Abstract
The mammalian hearing organ is a stereotyped cellular assembly with orderly innervation: two types of spiral ganglion neurons (SGNs) innervate two types of differentially distributed hair cells (HCs). HCs and SGNs evolved from single neurosensory cells through gene multiplication and diversification. Independent regulation of HCs and neuronal differentiation through expression of basic helix-loop-helix transcription factors (bHLH TFs: Atoh1, Neurog1, Neurod1) led to the evolution of vestibular HC assembly and their unique type of innervation. In ancestral mammals, a vestibular organ was transformed into the organ of Corti (OC) containing a single row of inner HC (IHC), three rows of outer HCs (OHCs), several unique supporting cell types, and a peculiar innervation distribution. Restoring the OC following long-term hearing loss is complicated by the fact that the entire organ is replaced by a flat epithelium and requires reconstructing the organ from uniform undifferentiated cell types, recapitulating both evolution and development. Finding the right sequence of gene activation during development that is useful for regeneration could benefit from an understanding of the OC evolution. Toward this end, we report on Foxg1 and Lmx1a mutants that radically alter the OC cell assembly and its innervation when mutated and may have driven the evolutionary reorganization of the basilar papilla into an OC in ancestral Therapsids. Furthermore, genetically manipulating the level of bHLH TFs changes HC type and distribution and allows inference how transformation of HCs might have happened evolutionarily. We report on how bHLH TFs regulate OHC/IHC and how misexpression (Atoh1-Cre; Atoh1f/kiNeurog1) alters HC fate and supporting cell development. Using mice with altered HC types and distribution, we demonstrate innervation changes driven by HC patterning. Using these insights, we speculate on necessary steps needed to convert a random mixture of post-mitotic precursors into the orderly OC through spatially and temporally regulated critical bHLH genes in the context of other TFs to restore normal innervation patterns.
Introduction
The inner ear of vertebrates consists of mechanosensory hair cells (HCs) arranged in discrete sensory patches to transduce various mechanical stimuli (gravity and other linear accelerations, angular accelerations, and sound [Lewis et al. 1985; Fritzsch and Elliott 2017b]). These sensory HCs are innervated by distinct populations of sensory neurons (three types of vestibular sensory neurons and two types of spiral ganglion [auditory] sensory neurons) that conduct the information to the vestibular and cochlear nuclei of the brainstem, respectively (Desai et al. 2005; Fritzsch and Elliott 2017b). In addition, HCs and sensory neurons are innervated by inner ear efferent (IEE) fibers that fine-tune the perception of mechanical stimuli (Simmons et al. 2011; Sienknecht et al. 2014; Fritzsch and Elliott 2017a). Among the various sensory epithelia of the inner ear, the mammalian auditory organ, or organ of Corti (OC), is unique in its detailed cellular architecture (Jahan et al. 2015a) and innervation (Dabdoub et al. 2016). This unique architecture is required for the fine tuning of the sound evoked movements of the basilar membrane (Shera 2015; Fettiplace 2017; Reichenbach and Hudspeth 2014) to enable, in various mammals, a hearing range much wider than that of other species (Lewis et al. 1985), ranging from over 200,000 Hz (bats, dolphins) to 16 Hz (elephants), and project it in a tonotopic way to the cochlear nuclei (Dabdoub et al. 2016). Importantly, inner hair cells (IHCs) of the OC function as hydrodynamic receptors (Richter et al. 2007) driven by the endolymph mobilized by sound evoked volume changes of the space between tectorial membrane and reticular lamina (arrows in Fig. 1A). Also unique to the OC are the outer hair cells (OHCs) that function as an amplifier using modified ion channels for their active length changes (Okoruwa et al. 2008). These molecular, cellular, and physiological features set the OC apart from other auditory organs found in sauropsids (Manley 2012) or amphibians (Fritzsch et al. 1988).
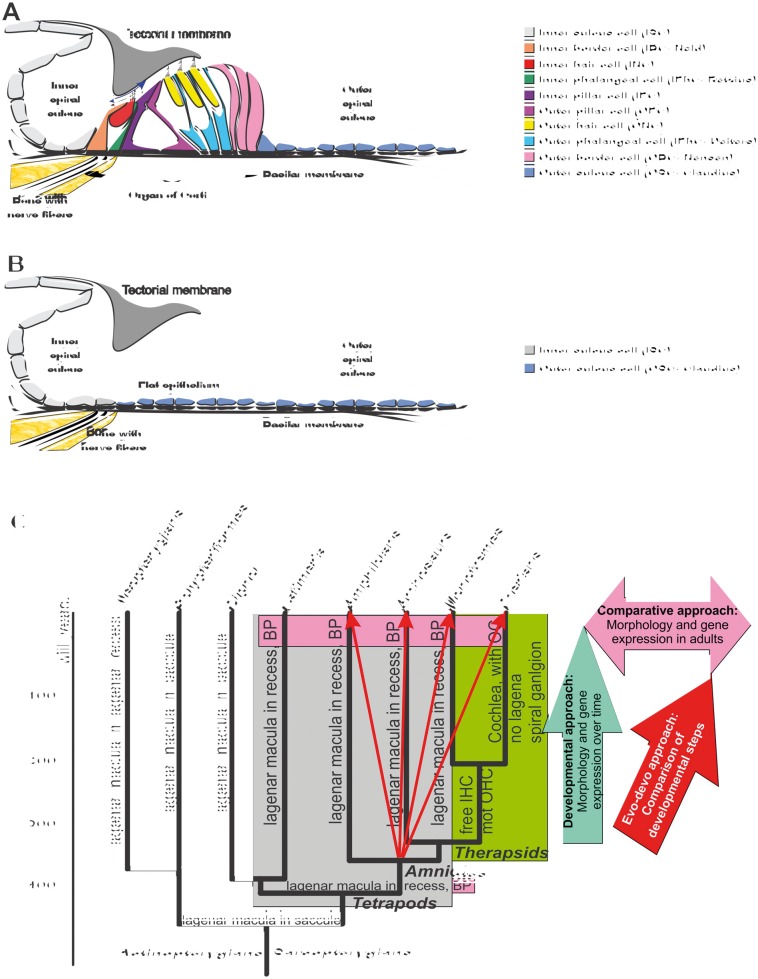
Fig. 1.
The illustration represents the normal organization of the OC (A) and residual flat epithelium after HCs and OC have degenerated (B). Inner hair cells IHCs function as hydrodynamic receptors driven by the endolymph flow between the sub-tectorial space and the inner spiral sulcus (blue arrow in A). Note that outer sulcus cells may form a sharp boundary near the entry of nerve fibers out of the bony lip on which the inner pillar cell sits. (C) The evolution of mammalian OC (green highlight) from the ancestral basilar papilla (BP) inside the lagenar duct (gray highlight) is depicted, possibly starting in sarcopterygians. Note that understanding the molecular basis of OC evolution out of the ancestral Therapsid BP requires a comparative approach across BP (pink highlight), developmental analysis (turquois arrow) comparison of developmental processes (red arrows) to deduce original state of the BP in tetrapods. Modified after Fritzsch et al. (2013) and Jahan et al. (2015b).
The mammalian hearing organ is not only unique in its cellular composition and distribution details, such as absence of kinocilia, but is particularly susceptible to loud sound, ototoxic drugs, and age related functional decline (Fattal et al. 2018). In fact, a major problem of aging societies is the increase of age related hearing loss, predicted to reach nearly 1 billion people worldwide by 2050 (WHO). Most of this hearing decline is in the range of ∼25 dB and can be compensated for by middle ear amplifiers. Nevertheless, hundreds of millions of people are suffering from neurosensory hearing loss, whereby first the HCs in auditory periphery die followed by loss of all cells of the OC over time (Taylor et al. 2012; Kersigo and Fritzsch 2015) leaving a featureless flat epithelium (Fig. 1B). Moreover, ototoxic drugs can unmask differential susceptibility of HCs between the apex and base, with basal HCs being more vulnerable (Sha et al. 2001; Booth et al. 2018). This variation is also evident after various genetic manipulations (Jahan et al. 2010b, 2015b; Nakano et al. 2012; Pan et al. 2012), indicating that locally variable gene profiles might need to be established to restore normal tonotopic hearing. Previous work has demonstrated that HCs in non-mammalian vertebrates either proliferate continuously or can regenerate following any means of destruction, whether it be mechanical, chemical, or genetic (Zine et al. 2014). In contrast to this ubiquitous ability of the non-mammalian inner ear HCs, that extends also to the lateral line HCs, electroreceptor cells (if present) (Fritzsch et al. 1990), and sensory cells of the taste buds (Fritzsch et al. 1998), the adult mammalian OC shows neither proliferation of new HCs nor is it capable of restoring lost HCs without extra manipulations. Most recent advances suggest some ability to restore lost HCs in mammalian vestibular sensory organs relevant for age related balance loss, such as the semicircular canal cristae and the gravistatic sensors (Burns and Stone 2016; Bucks et al. 2017). However, the mammalian OC is apparently unable to restart proliferation past early juvenile stages (White et al. 2006; Walters et al. 2017). Even if extreme measures are used, such as eliminating the proliferation regulation protein retinoblastoma (Mantela et al. 2005), the mammalian OC does not increase proliferation. This inability to jump-start proliferation is consistent with the fact that there is no inner ear tumor, except for schwannoma. However, schwannomas are related to neural crest derived Schwann cells (Cioffi et al. 2010).
Current attempts to restore lost HCs in the OC revolve around three basic cellular and molecular strategies:
Induce transdifferentiation of supporting cells: This can be achieved using either delta/notch inhibitors or single/multiple transcription factors (TFs) as initially shown by Weintraub and collaboratores (Lassar 2017). It should be pointed out that 30 years after the seminal finding of transdifferentiating fibroblasts into muscle fibers in vitro, this approach is still not able to achieve reliable transdifferentiation in vivo (Taylor 2017). This indicates that transdifferentiation is likely even more complicated in the OC with its intricate cellular mosaic and progressive loss of supporting cells after HC loss.
Transdifferentiation of postmitotic cells: Transdifferentiation is more effective in postmitotic compared with fully differentiated cells and would also have an advantage of not depleting supporting cells to reconstruct lost HCs. This approach is to induce proliferation followed by differentiation of the small number of capable cells in the OC (Bramhall et al. 2014; Shi et al. 2014). Developing this approach further to restore areas of flat epithelia, where all cells of the OC have been depleted, will be a major hurtle (Zine et al. 2014; Warnecke et al. 2017) that will in particular affect seniors with long term hearing loss that need that therapy the most.
Induction of stem cells: Following the successful generation of induced pluripotent stem cells in tissue culture (Takahashi and Yamanaka 2006), such culture based approaches have revealed that formation of ear-like organoids are possible in the dish and could lead to generation of HCs that could potentially be inserted to restore hearing (Liu et al. 2016; Koehler et al. 2017). However, the potassium rich endolymph of the scala media is toxic to cell survival and even flushing and replacing the endolymph with perilymph leads only to limited survival of inserted cells (Lee et al. 2017).
Given the current limitations of all these approaches, alternatives could be increasing the survival of or increase numbers of sensory neurons to conduct more information for hearing provided by a cochlear implant to the brain. Such approaches could require generation and insertion of sensory neurons into the ear and connecting them to both the OC and the central nervous system. Neither the peripheral guidance cues (Yang et al. 2011; Fritzsch et al. 2015; Goodrich 2016) nor the central guidance cues (Elliott et al. 2017; Yang et al. 2017) are currently understood to the extent that they can be used to connect reliably nerve fibers of implanted sensory neurons with cochlear nuclei and cochlear implants (Dabdoub et al. 2016).
In an attempt to refocus current activity centered on restoration after short-term loss of HCs, we present here some molecular cues that have emerged as an essential basis for OC development. More specifically, we present molecules that affect, in mutants, selectively the OC while sparing partially vestibular organ development and put those molecules into the background of OC evolution out of the ancestral basilar papilla (Fritzsch et al. 2013). Here we like to advance the idea that an understanding of the evolutionary and developmentally important molecular cues for OC development will enable the design of novel approaches for complete restoration of long lost OC with extensive formation of flat epithelium (Fig. 1C), as is typically the case in the increasing numbers of seniors suffering from hearing loss.
Nothing comes from nothing
As outlined above, the mammalian OC is a transformed vestibular sensory patch that arose in ancestral tetrapods, possibly already in sarcopterygians (Fig. 1C), transforming a stretch of the lagena recess epithelium into a sensory organ (Fritzsch 1987, 1992). Loss of HCs turns the OC into a flat epithelium, not unlike the epithelium of the lagenar recess wall (Fig. 1B). Rebuilding an OC requires a detailed understanding how to transform, during development and evolution, a flat non-sensory epithelium into a BP/OC sensory epithelium (Fig. 1C). Given the hundreds of genes already known to be involved in this process (Liu et al. 2014) and their incompletely understood complexity of interactions (Booth et al. 2018), additional steps need to be taken to filter out the most important genes needed to rebuild an OC from such a flat epithelium. One way is to analyze the progressive changes in gene expression profiles to identify those genes that correlate the most with specific steps in development. While such processes can seemingly identify relevant genes in cell fate determination (Ealy et al. 2016), they may not provide an easy understanding of how cell fate decision and organ development integrate (Fritzsch et al. 2006; Groves and Fekete 2012). Ultimately, such suggestions need to be confirmed in their significance through mutational analysis, complementing the large body of work that has already identified crucial transcription factors such as Sox2, Atoh1, Pou4f3, and others (Groves et al. 2013; Dvorakova et al. 2016). Comparison of various sensory organ development within a species such as mouse (Burns et al. 2015) could provide critical developmental genes but does not allow easy prioritization. Another approach, comparison of homologous organs such as the basilar papilla/OC (Fig. 1C) could allow identifying the most relevant genes expressed in adult organs, but will not identify critical developmental genes. However, comparing developmental gene expression profiles of the various auditory organs across tetrapods is a likely way to find all relevant genes (Fig. 1C) and should soon be possible using modern techniques.
With these considerations in mind, we propose here a strategy that builds on molecular and mutant analysis of mouse ear development but puts the effects of genes identified into the context of the emerging understanding of the OC evolution, as well as the unique physiology briefly outlined above. We argue that solving the problem of identifying the most relevant genes could benefit from an existing evolutionary understanding. Recent data identified critical steps that drive the evolutionary changes of an amniote basilar papilla in fossil Therapsids (Luo et al. 2011) toward the cellular mosaic and overall organization of monotremes (Schultz et al. 2017). Monotremes show physiological characteristics of Therian OC (Mills and Shepherd 2001) as well as cellular distinction of IHC and OHC and their distribution (Ladhams and Pickles 1996; Vater and Kössl 2011), but in a differently organized sensory patch than that of the OC (Fig. 2A). These evolutionary data suggest that patterning and positioning of the OC, formation of distinct cell types, and their overall distribution, but not the detailed cellular alignment, evolved already in Therapsid ancestors of monotremes and Therians (Figs. 1C and 2A). We suggest that understanding these evolutionary changes helps identifying relevant genes out of several hundreds of genes involved in OC development to pick the most pertinent ones to rebuild an OC from a flat epithelium. Evolution is mostly achieved through gene duplication to make ohnologs followed by mutation of cis and trans elements to diversify the function into paralogs and orthologs (Peter and Davidson 2011; Onimaru and Kuraku 2018). We argue that the OC can be best understood as duplication of a vestibular organ in early tetrapods that followed diversification through alteration of gene expressions and their interactions (Fritzsch and Elliott 2017b). We and others have dealt previously with the evolution of cell types (Arendt et al. 2016; Elliott et al. Forthcoming 2018) and have therefore excluded this aspect here.
Fig. 2.
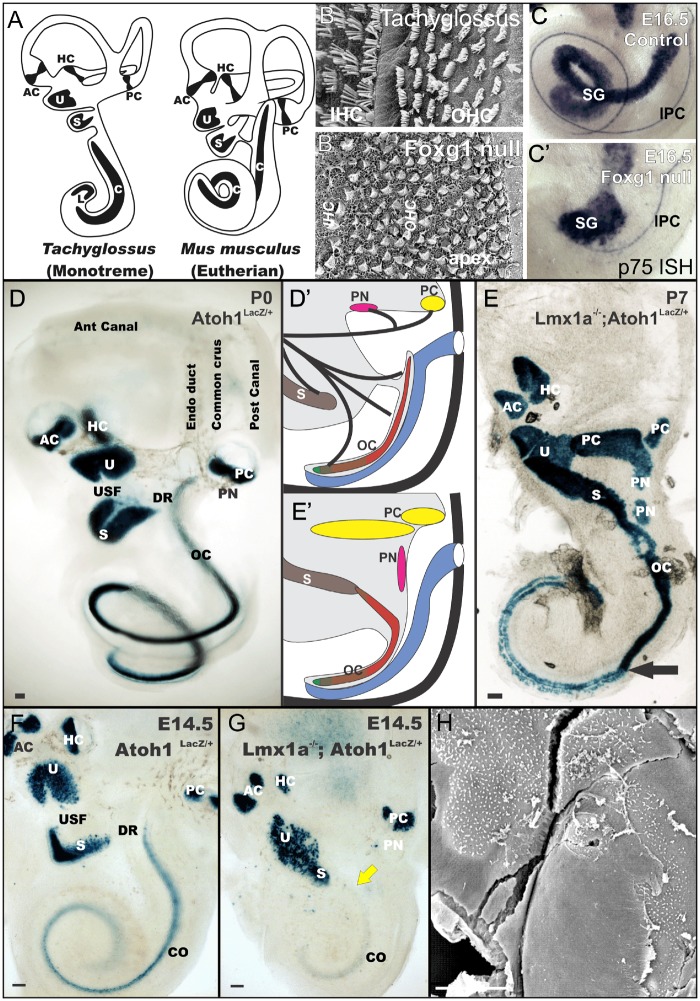
The illustration depicts the main differences in overall ear organization of monotreme and eutherian mammals, suggesting a correlation of the loss of the lagena and the coiled extension of the cochlea as well as the OC in Eutherians. Cellular details indicate a multiple step evolution with formation of IHCs and OHCs already in monotremes (A). Loss of Foxg1 partially converts the OC into a shorter and wider epithelium with multiple cellular rows (B, B′). Foxg1 null mice reveals that formation of four rows of HCs depend on the elongation of the cochlea that may, in turn depend on inner pillar cells that are restricted to the near normal basal part of the shortened cochlea and the fewer, aggregated spiral ganglion neurons revealed by a p75 in situ hybridization (C, C′). Atoh1 lacZ expression shows delay in upregulation of Atoh1 in the Lmx1a null basal turn cochlea that results in the transformation of basal HCs into vestibular-like HCs (D–H). The transformation of the basal turn of the OC (indicated by arrow in E) into a vestibular like epithelium partially covered by a tectorial membrane (H) seems to be the consequence of the delayed upregulation of Atoh1 in the base of Lmx1a null mutants (F, yellow arrow in G). AC, anterior crista; C/OC, organ of Corti; Co, cochlea; DR, ductus reuniens; HC, horizontal crista; IPC, inner pillar cell; L, lagena; PN, papilla neglecta; PC, posterior crista; S, saccule; SG, spiral ganglion; U, utricle. Bar indicates 10 µm. Modified after Fritzsch et al. (2013); Jahan et al. (2015b); Nichols et al. (2008); and Pan et al. (2012).
Lmx1a is essential for vestibular transformation and segregation from the saccule
Cell specification in the OC is in part dependent on the intracellular interactions of pro-proliferative versus pro-differentiation factors as revealed in Lmx1a mutants (Fig. 2). Lmx1a protein regulates numerous aspects of cell fate determination by promoting proliferation while inhibiting differentiation. This is particularly obvious in the development of the brainstem where entire areas may be absent in mice null for both Lmx1 genes (Glover et al. 2018). In the developing ear, Lmx1a expression is progressively reduced (Nichols et al. 2008), which in turn releases the cellular inhibition of e-proteins (Asbreuk et al. 2002). Availability of e-proteins is needed to initiate the heterodimerization with pro-differentiating basic helix-loop-helix (bHLH) proteins, such as Atoh1, that initiate differentiation. All Lmx1a mutants (Nichols et al. 2008; Koo et al. 2009; Steffes et al. 2012; Mann et al. 2017) show expansion of some sensory epithelia, loss of other sensory epithelia, and partial transformation of the basal turn of the OC into a vestibular type of HC organization (Fig. 2). In addition, Lmx1a cooperates with the delta/notch signaling to regulate the segregation of sensory epithelia (Nichols et al. 2008; Mann et al. 2017), possibly by changing the temporal progression of proliferation versus differentiation like in n-Myc mutants (Kopecky et al. 2011; Schimmang 2013). Eliminating expression of proteins that inhibit Lmx1a, such as LMO proteins, suffices to induce sensory epithelia formation (Hill et al. 2010; Deng et al. 2014), mimicking the prosensory induction by misexpression of delta/notch (Pan et al. 2010).
Consistent with Lmx1a protein function in interfering with proneural bHLH genes, available data show a profound delay in Atoh1 expression in the basal turn of the OC in Lmx1a mutants (Fig. 2) and this delay in expression of Atoh1 seems to relate to the phenotypic transformation of basal turn HCs mostly into vestibular HCs (Fig. 2). This transformation of HCs that topographically belong to the OC and are next to a tectorial membrane (Fig. 2) suggests that timing of proliferation versus onset of differentiation is tied into the cell fate specification as previously pointed out (Matei et al. 2005; Groves et al. 2013). Such effects of cell cycle exit versus differentiation have also been found in other mutants such as Neurod1 null mice where OHCs can turn into IHCs due to premature upregulation of Atoh1 (Jahan et al. 2013; Kopecky et al. 2013). Such systematic changes between cell cycle exit and upregulation of Atoh1 seem to be unique to the OC. Obviously, understanding the progressive downregulation of Lmx1a followed by downregulation of cell cycle progression factors such as n-Myc and coordinated upregulation of Atoh1 could be a way to drive both proliferation followed by differentiation of the flat epithelium. Finding regulators that allow to mimic the spatio-temporal progression of the various factors to ensure both proliferation followed by differentiation occur in a pattern that re-establishes a lost OC could provide one major step forward. In this context, it would be important to understand Lmx1a function in the formation of non-mammalian hearing organs of birds (Manley 2017a, 2017b) or amphibians (Fritzsch and Wake 1988) to reveal how differential regulation of Lmx1a drives the more vestibular organ-like basilar papilla developmental program of birds and amphibians into a mammalian OC program. For example, the molecular evolution of a temporal delay between cell cycle exit and onset of differentiation (Matei et al. 2005) correlates with the differentiation of more robust HCs in the apex known to be the least vulnerable HCs of the OC. It is possible that this effect evolved due to the inclusion of the monotreme lagena (Fig. 1C, 2A) into the growing OC (Fritzsch et al. 2013) providing apical HCs with nearly vestibular HC like resistance to ototoxic drugs, genetic manipulation of actin homeostasis, and denervation (Sha et al. 2001; Ueyama et al. 2014; Kersigo and Fritzsch 2015).
Altering proliferation progression affects OC cellular assembly
Loss of Foxg1 results in near complete loss of inner pillar cells with formation of multiple rows of HCs that have nearly lost distinctions between IHCs and OHCs (Fig. 2), while increasing greatly the number of rows of HCs to 12 or more rows, comparable to monotremes (Ladhams and Pickles 1996; Pauley et al. 2006). Labeling for the inner pillar cell marker p75 shows reduced expression in only some basal inner pillar cells (Fig. 2), suggesting that inner pillar cell formation is an essential step in the process that expands the much shorter and wider assembly of HCs in early development into the adult OC through convergent extension and cell movements (Driver et al. 2017). The shortening cochlear duct in Foxg1 mutants resembles that of monotremes, as does the organization of spiral ganglion neurons (SGNs) into a cluster instead of an elongated cell array (Pauley et al. 2006). Evolution shows that monotremes have the ancestral amniotic lagena epithelium at the tip of the lagenar recess (Figs. 1C, 2A) that was either lost or incorporated into the growing OC of the coiled cochlea in therian mammals (Luo et al. 2011; Fritzsch et al. 2013).
Consistent with the evolutionary incorporation of the lagena into the apex of the OC (Fig. 2) are the effects caused by the loss of the proliferation regulation bHLH gene, n-Myc. This mutation results in fusion of the base of the cochlea to the saccule, shortening of the cochlea, and gross abnormal development of the apex of the cochlea that resembles a vestibular sensory epithelium (Kopecky et al. 2011; Fritzsch et al. 2013). Interestingly, reconstruction of the perilymphatic sound conduction system reveals that the apical “vestibular-like” epithelium is segregated from the sound conducting system (Kopecky et al. 2011; Fritzsch et al. 2013), resembling the absence of sound conduction contacts described for the lagena of monotremes (Schultz et al. 2017). Thus, proliferation regulation seems to be not only essential to generate the many more HCs of the OC of mammals relative to the basilar papilla of Therapsid ancestors but also affects the cellular assembly and cell fate development. It is possible that these effects relate to the close relationship of pro-proliferative and pro-differentiation bHLH genes and their regulation by Lmx1a inside the prosensory epithelium that affects cell fate commitment within and between cells (Fritzsch et al. 2006; Kopecky and Fritzsch 2011). It would be important to combine n-Myc and Lmx1a mutations to establish that a combination of both can convert the entire OC into a vestibular-like sensory epithelium, establishing the crucial role of those genes in OC evolution. Such data could support or refute our notion that adding the evolutionary insights can help understand otherwise merely descriptive aspects of OC development as a transformational compromise needed to convert an ancestral vestibular organ into the apex of the OC.
Defining the position of the OC
The function of the OC depends on having all cellular components at the right position and the right level of differentiation and correct numbers (Jahan et al. 2015a). In addition, the organ needs to sit with specific cell types, such as the inner pillar cells, specifically on the bony lip of Rosenthal’s canal to function (Fig. 1A, B). Obviously, Lmx1a, Foxg1, and n-Myc are part of that signaling but it is presently unknown what regulates the precise position. Recent evidence provides additional clues not only about the molecular basis of the positioning but also about the molecular nature of the cells that replace the OC after degeneration. Investigations of molecular markers flanking the OC has led to the insight that Fgf10 is a marker for the future inner spiral sulcus cells whereas BMP4 is a marker for outer spiral sulcus (aka Claudius) cells (Pan et al. 2011). Interestingly enough, there is a molecularly unclear feedback loop between the OC and the Fgf10 positive future inner spiral sulcus cells (Fig. 3) such that Fgf10 will be downregulated in the absence of the OC (Pan et al. 2011). Moreover, comparing Fgf10 and BMP4 expression with vestibular organs or sensory epithelia of the chicken shows that only the OC has a segregated, non-overlapping expression of those two crucial transcription factors (Fritzsch et al. 2006; Groves and Fekete 2012), that combined with other diffusible factors and lateral inhibition to define the unique cellular mosaic of the mammalian OC (Jahan et al. 2015a).
Fig. 3.
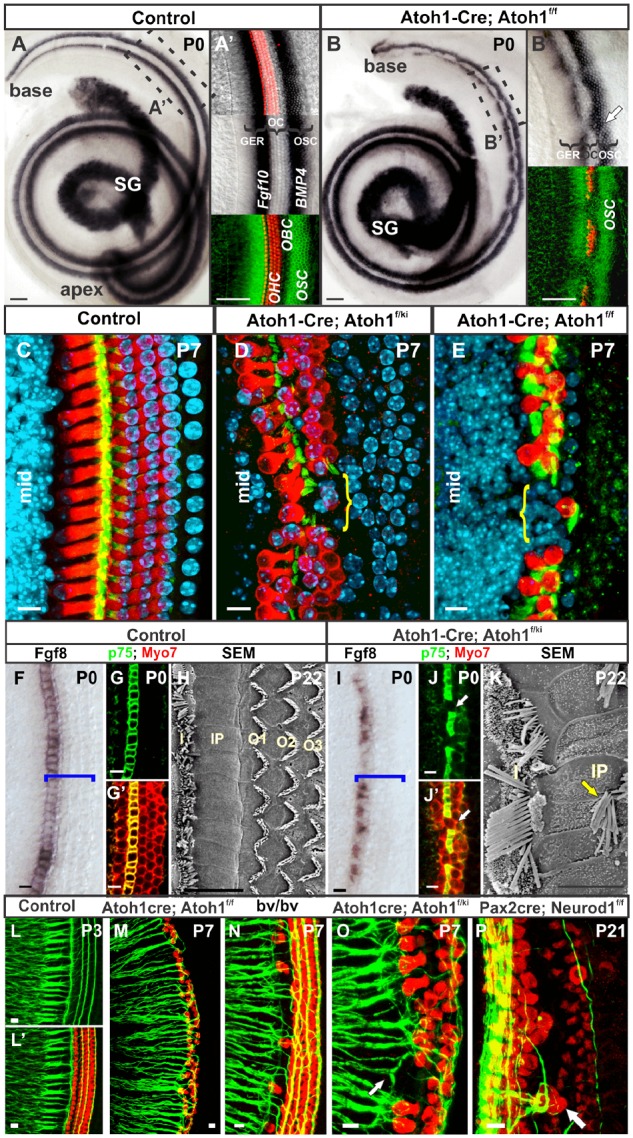
Double in situ hybridization demonstrates that Bmp4 and Fgf10 form lateral (OSC) and medial (GER) boundary of the control OC, respectively (A–B′). After incomplete loss of HCs in Atoh1-cre; Atoh1f/f mice (B, B′), Bmp4 positive outer spiral sulcus cells migrate medially between the Myo7a positive remaining HC patches, replacing the OC in areas devoid of HC with matching loss of Fgf10 in that area (B–B′, see arrow in B′). Misexpression of Neurog1 in Atoh1 locus in Atoh1-cre; Atoh1f/kiNeurog1 mice rescues HCs formation compared with Atoh1-cre; Atoh1f/f mice (C–E), without preserving the organization of OC or gaps in OC (brackets in D and E), shown with Myo7a, Tubulin immunohistochemistry, and Hoechst nuclear staining (C–E). Compared with controls, Fgf8 in situ hybridization shows patchy expression in the Atoh1-cre; Atoh1f/kiNeurog1 mice (F, I; brackets indicate OC). Myo7a positive HCs replace in the patchy loss of p75 positive inner pillar cells (G, G′, arrows in J and J′) that can also form stereocilia bundles in the inner pillar cells (arrow in K) demonstrated by the scanning electron microscopy (SEM) in P22 mice (H, K). Immunohistochemistry of Myo7a and neurofilament (NF) reveals the defect of innervation pattern in different types of HC defects (L–P). In loss of IHCs, as in Atoh1-cre; Atoh1f/f or in Bronx-Waltzer (bv/bv) mice, type I fibers appear to innervate the remaining OHCs or bifurcate to reach nearby IHCs (L–N). Fiber distribution pattern is disorganized to reach abnormally placed OHCs in the position of IHCs or bifurcate in the gaps of IHCs in Atoh1-cre; Atoh1f/kiNeurog1 mice (arrow in O) or to reach ectopic IHCs in OHCs position in Neurod1 null mice (arrow in P). GER, greater epithelial ridge; I, inner hair cell; IP, inner pillar cell; OBC, outer border cell (of Hensen); O/OHC, outer hair cell; OSC, outer sulcus cells (of Claudius); SG, spiral ganglion. Bar indicates 10 µm. Modified after Pan et al. (2012) and Jahan et al. (2015b).
Using a more delayed deletion of the HC differentiation factor Atoh1, it was shown that the Fgf10 signal remained only where some HCs survived in this OC, whereas in areas with complete loss of the OC, BMP4 positive cells expand medially toward OC with a concomitant loss of Fgf10 (Pan et al. 2012). These data (Fig. 3) suggest that the OC is replaced by outer spiral sulcus cells and that the approximation of BMP4 positive cells in the absence of an OC causes the downregulation of Fgf10. This idea is consistent with data showing that both Fgfs and BMPs are necessary to generate a sophisticated diffusion gradient profile that defines the different cell types of the OC (Fritzsch et al. 2006; Groves and Fekete 2012; Munnamalai and Fekete 2016). In fact, BMP4/Fgf interactions are among the best understood interactions in defining a number of cell fate changes (Neubüser et al. 1997) that are essential for early steps in neurogenesis (Fritzsch et al. 2006; Meinhardt 2015; Fritzsch and Elliott 2017b) and alterations in Fgf or Bmp signaling disrupt OC microstructure (Ohyama et al. 2010; Jahan et al. 2015a; Urness et al. 2015). Obviously, Monotremes have already the segregation of inner and outer HCs (Figs. 1C and 2A) indicating that the evolution of the segregated expression of Fgfs, Wnts, BMPs, and other factors evolved within ancestral Therapsids and may be correlated with the unique mammalian postnatal loss of kinocilia turning the mammalian IHC into a hydrodynamic sensor (Elliott et al. Forthcoming 2018).
Going beyond the effect of loss of an OC after its formation through targeted deletion of Atoh1, and thus HCs (Fig. 2), one can also eliminate formation of HCs through manipulation of essential genes that drive HC precursor development such as Sox2 or Eya1. Close examination of conditional deletion of Sox2 shows near complete absence of HCs with expansion of BMP4 positive cells to fill the position of the OC (Dvorakova et al. 2016). In combination, all these data indicate that, upon degeneration of the OC, a flat epithelium composed of BMP4 positive outer sulcus cells (Claudius cells) replaces the OC (Fig. 1), potentially providing a boundary at the location of the inner pillar cell (Figs. 1 and 3). It would be important to demonstrate how long this boundary of gene expression differences remains. Knowing the molecular marker of the cells that replace the OC can provide the means to target these cells, reduce their expression of BMP4 (if still present), and increase the presence of Foxg1, Lmx1a, n-Myc, and Fgf10 to initiate proliferation of these cells followed by coordinated upregulation of Atoh1 to drive differentiation (Dabdoub et al. 2008; Nishimura et al. 2017). Using known enhancer elements for BMP4 could allow driving the coordinated suppression of BMP4 combined with upregulation of Gata3, Pax2, Eya1, and Sox2, all needed for OC development (Bouchard et al. 2010; Duncan and Fritzsch 2013; Dvorakova et al. 2016; Zhang et al. 2017), to initiate sensory cell precursor development out of the distinctly non-sensory outer sulcus cells. Importantly, understanding the upstream regulators that drive Fgf10 and BMP4 from an overlapping vestibular epithelial to a segregated OC expression (Fig. 3) is needed to understand the evolution of such an arrangement and its use during development to enhance desired local cell fate decisions during regeneration.
Regulating the cell type development of the OC
Obviously, the above outlined approach will not suffice to regenerate an OC in all its complicated and numerically distinct cell types (Jahan et al. 2015a). Simply transforming an otic epithelium into a neurosensory patch will most likely result in a vestibular like sensory epithelium or possibly a basilar papilla used for hearing in most non-mammalian tetrapods (Thiede et al. 2014; Manley 2017a, 2017b) that was only converted into the OC in ancestral mammals (Luo et al. 2011; Fritzsch et al. 2013). This conversion required a number of changes that are poorly understood at the molecular level but are obvious at the cellular level. One crucial step is the evolution of two distinct types of HCs, the IHCs and OHCs in ancestral mammals, separated by two distinct supporting cells, the inner and outer pillar cells bordering at each other (Figs. 1A, C and 2A). This case of adjacent supporting cells without any HC interspaced is unique to the OC and is not found in any other sensory epithelium that all have a regular mosaic of alternating HCs and supporting cells (Desai et al. 2005). Notably, such an organization is present in the outer compartment of the OC consisting of a regular mosaic of OHCs and outer phalangeal cells (aka Deiters’ cells; Fig. 1).
A crucial step in OC evolution is the specification and differentiation of cochlear specific HCs that have to lose the kinocilium to allow the fluid movement of the endolymph to drive the IHCs as a hydrodynamic receptor (Richter et al. 2007). In addition, two distinct types of HCs with very different physiological properties need to be specified and located selectively to the inner and outer compartment on either side of the inner/outer pillar cells. Importantly, neither the functional segregation of IHCs (the nearly exclusive transducer of sound) nor the functional transformation of OHCs into a cochlear amplifier with limited sound transmission (Okoruwa et al. 2008; Tang et al. 2013) is understood in its regulation. What is clear, however, is that both the viability of HCs, as well as the types of HCs in the OC, depend on a spatio-temporal expression profile of the regulator of HC differentiation, Atoh1 (Bermingham et al. 1999; Fritzsch et al. 2005; Kopecky et al. 2013), which in turn depends on both diffusible factors and lateral inhibition via the delta/notch system (Groves and Fekete 2012; Jahan et al. 2015a) and the cross-regulation by Neurod1 for its spatio-temporal expression profile (Jahan et al. 2013). Since monotremes have the same basic HC organization, it appears that these processes evolved in Therapsids and can thus only be indirectly studied by comparing extant species or by comparing vestibular and OC development within one species, such as mice.
Even more distinct in its development is the inner pillar cell, a molecularly distinct supporting cell with essential properties for cochlear elongation (Driver et al. 2017). This cell is unique to the monotreme and therian OC (Vater and Kössl 2011; Fritzsch et al. 2013). Inner pillar cells are numerically distinct from other cells of the OC (Jahan et al. 2015a) and are not dependent on Fgf8 signaling derived from IHCs for their development, like the outer phalangeal (Deiters) cells (Puligilla et al. 2007; Jacques et al. 2012). The inner pillar cell expresses transiently the HC differentiation factor Atoh1 (Matei et al. 2005) and several distinct Hes/Hey factors to inhibit Atoh1 from transforming these cells into HCs (Doetzlhofer et al. 2009). The neurotrophin receptor p75 is also expressed in the inner pillar cells (Fig. 2), otherwise only expressed in sensory neurons (Von Bartheld et al. 1991). Replacing Atoh1 by Neurog1 (Fig. 3) or changing the spatio-temporal expression profile of Atoh1 by deleting Neurod1 leads to differentiation of inner pillar cells into HCs (Jahan et al. 2015b). The expression of the neurotrophin receptor p75 is partially lost after Neurog1 replacement of Atoh1 suggesting that it is tightly regulated by IHCs (Jahan et al. 2015b). Inner pillar cells that lose p75 are converted into HC like cells, upregulating the HC marker Myo7a (Fig. 3). The inner pillar cell is precisely positioned on the bony lip of Rosenthal’s canal (Fig. 1A) to allow the movement of the outer compartment sitting on the basilar membrane relative to the tectorial membrane to generate fluid movement between the tectorial membrane and the reticular lamina. Understanding the evolutionary origin of inner pillar cells and their expression of p75 as well as the molecular mechanism by which this cell type is selected and so precisely positioned is a crucial step in any attempt to restore hearing after complete loss of the OC and its transformation into a flat epithelium (Figs. 1A, B, 2A, and 3A). It would thus be important to establish that molecular markers identifying the outer sulcus cells that replace the OC by a flat epithelium are abutting the inner sulcus cells exactly where the inner pillar cells need to be placed (Figs. 1B and 3A), i.e., defining exactly the pivoting point of the OC.
As outlined above, understanding the distinct developmental programs of the OC can be facilitated by understanding how a general vestibular epithelium developmental program was changed following the formation of a novel cell patch that became the basilar papilla in ancestral tetrapods (Fritzsch et al. 2002) and evolved into the OC (Figs. 1 and 2). Using evolutionary consideration can facilitate extraction of the most meaningful data in such comparisons. Irrespective of this forward-looking approach, some TFs that are uniquely needed for OC development have already been identified through mouse mutant analysis. Still, adding the evolutionary dimension allows to categorize those TFs into some that are either essential for OC formation by regulating the segregation of the basilar papilla from the saccule (Nichols et al. 2008; Kopecky et al. 2011; Mann et al. 2017), are crucial for overall development (Bouchard et al. 2010; Kersigo et al. 2011; Duncan and Fritzsch 2013), or are essential for extension of the original population of HCs that form multiple rows (Pauley et al. 2006) through convergent extension (Dabdoub et al. 2008; Driver et al. 2017). How these and other factors integrate in normal OC development to ensure the differentiation of the right cell type at the right place, cause elongation of the initially much shorter OC (Driver et al. 2017), and cause loss or transformation of the monotreme lagena (Fritzsch et al. 2013; Schultz et al. 2017) in ancestral Therians (Fig. 1C) remain to be elucidated.
Order begets order: the effect of altered HC organization on OC innervation pattern
The OC is not only a highly ordered cellular assembly (Jahan et al. 2015a), but it also receives an equally highly regulated sensory afferent (Fritzsch et al. 2015; Goodrich 2016) and efferent (Simmons et al. 2011) innervation. The unusual distribution of ∼95% of all afferents to IHCs with convergence of 20 or more afferents onto a single IHC does not exist in any vestibular organ of vertebrates or non-mammalian hearing organ (Fritzsch et al. 1988; Manley 2017b) and may relate to the evolutionary novel expression of a second neurotrophin factor, Ntf3, in and around IHCs (Fritzsch et al. 2016). Multiple factors have been shown to affect the navigation of afferents to the distinct cell types of the OC (Coate and Kelley 2013; Mao et al. 2014) including the attraction toward neurotrophin sources (Yang et al. 2011; Fritzsch et al. 2016). It should be pointed out that navigation to HCs, while clearly influenced by neurotrophin expression (Tessarollo et al. 2004), is possible in the partial (Hellard et al. 2004) or complete absence of neurotrophins (Kersigo and Fritzsch 2015), indicating that multiple, potentially hierarchically organized molecular and cellular interactions are responsible for guidance (Fritzsch et al. 2015). It is noteworthy that factors affecting OC innervation have little effect on vestibular epithelia innervation (Mao et al. 2014) and rerouted vestibular fibers seemingly cannot innervate the OC (Tessarollo et al. 2004) indicating that entering the OC requires features not shared with vestibular organs that may relate to the evolution of unique SGNs in ancestral Therians (Luo et al. 2011). Here we focus on how the pattern of HC distribution that is so unique to the OC affects the pattern of innervation (Fig. 3).
Changing OHCs into IHCs through deletion of Neurod1 affects the pattern of innervation (Jahan et al. 2010a). More specifically, instead of having the typical type II fibers projecting along the three rows of OHCs, selective afferents can be traced to innervate the highly Myo7a and Fgf8 positive IHC-like OHCs that receive a direct innervation of fibers that end on those cells instead of forming spiral bundles (Fig. 3). This directed growth of fibers to these cells seems to be in part related to high expression of the neurotrophin Ntf3 that is more profound in the inner compartment, IHCs, and surrounding supporting cells (Fariñas et al. 2001) and is an evolutionary novel feature of the mammalian OC. Unfortunately, Neurod1 deletion also affects directly sensory neurons (Jahan et al. 2010a) making the interpretation of the innervation defects less straightforward.
To fully reveal the effect of miss-patterning of the OC on innervation requires analysis of mutations that primarily affect OC organization without directly affecting the innervating neurons such as Atoh1 manipulations. For example, navigation of fibers in Atoh1-cre; Atoh1f/f mutants that lose nearly all IHCs and retain mostly a single row of OHCs is also altered (Pan et al. 2012). The data in these mice show near complete absence of radial fibers passing along OHCs as overlapping layers of afferent fibers (Puligilla et al. 2007). Only in parts of the OC with two rows of cells and partial retention of IHCs could we find partial formation of the outer spiral bundles (Fig. 3). This reduction of outer spiral bundles is completely reversed in mice with near complete loss of IHCs such as Bronx-Waltzer mutants (Fig. 3). In these mutants, a gene that codes for the Srrm4 protein that mediates differential splicing is mutated causing the near complete loss of most IHCs without affecting OHCs (Nakano et al. 2012). Interestingly, in these mutants the density of outer spiral bundles is increased and many fibers normally targeted in their majority to IHCs seemingly expand to OHCs. Since Srrm4 is also expressed in sensory neurons it makes the conclusion that this fiber reorganization is exclusively dependent on the loss of IHCs somewhat tentative. However, in mice mutants in which one Atoh1 allele is replaced by Neurog1 (Jahan et al. 2015b) and the other floxed allele removed by Atoh1-cre mediated recombination (Atoh1-cre; Atoh1f/kiNeurog1) we found similar defects of innervation in areas without IHC formation (Fig. 3). In contrast to complete deletion of Atoh1 using the “self-terminating” approach of floxed Atoh1 alleles (Pan et al. 2012), the substitution of the Atoh1 allele by Neurog1 rescues not only many more HCs (Fig. 3) but also rescues functionality of remaining afferents (Tan et al. 2018). Much like in Srrm4 mutants, fibers were seen to bypass pillar cells to innervate in disorganized bundles the two–three remaining rows of disorganized OHCs (Fig. 3) suggesting that patterning of HCs directly translates into afferent organization. More work on alterations of neurotrophins and other factors regulating innervation is needed in those mutants to fully understand the mechanisms of this obvious reorganization of innervation pattern. These data demonstrate that the pattern of innervation depends on the pattern of HCs and thus is a consequence of HC patterning events.
Summary and conclusion
Here we present an overview of major molecular and cellular steps to transform a vestibular like cellular organization of ancestral Therapsid pre-mammals into the unique organization of the mammalian hearing organ, the OC. We suggest that these changes come about through altered changes in proliferation versus differentiation regulation by multiple factors. We highlight how mutations of some of these factors not only affect the cellular patterning of the OC but mimic the needed evolutionary changes to transform the cellular mosaic of a basilar papilla of most tetrapods into the unique OC distribution exclusive to mammals. We argue that more detailed comparison of developmental and evolutionary processes will allow selecting among the emerging multitude of molecular data those most relevant to evolve a unique OC developmental module. Such a restricted set of transcription factors could provide needed information to guide restoration of this organ from a flat epithelium in millions of seniors that have lost this sensory epithelium for years, recapitulating the evolution of the OC. Investigating the detailed pattern of innervation suggests that the distribution of HC types is driving the distinct pattern of innervation of the OC, relegating the innervation organization to a downstream effect that can only be addressed after managing the reconstitution of the cellular pattern of the OC.
Acknowledgments
We wish to thank Central Microscopy Research Facility for SEM and Roy J. Carver Center for Imaging for using Leica TCS SP5 confocal microscopy.
Funding
This work was supported by a National Institute on Deafness and Other Communication Disorders NIH grant [DC013655 to I.J.].
References
- Arendt D, Musser JM, Baker CV, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD.. 2016. The origin and evolution of cell types. Nat Rev Genet 17:744. [DOI] [PubMed] [Google Scholar]
- Asbreuk CH, Vogelaar CF, Hellemons A, Smidt MP, Burbach JPH.. 2002. CNS expression pattern of Lmx1b and coexpression with ptx genes suggest functional cooperativity in the development of forebrain motor control systems. Mol Cell Neurosci 21:410–20. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY.. 1999. Math1: an essential gene for the generation of inner ear hair cells. Science 284:1837–41. [DOI] [PubMed] [Google Scholar]
- Booth KT, Azaiez H, Smith RJH, Jahan I, Fritzsch B.. Forthcoming 2018. Intracellular regulome variability along the organ of Corti: evidence, approaches, challenges and perspective. Front Genet 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, de Caprona D, Busslinger M, Xu P, Fritzsch B.. 2010. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol 10:89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS.. 2014. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports 2:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks SA, Cox BC, Vlosich BA, Manning JP, Nguyen TB, Stone JS.. 2017. Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. eLife 6:e18128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Stone J.. 2016. Development and regeneration of vestibular hair cells in mammals. Semin Cell Dev Biol 65:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW.. 2015. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun 6:8557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi JA, Yue WY, Mendolia-Loffredo S, Hansen KR, Wackym PA, Hansen MR.. 2010. MicroRNA-21 over-expression contributes to vestibular schwannoma cell proliferation and survival. Otol Neurotol 31:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate TM, Kelley MW.. 2013. Making connections in the inner ear: recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells. Semin Cell Dev Biol 24:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Fritzsch B, Popper AN, Fay RR.. 2016. The primary auditory neurons of the mammalian cochlea. New York (NY): Springer; p. 286. [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW.. 2008. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A 105:18396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Luo X-J, Pan L, Yang H, Xie X, Liang G, Huang L, Hu F, Kiernan AE, Gan L.. 2014. LMO4 functions as a negative regulator of sensory organ formation in the mammalian cochlea. J Neurosci 34:10072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A.. 2005. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol 93:251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N.. 2009. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell 16:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Northrop A, Kelley MW.. 2017. Cell migration, intercalation and growth regulate mammalian cochlear extension. Development 144:3766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B.. 2013. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One 8:e62046.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M, Jahan I, Macova I, Chumak T, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G.. 2016. Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Sci Rep 6:38253.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealy M, Ellwanger DC, Kosaric N, Stapper AP, Heller S.. 2016. Single-cell analysis delineates a trajectory toward the human early otic lineage. Proc Natl Acad Sci U S A 113:8508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K, Kersigo J, Pan N, Jahan I, Fritzsch B.. 2017. Spiral ganglion neuron projection development to the hindbrain in mice lacking peripheral and/or central target differentiation. Front Neural Circuit 11:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Fritzsch B, Duncan JS.. Forthcoming 2018. Evolutionary and developmental insights provide the necessary molecular steps to regenerate organ of Corti hair cells. Front Cell Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, et al. 2001. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci 21:6170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal D, Hansen MR, Fritzsch B.. 2018. Age related balance impairment and hearing loss In: Rizzo M, Anderson S, Fritzsch B, editors. The Wiley handbook on the aging mind and brain. Chichester: wiley; p. 848. [Google Scholar]
- Fettiplace R. 2017. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr Physiol 7:1197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. 1987. Inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature 327:153–4. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. 1992. The water-to-land transition: evolution of the tetrapod basilar papilla, middle ear, and auditory nuclei. In: Webster DB, Popper AN, Fay RR, editors. The evolutionary biology of hearing. New York (NY): Springer; p. 351–75. [Google Scholar]
- Fritzsch B, Barbacid M, Silos‐Santiago I.. 1998. Nerve dependency of developing and mature sensory receptor cells. Ann N Y Acad Sci 855:14–27. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel K, Jones K, Farinas I, Maklad A, Lee J, Reichardt L.. 2002. Development and evolution of inner ear sensory epithelia and their innervation. Dev Neurobiol 53:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA.. 2006. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration?. Bioessays 28:1181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL.. 2017. Evolution and development of the inner ear efferent system: transforming a motor neuron population to connect to the most unusual motor protein via ancient nicotinic receptors. Front Cell Neurosci 11:114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL.. 2017. Gene, cell, and organ multiplication drives inner ear evolution. Dev Biol 431:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Kersigo J, Yang T, Jahan I, Pan N.. 2016. Neurotrophic factor function during ear development: expression changes define critical phases for neuronal viability In: Dabdoub A, Fritzsch B, Popper AN, Fay RR, editors. The primary auditory neurons of the mammalian cochlea. New York (NY): Springer; p. 49–84. [Google Scholar]
- Fritzsch B, Matei V, Nichols D, Bermingham N, Jones K, Beisel K, Wang V.. 2005. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn 233:570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T.. 2013. Evolution and development of the tetrapod auditory system: an organ of Corti‐centric perspective. Evol Dev 15:63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Elliott KL.. 2015. Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Res 361:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W.. 1988. Evolution of the amphibian auditory system. New York (NY: ): Wiley. [Google Scholar]
- Fritzsch B, Wake M.. 1988. The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system (Amphibia, Gymnophiona). Zoomorphology 108:201–17. [Google Scholar]
- Fritzsch B, Zakon HH, Sanchez DY.. 1990. Time course of structural changes in regenerating electroreceptors of a weakly electric fish. J Comp Neurol 300:386–404. [DOI] [PubMed] [Google Scholar]
- Glover JC, Elliott KL, Erives A, Chizhikov VV, Fritzsch B.. 2018. Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev Biol published online (doi:10.1016/j.ydbio.2018.02.001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV. 2016. Early development of the spiral ganglion In: Dabdoub A, Fritzsch B, Popper AN, Fay RR, editors. The primary auditory neurons of the mammalian cochlea. New York (NY): Springer; p. 11–48. [Google Scholar]
- Groves AK, Fekete DM.. 2012. Shaping sound in space: the regulation of inner ear patterning. Development 139:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, Zhang KD, Fekete DM.. 2013. The genetics of hair cell development and regeneration. Annu Rev Neurosci 36:361–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellard D, Brosenitsch T, Fritzsch B, Katz DM.. 2004. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice. Dev Biol 275:34–43. [DOI] [PubMed] [Google Scholar]
- Hill JK, Gan L, Wu DK.. 2010. Genetic interaction of Lmx1a and Lmo4 in the mouse inner ear. Dev Biol 344:455. [Google Scholar]
- Jacques BE, Dabdoub A, Kelley MW.. 2012. Fgf signaling regulates development and transdifferentiation of hair cells and supporting cells in the basilar papilla. Hear Res 289:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B.. 2010. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res 341:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Elliott KL, Fritzsch B.. 2015. The quest for restoring hearing: understanding ear development more completely. Bioessays 37:1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B.. 2010. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One 5:e11661.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B.. 2013. Beyond generalized hair cells: molecular cues for hair cell types. Hear Res 297:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B.. 2015. Neurog1 can partially substitute for Atoh1 function in hair cell differentiation and maintenance during organ of Corti development. Development 142:2810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J, D’Angelo A, Gray BD, Soukup GA, Fritzsch B.. 2011. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 49:326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J, Fritzsch B.. 2015. Inner ear hair cells deteriorate in mice engineered to have no or diminished innervation. Front Aging Neurosci 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler KR, Nie J, Longworth-Mills E, Liu X-P, Lee J, Holt JR, Hashino E.. 2017. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol 35:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK.. 2009. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol 333:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Fritzsch B.. 2011. Regeneration of hair cells: making sense of all the noise. Pharmaceuticals 4:848–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B.. 2011. Conditional deletion of N‐Myc disrupts neurosensory and non‐sensory development of the ear. Dev Dyn 240:1373–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky BJ, Jahan I, Fritzsch B.. 2013. Correct timing of proliferation and differentiation is necessary for normal inner ear development and auditory hair cell viability. Dev Dyn 242:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhams A, Pickles J.. 1996. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol 366:335–47. [DOI] [PubMed] [Google Scholar]
- Lassar AB. 2017. Finding MyoD and lessons learned along the way. Semin Cell Dev Biol 72:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Hackelberg S, Green KL, Lunghamer KG, Kurioka T, Loomis BR, Swiderski DL, Duncan RK, Raphael Y.. 2017. Survival of human embryonic stem cells implanted in the guinea pig auditory epithelium. Sci Rep 7:46058.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS.. 1985. The vertebrate inner ear. Boca Raton (FL): CRC Press, Inc. [Google Scholar]
- Liu H, Pecka JL, Zhang Q, Soukup GA, Beisel KW, He DZ.. 2014. Characterization of transcriptomes of cochlear inner and outer hair cells. J Neurosci 34:11085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-P, Koehler KR, Mikosz AM, Hashino E, Holt JR.. 2016. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat Commun 7:11508.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z-X, Ruf I, Schultz JA, Martin T.. 2011. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc R Soc Lond B Biol Sci 278:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. 2012. Evolutionary paths to mammalian cochleae. J Assoc Res Otolaryngol 13:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. 2017a. The cochlea: what it is, where it came from, and what is special about it In: Manley GA, Gummer AW, Popper AN, Fay RR, editors. Understanding the cochlea. New York (NY): Springer; p. 17–32. [Google Scholar]
- Manley GA. 2017. Comparative auditory neuroscience: understanding the evolution and function of ears. J Assoc Res Otolaryngol 18:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann ZF, Gálvez H, Pedreno D, Chen Z, Chrysostomou E, Żak M, Kang M, Canden E, Daudet N.. 2017. Shaping of inner ear sensory organs through antagonistic interactions between Notch signalling and Lmx1a. eLife 6:pii: e33323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U.. 2005. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 132:2377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Reiprich S, Wegner M, Fritzsch B.. 2014. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS One 9:e94580.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B.. 2005. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234:633–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. 2015. Models for patterning primary embryonic body axes: the role of space and time. Semin Cell Dev Biol 42:103–17. [DOI] [PubMed] [Google Scholar]
- Mills DM, Shepherd RK.. 2001. Distortion product otoacoustic emission and auditory brainstem responses in the echidna (Tachyglossus aculeatus). J Assoc Res Otolaryngol 2:130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V, Fekete DM.. 2016. Notch–Wnt–Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 143:4003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Jahan I, Bonde G, Sun X, Hildebrand MS, Engelhardt JF, Smith RJ, Cornell RA, Fritzsch B, Bánfi B.. 2012. A mutation in the Srrm4 gene causes alternative splicing defects and deafness in the Bronx waltzer mouse. PLoS Genet 8:e1002966.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubüser A, Peters H, Balling R, Martin GR.. 1997. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90:247–55. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B.. 2008. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res 334:339–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Noda T, Dabdoub A.. 2017. Dynamic expression of Sox2, Gata3, and Prox1 during primary auditory neuron development in the mammalian cochlea. PLoS One 12:e0170568.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK.. 2010. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci 30:15044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoruwa OE, Weston MD, Sanjeevi DC, Millemon AR, Fritzsch B, Hallworth R, Beisel KW.. 2008. Evolutionary insights into the unique electromotility motor of mammalian outer hair cells. Evol Dev 10:300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru K, Kuraku S.. 2018. Inference of the ancestral vertebrate phenotype through vestiges of the whole-genome duplications. Brief Funct Genomics published online (doi:10.1093/bfgp/ely008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B.. 2012. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One 7:e30358.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B.. 2011. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res 275:66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE.. 2010. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A 107:15798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B.. 2006. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn 235:2470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH.. 2011. Evolution of gene regulatory networks controlling body plan development. Cell 144:970–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW.. 2007. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn 236:1905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach T, Hudspeth AJ.. 2014. The physics of hearing: fluid mechanics and the active process of the inner ear. Rep Prog Phys 77:076601. [DOI] [PubMed] [Google Scholar]
- Richter C-P, Emadi G, Getnick G, Quesnel A, Dallos P.. 2007. Tectorial membrane stiffness gradients. Biophys J 93:2265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T. 2013. Transcription factors that control inner ear development and their potential for transdifferentiation and reprogramming. Hear Res 297:84–90. [DOI] [PubMed] [Google Scholar]
- Schultz JA, Zeller U, Luo ZX.. 2017. Inner ear labyrinth anatomy of monotremes and implications for mammalian inner ear evolution. J Morphol 278:236–63. [DOI] [PubMed] [Google Scholar]
- Sha S-H, Taylor R, Forge A, Schacht J.. 2001. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res 155:1–8. [DOI] [PubMed] [Google Scholar]
- Shera CA. 2015. The spiral staircase: tonotopic microstructure and cochlear tuning. J Neurosci 35:4683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge AS.. 2014. β-Catenin is required for hair-cell differentiation in the cochlea. J Neurosci 34:6470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienknecht UJ, Köppl C, Fritzsch B.. 2014. Evolution and development of hair cell polarity and efferent function in the inner ear. Brain Behav Evol 83:150–61. [DOI] [PubMed] [Google Scholar]
- Simmons D, Duncan J, de Caprona DC, Fritzsch B.. 2011. Development of the inner ear efferent system In: Ryugo D, Popper AN, Fay RR, editors. Auditory and vestibular efferents. New York (NY): Springer; p. 187–216. [Google Scholar]
- Steffes G, Lorente-Cánovas B, Pearson S, Brooker RH, Spiden S, Kiernan AE, Guénet J-L, Steel KP.. 2012. Mutanlallemand (mtl) and belly spot and deafness (bsd) are two new mutations of Lmx1a causing severe cochlear and vestibular defects. PLoS One 7:e51065.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S.. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–76. [DOI] [PubMed] [Google Scholar]
- Tan X, Jahan I, Xu Y, Stock S, Kwan CC, Soriano C, Xiao X, García-Añoveros J, Fritzsch B, Richter C-P.. 2018. Auditory neural activity in congenitally deaf mice induced by infrared neural stimulation. Sci Rep 8:388.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Pecka JL, Fritzsch B, Beisel KW, He DZ.. 2013. Lizard and frog prestin: evolutionary insight into functional changes. PLoS One 8:e54388.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MV. 2017. Skeletal muscle development on the 30th Anniversary of MyoD. Semin Cell Dev Biol 72:1–2. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, Forge A.. 2012. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One 7:e30577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B.. 2004. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci 24:2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede BR, Mann ZF, Chang W, Ku Y-C, Son YK, Lovett M, Kelley MW, Corwin JT.. 2014. Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat Commun 5:3840.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Sakaguchi H, Nakamura T, Goto A, Morioka S, Shimizu A, Nakao K, Hishikawa Y, Ninoyu Y, Kassai H, et al. 2014. Maintenance of stereocilia and apical junctional complexes by Cdc42 in cochlear hair cells. J Cell Sci 127:2040–52. [DOI] [PubMed] [Google Scholar]
- Urness LD, Wang X, Shibata S, Ohyama T, Mansour SL.. 2015. Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev Biol 400:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater M, Kössl M.. 2011. Comparative aspects of cochlear functional organization in mammals. Hear Res 273:89–99. [DOI] [PubMed] [Google Scholar]
- Von Bartheld CS, Patterson SL, Heuer JG, Wheeler EF, Bothwell M, Rubel EW.. 1991. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development 113:455–70. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J.. 2017. In vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep 19:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke A, Mellott AJ, Römer A, Lenarz T, Staecker H.. 2017. Advances in translational inner ear stem cell research. Hear Res 353:76–86. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N.. 2006. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441:984.. [DOI] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B.. 2011. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res 278:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Wu S, Fritzsch B, Bassuk AG.. 2017. Prickle1 regulates neurite outgrowth of apical spiral ganglion neurons but not hair cell polarity in the murine cochlea. PLoS One 12:e0183773.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xu J, Maire P, Xu P-X.. 2017. Six1 is essential for differentiation and patterning of the mammalian auditory sensory epithelium. PLoS Genet 13:e1006967.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, Löwenheim H, Fritzsch B.. 2014. Toward translating molecular ear development to generate hair cells from stem cells In: Turksen K, editor. Adult stem cells. New York (NY): Humana Press; p. 111–61. [Google Scholar]