, Larsson A, Manivel VA, et al. Cathepsin S and cathepsin L in serum and synovial fluid in rheumatoid arthritis with and without autoantibodies. Rheumatology 2015;54:1923–8. 10.1093/rheumatology/keu486 [DOI] [PubMed] [Google Scholar]
- 43. Ahlin E, Mathsson L, Eloranta ML, et al. Autoantibodies associated with RNA are more enriched than anti-dsDNA antibodies in circulating immune complexes in SLE. Lupus 2012;21:586–95. 10.1177/0961203311434938 [DOI] [PubMed] [Google Scholar]
- 44. Singh KV, Kaur J, Raje M, et al. An ELISA-based approach to optimize elution conditions for obtaining hapten-specific antibodies. Anal Bioanal Chem 2003;377:220–4. 10.1007/s00216-003-2066-z [DOI] [PubMed] [Google Scholar]
- 45. Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods 2005;304:126–36. 10.1016/j.jim.2005.06.017 [DOI] [PubMed] [Google Scholar]
- 46. Maccioni M, Zeder-Lutz G, Huang H, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med 2002;195:1071–7. 10.1084/jem.20011941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mullazehi M, Wick MC, Klareskog L, et al. Anti-type II collagen antibodies are associated with early radiographic destruction in rheumatoid arthritis. Arthritis Res Ther 2012;14:R100 10.1186/ar3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manivel VA, Sohrabian A, Wick MC, et al. Anti-type II collagen immune complex-induced granulocyte reactivity is associated with joint erosions in RA patients with anti-collagen antibodies. Arthritis Res Ther 2015;17:8 10.1186/s13075-015-0523-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Manivel VA, Woude DV, Toes R, et al. Antibodies against collagen type II are not a general marker of acute arthritis onset. Ann Rheum Dis 2018;77:954–6. 10.1136/annrheumdis-2017-211974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-212627supp001.docx (10.1MB, docx)