Abstract
During rapid locomotion, the vestibular inner ear provides head-motion signals that stabilize posture, gaze, and heading. Afferent nerve fibers from central and peripheral zones of vestibular sensory epithelia use temporal and rate encoding, respectively, to emphasize different aspects of head motion: central afferents adapt faster to sustained head position and favor higher stimulus frequencies, reflecting specializations at each stage from motion of the accessory structure to spike propagation to the brain. One specialization in amniotes is an unusual nonquantal synaptic mechanism by which type I hair cells transmit to large calyceal terminals of afferent neurons. The reduced synaptic delay of this mechanism may have evolved to serve reliable and fast input to reflex pathways that ensure stable locomotion on land.
The vestibular labyrinth of the vertebrate inner ear is an ensemble of sensory organs, each including an accessory structure that moves in response to a particular dimension of head motion (rotational or linear in a particular plane (Carriot et al. 2017b)), receptor cells that detect that motion, and afferent neurons that convey the signals to the brain. The receptor cells are called hair cells for their apical bundles of mechanosensitive microvilli (Fig. 1). Motion of the overlying accessory structure deflects the hair bundle, initiating electrical signals that propagate centrally to drive reflexes controlling eye, neck, and body position, and to contribute to a sense of orientation and heading. The ability to detect and compensate for our head motions is essential to a normal, mobile life, as poignantly revealed when vestibular inner ear function is lost through disease, trauma, or aging (Agrawal et al. 2013). Recent research has uncovered specializations of vestibular hair cells, afferent neurons, and their synaptic contacts that may enhance stability during rapid head motions.
Fig. 1.
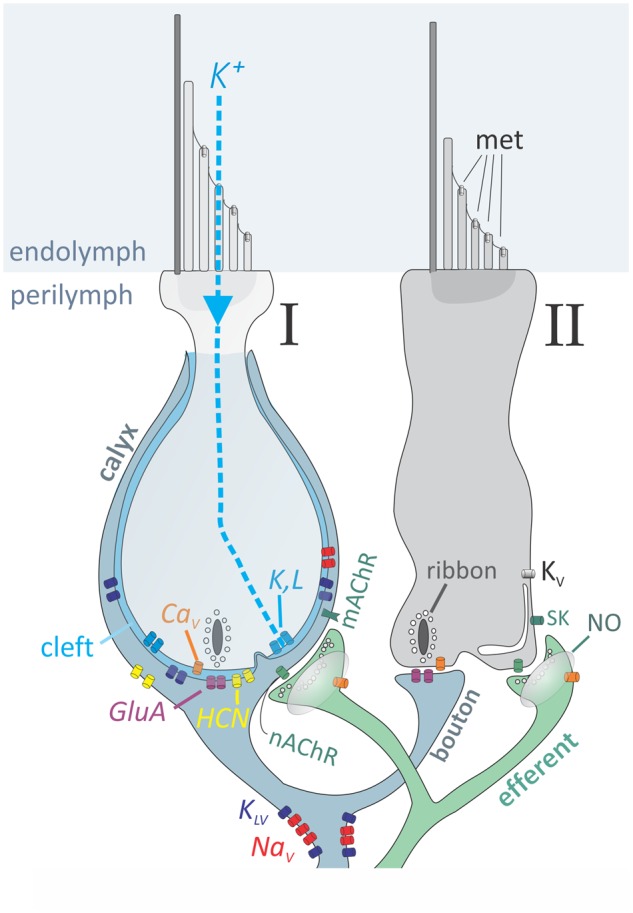
Elements in mechanoelectrical transduction and synaptic transmission cascades in amniote vestibular hair cells. Schematic cross-section through a type I hair cell and a type II hair cell, innervated by a dimorphic afferent (blue-grey) that forms a calyx around the basolateral membrane of the type I cell and a bouton on the type II cell. An efferent fiber (green) originating in the brainstem contacts the type II cell and the calyx. During transduction, K+, which is enriched in endolymph bathing the hair bundles, enters met channels in the hair bundle. In type II cells, the depolarizing current produces large depolarizations (receptor potentials) that open CaV channels (orange) and cause Ca2+-mediated quantal transmission: exocytosis of glutamate from vesicles (quanta) arrayed around synaptic ribbons. Glutamate opens postsynaptic GluA channels (purple) to produce epscs, which make epsps that trigger spikes at spike initiation zones: aggregates of NaV (red) channels. In type I cells, K+ exits the hair cell via open K,L channels, raising [K+] in the synaptic cleft and depolarizing the pre-synaptic hair cell membrane. The elevated cleft K+ causes non-quantal transmission, which requires HCN channels (yellow) and involves K+ efflux from KLV channels (dark blue) in the afferent postsynaptic membrane. KLV channels are also located next to NaV channels at spike initiation zones and influence the regularity of spike intervals. Efferents (green) release multiple neurotransmitters, including acetylcholine onto nicotinic (nAChR) and muscarinic (mAChR) receptors, and possibly nitric oxide (NO), which can permeate membranes to act intracellularly on nearby cells.
To compute static head position and low-frequency head motions, the brain has access to information from the vestibular inner ear, neck proprioceptors, and retinal photoreceptors. Because proprioceptors and photoreceptors are relatively slow to respond, signals from these sensors are useful only for slow (low-frequency) head motions—in the squirrel monkey, the lag in visual following of a moving stimulus increases steeply above ∼1 Hz (Paige 1983). Only vestibular hair cells are fast enough to drive compensatory reflexes at the higher frequencies present in normal head motions (up to tens of Hertz; Carriot et al. 2017a). Acute damage to the vestibular inner ear can lead to a bouncing visual field (oscillopsia) as the fast, compensatory eye motions needed during normal locomotion are lost; imagine the difference between the movie captured by a camera mounted on your head and the smoothly changing visual world that you experience.
The horizontal angular vestibulo-ocular reflex (VOR), which stabilizes gaze by counter-rotating the eyes during a horizontal head rotation (yaw), is the fastest known reflex: the latency is as short as ∼5 ms in the rhesus monkey (Huterer and Cullen 2002). Furthermore, the horizontal angular VOR maintains gain near 1 and phase near 0 for steady sinusoidal head motions up to at least 25 Hz (Huterer and Cullen 2002). This near-perfect performance exceeds that predicted by the fixed minimal latency of the reflex. Sensory mechanisms that advance the phase (timing) of vestibular signals as they flow through the afferent pathway are the topic of this review.
The frequency content of head motions
In non-mammals, it has long been known that the otolith organs, which respond to linear accelerations, serve as vibration detectors (e.g., saccule and lagena of frogs and reptiles) or sound detectors (saccule of fish and frogs), with frequency ranges extending to 100 s of Hz. Until recently, however, studies of mammalian vestibular function focused on stimulus frequencies <10 Hz, possibly reflecting both technical limitations and scientific expectations. It is technically challenging to stimulate whole animals and record with stability at high frequencies. There may also have been an assumption that the large heads of humans and other primates filter out mechanical energy at frequencies much above the dominant walking frequency (2–3 Hz) (Grossman et al. 1988). In any case, for a long time both clinical and animal tests of vestibular function challenged only low-frequency performance, where there can be redundancy with other modalities (vision and proprioception).
Since 1990, however, attention has turned toward vestibular function at head motion frequencies exceeding 10 Hz, either applied or as experienced in spontaneous complex head motions during daily life. For humans, macaques, and mice, accelerometer readings collected during daily activities reveal that 5–25% of head motion power is at frequencies >7 Hz, with the percentage varying across species and motion dimension (Carriot et al. 2017a). In experiments applying sinusoidal head motions to rodents and primates, the upper stimulus frequencies have increased to 10–30 Hz (Hullar et al. 2005; Lasker et al. 2008; Sadeghi et al. 2009a; Jamali et al. 2013). Curthoys et al. (2006) have described the sensitivity of guinea pig vestibular afferents to bone-conducted vibrations at 100 s of Hz. Vestibular function in mutant mice is now routinely screened with standardized scalp recordings of inner ear potentials evoked by linear acceleration transients (jerk) (Jones and Jones 1999, 2014). In recognition of the need to test high frequency vestibular function, clinical test stimuli now include abrupt angular head motions (Halmagyi et al. 2008) and bone-conducted vibrations and sounds (McCue and Guinan 1994; Curthoys 2017).
Rapid head motions are represented in the central zones of mammalian vestibular sensory epithelia
The vestibular epithelia of rodents have central and peripheral zones with distinct physiological, molecular, genetic, and anatomical markers, affecting each layer of processing from accessory structures to afferent terminals (reviewed in Goldberg 1991; Peterson 1998; Eatock and Songer 2011). In the epithelia (maculae) of otolith organs, the central and peripheral zones are called the striola and extrastriola, respectively. Vestibular afferents that innervate central zones of semicircular canal cristae and striolar zones of saccular or utricular maculae have highly irregular spike timing and adapting responses to head motion stimuli. In sharp contrast, afferents to the peripheral and extrastriolar zones have highly regular spike timing and less adapting (more tonic) responses. These adaptation properties mean that the central zones emphasize rapid head motions and the peripheral zones slower head motions. The greater adaptation of central and striolar afferents to sustained (step) stimuli manifests as high-pass filtering for sinusoidal stimuli: the response at low frequencies leads the stimulus and the response gain (output/input) increases as the stimulus frequency increases. These relationships were established for head motion frequencies below several Hertz in the vestibular epithelia of a rodent, the chinchilla, in landmark papers by Goldberg, Fernández and colleagues (Baird et al. 1988; Goldberg et al. 1990).
The irregular, adapting afferents of the central and striolar zones are believed to be largely responsible for the detection of high-frequency stimuli, including bone-conducted vibrations (reviewed in Curthoys et al. 2017), sounds of moderate intensity in the 500–1000 Hz frequency range (McCue and Guinan 1994), and the jerk stimulus (transient linear acceleration) used to evoke the short-latency linear vestibular sensory evoked potential (VsEP) in mice and chicks (Jones et al. 2016).
The dichotomy in spike timing regularity is a striking feature of the vestibular afferent nerve, especially in mammals. It is interesting both in terms of underlying mechanisms and because of its strong correlation with salient response properties (reviewed in Goldberg 2000). Cullen, Chacron and colleagues have been investigating how the different spike patterns encode information and have suggested that the regular and irregular discharge patterns are well suited for rate coding and precise temporal coding, respectively (Jamali et al. 2016).
When similar physiological distinctions are seen in central vestibular neurons (Beraneck and Straka 2011; Rossert and Straka 2011), it is not clear whether they are conferred by afferent inputs or arise de novo because we often have limited comparative information on the central projections of irregular and regular afferents (see commentary by Peterson 1998). In mouse otolith organs, neurons from particular zones project in a strikingly segregated fashion to either the vestibular nuclei or to the cerebellum (Maklad et al. 2010), but it is not clear how the zones relate to striolar and extrastriolar zones—they may instead reflect an abrupt transition in hair bundle orientation, the “line of polarity reversal,” which runs parallel to the striolar–extrastriolar border. Explicit comparison of central projections of irregular and regular central projections has been done in two ways. By combining physiological characterization with anatomical reconstruction of individual afferents, Sato and Sasaki (1993) showed that each neuron projects widely throughout the vestibular brainstem nuclei but tends to have different anatomical targets depending on its spike timing, with irregular afferents contacting larger neurons closer to their cell bodies (Sato et al. 1988, 1989; Sato and Sasaki 1993), consistent with their larger diameters and conduction velocities. Highstein, Goldberg and colleagues used an electrophysiological method, relying on differential sensitivity of irregular and regular afferents to electrical shocks delivered to the inner ear (Goldberg et al. 1987; Highstein et al. 1987; Boyle et al. 1992). The results are consistent with some segregation of irregular and regular inputs at the level of secondary vestibular neurons, and also substantial convergence, with 50–60% of secondary neurons receiving significant (>10%) input from both kinds of afferent.
Although it is not clear whether parallel pathways persist centrally, peripherally the transient (or high-frequency) and sustained (or low-frequency) components of head motions (Curthoys et al. 2017) are represented on distinct epithelial zones. Such maps are a common organizing principle in sensory epithelia. What are the specialized properties of each zone that contribute to this analysis of head motions? In otolith organs, macromechanical specializations of the otolithic structure and hair bundle enhance adaptation and high-pass filtering of the hair bundle motion in the striola. Long extrastriolar hair bundles insert into the gel layer that supports the calcareous crystals, such that a step change in linear acceleration evokes a step displacement of the otolith and of the hair bundles’ well-coupled apical tips. In the striola, hair bundles are less firmly coupled to a more porous gel layer and more subject to fluid motion; they are modeled as being particularly sensitive to fluid forcing (Nam et al. 2005). There are also striking zonal differences in hair cells and afferent contacts, which are related in a complex way to the epithelial distribution of the types of hair cells and afferents recognized in the literature.
Two classes of hair cell and afferent terminal
A striking feature of the vestibular epithelia of amniotes is the calyceal synaptic contacts between certain hair cells and the distal terminals of primary afferent neurons. Such postsynaptic calyces are not reported anywhere else. In addition to afferent calyces, amniote vestibular epithelia have compact “bouton” afferent contacts that are common to all vertebrate hair cell epithelia. Each calyx enwraps one or more type I hair cells and each bouton terminal contacts a type II hair cell, opposite a presynaptic ribbon with associated synaptic vesicles (Fig. 1). While the form of the postsynaptic afferent contact is the defining difference between type I and II hair cells, there are clear differences between the hair cells themselves in morphology (hair bundle and cell shape) and expression of Ca2+-binding proteins, voltage-dependent ion channels, and transcription factors (Sox2).
How do the hair cell/synapse categories relate to the epithelial zones? In the utricular epithelia of reptiles (Huwe et al. 2015; Jorgensen 1974) and birds (Si et al. 2003), the relationship is simple: type I hair cells and their calyceal endings are specific to the striolar zone; type II hair cells and their bouton contacts are found in both zones. In the lagena and saccule of birds, the presence of type I hair cells and calyces has been used to define the striola, producing a zone that is a much broader swath of the epithelium than the striola of avian utricle. It is not clear how these zones compare to the mammalian striola, which can be identified by a large number of co-varying properties, including accessory structures, Ca2+-binding proteins, afferent physiology (reviewed in Eatock and Songer 2011) and gene expression (e.g., Burns et al. 2015; Jiang et al. 2017).
In mammals, both types of hair cells and afferent terminals occur throughout the epithelium, although in varying percentages across species, and most afferents (60–80%) form both kinds of synaptic contact. In the chinchilla cristae and utricle, many regular, tonic afferents and many irregular, adapting afferents get input from both type I and type II hair cells (Baird et al. 1988; Goldberg et al. 1990). This unexpected finding complicated the quest to understand the role(s) of the unique vestibular calyx. Furthermore, it is becoming clear that epithelial zone (Burns et al. 2015), hair cell type (Oesterle et al. 2008), and hair bundle orientation (Jiang et al. 2017) are regulated by distinct genes.
Nevertheless, even in mammals there are correlations between hair cell/synapse categories and vestibular epithelial zones, although they are subtler than those in avian and reptilian utricles. Afferents of central/striolar zones form either calyces only or both calyces and boutons, such that all irregular afferents get input from type I hair cells. Afferents from peripheral/extrastriolar zones form either boutons only or both calyces and boutons, such that all regular afferents contact type II hair cells. Afferents that form both calyces and bouton terminals—i.e., innervate both type I and type II hair cells, as illustrated schematically in Fig. 1—have been called “dimorphic,” but the single category is misleading given significant zonal differences in their morphology and physiology. Compared to peripheral-zone afferents, both calyceal and dimorphic central-zone afferents tend to have more variable spike timing, slightly thicker axonal diameters and consequently faster impulse conduction (Goldberg and Fernández 1977), and more compact terminal arbors that innervate smaller territories (Fernández et al. 1988, 1990). The spike initiation zones of central-zone afferents are found closer to the calyces, sometimes even within the sensory epithelium (Lysakowski et al. 2011). These specializations may reduce the time to initiate spikes and the time to propagate spikes to the brain. In contrast, the extended and finer arbors of peripheral-zone, regularly discharging, afferents may enhance spatiotemporal integration of postsynaptic potentials, which travel electrotonically from distributed synapses to a more distant spike initiation zone located below the epithelium (Lysakowski et al. 2011).
Thus, the accessory structures and afferent innervation to the central/striolar zones seem to favor adaptation and high-pass filtering—that is, emphasizing transient rather than sustained stimuli (Curthoys et al. 2017). The irregular afferents of mammals all contact type I hair cells with calyceal endings—and in birds and reptiles, calyces are thought to be restricted to central/striolar zones. Consistent with this association, type I hair cells and calyces have specialized ion channels and synaptic transmission that favor the speed of signal transfer, as discussed in the next section.
Non-quantal transmission at the type I-calyx synapse
In hair cells, positive deflection of the apical hair bundle leads to spiking (action potentials) in primary afferent neurons by a cascade involving mechanosensitive channels in the hair cell, voltage-gated channels in the hair cell and neuron, and quantal transmission: the release of excitatory neurotransmitter from presynaptic vesicles (quanta) onto afferent synaptic contacts (reviewed in Fuchs and Parsons 2006). Excitatory bundle deflection opens mechanoelectrical transduction (met) channels at the tips of individual bundle microvilli (stereocilia), increasing the influx of cations (Imet). Because the bundle is bathed in a high-K+ saline (endolymph), Imet is carried mostly by K+ ions (Fig. 1). The ensuing depolarization of the hair cell membrane activates potassium (KV) channels (which may be gated by voltage and/or Ca2+) and voltage-gated calcium (CaV) channels and the resulting K+ and Ca2+ currents combine with Imet to shape the receptor potential. The most numerous channels pass outward K+ current (reviewed in Meredith and Rennie 2016), which repolarizes the hair cell membrane. Ca2+ current through CaV channels mediates release of glutamate from synaptic vesicles arrayed around presynaptic electron-dense structures called ribbons. Ca2+-mediated fusion of a synaptic vesicle with the hair cell membrane releases a bolus (“quantum”) of glutamate into a narrow synaptic cleft; glutamate diffuses across the cleft to bind and open fast glutamate receptor-channels (GluA receptors) in the postsynaptic membrane. Na+ ions enter open GluA receptor channels to produce an excitatory postsynaptic current (epsc) in the postsynaptic terminal, in turn evoking an excitatory postsynaptic potential (epsp) which propagates electrotonically to the spike initiation zone and activates concentrated voltage-gated sodium (NaV) and KV channels to produce a spike. The larger the hair cell receptor potential, the higher the rates of released vesicles and subsequent spiking in the afferent neuron (rate encoding). The timing of spikes can also reveal precise information about temporal features of the stimulus (temporal encoding).
When type I hair cells and calyces were first described (Wersäll 1956), the quantal theory of neurotransmission (Del Castillo and Katz 1954) was new. The tight calyceal enwrapping of the hair cell’s basolateral surface stimulated speculation that the calyx serves more direct transmission, possibly electrical in nature. There is now, however, abundant morphological and electrophysiological evidence for quantal transmission. Serial reconstruction of the chinchilla crista revealed that type I hair cells have on average 15–20 presynaptic ribbons and many associated clear vesicles (Lysakowski and Goldberg 1997). Epscs and epsps, the electrical signatures of quantal transmission, have been recorded from calyces in excised preparations of rodents and turtles (Rennie and Streeter 2006; Dulon et al. 2009; Songer and Eatock 2013; Sadeghi et al. 2014; Contini et al. 2016) and are blocked by GluA receptor blockers. For spontaneous activity in semi-intact preparations, quantal events recorded in the calyx may reflect transmission from type II hair cells connecting to the same afferent terminal. In other cases, when hair cells and calyces are isolated (Rennie and Streeter 2006), or when epscs are driven by motion of a visually identified hair bundle (Songer and Eatock 2013) or by voltage clamping the presynaptic type I hair cell (Contini et al. 2017), we can be confident that epscs originated in quantal transmission from a type I hair cell.
In parallel with these convincing demonstrations of quantal transmission, intracellular recordings from type I-innervating afferents provided evidence for unconventional, non-quantal transmission. In afferents innervating type I hair cells in chick crista (Yamashita and Ohmori 1990) and turtle crista (Holt et al. 2007), sinusoidal fluid motions evoked graded potentials that did not resemble the distinctive α-shaped epsps but appeared more similar to hair cell receptor potentials. Yamashita and Ohmori (1991) initially proposed that the graded calyceal responses arose via electrical transmission through gap junctions with hair cells, but electron microscopic examination revealed no gap junctional contacts. At multiple sites, the calyx forms a shallow “invagination” into the hair cell where the membranes are relatively closely apposed (Lysakowski and Goldberg 1997) but free of gap junctional particles (Gulley and Bagger-Sjöbäck 1979). Moreover, dyes injected into either the hair cell or the afferent have never been reported to transfer across the synapse, as would be expected if there were substantial gap junctional contacts (Yamashita and Ohmori 1991; Songer and Eatock 2013; Highstein et al. 2014; Contini et al. 2017).
Instead, non-quantal transmission from type I hair cells onto the calyx involves ion channels in the pre- and post-synaptic membranes facing the synaptic cleft and K+ accumulation in the cleft itself (Fig. 1). Type I hair cells acquire low-voltage-gated ion channels in large numbers as they mature, in parallel with but independently of the expansion of calyceal contacts (Rüsch et al. 1998; Géléoc et al. 2004; Hurley et al. 2006), giving them a very different electrophysiological profile from type II hair cells. These channels (collectively “gK,L” for low-voltage-activated K+ conductance) are significantly activated at resting potential, such that type I cells have low input resistances (Correia and Lang 1990; Rennie et al. 1996; Rüsch and Eatock 1996). K+ efflux through gK,L can raise the K+ concentration in the synaptic cleft, feeding back negatively on hair cell’s K+ current by reducing the driving force (Wong et al. 2004; Lim et al. 2011; Contini et al. 2012). In the early postnatal period, postsynaptic afferents also acquire low-voltage-activated K+ (KLV) channels; these are in the KV1 and KV7 channel families (Hurley et al. 2006; Iwasaki et al. 2008; Kalluri et al. 2010). Immunolocalization shows intense concentrations of these channels in the “inner-face” calyceal membrane (facing the cleft), and at the nearby hemi-node where spikes initiate (Lysakowski et al. 2011). Postsynaptic calyceal terminals also bear HCN (hyperpolarization-activated cyclic-nucleotide-gated) channels, which are permeable to both K+ and Na+ and activate at voltages negative to –60 mV in the presence of intracellular cAMP (Horwitz et al. 2011; Meredith et al. 2012). Contini et al. (2017) could block non-quantal transmission by replacing hair cell K+ with other cations or by blocking HCN channels, implicating both K+ efflux and HCN channels in the non-quantal mechanism. The rise time of the transmitted current matches the expected time course of K+ accumulation in the cleft (Contini et al. 2017; Chen 1995).
Without having all the details to allow an accurate model, we can partly visualize the flow of ions during non-quantal transmission. Multiple lines of evidence show that in the intact inner ear, Imet enters the hair cell’s apical met channels principally as K+ and drives K+ efflux through open basolateral gK,L channels into the thin synaptic cleft, increasing K+ concentration in the small volume of cleft saline. This diminishes trans-membrane EK across both hair cell and calyx inner-face membranes, a depolarizing influence that will be especially strong in the hair cell membrane, which is dominated by gK,L and enwrapped along nearly its entire basolateral extent by the calyx. The calyx also depolarizes passively in response to the gradient change across the calyceal inner-face membrane, but the impact is tempered by a normal K+ gradient across the outer-face calyx membrane and afferent dendrite. In the resting inner-face membrane of the calyx, open KLV channels (Songer and Eatock 2013) reduce input resistance. These open more with depolarization and provide another path for K+ accumulation in the synaptic cleft (Contini et al. 2017). Afferent KLV channels also increase the variability (irregularity) of firing (Iwasaki et al. 2008; Kalluri et al. 2010) by hyperpolarizing the nerve fiber membrane and reducing intrinsic excitability, such that spike timing reflects the noisy arrival of synaptic quanta (Smith and Goldberg 1986). In addition to K+-selective channels, there are HCN and NaV channels for Na+ ions into the calyx. In immature mouse vestibular afferent neurons, HCN channels are active at resting potential, as evident from their impact on spontaneous spiking (Horwitz et al. 2014), and close with depolarization (Horwitz et al. 2011). The channel subunits have been immunolocalized to afferent neuronal cell bodies but calyceal localization is not reported; in Fig. 1, they are depicted on both faces. Immunocytochemistry places NaV channels in strength at hemi-nodes, as expected, and, more curiously, in the calyx inner-face membrane (Wooltorton et al. 2007; Lysakowski et al. 2011). Finally, protons have been proposed to carry the non-quantal current (Highstein et al. 2014) although it is possible that the effects of manipulating pH on transmission are indirect (Contini et al. 2017). Thus, the non-quantal voltage response of the calyx membrane depends on the K,L channels in hair cells and HCN channels in afferents, while the roles of diverse other channels remain unclear.
Why non-quantal transmission?
Lysakowski and Goldberg (2008) found that available comparisons across extant amniotes and anamniotes of different vestibular lifestyles did not point to a particular evolutionary rationale for the type I-calyx synapse. In the intervening decade, significant information has emerged on the special nature of transmission, the encoding potential of calyx-bearing afferents, and the natural vestibular environment. Armed with these new insights, future comparative analyses combined with experimental manipulations may reveal why amniotes have type I hair cells and calyceal endings. Two questions to address are (1) how non-quantal and quantal transmission work together to serve vestibular function, and (2) what new constraints and pressures accompanied the move from water to land.
How do non-quantal and quantal transmission work together in vestibular signaling?
In addition to the mixing of quantal and non-quantal transmission at individual type I-calyx contacts, many calyx-bearing afferents receive quantal transmission via type II inputs. Even in “calyx-only” afferents of the central/striolar zones, the outer faces of calyces are postsynaptic to ribbon synapses of adjacent type II hair cells (Lysakowski and Goldberg 1997). Most head motions should lead to widespread co-activation of hair cells, especially neighboring hair cells which have similar hair bundle orientations and similar coupling to overlying accessory structures. Indeed, spikes in calyx-bearing afferents arise from the combined actions of quantal and non-quantal transmission, as seen in intracellular recordings from turtle canal afferents (Holt et al. 2007). Contini et al. (2017) note that, given the large number of K channels in the hair cell membrane, extracellular K+ accumulation may be needed to drive the type I hair cell’s membrane potential into the activation range of CaV channels that mediate quantal transmitter release.
Non-quantal transmission, which is fast, seems an appropriate adaptation for the calyceal synapses of central and striolar zones, which have other adaptations to favor speed and high frequency signaling. Mammalian vestibular epithelia, however, have numerous extrastriolar and peripheral-zone calyces that provide input to dimorphic, regularly firing afferents with relatively tonic response properties. Preliminary observations (O. López Ramírez, A. González-Garrido, R. A. Eatock) show non-quantal transmission in extrastriolar calyces of the mouse utricle; thus, while all central/striolar afferents are likely to receive non-quantal transmission, so do many peripheral/extrastriolar afferents.
Sensory history may favor one or the other transmission mode. Quantal transmission at hair cell synapses adapts during steady-state sinusoidal stimulation following depletion of readily releasable vesicle pools (e.g., Furukawa and Matsuura 1978; Schnee et al. 2011). In contrast, non-quantal transmission does not obviously adapt to such stimuli (Songer and Eatock 2013) and may therefore come to dominate during sustained stimulation. Afferent signals activate vestibular efferent neurons (Plotnik et al. 2002; Sadeghi et al. 2009b) which synapse on type II hair cells and afferent terminals, including calyces (Fig. 1). The efferent terminals release acetylcholine (Jordan et al. 2015) and possibly nitric oxide (NO) (Flores et al. 1996; Singer and Lysakowski 1996), both of which have been shown in vitro to turn off low-voltage-activated K channels in type I hair cells and calyces. Acetylcholine acts through muscarinic receptors to turn off KV7 channels in vestibular afferents (Holt et al. 2017; Pérez et al. 2010). NO, which passes through membranes, can turn off gK,L (Chen and Eatock 2000). Turning off gK,L would convert the type I-calyx synapse into a more conventional chemical synapse in two ways: by reducing or eliminating K+ efflux from the hair cell into the cleft, and by increasing the type I hair cell’s input resistance and consequently its receptor potential and voltage-driven quantal transmission. By pushing transmission toward one or the other mode, synaptic adaptation and efferent input could regulate the gain and time course of afferent transmission.
The move from water to land
We can speculate that the type I-calyx synapse evolved in stem amniotes of the Carboniferous period, possibly as an adaptation to terrestrial locomotion. Migration from water to land drove the emergence of middle ear and inner ear structures adapted for airborne sound (reviewed in Manley 2017). The stimulus environment also changed for the vestibular apparatus, in part reflecting dramatic changes in locomotion: elongation of limbs and necks facilitated independence of head and trunk motion, and on land there is the danger of falling. Such differences may have driven the evolution of a synapse to support fast reflexes in response to abrupt head motions. Even in a single afferent, quantal and non-quantal responses to type I hair bundle motions differ: the non-quantal response is faster and more broadly tuned, extending to higher frequencies (Songer and Eatock 2013). Factors in these differences include the pre- and post-synaptic expression of channels (low-voltage-activated K+ and HCN) that reduce membrane charging times and, by allowing non-quantal transmission, eliminate the synaptic delay inherent in quantal transmission (Songer and Eatock 2013). McCue and Guinan (1994) obtained in vivo support for remarkably fast transmission at type I-calyceal synapses by taking advantage of the acoustic sensitivity of saccular irregular (striolar) afferents. Saccular afferents had even shorter latencies to acoustic clicks than did cochlear afferents (0.7 ms vs. 1 ms), with the difference attributable in part to the calyceal synapse. As for gap-junction transmission in startle reflexes (Korn and Faber 2005; Bierbower and Cooper 2013), the primary impact of non-quantal transmission at the type I-calyx synapse may be to shave critical fractions of a millisecond from reflexes that drive fast compensatory actions.
Acknowledgments
Related research in the author’s laboratory is supported by the National Institute on Deafness and Other Communication Disorders.
Funding
Related research in the author's laboratory has been supported by grants R01 DC002290 and R01 DC012347 from the National Institute on Deafness and Other Communication Disorders.
References
- Agrawal Y, Ward BK, Minor LB.. 2013. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res 23:113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM.. 1988. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60:182–203. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Straka H.. 2011. Vestibular signal processing by separate sets of neuronal filters. J Vestib Res 21:5–19. [DOI] [PubMed] [Google Scholar]
- Bierbower SM, Cooper RL.. 2013. The mechanistic action of carbon dioxide on a neural circuit and NMJ communication. J Exp Zool 319:340–54. [DOI] [PubMed] [Google Scholar]
- Boyle R, Goldberg JM, Highstein SM.. 1992. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in squirrel monkey vestibular nuclei. III. Correlation with vestibulospinal and vestibuloocular output pathways. J Neurophysiol 68:471–84. [DOI] [PubMed] [Google Scholar]
- Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW.. 2015. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun 6:8557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriot J, Jamali M, Chacron MJ, Cullen KE.. 2017a. The statistics of the vestibular input experienced during natural self-motion differ between rodents and primates. J Physiol 595:2751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriot J, Jamali M, Cullen KE, Chacron MJ.. 2017b. Envelope statistics of self-motion signals experienced by human subjects during everyday activities: implications for vestibular processing. PLoS One 12:e0178664.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JWY, Eatock RA.. 2000. Major potassium conductance in type I hair cells from rat semicircular canals: characterization and modulation by nitric oxide. J Neurophysiol 84:139–51. [DOI] [PubMed] [Google Scholar]
- Chen JWY. 1995. The properties and functions of a low-voltage activated K current in type I hair cells of rat semicircular canal organs [PhD thesis]: University of Rochester, Rochester NY.
- Contini D, Price SD, Art JJ.. 2017. Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. J Physiol 595:777–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D, Zampini V, Tavazzani E, Magistretti J, Russo G, Prigioni I, Masetto S.. 2012. Intercellular K(+) accumulation depolarizes Type I vestibular hair cells and their associated afferent nerve calyx. Neuroscience 227:232–46. [DOI] [PubMed] [Google Scholar]
- Correia MJ, Lang DG.. 1990. An electrophysiological comparison of solitary type I and type II vestibular hair cells. Neurosci Lett 116:106–11. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. 2017. The new vestibular stimuli: sound and vibration-anatomical, physiological and clinical evidence. Exp Brain Res 235:957–72. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ.. 2006. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175:256–67. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, MacDougall HG, Vidal PP, de Waele C.. 2017. Sustained and Transient Vestibular Systems: a Physiological Basis for Interpreting Vestibular Function. Front Neurol 8:117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B.. 1954. Quantal components of the end-plate potential. J Physiol 124:560–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C.. 2009. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci 29:10474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE.. 2011. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34:501–34. [DOI] [PubMed] [Google Scholar]
- Fernández C, Baird RA, Goldberg JM.. 1988. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol 60:167–81. [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM, Baird RA.. 1990. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63:767–80. [DOI] [PubMed] [Google Scholar]
- Flores A, Le¢n-Olea M, Vega R, Soto E.. 1996. Histochemistry and role of nitric oxide synthase in the amphibian (Ambystoma tigrinum) inner ear. Neurosci Lett 205:131–4. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Parsons TD.. 2006. The synaptic physiology of hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 249–312. [Google Scholar]
- Furukawa T, Matsuura S.. 1978. Adaptive rundown of excitatory post-synaptic potentials at synapses between hair cells and eighth nerve fibres in the goldfish. J Physiol 276:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GS, Risner JR, Holt JR.. 2004. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J Neurosci 24:11148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. 1991. The vestibular end organs: morphological and physiological diversity of afferents. Curr Opin Neurobiol 1:229–35. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. 2000. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130:277–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C.. 1990. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63:791–804. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C.. 1977. Conduction times and background discharge of vestibular afferents. Brain Res 122:545–50. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Highstein SM, Moschovakis AK, Fernández C.. 1987. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. I. An electrophysiological analysis. J Neurophysiol 58:700–18. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE.. 1988. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res 70:470–6. [DOI] [PubMed] [Google Scholar]
- Gulley RL, Bagger-Sjöbäck D.. 1979. Freeze-fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea-pig vestibular system. J Neurocytol 8:591–603. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Weber KP, Aw ST, Todd MJ, Curthoys IS.. 2008. Impulsive testing of semicircular canal function. Prog Brain Res 171:187–94. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Goldberg JM, Moschovakis AK, Fernández C.. 1987. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. II. Correlation with output pathways of secondary neurons. J Neurophysiol 58:719–38. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR, Mann MA, Rabbitt RD.. 2014. Evidence that protons act as neurotransmitters at vestibular hair cell-calyx afferent synapses. Proc Natl Acad Sci U S A 111:5421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Chatlani S, Lysakowski A, Goldberg JM.. 2007. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol 98:1083–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Jordan PM, Lysakowski A, Shah A, Barsz K, Contini D.. 2017. Muscarinic acetylcholine receptors and M-currents underlie efferent-mediated slow excitation in calyx-bearing vestibular afferents. J Neurosci 37:1873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Holt JR.. 2014. Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons. J Gen Physiol 143:481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Jones SM, Holt JR.. 2011. HCN channels expressed in the inner ear are necessary for normal balance function. J Neurosci 31:16814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB.. 2005. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93:2777–86. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA.. 2006. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26:10253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huterer M, Cullen KE.. 2002. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol 88:13–28. [DOI] [PubMed] [Google Scholar]
- Huwe JA, Logan GJ, Williams B, Rowe MH, Peterson EH.. 2015. Utricular afferents: morphology of peripheral terminals. J Neurophysiol 113:2420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Chihara Y, Komuta Y, Ito K, Sahara Y.. 2008. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol 100:2192–204. [DOI] [PubMed] [Google Scholar]
- Jamali M, Carriot J, Chacron MJ, Cullen KE.. 2013. Strong correlations between sensitivity and variability give rise to constant discrimination thresholds across the otolith afferent population. J Neurosci 33:11302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Chacron MJ, Cullen KE.. 2016. Self-motion evokes precise spike timing in the primate vestibular system. Nat Commun 7:13229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Kindt K, Wu DK.. 2017. Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. elife 6 (doi: 10.7554/eLife.23661). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA.. 2014. Genetics of peripheral vestibular dysfunction: lessons from mutant mouse strains. J Am Acad Audiol 25:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM.. 1999. Short latency compound action potentials from mammalian gravity receptor organs. Hear Res 136:75–85. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C.. 2016. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear Res 280:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Fettis M, Holt JC.. 2015. Efferent innervation of turtle semicircular canal cristae: comparisons with bird and mouse. J Comp Neurol 523:1258–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM. 1974. The sensory epithelia of the inner ear of two turtles, Testudo graeca L. and Pseudemys scripta (Schoepff). Acta Zool 55:289. [Google Scholar]
- Kalluri R, Xue J, Eatock RA.. 2010. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol 104:2034–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS.. 2005. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47:13–28. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Han GC, Park HJ, Minor LB.. 2008. Rotational responses of vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. J Assoc Res Otolaryngol 9:334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Kindig AE, Donne SW, Callister RJ, Brichta AM.. 2011. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp Brain Res 210:607–21. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Gaboyard-Niay S, Calin-Jageman I, Chatlani S, Price SD, Eatock RA.. 2011. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J Neurosci 31:10101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM.. 1997. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol 389:419–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM.. 2008. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B.. 2010. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res 340:303–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. 2017. Comparative auditory neuroscience: understanding the evolution and function of ears. J Assoc Res Otolaryngol 18:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Jr.. 1994. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci 14:6058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Benke TA, Rennie KJ.. 2012. Hyperpolarization-activated current (I(h)) in vestibular calyx terminals: characterization and role in shaping postsynaptic events. J Assoc Res Otolaryngol 13:745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Rennie KJ.. 2016. Channeling your inner ear potassium: k (+) channels in vestibular hair cells. Hear Res 338:40–51. [DOI] [PubMed] [Google Scholar]
- Nam JH, Cotton JR, Grant JW.. 2005. Effect of fluid forcing on vestibular hair bundles. J Vestib Res 15:263–78. [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR.. 2008. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol 9:65–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD. 1983. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. I. Response characteristics in normal animals. J Neurophysiol 49:134–51. [DOI] [PubMed] [Google Scholar]
- Pérez C, Vega R, Soto E.. 2010. Phospholipase C-mediated inhibition of the M-potassium current by muscarinic-receptor activation in the vestibular primary-afferent neurons of the rat. Neurosci Lett 468:238–42. [DOI] [PubMed] [Google Scholar]
- Peterson EH. 1998. Are there parallel channels in the vestibular nerve? News Physiol Sci 13:194–201. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM.. 2002. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol 88:1234–44. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Ricci AJ, Correia MJ.. 1996. Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol 75:2117–23. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Streeter MA.. 2006. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol 95:26–32. [DOI] [PubMed] [Google Scholar]
- Rossert C, Straka H.. 2011. Interactions between intrinsic membrane and emerging network properties determine signal processing in central vestibular neurons. Exp Brain Res 210:437–49. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Eatock RA.. 1996. Voltage responses of mouse utricular hair cells to injected currents. Ann N Y Acad Sci 781:71–84. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA.. 1998. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18:7487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE.. 2009. Effects of canal plugging on the vestibuloocular reflex and vestibular nerve discharge during passive and active head rotations. J Neurophysiol 102:2693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE.. 2009. Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J Neurophysiol 101:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E.. 2014. Glutamatergic signaling at the vestibular hair cell calyx synapse. J Neurosci 34:14536–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Sasaki H.. 1993. Morphological correlations between spontaneously discharging primary vestibular afferents and vestibular nucleus neurons in the cat. J Comp Neurol 333:554–66. [DOI] [PubMed] [Google Scholar]
- Sato F, Sasaki H, Ishizuka N, Sasaki S, Mannen H.. 1989. Morphology of single primary vestibular afferents originating from the horizontal semicircular canal in the cat. J Comp Neurol 290:423–39. [DOI] [PubMed] [Google Scholar]
- Sato F, Sasaki H, Mannen H.. 1988. Electron microscopic comparison of the terminals of two electrophysiologically distinct types of primary vestibular afferent fibers in the cat. Neurosci Lett 89:7–12. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Santos-Sacchi J, Castellano-Munoz M, Kong JH, Ricci AJ.. 2011. Calcium-dependent synaptic vesicle trafficking underlies indefatigable release at the hair cell afferent fiber synapse. Neuron 70:326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Zakir MM, Dickman JD.. 2003. Afferent innervation of the utricular macula in pigeons. J Neurophysiol 89:1660–77. [DOI] [PubMed] [Google Scholar]
- Singer M, Lysakowski A.. 1996. Nitric oxide synthase localized in a subpopulation of vestibular efferents with NADPH diaphorase histochemistry. Ann N Y Acad Sci 781:658–62. [DOI] [PubMed] [Google Scholar]
- Smith CE, Goldberg JM.. 1986. A stochastic afterhyperpolarization model of repetitive activity in vestibular afferents. Biol Cybern 54:41–51. [DOI] [PubMed] [Google Scholar]
- Songer JE, Eatock RA.. 2013. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33:3706–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersäll J. 1956. Studies on the structure and innervation of the sensory epithelium of the cristae ampullares in the guinea pig; a light and electron microscopic investigation. Acta Otolaryngol Suppl 126:1–85. [PubMed] [Google Scholar]
- Wong WH, Hurley KM, Eatock RA.. 2004. Differences between the negatively activating potassium conductances of Mammalian cochlear and vestibular hair cells. J Assoc Res Otolaryngol 5:270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, Lysakowski A, Eatock RA.. 2007. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol 97:1684–704. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ohmori H.. 1990. Synaptic responses to mechanical stimulation in calyceal and bouton type vestibular afferents studied in an isolated preparation of semicircular canal ampullae of chicken. Exp Brain Res 80:475–88. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ohmori H.. 1991. Synaptic bodies and vesicles in the calix type synapse of chicken semicircular canal ampullae. Neurosci Lett 129:43–6. [DOI] [PubMed] [Google Scholar]


