Bacillus anthracis is a Gram-positive spore-forming bacterium that resides in the soil and causes the disease anthrax. B. anthracis predominantly affects livestock and is endemic worldwide. The incidence of infection in the developed countries is low. Most commonly, humans become infected due to contact with infected animals or contaminated animal products. Anthrax continues to be of importance to human health as a bio-threat. The infection is typically initiated by one of 3 routes: cutaneous, gastrointestinal and inhalational. Without an appropriate treatment, bacteria from the sites of entry can disseminate to other organs ultimately causing septic shock and death. The inhalation form of anthrax is the most deadly. While antibiotic therapeutics such as ciprofloxacin are available, patients with inhalation anthrax have only a 50% chance of survival,1 thus new therapies are needed.
During its vegetative growth phase, B. anthracis produces several virulence factors including Lethal and Edema toxins (encoded by plasmid XO1) as well as a poly-γ-D-glutamic acid capsule (encoded by plasmid XO2).2 In this work, we used B. anthracis Sterne, a strain of anthrax lacking the pXO2. As a result the Sterne strain is strongly attenuated in humans, but displays residual virulence in mice due to the presence of pXO1.
Cationic antimicrobial peptides (CAMPs) are produced as a part of the innate immunity and often display an amphipathic secondary structure. Their overall positive charge allows association with negatively charged outer leaflet of bacterial membrane3 while leaving eukaryotic membranes intact.4 We previously determined the activity and some mechanistic features for many native and synthetic CAMPs against Francisella,5-8 Pseudomonas,9 Staphylococcus aureus10 and Burkholderia thailandensis.11 We sought to find peptide-based antimicrobials that could be developed further as potential therapeutics for B. anthracis infection, or as compounds to be used in conjunction with antibiotics to increase the overall survival from this infection.
Several CAMPs were previously shown to be effective against the capsulated and non-capsulated B. anthracis strains.12,13 Interestingly, most of these peptides did not function by directly exerting their antimicrobial action on the bacterial membrane as has been described previously for other AMPs against other bacteria.14 For example, protegrin-1 (PG-1), a porcine cathelicidin, functions by altering vegetative outgrowth process.12 Theta-defensin retrocyclin displays an immunomodulatory effect to increase macrophage performance13,15 and thus achieves a “host-directed” action against B. anthracis. In this study, we sought to characterize 8 CAMPs from the cathelicidin family with regard to their antibacterial and sporicidal effects upon B. anthracis.
This work introduces an in vivo model of testing the activity of CAMPs against B. anthracis injected into the hemocoel of the waxworm G. mellonella. This provides an opportunity to perform in vivo testing in an invertebrate model before moving lead candidates forward to established animal models of infection. Use of G. mellonella has been published as an alternative infection model system for a variety of bacterial infections.8,9,16-24 Further, many bacterial virulence factors required for bacterial infection in mice were shown to be also required for infection in G. mellonella.25 This G. mellonella model of infection, by injection into the hemocoel, may provide a model of disseminated anthrax infection, as the hemocoel is the circulation system of the insect and contains phagocytic hemocytes. Vegetative cells and spores can be distributed throughout the organism, as in disseminated anthrax.26,27 We used G. mellonella as the in vivo assay to down-select our lead antimicrobial peptides in preparation for future testing in the mouse model of B. anthracis infection. This waxworm model allows for testing the antibacterial activity of numerous antimicrobial peptides, which would be impractical in a mouse model.
B. anthracis Sterne strain was grown as described in the Supplemental text, and exposed to a panel of CAMPs to test their antimicrobial activity including LL-37 (human cathelicidin); D-LL-37 (D-enantiomer of LL-37)9; SMAP-29 (sheep cathelicidin)28; PG-1 (porcine protegrin-1)12; mCRAMP (murine cathelicidin)29; BMAP-28 (bovine cathelicidin)28; NA-CATH (Chinese cobra cathelicidin)5; and CAP-18 (rabbit cathelicidin).30
The EC50 antimicrobial assay was performed against vegetative cells (Fig. 1) followed by a standard minimum inhibitory concentration (MIC) determination (Table 1) following our previous publications (Please refer to Supplemental text for detailed methods). Seven of the 8 cathelicidins tested had a similar EC50 value to ciprofloxacin (p>0.05), with the exception being BMAP-28, which was 100x less effective.
Figure 1.
Antimicrobial peptides exert activity against vegetative bacilli and endospores. B. anthracis Sterne bacilli (A, B) or spores (C, D) were incubated for 3 h with a range of the log10 peptide concentrations in 10 mM phosphate buffer and percent (%) survival was determined (EC50). Standard deviations of the mean are shown on each graph as error bars.
Table 1.
Activity of Antimicrobial peptides against vegetative B. anthracis. Antibacterial and Sporicidal activity of CAMPs against B. anthracis Sterne strain vegetative activity.
| EC50 (95% Confidence Interval) |
Sporicidal EC50 (95% Confidence Interval) |
MIC |
||||
|---|---|---|---|---|---|---|
| Peptide | (µg/ml) | (µM) | (µg/ml) | (µM) | (µg/ml) | (µM) |
| Ciprofloxacin | 0.01(0.003–0.06) | 0.02 (0.01–0.18) | 70.8 (28.7–175) | >100 | 0.01 | 0.04 |
| SMAP-29 | 0.03 (0.01–0.08) | 0.01 (0.003–0.025) | >100 | >100 | 8 | 2.46 |
| NA-CATH | 0.29 (0.21–0.45) | 0.07 (0.05–0.11) | >100 | >100 | 16 | 3.83 |
| PG-1 | 0.22 (0.14–0.32) | 0.10 (0.06–0.15) | 3.41 (1.16–10.1) | 1.58 (0.54–4.63) | 16 | 7.41 |
| D-LL-37 | 0.13 (0.07–0.34) | 0.03 (0.02–0.08) | 81.8 (41.8–160) | 18.2 (9.30–35.6) | 32 | 7.12 |
| LL-37 | 0.22 (0.11–0.47) | 0.05 (0.02–0.10) | 51.1 (23.9–109) | 11.4 (5.32–24.3) | 32 | 7.12 |
| mCRAMP | 0.23 (0.14–0.43) | 0.06 (0.04–0.11) | >100 | >100 | 64 | 16.5 |
| CAP-18 | 0.31 (0.24–0.95) | 0.07 (0.05–0.21) | >100 | >100 | >150 | >150 |
| BMAP-28 | 7.89 (3.41–20.3) | 2.52 (1.09–6.48) | 82.1 (40.8–165) | 26.2 (13.0–52.7) | >150 | >150 |
| Scrambled LL-37 | >100 | >100 | >100 | >100 | >150 | >150 |
Six of the cathelicidins demonstrated significant MIC activity (Table 1), with only CAP-18 and BMAP-28 found to be inactive. The range of MICs for active peptides was from 8–64 µg/ml (Table 1). The most effective peptide against B. anthracis was SMAP-29, the sheep myeloid antimicrobial peptide (MIC = 8 µg/ml). Ciprofloxacin, an antibiotic used clinically to treat anthrax infections in humans, was used as a positive control and demonstrated a MIC of 0.01 µg/ml, similar to published results.31 The lack of activity of CAP-18 and BMAP-28 under MIC conditions was notable, as other cathelicidins were active. The overall lack of in vitro activity of BMAP-28 against the vegetative cells was interesting, given that this peptide has activity against other organisms.32-34
Inoculation by B. anthracis endospores causes anthrax in mammalian hosts, thus we also examined the sporicidal activity of these peptides (Table 1). While all peptides had various killing activity against B. anthracis bacilli only 3 peptides had activity against B. anthracis spores; PG-1, BMAP-28 and LL-37 (Fig. 1C, D). PG-1 had the strongest activity in alignment with published reports of PG-1 being capable of killing spores before vegetative outgrowth.12 We demonstrated sporicidal activity of PG-1 with an EC50 of 1.58 µM (95% CI: 0.54 µM–4.63 µM). Less effective were the human cathelicidin LL-37 with an EC50 of 11.4 µM (95% CI: 5.32 µM–24.3 µM) and bovine cathelicidin BMAP-28 with an EC50 of 26.2 µM (95% CI: 13.0 µM–52.7 µM). It was determined that B. anthracis Sterne strain spores do not germinate after 3 hrs incubation in 10 mM phosphate buffer (data not shown).
For characterization of the in vivo invertebrate model Galleria mellonella, we first performed a standard kill curve to determine the lethal dose of B. anthracis Sterne strain injected into the hemocoel of the larvae (Fig. S1). G. mellonella larvae were injected with 10 µl of various concentrations of B. anthracis spores or vegetative cells and the amount that kills all larvae by 48 h was used as the LD99 for future experiments. It was found that 105 spores were sufficient to cause death of all larvae by 48 h and this was used as our infectious dose for future experiments (LD50 = 3 × 103) (Fig. S1A). Vegetative cells were also tested and the LD99 was determined to be 109 cells, much higher than that for spores (LD50 = 107) (Fig. S1B). As the usual infecting form is spores,26 we infected with spores for these in vivo experiments. Each experiment was performed with 10 randomly selected G. mellonella larvae and each experiment was performed 3 times with a representative experiment shown.
Waxworms were infected with 1 × 105 spores per larvae B. anthracis Sterne spores and then treated with a single injection of 10 µg (in 10 µl) of peptide injected into the hemocoel 1 h after the infection. Controls were treated with PBS or ciprofloxacin (Fig. 2). The negative control group (PBS-treated) did not survive past 48 h. An antibiotic control was used to verify the performance of this in vivo model (Fig. 2A). Ciprofloxacin (1 µg/10 µl) was injected one time only into the hemocoel to treat G. mellonella infected with the B. anthracis Sterne spores, and was able to rescue 100% of infected waxworms (1 µg of ciprofloxacin per larva following 3 h of infection). As another control, we used scrambled LL-37, which is a peptide that has the same molecular weight, amino acids and charge but the order of the amino acids is changed. This control also did not survive past 48 h, consistent with our previous findings that scrambled LL-37 peptide does not confer antibacterial activity9 (Fig. 2A).
Figure 2.
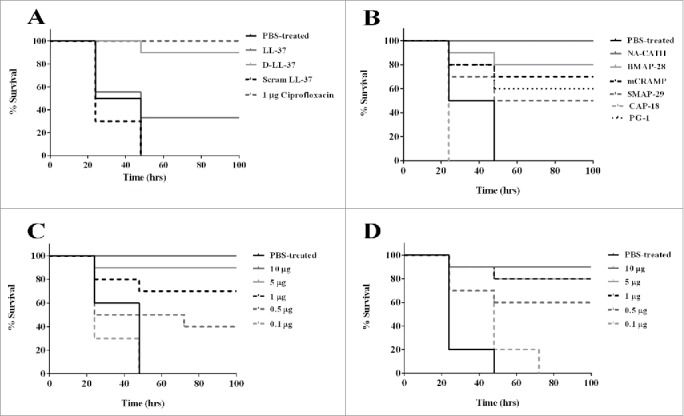
Antimicrobial peptide treatment of B. anthracis Sterne spore challenge of G. mellonella. Galleria mellonella survival curve was performed for B. anthracis Sterne spore infected waxworms and treated with (A) LL-37, D-LL-37 and scrambled LL-37; (B) PBS-treated, NA-CATH, BMAP-28, mCRAMP, SMAP-29, CAP-18 and PG-1; (C) dose dependence in NA-CATH and (D) dose dependence in D-LL-37. Significant rescue was observed for all peptides except CAP-18 and scrambled LL-37 while NA-CATH and D-LL-37 had survival that was statistically similar to that of the antibiotic control ciprofloxacin. For dose dependence, both peptides were able to rescue a portion G. mellonella at peptide concentrations as low as 0.5 µg per larva in 10 µl. NA-CATH was able to rescue a statistically similar number of larvae as ciprofloxacin at 5 µg per larvae while D-LL-37 was able to rescue a statistically similar population as ciprofloxacin with 1 µg per larvae. Kaplan-Meier statistics was performed to determine statistical significance of peptide treated versus PBS treated and p-values are shown in Table S2.
The experimental groups treated with various CAMPs demonstrated a prolonged survival time (p<0.05) for all cathelicidin peptides except CAP-18. Peptide NA-CATH treated waxworms demonstrated 100% survival and peptide D-LL-37 treated waxworms demonstrated 90% survival, which is similar to that of the antibiotic control ciprofloxacin (p > 0.05), according to Kaplan-Meier statistics estimator.35 Other peptides BMAP-28, mCRAMP, SMAP-29, PG-1 and LL-37 demonstrated some rescue of B. anthracis-infected G. mellonella; however, the survival was statistically different and less than that of the antibiotic control ciprofloxacin (p < 0.05) (Fig. 2A,B). Of interest, BMAP-28 treatment led to significant rescue, despite its poor in vitro performance against vegetative bacilli.
Peptides NA-CATH and D-LL-37, which were highly effective and comparable to ciprofloxacin in survival, were further analyzed to determine the lowest peptide concentration required for rescue of B. anthracis infected G. mellonella (Fig. 2C,D). For peptide NA-CATH, 5 µg (in 10 µl) of peptide per larva (approx. 0.3 g each) demonstrated high levels of survival (statistically similar to the antibiotic control, p < 0.001) while treatment with as low as 0.5 µg of peptide per larva still rescued a significant portion of the population. Peptide D-LL-37 rescued B. anthracis-infected G. mellonella at 1 µg of peptide per larva, (statistically similar to the antibiotic control, p < 0.001) while doses as low as 0.5 µg peptide per larva still rescued a significant portion of the population (Fig. 2C,D) (p < 0.001).
Cytotoxicity and hemolysis testing was performed for these peptides (Fig. S2). Peptide concentrations used for these experiments were 100 µg/ml, 100x higher than the concentration being used to treat B. anthracis infected G. mellonella. Defibrinated sheep blood was used for hemolytic testing and no peptide demonstrated statistically significant hemolytic activity (p < 0.001), consistent with our previous result for NA-CATH.5 For cytotoxicity testing, A549 human lung epithelial cells were used as they have been previously shown to be a target of internalization by B. anthracis.36 A549 cells treated with 100 µg/ml of all peptides demonstrated statistically similar cell survival (p < 0.001) compared with the PBS-treated control, while all were different from the Triton X treated control (p < 0.001), which represented 100% lysis. From these experiments, we conclude that the concentrations we were using to treat B. anthracis infected G. mellonella were not causing cytotoxicity. We also performed toxicity testing of peptides injected into the larvae. We found that peptide-only injections did not adversely affect waxworm survival (data not shown).
This study established an in vivo model for B. anthracis infection in the waxworm that may be useful for screening and testing novel antibiotics as they are developed and may enable down-selection before murine testing. This model involves injecting B. anthracis spores into the hemocoel of the G. mellonella via a pro-leg, followed 3 h later by injection of the potential therapeutic in the opposite pro-leg. This in vivo model allows for time-efficient and inexpensive experiments to be performed when testing a large panel of therapeutics, such as was done in this study, where the large number of in vitro active compounds would take considerable time and resources in a murine model. Other invertebrate models do exist for B. anthracis infection; however, they use other routes of infection such as gastrointestinal in the cases of G. mellonella and C. elegans.37,38 In the case of our G. mellonella model, we are able to test therapeutic agents against hemocoel anthrax infections, which may model disseminated anthrax. While the number of treatments can be adjusted to suit experimental needs,8-10,16 in this case we tested a single treatment of 10 ug of peptide per larvae injected into the hemocoel. This assay suggests that the survival-promoting peptides are strongly active to affect waxworm survival outcomes with a single treatment dose. This model enabled us to down-select from 7 CAMPS with in vitro activity for B. anthracis to 2 CAMPs with strong in vivo activity (D-LL-37 and NA-CATH), and 4 CAMPs (BMAP-28, mCRAMP, SMAP-29, PG-1) with moderate survival between 40–60%.
Protegrin-1 (PG-1), which was previously shown to be sporicidal, was able to rescue 60% of the waxworms in a single treatment of 10 ug (23 µM) of PG-1 peptide in our model. This compares with the published results in mice that treatment with 50 uM PG-1 given subcutaneously 4 h post infection results in 80% survival,12 further supporting the relevance of our G. mellonella model.
Our test panel of CAMPs represents a diverse set of cathelicidin peptides that could potentially exert an antimicrobial effect upon B. anthracis. This work examined these antibacterial abilities against B. anthracis Sterne, 6 of which had MIC activity, and the snake-derived cathelicidin peptide, NA-CATH, rescued 100% of waxworms following B. anthracis infection. Our model corroborated published data with PG-1 demonstrating activity against both vegetative bacilli and spores while LL-37 and mCRAMP were antimicrobial but not effective against the spores.12,13 Additionally, we have shown that these peptides were not cytotoxic, hemolytic or toxic.
In vivo experiments were performed in G. mellonella as a new in vivo model organism for B. anthracis infection. A large difference was observed between the infectious dose of vegetative bacilli and spores in the G. mellonella model (Fig. S1A,B). Spores have an exosporium that protects them from toxic molecules such as host-derived proteases and lysozyme, while vegetative bacterial cells would be more susceptible to assault from these molecules. Thus, in our model we used the spores to initiate the infection, although we demonstrated that waxworms are also susceptible to infection by vegetative cells, and that vegetative cells are susceptible to these CAMPs.
Both host-derived and pathogen-expressed proteases are known to play a large role in B. anthracis infection.39 We observed a significant difference (p<0.001) between the D- and L-enantiomers (90% vs. 30% survival) of LL-37 treatment (Fig. 2A) indicating that the D-enantiomer, which is resistant to protease degradation,9 is superior to its L-enantiomer in in vivo performance, although the in vitro MICs are statistically identical. This result agrees with our previously published results demonstrating a survival advantage for protease-resistant peptide D-LL-37 against Pseudomonas aeruginosa infection in G. mellonella.9
G. mellonella has numerous features that make them an appropriate surrogate for mammalian infection, including that: (1) as insects they are invertebrates and do not need IACUC approval, (2) they can be incubated at 37°C, (3) they can be easily injected via the prolegs, (4) they are ethically acceptable, and (5) have been developed as an infection model for several other bacteria. Additionally, the insect immune system is related to that of mammals.40 Insects have phagocytic cells that engulf bacteria and produce bactericidal compounds.41 Other advantages that we are continuing to explore in this model include performing synergy experiments with antibiotics, which can be easily achieved in this in vivo model. This study establishes G. mellonella as a useful new in vivo model for rapidly and efficiently testing potential therapeutic agents against B. anthracis infection.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Evelyn Hrifko for technical assistance with some experiments.
Funding
RJB and MVH were supported by HDTRA1–12-C-0039 “Translational Peptide Research for Personnel Protection.”
References
- [1].Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, et al.. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002; 287:2236-52; PMID:11980524; http://dx.doi.org/ 10.1001/jama.287.17.2236 [DOI] [PubMed] [Google Scholar]
- [2].Guichard A, Nizet V, Bier E. New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect 2012; 14:97-118; PMID:21930233; http://dx.doi.org/ 10.1016/j.micinf.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hancock RE, Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett 2002; 206:143-9; PMID:11814654; http://dx.doi.org/ 10.1111/j.1574-6968.2002.tb11000.x [DOI] [PubMed] [Google Scholar]
- [4].De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 2005; 27:1337-47; PMID:16215847; http://dx.doi.org/ 10.1007/s10529-005-0936-5 [DOI] [PubMed] [Google Scholar]
- [5].de Latour FA, Amer LS, Papanstasiou EA, Bishop BM, van Hoek ML. Antimicrobial activity of the Naja atra cathelicidin and related small peptides. Biochem Biophys Res Commun 2010; 396:825-30; PMID:20438706; http://dx.doi.org/ 10.1016/j.bbrc.2010.04.158 [DOI] [PubMed] [Google Scholar]
- [6].Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 2008; 76:4176-82; PMID:18591225; http://dx.doi.org/ 10.1128/IAI.00318-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung MC, Dean SN, van Hoek ML. Acyl carrier protein is a bacterial cytoplasmic target of cationic antimicrobial peptide LL-37. Biochem J 2015; 470:243-53; PMID:26188040; http://dx.doi.org/ 10.1042/BJ20150432 [DOI] [PubMed] [Google Scholar]
- [8].Propst CN, Pylypko SL, Blower RJ, Ahmad S, Mansoor M, van Hoek ML. Francisella philomiragia infection and lethality in mammalian tissue culture cell models, galleria mellonella, and BALB/c Mice. Front Microbiol 2016; 7:696; PMID:27252681; http://dx.doi.org/ 10.3389/fmicb.2016.00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dean SN, Bishop BM, van Hoek ML. Susceptibility of pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front Microbiol 2011; 2:128; PMID:21772832; http://dx.doi.org/ 10.3389/fmicb.2011.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dean SN, Bishop BM, van Hoek ML. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol 2011; 11:114; PMID:21605457; http://dx.doi.org/ 10.1186/1471-2180-11-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blower RJ, Barksdale SM, van Hoek ML. Snake cathelicidin NA-CATH and smaller helical antimicrobial peptides are effective against burkholderia thailandensis. PLoS Negl Trop Dis 2015; 9:e0003862; PMID:26196513; http://dx.doi.org/ 10.1371/journal.pntd.0003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lisanby MW, Swiecki MK, Dizon BL, Pflughoeft KJ, Koehler TM, Kearney JF. Cathelicidin administration protects mice from Bacillus anthracis spore challenge. J Immunol 2008; 181:4989-5000; http://dx.doi.org/ 10.4049/jimmunol.181.7.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu W, Bradley KA, et al.. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J Biol Chem 2006; 281:32755-64; PMID:16790431; http://dx.doi.org/ 10.1074/jbc.M603614200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].O'Driscoll NH, Labovitiadi O, Cushnie TP, Matthews KH, Mercer DK, Lamb AJ. Production and evaluation of an antimicrobial peptide-containing wafer formulation for topical application. Curr Microbiol 2013; 66:271-8; PMID:23183933; http://dx.doi.org/ 10.1007/s00284-012-0268-3 [DOI] [PubMed] [Google Scholar]
- [15].Welkos S, Cote CK, Hahn U, Shastak O, Jedermann J, Bozue J, Jung G, Ruchala P, Pratikhya P, Tang T, et al.. Humanized theta-defensins (retrocyclins) enhance macrophage performance and protect mice from experimental anthrax infections. Antimicrob Agents Chemother 2011; 55:4238-50; PMID:21768520; http://dx.doi.org/ 10.1128/AAC.00267-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ahmad S, Hunter L, Qin A, Mann BJ, van Hoek ML. Azithromycin effectiveness against intracellular infections of Francisella. BMC Microbiol 2010; 10:123; PMID:20416090; http://dx.doi.org/ 10.1186/1471-2180-10-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aperis G, Fuchs BB, Anderson CA, Warner JE, Calderwood SB, Mylonakis E. Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain. Microbes Infect 2007; 9:729-34; PMID:17400503; http://dx.doi.org/ 10.1016/j.micinf.2007.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McKenney ES, Sargent M, Khan H, Uh E, Jackson ER, San Jose G, Couch RD, Dowd CS, van Hoek ML.. Lipophilic prodrugs of FR900098 are antimicrobial against Francisella novicida in vivo and in vitro and show GlpT independent efficacy. PloS One 2012; 7:e38167; PMID:23077474; http://dx.doi.org/ 10.1371/journal.pone.0038167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Champion OL, Cooper IA, James SL, Ford D, Karlyshev A, Wren BW, Duffield M, Oyston PC, Titball RW.. Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis. Microbiology 2009; 155:1516-22; PMID:19383703; http://dx.doi.org/ 10.1099/mic.0.026823-0 [DOI] [PubMed] [Google Scholar]
- [20].Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 2010; 76:310-7; PMID:19897755; http://dx.doi.org/ 10.1128/AEM.01301-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr., Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 2009; 53:2605-9; PMID:19332683; http://dx.doi.org/ 10.1128/AAC.01533-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thomas RJ, Hamblin KA, Armstrong SJ, Muller CM, Bokori-Brown M, Goldman S, Atkins HS, Titball RW. Galleria mellonella as a model system to test the pharmacokinetics and efficacy of antibiotics against Burkholderia pseudomallei. Int J Antimicrob Agents 2013; 41:330-6; PMID:23402703; http://dx.doi.org/ 10.1016/j.ijantimicag.2012.12.009 [DOI] [PubMed] [Google Scholar]
- [23].Ramarao N, Nielsen-Leroux C, Lereclus D. The insect galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp 2012:e4392; PMID:23271509; http://dx.doi.org/ 10.3791/4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, et al.. AB5075, a highly virulent isolate of acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. MBio 2014; 5:e01076-14; PMID:24865555; http://dx.doi.org/ 10.1128/mBio.01076-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harding CR, Schroeder GN, Collins JW, Frankel G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. J Vis Exp 2013: (81)e50964; PMID:24299965; http://dx.doi.org/ 10.3791/50964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Plaut RD, Kelly VK, Lee GM, Stibitz S, Merkel TJ. Dissemination bottleneck in a murine model of inhalational anthrax. Infect Immun 2012; 80:3189-93; PMID:22753373; http://dx.doi.org/ 10.1128/IAI.00515-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Popova TG, Teunis A, Espina V, Liotta LA, Popov SG. Chemokine-releasing microparticles improve bacterial clearance and survival of anthrax spore-challenged mice. PLoS One 2016; 11:e0163163; PMID:27632537; http://dx.doi.org/ 10.1371/journal.pone.0163163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brogden KA, Nordholm G, Ackermann M. Antimicrobial activity of cathelicidins BMAP28, SMAP28, SMAP29, and PMAP23 against Pasteurella multocida is more broad-spectrum than host species specific. Vet Microbiol 2007; 119:76-81; PMID:16997510; http://dx.doi.org/ 10.1016/j.vetmic.2006.08.005 [DOI] [PubMed] [Google Scholar]
- [29].Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB Jr, Lehrer RI, Welsh MJ, Tack BF.. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 2000; 68:2748-55; PMID:10768969; http://dx.doi.org/ 10.1128/IAI.68.5.2748-2755.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Larrick JW, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright SC. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob Agents Chemother 1993; 37:2534-9; PMID:8109914; http://dx.doi.org/ 10.1128/AAC.37.12.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luna VA, King DS, Gulledge J, Cannons AC, Amuso PT, Cattani J. Susceptibility of Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus thuringiensis to 24 antimicrobials using Sensititre automated microbroth dilution and Etest agar gradient diffusion methods. J Antimicrob Chemother 2007; 60:555-67; PMID:17586563; http://dx.doi.org/ 10.1093/jac/dkm213 [DOI] [PubMed] [Google Scholar]
- [32].Takagi S, Hayashi S, Takahashi K, Isogai H, Bai L, Yoneyama H, Ando T, Ito K, Isogai E. Antimicrobial activity of a bovine myeloid antimicrobial peptide (BMAP-28) against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Anim Sci J 2012; 83:482-6; PMID:22694332; http://dx.doi.org/ 10.1111/j.1740-0929.2011.00979.x [DOI] [PubMed] [Google Scholar]
- [33].Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, Gennaro R, Di Bonaventura G.. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011; 32:1807-14; PMID:21849157; http://dx.doi.org/ 10.1016/j.peptides.2011.08.002 [DOI] [PubMed] [Google Scholar]
- [34].Giacometti A, Cirioni O, Ghiselli R, Bergnach C, Orlando F, D'Amato G, Mocchegiani F, Silvestri C, Del Prete MS, Skerlavaj B, et al.. The antimicrobial peptide BMAP-28 reduces lethality in mouse models of staphylococcal sepsis. Crit Care Med 2004; 32:2485-90; PMID:15599155; http://dx.doi.org/ 10.1097/01.CCM.0000148221.09704.22 [DOI] [PubMed] [Google Scholar]
- [35].Kaplan ELM, P. Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958; 53:457-81; ; http://dx.doi.org/ 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- [36].Russell BH, Vasan R, Keene DR, Koehler TM, Xu Y. Potential dissemination of Bacillus anthracis utilizing human lung epithelial cells. Cell Microbiol 2008; 10:945-57; PMID:18067609; http://dx.doi.org/ 10.1111/j.1462-5822.2007.01098.x [DOI] [PubMed] [Google Scholar]
- [37].Franks SE, Ebrahimi C, Hollands A, Okumura CY, Aroian RV, Nizet V, McGillivray SM. Novel role for the yceGH tellurite resistance genes in the pathogenesis of Bacillus anthracis. Infect Immun 2014; 82:1132-40; PMID:24366250; http://dx.doi.org/ 10.1128/IAI.01614-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fedhila S, Buisson C, Dussurget O, Serror P, Glomski IJ, Liehl P, Lereclus D, Nielsen-LeRoux C. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J Invertebr Pathol 2010; 103:24-9; PMID:19800349; http://dx.doi.org/ 10.1016/j.jip.2009.09.005 [DOI] [PubMed] [Google Scholar]
- [39].Chung MC, Jorgensen SC, Tonry JH, Kashanchi F, Bailey C, Popov S. Secreted Bacillus anthracis proteases target the host fibrinolytic system. FEMS Immunol Med Microbiol 2011; 62:173-81; PMID:21395696; http://dx.doi.org/ 10.1111/j.1574-695X.2011.00798.x [DOI] [PubMed] [Google Scholar]
- [40].Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101-12; PMID:14975532; http://dx.doi.org/ 10.1016/j.femsre.2003.09.002 [DOI] [PubMed] [Google Scholar]
- [41].Marmaras VJ, Lampropoulou M. Regulators and signalling in insect haemocyte immunity. Cell Signal 2009; 21:186-95; PMID:18790716; http://dx.doi.org/ 10.1016/j.cellsig.2008.08.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


