Abstract
Sensory hair cells are specialized secondary sensory cells that mediate our senses of hearing, balance, linear acceleration, and angular acceleration (head rotation). In addition, hair cells in fish and amphibians mediate sensitivity to water movement through the lateral line system, and closely related electroreceptive cells mediate sensitivity to low-voltage electric fields in the aquatic environment of many fish species and several species of amphibian. Sensory hair cells share many structural and functional features across all vertebrate groups, while at the same time they are specialized for employment in a wide variety of sensory tasks. The complexity of hair cell structure is large, and the diversity of hair cell applications in sensory systems exceeds that seen for most, if not all, sensory cell types. The intent of this review is to summarize the more significant structural features and some of the more interesting and important physiological mechanisms that have been elucidated thus far. Outside vertebrates, hair cells are only known to exist in the coronal organ of tunicates. Electrical resonance, electromotility, and their exquisite mechanical sensitivity all contribute to the attractiveness of hair cells as a research subject.
Introduction
Sensory hair cells are highly specialized mechanosensitive cells found in all vertebrate animals in some related chordates (tunicates). The structure of hair cells makes them highly sensitive to displacement of the fluid environment that surrounds their apical microvilli, or stereocilia. The stereocilia are linked together and usually referred to as a hair bundle or hair cell bundle. By developing arrays of hair cells in their integument, animals can be highly sensitive to pressure waves or movement in the fluid environment surrounding the animal. In tunicates, hair cells called coronal cells are present on the velum that rings the inner surface of the oral (incurrent) siphon and may serve a protective function by sensing large particles (Caicci et al. 2007; Rigon et al. 2013). In fish, salamanders, and some anuran amphibians (e.g., Xenopus and other pipid frogs), a lateral line system of hair cells is sensitive to movements in the surrounding water; this is important in predator avoidance, prey detection, swimming coordination and courtship (reviewed in Ghysen and Dambly-Chaudiere 2007). The lateral line system is widely present in aquatic larval forms of frogs and salamanders; in newts it is present during the juvenile aquatic stage, disappears during the terrestrial stage, and then reappears during the adult aquatic stage (Duellman and Trueb 1994).
Terrestrial vertebrates (including land-going post-larval amphibians) lack the lateral line system but retain a highly developed vestibular system (Duellman and Trueb 1994; Hill et al. 2016). In the vestibular system, multiple sensory epithelia contribute sensitivity to seismic vibration, linear acceleration (movements generating translation in space), and angular acceleration (rotational movements of the head) (Smotherman and Narins 2004). The utricle and saccule sense linear acceleration and the semicircular canals sense angular rotation of the head (Hill et al. 2016). The amphibian saccule is also highly sensitive to seismic and auditory vibrations below about 100 Hz (Koyama et al. 1982; Smotherman and Narins 2004). Displacement of the hair bundle in the utricle and saccule results from the inertia of an overlying membrane containing a gelatinous matrix in which crystals of calcium carbonate are embedded (the otoconial membrane). The inertial mass of the matrix with its mineral content induces lateral bending of the stereocilia when the hair cell epithelium is displaced along the orthogonal axis. The utricular epithelium is approximately horizontal, making it most sensitive to accelerations forward, backward, or sideways. The saccular epithelium orientation is approximately parasagittal (vertical to the ground), making it most sensitive to accelerations forward, backward, upward, or downward. The otoconial membrane also induces stereociliar bending if the epithelium is displaced by tilting, and in this way hair cells of the utricle and saccule can sense postural changes of head position by the effect of gravity (Goldberg et al. 2012).
Hair cells of the semicircular canals are located in three ampullae, one for each of the canals. The tips of the hair bundles are embedded in a gelatinous cupula. Rotation of the head induces inertial pressure by the fluid within the canal against the cupula, which in turn causes displacement of the hair bundle (Goldberg et al. 2012). As an example of the sensory capability of the semicircular canal system, a housecat is able to right itself and land gracefully after being dropped from an upside-down position in less than the time it takes to fall 1.5 m. At a gravitational acceleration of 9.8 m/s2, a cat falls 1.5 m in about 550 ms. The righting reflex which requires transduction by the vestibular receptors and rapid conversion of the transduced signal into changes of primary afferent firing frequency, followed by rapid central processing and activation of appropriate motor systems. Our understanding of the cellular mechanisms of hair cell mechanotransduction has grown rapidly in the past decade. Recent progress has been reviewed in depth by Fettiplace and Kim (2014) and Fettiplace (2017).
To summarize so far, sensory hair cells are responsible for our ability to sense angular acceleration of the head (rotational movements) by way of the semicircular canal system, linear acceleration (translational movements), and postural orientation with respect to gravity by way of the macular epithelia (in mammals, the utricle, and saccule), air-borne sound by way of the cochlea, and water displacements by the lateral line system in fish and amphibians, and water displacements by the coronal cells in tunicates. Taken together, this is an astonishing diversity of sensory functions for a single cell type, and invites a deeper exploration of the structure and function of hair cells. The remainder of this review is intended to provide an overview of the anatomy and physiology of hair cells so that a non-specialist reader may more fully appreciate the specialized reviews that follow in this collection.
Structural features of sensory hair cells
The diagnostic feature of sensory hair cells is the presence of an apical bundle of stereocilia, known as the hair bundle. Stereocilia are structurally similar to microvilli as they are packed with actin filaments in a semi-crystalline array, but stereocilia are generally larger (∼200 nm diameter) than the microvilli found in other animal cells. The stereocilia number in the range of 50–100 per cell, are tightly clustered and are graded in length from one end of the bundle to the other, giving the apical membrane an axis of symmetry (Schwander et al. 2010). A single true cilium containing a 9 + 2 array of microtubules is usually present just beyond the tallest stereocilia on the short-to-long axis. The kinocilium plays an important role in the development and orderly arrangement of the stereocilia, and the stereocilia closest to the kinocilium are linked to it (Fig. 1). In some hair cells, such as those in the mammalian cochlea and in the auditory papilla of some birds, there is no kinocilium in the mature hair cell. In all cases, however, a kinocilium is present during embryological development of the hair cell, and this is shown by the persistence of the ciliary basal body in the mature hair cell even in the absence of a cilium. In physiological experiments in which the kinocilium was removed from bullfrog saccular hair cells, hair cell function was not disrupted (Hudspeth and Jacobs 1979). Together these results indicate that the kinocilium is necessary for normal development of the stereocilia array but that it is not required for normal function (Schwander et al. 2010; Barr-Gillespie 2015).
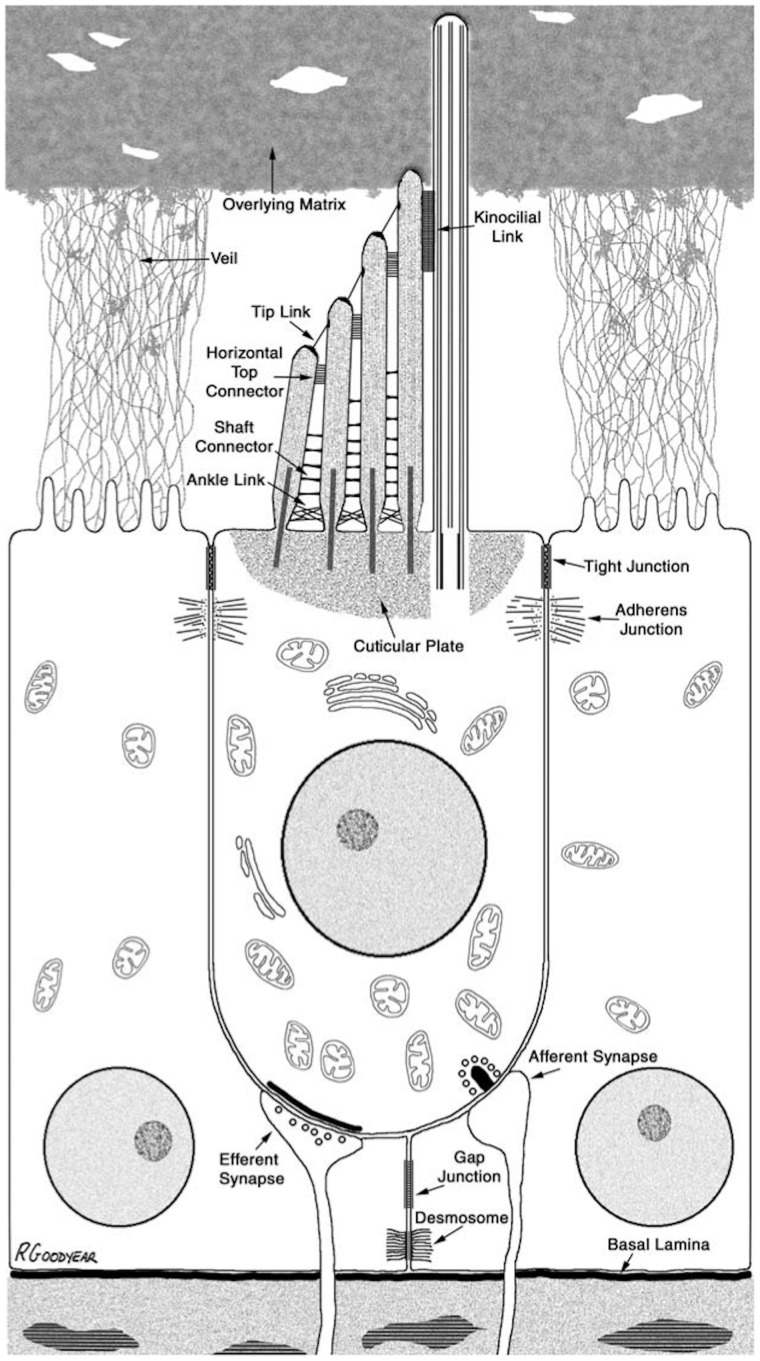
Fig. 1.
Diagram of a stereotypical vertebrate hair cell, showing the major features of the cell and its relationship to the supporting cells around it. Note the presence of tight junctions linking all the cells at their apical poles and forming a tight epithelium. Note that supporting cells communicate with each other via gap junctions, but not with the hair cell. Reproduced with permission from Goodyear et al. (2006).
The stereocilia are packed with actin filaments, as mentioned above, and the arrangement of the actin filaments is highly regular, with all of the “plus” (barbed) ends toward the stereocilium tip, which would allow the stereocilium to grow longer by addition of actin monomers at the tip. As in other cellular systems, there is turnover of actin subunits, but the rate of actin turnover is relatively low (Zhang et al, 2012). This has led to a tip-turnover model in which actin monomers at the stereocilium tip (the barbed end of the actin filaments) turn over dynamically with F-actin, while the remaining F-actin is stable and not subject to turnover (Drummond et al. 2015; Narayanan et al. 2015). Stabilization of the actin core of stereocilia involves a number of proteins believed to crosslink actin filaments and bundles of filaments together, including plastin-1, fascin-2, espin-3A, espin-1, and XIRP2 (Sekerkova et al. 2006; Shin et al. 2010; Taylor et al. 2015; Francis et al. 2015; Scheffer et al. 2015). Mutation or deletion of the genes for plastin-1, fascin-2, and XIRP2 result in stereocilia degeneration, confirming their importance (McGrath et al. 2017).
Importantly, the length of each stereocilium is tightly controlled to preserve the staircase-like array of stereocilia lengths, indicating that there is some sort of feedback system regulating their length (McGrath et al. 2017). Several proteins have been identified that contribute to the control of stereocilia length, including EPS8, which acts as both a bundler and a capping protein for actin filaments (Hertzog et al. 2010; Zampini et al. 2011). As expected, EPS8 is most abundant at the tips of stereocilia, as is the related protein EPS8L2 (McGrath et al. 2017). Two other proteins, myosin XVa and whirlin, are also located at stereocilia tips, and all three are required for normal stereocilia length regulation. Myosin XVa functions in part by transporting EPS8 to the stereocilia tips (Belyantseva et al. 2005; Manor et al. 2011).
At the other end, beneath the bases of the stereocilia lies a dense layer of actin filaments that form the cuticular plate, to which the stereocilia are anchored; unlike the actin in stereocilia, actin filaments in the cuticular plate appear to lack any consistent pattern of orientation. Other components of the cuticular plate include alpha-actinin, myosin Ie, myosin VI, tropomyosin, and spectrin (Goodyear et al. 2006). The protein ELMOD1 is necessary for normal development of the cuticular plate and apical membrane of hair cells; it functions by stimulating GTP hydrolysis by an ADP-ribosylation factor, ARF6 (Krey et al. 2018). Mutations in the Elmod1 gene disrupt formation of hair bundles and lead to deafness and vestibular dysfunction in mice. The current data suggest that conversion of AFR6-GTP to ARF6-GDP stabilizes actin structures in the apical region (Krey et al. 2018).
Each stereocilium tapers to a narrow ankle region where it joins the cuticular plate, a a central group of stereocilia actin filaments extends into the cuticular plate, forming a rootlet that anchors that stereocilium (Goodyear et al. 2006). The ankle region is more flexible than the more distal regions of the stereocilium and thus forms a hinge about which the stereocilium can be deflected toward or away from the kinocilium (or toward/away from the basal body that remains where there was once a kinocilium). Lateral deflections of the hair bundle (i.e., orthogonal to the short-long axis) are also possible, at least experimentally, but such deflections do not generate a receptor potential (Fettiplace and Kim 2014).
In the extracellular space, adjacent stereocilia are linked together by a variety of connections (Goodyear et al. 2005; Fettiplace and Kim 2014). Those at the base are termed ankle links; beyond those are a series of so-called shaft connectors, and distal to the shaft connectors there are horizontal top connectors. The stereocilia adjacent to the kinocilium are linked to it by distinct kinocilial connectors. The functional effect of these multiple links is that the entire hair bundle moves as a single unit in response to mechanical stimuli. Between the tip of one stereocilium and the shaft of its adjacent, taller neighbor there is a different mechanical link, termed a tip link (Goodyear et al. 2005). The tip links are vital to mechanotransduction and disruption of them, which can be achieved by reducing the extracellular Ca2+ concentration to a sub-micromolar level, results in loss of hair cell sensitivity (Assad et al. 1991). This manipulation led in turn to the hypothesis that the mechanotransducer channel is located at the tips of the stereocilia rather than at the base, a hypothesis which was eventually confirmed by high-speed, high-resolution calcium imaging (Beurg et al. 2009).
Subsequent studies have revealed the identity of many of the proteins that contribute to tip links, illustrated in Figure 2. The tip link itself is formed by cadherin 23 (CDH23) together with protocadherin 15 (PCDH15) (Siemens et al. 2004; Kazmierczak et al. 2007; Müller 2008; Alagramam et al. 2011). The upper portion of the tip link is a parallel dimer of CDH23, while the lower portion is a parallel dimer of PCDH15 (Narui and Sotomayor 2018). Multiple isoforms of PCDH15 can be created by alternative splicing and they have different spatial and developmental distributions in hair bundles. The PCDH15-CD2 isoform is functionally essential for mature tip links (Pepermans and Petit 2015). At the upper end of the tip link, CDH-23 is connected to a complex of harmonin and myosin VIIa (Fettiplace and Kim 2014). Mutations of CDH-23, harmonin, or myosin VIIa disrupt mechanotransduction (Di Palma et al. 2001; Grillet et al. 2009; Yu et al. 2017).
Fig. 2.
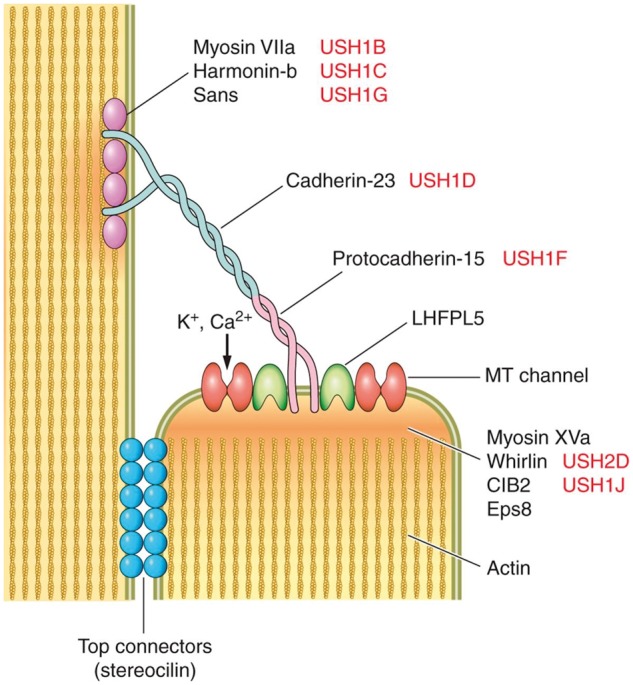
Schematic depiction of two adjacent stereocilia to illustrate our current understanding of the molecules that contribute to the tip link. The “USH” designations to the right of the protein names indicate that mutations in those proteins are associated with Usher syndrome in humans. MT channel = mechanotransduction channel. Reproduced with permission from Fettiplace and Kim (2014).
At the other end of the tip link, PCDH15 interacts with proteins TMIE and LHFPL5 (formerly known as TMHS). These two proteins have multiple transmembrane domains and may contribute to the functional mechanotransducer channel (Xiong et al. 2012; Zhao et al. 2014). Another protein, calcium- and integrin-binding protein 2 (CIB2), interacts with whirlin and myosin VIIa; mutations in CIB2 cause postnatal regression of stereocilia and hair cell death in the mammalian cochlea but not in the mammalian vestibular system (Michel et al. 2017).
The epithelium which contains vestibular hair cells is structurally simple and is dominated by two cell types: hair cells and supporting cells (Goodyear et al. 2006). Supporting cells span the epithelium from its apical border to the underlying basement membrane. Each hair cell is surrounded by supporting cells and is thus physically isolated from nearby hair cells. In addition, the basolateral membrane of each hair cell is separated from the basement membrane by lateral projections from the supporting cells. Supporting cells and hair cells are linked by tight junctions at the apical side of the epithelium, and by adherens junctions more basally. Supporting cells are linked to one another by gap junctions, which may provide a pathway for potassium transport as well as other cell–cell communications. In addition, supporting cells are linked by desmosomes, which contribute the necessary mechanical strength to the epithelium. Supporting cells in the organ of Corti are significantly more differentiated than in the vestibular system, and include many distinct types. The basal side of each inner hair cell is surrounded by processes of an inner border cell on the medial side and an inner phalangeal cell on the lateral (abneural) side. These processes form a calyx structure around both the inner hair cell body and its afferent nerve fiber, isolating it from the basilar membrane. On its lateral side, the phalangeal cell meets the medial side of an inner pillar cell; adjacent inner pillar cells form the medial wall of the tunnel of Corti. The lateral wall of the tunnel of Corti is formed, in turn, by a row of outer pillar cells. Beyond the outer pillar cells lie three rows of Deiter’s cells, each of which supports an outer hair cell in a cup-like invagination at its upper end. Analogous to the inner hair cells, each outer hair cell is isolated from the basilar membrane on its basolateral surface by processes of Deiter’s cells. Lateral to the last row of Deiter’s cells is a population of Hensen’s cells, characterized by their large lipid-filled vacuoles. Unlike Deiter’s cells, Hensen’s cells do not make distinct contact with the basilar membrane. At the lateral extreme of the organ of Corti is a group of squamous epithelial cells called Claudius’ cells; these cells are directly attached to the basilar membrane (Gale and Jagger 2010).
Hair cell physiology
Resting potentials
Compared to neurons in the central nervous system, the resting membrane potentials of hair cells are moderately depolarized, lying in the range of −70 to −50 mV. On the basolateral side of the epithelium, hair cells are exposed to the typical extracellular fluid of the animal; it has a high sodium concentration (120–150 mM), a low potassium concentration (2–4 mM), and a similarly low calcium concentration (2–5 mM). The primary anion in the extracellular fluid is chloride (80–110 mM). The hair cell cytosol has a low concentration of sodium (10–15 mM), a high concentration of potassium (125–140 mM), and a negligible concentration of free calcium (≪1 μM) (Bosher and Warren, 1978; Sauer et al. 1999). The intracellular chloride concentration is also low (1.5–4 mM) and intracellular charge balance is due mainly to the presence of the fixed negative charges which are present on free amino acids and macromolecules (proteins and nucleic acids) at physiological pH. Assuming an intracellular K+ concentration of 130 mM and an extracellular K+ concentration of 3 mM, such a cell would have a resting membrane potential of about −98 mV if it behaved as a perfectly K+-selective Nernstian membrane (where the Nernst equilibrium potential = 60*log10{[K+]out/[K+]in} in mV at 20°C). That the resting potential is so far depolarized from the K+ equilibrium potential suggests the presence of a resting permeability to one or more additional ions which have a more depolarized equilibrium potential. Crawford and Fettiplace observed an average resting potential of about −50 mV in auditory hair cells and estimated the K+ equilibrium potential to be −80 mV for those cells (Crawford and Fettiplace 1980). Studies of frog saccular hair cells indicate two opposing ion currents at rest, one an inward rectifier K+ current (IK1) and the other a hyperpolarization-activated inward current (Ih) (Holt and Eatock 1995). The K+ current was activated at potentials negative to −60 mV and the inward current was activated at potentials negative to −50. Saccular hair cells fell into two morphological populations, spherical and cylindrical, which also differed in their resting potentials. Spherical cells had more depolarized potentials (ca. −50 mV) and lacked IK1 while possessing Ih. Cylindrical cells had more negative resting potentials (ca. −68 mV) and possessed both IK1 and Ih (Holt and Eatock 1995). The hyperpolarization-activated inward current Ih is a member of the HCN channel family and a recent report suggests that this type of channel is necessary for normal vestibular function (Horwitz et al. 2011). In mouse cochlear hair cells, which have a resting potential around −72 mV, voltage-sensitive K+ channels of KCNQ type contribute strongly to the resting potential (Oliver et al. 2003). Vestibular neurons express a variety of voltage-gated, inward rectifier, and Ca2+-activated channels, which contribute to the functional specialization of type I and type II hair cells (Meredith and Rennie 2016).
On the apical side of the hair cell membrane, the ionic composition of extracellular fluid in the inner ear more closely resembles the cytosol (Bosher and Warren 1978; Sauer et al. 1999). This fluid, called endolymph, is actively secreted by the stria vascularis of the scala media of the mammalian cochlea. The scala media is a fluid-filled duct which is continuous with the semicircular canals and with the fluid space that overlies hair cells in the other macular epithelia, such as the mammalian saccule and utricle. In those regions, an epithelium similar to the stria vascularis secretes endolymph (Wilms et al. 2016). The K+ concentration of cochlear endolymph in mammals is about 150 mM and the concentration of Na+ is about 1 mM; the Ca2+ concentration is about 30 μM, which is about 1% of the normal extracellular value but at the same time much higher than the normal intracellular value, which is less than 1 μM (Fettiplace 2017). Because the K+ concentrations are similar on both sides of the apical membrane, the Nernst potential for K+ across that membrane is close to zero (EK = 60*log10{[K+]out/[K+]in}. Consequently, if the resting membrane potential is −50 mV (due to resting permeabilities on the basolateral side), there exists an inward driving force for K+ to enter the cell on the apical side. Because of the low Na+ concentration in endolymph, the driving force for Na+ is actually outward across the apical membrane (ENa = 60*log10(1/10)=-60 mV), while the driving force for Ca2+ remains inward [ECa=(60/2)*log10(30 μM/1 μM) = +44 mV]. In mammals and in some birds, there is a significant positive electrical potential between endolymph and the basolateral extracellular fluid, as a result of the active ion transport that takes place in the endolymph-secreting epithelium. In mammals, this potential is about +80 mV in the cochlea and therefore adds to the inward driving force for cations across the hair cell apical membrane (Zdebik et al. 2009; Wilms et al. 2016). By adding to the driving force for the inward flow of cationic current, this is thought to enhance the response of hair cells to high-frequency tones (Nin et al. 2008, 2016; Fettiplace 2017).
Mechanotransduction
The appropriate mechanical stimulus for a hair cell is one that displaces the stereocilia bundle in the direction of the tallest stereocilia. This was demonstrated by Hudspeth and Corey using an isolated bullfrog saccular epithelium preparation (Hudspeth and Corey 1977). Minute displacements of the hair bundle produced depolarizing receptor potentials of several millivolts, and displacement in the opposite direction caused a hyperpolarization, though it was smaller in amplitude. Similar experiments on mammalian outer hair cells indicate that a 250 nm deflection of the hair bundle generates a half-maximal activation of the inward current and a 500 nm deflection is saturating (Fettiplace and Kim 2014). The hyperpolarizing response observed in bullfrog saccular hair cells indicates that some of the mechanosensitive channels must be open at rest, but they can only a small fraction of the total number since the cells were much more responsive in the depolarizing direction (Hudspeth and Corey 1977).
The ionic current activated by hair bundle displacement is carried by cations, and the channel is permeable to Na+, K+, and Ca2+ (Corey and Hudspeth 1979). Potasssium ions carry most of the current, because they are the most abundant cation in the endolymph; however, Ca2+ may contribute up to 10% of the inward current despite its low concentration (20–30 μM in cochlear endolymph, 200–250 μM in vestibular endolymph) (Ricci and Fettiplace 1998). As noted above, current carried by Na+ will be in the outward direction; this would not contribute to the depolarizing receptor potential but might assist in Na+ homeostasis by allowing outward diffusion of Na+ that diffused inward on the basolateral side. Depolarization caused by inward current across the apical membrane increases the already-high conductance to K+ in the basolateral membrane by opening voltage-gated K+ channels; these appear to include KCNQ4 channels, and mutations of the KCNQ4 gene cause a dominant form of hereditary deafness (Kharkovets et al. 2000).
The large outward driving force for K+ on the basolateral side of the hair cell means that no energy expenditure is required to restore the K+ ion concentration gradient during and after the mechanotransduction process. This is a remarkable efficiency of cellular energetics and a prime example of the physiological power of epithelia. By a combination of K+ diffusion through the perilymph and cell-to-cell diffusion through gap junctions connecting supporting cells to each other and to other cells, the K+ that entered through the apical membrane eventually travels back to the stria vascularis to be recycled into more endolymph (Zdebik et al. 2009; Fettiplace 2017).
Synaptic transmission
Mature hair cells do not produce action potentials, so all synaptic transmission is based on graded receptor potentials. Hair bundle displacement produces inward currents as large as 10 pA for 1 nm of displacement, which would lead to a depolarization of 1 mV for a hair cell having an input resistance of 100 MΩ (Fettiplace and Ricci 2006). The physiological range for receptor potentials is from the resting potential to about −20 mV (Glowatzki et al. 2008), corresponding to hair bundle displacements of up to 50 nm under physiological conditions.
Neurotransmitter release at the afferent synapse is Ca2+-dependent, as at other neuronal synapses, but there are several important specializations in the hair cell synapse. Structurally, the presynaptic zone contains a prominent, oblong, electron-dense structure around which synaptic vesicles are clustered; this leads it to be termed a ribbon synapse (Fig. 1), and the appearance in transmission electron micrographs is similar to that seen in retinal photoreceptors and bipolar cells (Wichmann and Moser 2015). The release of neurotransmitter is graded in relation to membrane depolarization and the relationship of transmitter release to Ca2+ influx is linear (Glowatzki et al. 2008; Rutherford and Pangrsic 2012; Fettiplace 2017). This is rather surprising, because the Ca2+-dependence of vesicular exocytosis at the ribbon synapse, when measured by photolysis of photo-sensitive Ca2+-buffers, seems to display the same 4th or 5th power relationship as that seen at other neuronal synapses (Glowatzki et al. 2008; Johnson et al. 2017; Rutherford and Pangrsic 2012). However it occurs, the net effect of the observed linear relationship at the ribbon synapse is to make transmitter release more sensitive to small depolarizations, thereby enhancing the overall sensitivity of the system.
In the absence of stimulus-induced hair cell depolarization, neurotransmitter is continually released due to the tonic open state of L-type voltage-dependent Ca2+ channels at the active zone of the presynaptic membrane at the normal resting potential (Cho and von Gersdorff 2012). The neurotransmitter released is glutamate, and the postsynaptic receptors are fast, excitatory AMPA receptors (Sadeghi et al. 2014; Kirk et al. 2017). The result of the tonic release of glutamate is that the primary afferent fibers are firing spontaneously in the absence of hair cell activation and increase their firing rate in proportion to the graded increase of glutamate caused by graded hair cell depolarization.
The hair cell ribbon synapse is specialized to release neurotransmitter with minimal delay; this is important because many processes, such as sound source localization, require precise assessment of the interaural latency of sound arrival. How this is accomplished is not completely understood, but several key components of the ribbon synapse have been identified. Prominent among these is the protein ribeye, which forms the bulk of the ribbon structure (Wichmann and Moser 2015). Ribeye is anchored to the active zone by the protein bassoon (Fig. 3), and mice with bassoon mutations have impaired ribbon synapse function (Wichmann and Moser 2015). The identity of the proteins that tether synaptic vesicles to ribeye is not known, but may involve the B domain of ribeye (Wichmann and Moser 2015). Knockout of ribeye has recently been reported in mice, with the result that temporal precision of sound encoding was disrupted, though synaptic transmission continued (Jean et al. 2018).
Fig. 3.
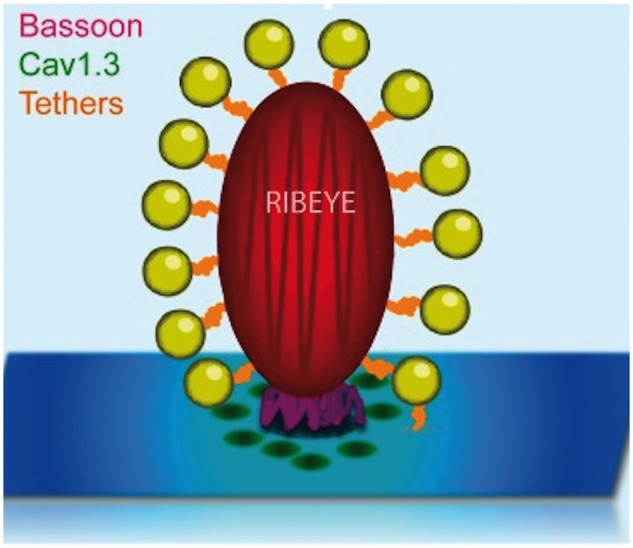
Schematic depiction of the ribbon synapse in a mature inner hair cell. Synaptic vesicles (small spheres) are clustered around the ribeye protein (large oval) and are linked to it by as-yet undescribed tethers. The bassoon protein (pedestal below ribeye) is positioned between ribeye and the presynaptic membrane and may serve as an anchor for ribeye. Voltage-sensitive Cav1.3 Ca2+ channels (dark spots near bassoon) are clustered tightly around the ribeye-bassoon complex, minimizing the diffusion time between Ca2+ entry and Ca2+-triggered exocytosis. Modified with permission from Wichmann and Moser (2015).
Besides the structural differences from central synapses, there are significant functional differences in ribbon synapses: vesicle priming factors such as Munc13 are not involved, SNARE proteins do not appear to be necessary, and synaptotagmin, the Ca+2-binding protein that triggers exocytosis at central synapses, is completely absent from hair cell ribbon synapses (Rutherford and Pangrsic 2012; Pangrsic et al. 2012). In place of synaptotagmin, ribbon synapses contain a related protein, otoferlin, which, like synaptotagmin, has multiple C2 domains which could bind Ca2+ and promote vesicle exocytosis (Cho and von Gersdorff 2012; Fettiplace, 2017; Michalski et al. 2017). Otoferlin is necessary for exocytosis from hair cell ribbon synapses, as knockout of the protein in mice causes deafness, and mutation of the otoferlin gene is associated with human deafness (Roux et al. 2006). Further evidence in support of otoferlin as a Ca2+ sensor and promoter of vesicle fusion comes from experiments using a knock-in mouse with a modified otoferlin having lower Ca2+ affinity in its C2 domains (Michalski et al. 2017). These mice had normal ribbon synapse structure and presynaptic Ca2+ currents, but synaptic exocytosis was delayed and brainstem auditory responses were smaller (Michalski et al. 2017). Otoferlin may also contribute to recruitment of vesicles to the ribbon synapse (Michalski et al. 2017). Many questions remain regarding the hair cell ribbon synapse, and this system will certainly be a focus of productive research activity for many years to come.
Another specialized synapse is found in Type I vestibular hair cells, which are surrounded on the basolateral side by a calyx-like expansion of the primary afferent nerve ending (Fig. 4). Both type I and type II hair cells form ribbon synapses onto the primary afferent, and it is notable that one primary afferent may form synapses with both type I and type II hair cells (Eatock and Lysakowski 2006). However, the presence of a calyx constrains the outward diffusion of K+ from the basolateral membrane of the type I cell, leading to accumulation of K+ in the synaptic cleft. While this seems disadvantageous to the hair cell, which must eliminate on the basolateral side the K+ that enters through the stereocilia, it also has the potential to directly depolarize the primary afferent membrane by the resulting charge accumulation and/or by Nernstian depolarization, producing thereby a direct, non-quantal excitation (Songer and Eatock 2013). Consistent with this idea, the calyceal afferent membrane contains voltage-sensitive KCNQ-type channels, providing both a Nernstian permeability pathway and an exit channel from the synaptic cleft for accumulated K+ (Songer and Eatock 2013).
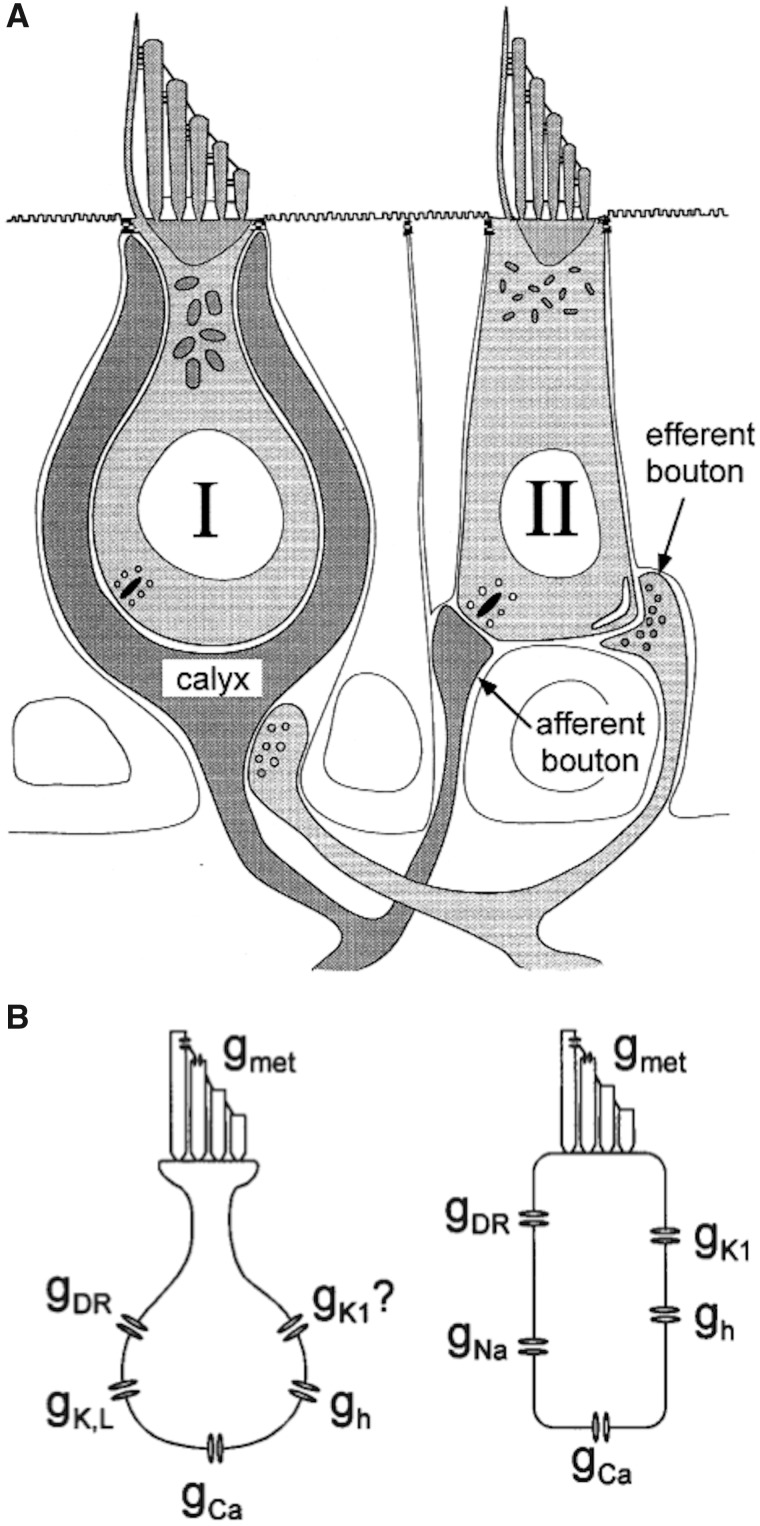
Fig. 4.
Diagram of stereotypical type I and type II hair cells from a mammalian vestibular organ. (A) Note that a single primary afferent may synapse with both a type I and a type II hair cell. A single efferent neuron may synapse onto a type I afferent ending (a postsynaptic synapse) and may synapse onto a type II hair cell (a presynaptic synapse) or onto the afferent axon (a postsynaptic synapse, not illustrated). But an efferent neuron cannot synapse onto a type I hair cell directly. (B) Ionic conductances that have been identified in vestibular hair cells. GDR, delayed rectifier K+ conductance; GK, L, K+ leak conductance; GCa, voltage-sensitive, noninactivating Ca2+ conductance; GNa, TTX-sensitive, voltage-sensitive Na+ conductance; GKI, K+- selective inward rectifier; Gh, hyperpolarization-activated inward current; GA, voltage-sensitive, rapidly inactivating K+ conductance, or A-current; GDRI, delayed rectifier K+ conductance; GDRII, delayed rectifier K+ conductance; GMET, mechanoelectrical transduction conductance. Reproduced with permission from Eatock et al. (1998).
The mechanotransducer channel
Although the mechanotransducer channel has not yet been fully isolated and characterized, much is known about it. Early experiments used low extracellular Ca2+ (less than 1 μM) to cause partial separation of the stereocilia tip links. Under fortunate conditions, this leaves enough intact tip links to allow electrical recording from one or a few channels at a time, and thus measurement of the channel conductance. When the extracellular Ca2+ concentration was subsequently raised to about 3 mM, the channel conductance measured was on the order of 100 picosiemens (100 pS) (Ohmori 1985; Crawford et al. 1991; Beurg et al. 2006). In contrast, most voltage-gated ion channels have conductances in the range of 10–30 pS (Hille 2001). Even more surprising, the conductance of the mechano-sensitive channel nearly doubled when the extracellular Ca2+ was lowered to its normal (sub-millimolar) endolymph concentration (Crawford et al. 1991). The increased conductance in low Ca2+ suggests that Ca2+ has an inhibitory effect on the channel (Beurg et al. 2010).
The mechanosensitive ion channel was conclusively localized to the tips of stereocilia by calcium imaging with fluorescent dyes (Lumpkin and Hudspeth 1995; Beurg et al. 2009). This result is consistent with the presence of tip links between adjacent stereocilia, which provide a possible mechanism for the activation of tip-located mechanoreceptors: they could act as a gating spring. Several components of the tip links have been identified, and among them two essential proteins are cadherin23 (CDH23) and protocadherin 15 (PCDH15) (Siemens et al. 2004; Narui and Sotomayor 2018). Both CDH23 and PCDH15 are calcium-binding proteins that form calcium-dependent links, so their presence is consistent with the earlier observation that low extracellular Ca2+ disrupts tip links and blocks mechanotransduction (Assad et al. 1991).
It is now well-established that CDH23 forms the upper region of the tip link and PCDH15 forms the lower (Zhao and Müller 2015). Subsequent searches, based on genes responsible for a severe deafness syndrome (Usher Syndrome Type 1, or USH1), have led to the description of proteins that engage with CDH23 or PCDH15 and could possibly form the mechanotransducer channel. These searches were productive and yielded important components of the stereocilia tip-link complex, including harmonin, sans, and myosin VIIa, which form a complex at the upper end of the tip-link and interact with CDH23. Also identified were CIB2, TMIE, and LHFPL5 (also known as TMHS), which form a complex at the lower end of the tip link and interact with PCDH15 (Zhao and Müller 2015). Both TMIE and LHFPL5 are integral membrane proteins and could possibly contribute to a transmembrane ion channel. However, experiments with a mouse knock-out of LHFPL5, while showing reduction of the mechanotransduction conductance, indicated not that LHFPL5 was a component of the mechanotransducer channel, but rather that disruption of LHFPL5 acts by downregulating expression of another potential channel protein, TMC1 (Beurg et al. 2015).
Multiple lines of evidence suggest that TMC1 is part of the mechanotransducer channel (Holt et al. 2014). Like TMIE and LHFPL5, TMC1 is localized to the stereocilia tips (Kurima et al. 2015). Also, TMC1 interacts with CIB2, which itself is necessary for normal mechanotransduction (Giese et al. 2017). Experiments with mice expressing TMC1, the related protein TMC2, or a mutated TMC1 showed that the votage-clamp recording from inner hair cells or vestibular hair cells yielded normal-appearing currents with either TMC1, TMC2, or a mixture of the two, while the mice expressing mutated TMC1 only showed reduced currents (Pan et al. 2013). These data do not prove that TMC1 or TMC2 can form a complete channel, but they strongly suggest that TMC proteins contribute to the channel pore (Pan and Holt 2015). However, this does not necessarily imply that they are the only components of the channel pore. Many questions remain and the issue is unlikely to be fully resolved until a functional mechanotransducer channel can be reconstituted in a heterologous expression system (Fettiplace 2016; Corey and Holt 2016; Wu and Müller 2016).
Reverse-polarity transduction
In hair cells lacking both TMC1 and TMC2, or in hair cells in which the tip links have been thoroughly disrupted, it is possible to observe a reverse-polarity current when the hair bundle is displaced in the negative direction (away from the tallest stereocilia). This outward current results from deformation of the apical plasma membrane of the cell (Beurg et al. 2016). Subsequent investigation determined that the mechanosensitive channel responsible is piezo2, which has previously been shown to underlie mechanotransduction in mammalian touch sensation (Ranade et al. 2014; Beurg and Fettiplace 2017). However, the normal role of this channel in the apical membrane of hair cells is not known.
Adaptation
All sensory systems exhibit adaptation, which is a decline in sensory response to a steady or unchanging stimulus. Adaptation may occur at any level of the system, from peripheral receptor to central integrating neural circuit, and it often occurs at multiple levels. From the organism’s point of view, adaptation allows the sensory system to remain sensitive to changes or differences in the environment, as those are the locus of information that is useful for survival and reproduction. Like many other mechanosensory cells, hair cells show rapid adaptation to tonic displacement of the stereocilia bundle. This is functionally important in the vestibular system because it allows hair cells to sense changes of acceleration against a background of constant velocity. Additionally, in auditory hair cells of amphibians, reptiles, and birds, adaptation contributes to frequency sensitivity. Two general types of hair cell adaptation have been described: fast and slow. Both were originally described in amphibians (frog saccule) or reptiles (turtle auditory papilla), and both types depend on Ca2+ influx through the mechanosensitive channel (Assad et al. 1989; Wu et al. 1999; Eatock 2000; Colclasure and Holt 2003; Corns et al. 2014)
Fast adaptation occurs very rapidly, with a time constant of <2 ms, and it includes a rapic, active movement of the stereocilia against the direction of the stimulus (i.e., toward the shorter stereocilia) (Crawford et al. 1989). The recoil has the same time course as the decay of inward current following stereocilia displacement, and the most likely mechanism is that entering Ca2+ binds to the mechanotransducer channel complex and causes rapid channel closing (Crawford et al. 1989; Ricci et al. 2000a; Fettiplace and Ricci 2003; Stepanyan and Frolenkov 2009). Consistent with this idea, turtle auditory hair cells contain a high concentration of endogenous Ca+2 buffer, equivalent to 0.1–0.4 mM BAPTA, and the concentration of endogenous buffer is higher in high-frequency responding hair cells than in low-frequency cells. In parallel, the time constant of fast adaptation is longer in high-frequency cells than in low-frequency cells (Ricci et al. 1998).
Fast adaptation has also been observed in outer hair cells of the mammalian cochlea. The kinetics of fast adaptation were more rapid than in turtle hair cells, even when both were at room temperature, with the average time constant for fast adaptation in mouse cells around 154 μs when the extracellular Ca2+ concentration was 1.5 mM. When extracellular Ca2 was reduced to 50 μM, a physiological concentration, the time constant was slower (620 μs) and the total inward current was greater (Kennedy et al. 2003). These differences reflect the fact that the mechanotransducer channel is blocked by millimolar Ca2+, which was also observed in turtle hair cells (Crawford et al. 1991). Similar results were obtained by Corns and colleagues when using millimolar Ca2+, but when Ca2+ was lowered to a physiological concentration, both fast and slow adaptation were abolished (Corns et al. 2014). It has also been reported that fast adaptation can occur independent of Ca2+ entry in mammalian cochlear hair cells (Peng et al. 2013).
Slow adaptation occurs with a longer time constant (5–50 ms). This adaptation involves a passive adjustment of the hair bundle in the direction of the excitatory displacement (i.e., toward the longer stereocilia). In the process, the channels become reset to respond to further displacement, and this is thought to involve adjustment of the resting tension on the channels. One model for this is that Ca2+ binds to myosin and causes its detachment from an actin filament in the stereocilium. The myosin is hypothesized to be attached to an elastic element, or “gating spring,” which is also attached to the channel, so that detachment of the myosin from actin would allow the complex to slip in the direction that reduces tension on the gating spring. Following channel closure, intracellular Ca2+ returns to normal and the myosin could again bind to actin and migrate in the opposite (tension-increasing) direction. Once tension reaches the point for channel opening, Ca2 influx would again act to reduce the tension. In this way, a negative feedback loop would keep the channels optimally positioned for sensitivity (Hudspeth et al. 2000; Farris et al. 2006). Recent evidence in support of this model is that directed mutation of the myosin1c gene, combined with an ADP analog that interferes with the mutated myosin, selectively blocks slow adaptation in the transgenic mice carrying the mutation (Holt et al. 2002). It has also been reported that myosin VIIa is necessary for slow adaptation in mouse cochlear hair cells (Kros et al. 2002). Myosin VI has also been implicated in adaptation (Marcotti et al. 2016).
How do auditory hair cells distinguish tones?
Through hair cells, the auditory system encodes sound intensity (loudness) and sound frequency (tone). In contrast to the lateral line and vestibular systems, in which water or endolymph translates in the plane of the epithelium, auditory transduction involves vertical displacements of the hair cell (i.e., along the basolateral-to-apical axis) caused by sound-induced oscillation of the epithelium. It is straightforward to see how larger amplitudes of oscillation might be induced by louder sounds, and how this could create greater depolarization of the hair cell. But how does the ear distinguish tones? Two fundamentally different mechanisms have evolved for tone discrimination; one which employs electrical resonance in the basolateral membrane of the hair cell, while the other makes use of tonotopic differences in the mechanical resonance of the basilar membrane. There are also tonotopic differences in the stiffness of hair bundles.
In amphibians, reptiles, and birds, tone discrimination derives primarily from resonant electrical properties of the hair cells; some resonate at lower frequencies and some at higher frequencies (Art et al. 1995; Art and Fettiplace 1987; Fuchs et al. 1988; Smotherman and Narins 1999a, 1999b). The physiological mechanism for this resonance involves an intriguing interplay of voltage-activated Ca2+ channels and Ca2+-activated K+ channels. This was initially described by Fettiplace and colleagues (Crawford and Fettiplace 1981; Art and Fettiplace 1987, 2006; Goodman and Art 1996). The electrical resonance manifests as a damped oscillation of membrane potential in responses to an applied current pulse, or a sustained oscillation of membrane potential in response to pure tone stimulation of the intact cochlea. The resonant response to pure tones is not an all-or-none phenomenon; each cell displays a tuning curve with maximal resonance at a characteristic frequency and a graded decline with frequencies on either side (Art et al. 1985). What is remarkable is that the same characteristic frequency can be seen in the isolated hair cell: it is an intrinsic property of the cell membrane.
While differences in the density and kinetics of channels bearing inward or outward current could influence a resonant feedback loop, the experimental evidence indicates that most of the differences lie in the density and kinetics of BK potassium channels, which are sensitive to both voltage and Ca2+ (Art and Fettiplace 1987; Art et al. 1995). Cells with a lower resonant frequency have a lower density of BK channels and those channels are composed of subunits that result in slower activation of the channel. Thus, the membrane response to both depolarization and Ca2+ influx are slowed, resulting in a lower resonant frequency. Cells which have a higher density of BK channels, and in which the channel subunits yield faster kinetics, repolarize more rapidly after an initial depolarization and Ca2+ influx. Buffering of the cytosolic Ca2+ then allows the BK channels to close, causing a rebound depolarization and Ca2+ channel opening. In the presence of tones that match the resonant frequency range of the cell, this would yield enhanced receptor potentials compared to a cell lacking resonance (Wu et al. 1995).
In addition to membrane electrical resonance of individual cells, there is an overall tonotopic organization in the basilar (auditory) papilla of reptiles such as turtles (from which many of these studies are drawn). Hair cells with high-frequency resonance reside at the basal end and cells with progressively lower-frequency resonance lie toward the apical end of the epithelium. This is similar to the the organization of the mammalian cochlea, and raises the question of whether the basilar papilla membrane itself has mechanical resonance properties like the basilar membrane in the mammalian organ of Corti (Ricci et al. 2000). If that were the case, the electrical resonance of the hair cells could be viewed as increasing the ear’s existing sensitivity to specific tones, effectively acting as a gain amplifier. The question was explored in the turtle ear by laser interferometry, the result that the turtle basilar membrane appears to be broadly tuned and does not display intrinsic tonotopic resonance differences (O’Neill and Bearden 1995). Similar results have been reported from the chick cochlea (Xia et al. 2016). These results are consistent with earlier experiments in alligator lizards which used the Mossbauer source method (Peake and Ling 1980).
While the basilar membrane is not tonotopically tuned in non-mammalian species, there are differences in the morphology of stereocilia along the length of the basilar papilla, one of which is stereocilia length (Howard and Ashmore 1986; Fettiplace and Fuchs 1999; Fettiplace 2017). These differences affect the passive compliance of the stereocilia, to which the opening and closing of the mechanosensitivie channels contribute an active component (i.e., greater compliance when the channels open and reduced compliance when the channels close). The features contribute mechanical tuning to hair cells that adds to their electrical tuning (Fettiplace and Ricci 2006).
Besides enhancing frequency discrimination, membrane electrical resonance can also amplify hair cell responses by inducing oscillatory movements of the hair bundle (Martin and Hudspeth 1999; Ricci et al. 2000). Consistent with this, short hair cells of the bird auditory papilla display electrical tuning although they have no afferent function (Tan et al. 2013). The time constant of fast adaptation is related to the resonant frequency, and the active movement of the hair bundle caused by fast adaptation could generate enough force to enhance displacement of hair bundles in long hair cells (the bird analog of inner hair cells) (LeMasurier and Gillespie 2005; Tan et al. 2013).
It is likely that electrical resonance first evolved in vestibular hair cells and was further refined in the tetrapod evolution of auditory papillae. In that light it is worth noting that electrical resonance properties are also found in the vestibular neurons of mammals, where they contribute importantly to rapid perception of head movements (rotational or translational) at physiological frequencies (Vollrath and Eatock 2003; Eatock and Lysakowski 2006; Fisher et al. 2011; Songer and Eatock 2013; Venturino et al. 2015).
Auditory hair cells also have structural features that vary tonotopically along the length of the basilar membrane in the mammalian cochlea. Chief among these is the length of the stereocilia. Hair cells toward the apex, which are most sensitive to low frequencies, have longer stereocilia, and hair cells toward the basal end, sensitive to high frequencies, have shorter stereocilia. In rat cochlea, inner hair cells toward to apex with an expected best frequency of 4 kHz had stereocilia that were twice as long as those in hair cells with an expected best frequency of 30 kHz (Furness et al. 2008). These differences are likely to enhance frequency sensitivity, as the stiffness of stereocilia is inversely proportional to the square of their length in frog sacculus and turtle cochlear hair cells (Crawford and Fettiplace 1985; Howard and Ashmore 1986).
In contrast to vestibular hair cells and non-mammalian auditory hair cells, cochlear hair cells of mammals do not display electrical resonance. In the absence of electrical resonance, mammalian inner ears have evolved two complementary mechanical features to enhance tone discrimination and sound sensitivity. The first is that the basilar membrane changes in stiffness along its length, being more stiff toward the basal end and less stiff toward the apical end. This arises from differences in the width of the basilar membrane and also differences in its structure, which is thicker at the base and thinner toward the apex. As initially observed by von Bekesy on the inner ears of human cadavers, sound entering the cochlea generates traveling waves along the length of the basilar membrane, and the amplitude of displacement is tonotopically organized; the apical end resonates with lower frequencies and the basal end at higher frequencies (von Bekesy 1960). Traveling waves of this sort have not been observed in fish, amphibians, reptiles, or birds.
The second mammalian innovation is the introduction of a second type of hair cell to amplify the basilar membrane’s oscillation at specific sound frequencies. These are the outer hair cells. The cochleas of eutherian mammals comprise one row of primary sensory hair cells (inner hair cells, IHCs) and three rows of modulatory hair cells with little or no afferent function (outer hair cells, OHCs). Each inner hair cell receives afferent synapses from 10 to 15 primary afferent nerve fibers, which amounts to 90–95% of the primary afferent fibers. The outer hair cells share synaptic connection to the remaining 5–10% of afferent axons. The upshot is that the CNS auditory system oversamples the activity of IHCs by a many-to-one mapping, while averaging the activity of OHCs by a one-to-many mapping. This arrangement indicates that IHCs are the primary mediators of auditory information.
Structurally, the OHCs have more stereocilia and the W-shaped arrangement of the stereocilia is more pronounced (Furness and Hackney 2006). In addition, the tips of OHC stereocilia are physically embedded in the overlying tectorial membrane, while those of IHCs are not. The functional significance of this arrangement became apparent when William Brownell and colleagues discovered that OHCs could shorten along their base-to-apical axis when depolarized (Brownell et al. 1985). This shortening is extremely rapid and can be observed in response to imposed depolarizations at frequencies up to 100 kHz, near the top end of frequency sensitivity in bats and cetaceans (whales and dolphins). Impressively, the response amplitude and response phase of OHCs display high fidelity out to 50 kHz in vitro (Frank et al. 1999). By shortening, the OHCs increase the shearing motion of the tectorial membrane over the surface of the IHCs, thereby amplifying the displacement of the IHC stereocilia bundle.
Shortening of the OHC is accompanied by proportional increase of cell radius; the cell volume remains constant. Uniquely among vertebrate cells, OHCs have a positive turgor pressure; this is made possible by structural specializations of the cuticular plate at the cell’s apical pole and a highly regular, thick cytoskeletal layer lining the lateral and basolateral plasma membrane (Brownell 2006). Shortening of the OHC under natural conditions begins with displacement of the stereocilia bundle and opening of mechanosensitive channels at their tips. Depolarization-induced shortening results from activation of a piezoelectric effect, meaning that electrical depolarization and physical shortening are directly coupled. The protein responsible for this effect has been cloned and identified and named prestin (Dallos and Fakler 2002; Dallos et al. 2006; Dallos 2008). Prestin belongs to a family of sulfate transporters called Slc26A (Vincourt et al. 2003). Like its family members, prestin has a large number of putative transmembrane domains predicted to form alpha helices with mostly hydrophobic amino acid residues. But unlike its cousins, prestin preferentially binds Cl−, not , and it does not function as a transporter. Our current knowledge of prestin structure and function has been recently reviewed (He et al. 2014). Intracellular Cl− is required for normal OHC function, consistent with a model in which Cl- ions bind to prestin and act as the voltage sensor. Chloride ions would be predicted to move away from the cell interior during hyperpolarization and toward the cell interior during depolarization. The close coupling between charge movement and cell shortening (actuation) remains unresolved. Prestin has also been reported to contribute to amplification by short hair cells in the bird auditory papilla (Beurg et al. 2013).
One constraint of the prestin-based cochlear amplifier is that the ability of an OHC to keep pace with high frequency tones is limited by the time constant of the OHC membrane, because OHC shortening must be preceded by OHC depolarization. Since the membrane time constant is the product or membrane resistance and membrane capacitance (τ = Rm × Cm), one approach to solve this problem is to reduce Rm. This is accomplished in OHCs by increasing the concentration of endogenous intracellular Ca2+ so that about 50% of mechanotransducer channels are open at rest, which in turn depolarizes the OHC membrane potential to about −40 mV (Johnson et al, 2011). An additional effect reducing Rm is that, at −40 mV, voltage-dependent KCNQ4 channels on the OHC basolateral membrane are fully activated. This creates a “silent current” of K+ through the OHC, which may be energetically expensive but which yields high sensitivity across the frequency spectrum (Johnson et al. 2011; Nam and Fettiplace 2012; Fettiplace 2017).
Efferent modulation of the cochlear amplifier
Hair cells generally receive both afferent and efferent innervation. In mammals, the OHCs receive about 90% of the efferent innervation. Whether OHCs even contribute an afferent signal is an open question, as attempts to record afferent activity in response to OHC activation have yielded negative results. In bird cochlea, the short hair cells, which appear analogous to OHCs, receive only efferent innervation. So, what is the function of efferent synapses on hair cells?
The transmitter released at the efferent synapse has long been known to be acetylcholine (ACh), and the postsynaptic receptor for ACh in hair cells is of the nicotinic type, which has a ligand-gated channel. In most cells, such as skeletal muscle, the ACh-gated channel is a non-selective cation channel, permeable to Na+, K+, and Ca2+. The efferent synapses are on the basolateral membrane of hair cells, where the predominant extracellular cations are Na+ and Ca2+, both of which would produce inward, depolarizing currents (Glowatzki and Fuchs 2000; Oliver et al. 2000). Yet, the effect of efferent signaling is a hyperpolarizing, inhibitory synaptic potential (Brown and Nuttall 1984). How does this come about?
Like other nicotinic receptors, the receptor in hair cells is a pentamer. It has five subunits but they need not be identical. Two nicotinic subunit types, alpha-9 and alpha-10, have been identified in hair cells, and when expressed together in Xenopus oocytes they form an ACh receptor that preferentially allows Ca2+ to permeate. In fact, it is about 10 times more permeable to Ca2+ than to Na+ (Weisstaub et al., 2002). The key to the puzzle of hyperpolarization produced by inward current is the presence of Ca2+-activated K+ channels in close proximity to the ACh receptors. These channels belong to the SK2 family of potassium channels, which are sensitive to micromolar concentrations of Ca2+ and insensitive to voltage. That they lie in very close proximity to the ACh channels was demonstrated by the observation that intracellular injection of BAPTA, a calcium chelator with fast kinetics, could prevent activation of the K+ channels, while injection of EGTA, a calcium chelator with similar affinity but slow kinetics, could not block their activation. Once activated, these Ca2+-activated K+ channels remain open for a long time, due to the time required to bring intracellular Ca2+ down below the concentration that activates them (Fuchs and Murrow 1992; Rohmann et al. 2015).
The net effect of efferent synaptic activity on OHCs is to counteract the depolarizing effect of mechanical activation, thereby inhibiting shortening and reducing their amplifying effect on IHCs. Direct experiments to demonstrate this sequence of events have remained elusive, however, due to the technical difficulty of making electrical recordings from OHCs in an intact cochlea preparation. In the future, this may be an area amenable to the use of voltage-sensitive dyes rather than microelectrode recording.
The effect of efferent modulation on IHCs is more clear-cut; hyperpolarization reduces transmitter release at the afferent synapse, allowing the central auditory system to attenuate or filter out those sound frequencies. This can improve detection of specific sound frequencies against background noise (the “cocktail party” effect) and also protects the cochlea from acoustic trauma (May and McQuone 1995; Lauer and May 2011; Fuente 2015). Developmentally, cholinergic efferents synapse onto both IHCs and OHCs prior to the onset of hearing (Simmons 2002). Efferent synapses develop first on IHCs, where they are initially excitatory due to an absence of Ca2+-activated K+ channels (SK channels); efferent synapses appear several days later (P6–P8) on OHCs (Roux et al. 2011). There are also differences in the distribution of Ca2-activated K+ channels on OHCs; OHCs in the high frequency region have a higher density of large-conductance BK channels, which have faster kinetics (Rohmann et al. 2015).
What, then, does efferent modulation contribute to hearing in non-mammalian vertebrates? The answer seems to be a combination of increased overall sensitivity to sound volume and decreased sensitivity to tone. This was initially demonstrated by stimulating efferent axons while recording the electrical responses to pure tone acoustic stimuli in hair cells of the turtle cochlea (Art et al. 1985). This experimental paradigm allowed identification of the characteristic resonant frequency of the hair cell. Stimulation of the efferent axons strongly inhibited the hair cell response to tones near its characteristic frequency, as would be expected if the increased conductance to K+ acted to lower the impedance of the resonant components (voltage-gated Ca2+ channels, Ca2+- and voltage-gated K+ channels). At the same time, however, the hair cell response to lower frequencies was enhanced, but in a flat, frequency-independent manner. This can be explained by an overall increase in the cell input resistance, as the hyperpolarization induced by the efferent synapse caused closure of voltage-gated K+ and voltage-gated Ca2+ channels that were open in the resting cell (Fuchs and Parsons 2006).
In contrast to the inhibitory effects of efferent modulation in the auditory system, efferent modulation of vestibular hair cells has both excitatory and inhibitory effects (Jordan et al. 2013). Calyx-bearing afferents (similar to type I cells) are excited by efferent fibers, which synapse postsynpatically onto the afferent nerve ending (Holt et al. 2015). Bouton afferents (similar to type II cells) receive both presynaptic efferent innervation (directly onto the hair cell) and postsynaptic innervation onto the primary afferent. Efferent stimulation of bouton afferent cells is initially inhibitory, followed by an extended postinhibitory excitation (Holt et al. 2006). As in the cochlea, acetylcholine is the neurotransmitter and fast synaptic effects are mediated by alpha-9 containing ACh receptors, with inhibitory effects mediated by SK Ca2+-activated K+ channels (Parks et al. 2017). In addition, muscarinic acetylcholine receptors mediate a slower excitation in calyx-bearing afferents, probably by inhibition of an M-current (Holt et al. 2017).
Evolution of hair cells
Are hair cells a vertebrate innovation? Or do homologous cells exist among invertebrates? This has been an active topic of research and discussion, and has been reviewed in depth elsewhere (Coffin et al. 2004; Burighel et al. 2011). While many invertebrates have mechanosensitive cells bearing cilia and/or microvilli, it has been difficult to identify candidate homologs to vertebrate hair cells. Mechanosensory cells on the tentacles of the sea anemone Nematostella vectensis have many interesting properties, including bundles of actin-filled stereocilia bound together by Ca+-dependent links (Tang and Watson 2014; Menard and Watson 2017). Closer to home in Chordata, Manni and colleagues have described mechanosensitive coronal cells on the oral (incurrent) siphon of tunicates that satisfy multiple criteria for homology with vertebrate hair cells (Caicci et al. 2007; Rigon et al. 2013). These characters include embryological development from placodes, differentiation as secondary sensory cells which synapse onto primary afferents and also receive efferent innervation, and an arrangement of microvilli or stereovilli with graded length, and one or two true cilia, usually located eccentrically (Caicci et al. 2007).
Summary and future directions
Great progress has been made in our understanding of sensory hair cell structure and function. We have a functional understanding of cochlear amplification in mammals and electrical tuning of hair cells in non-mammals, both of which contribute strongly to frequency sensitivity. The motor protein for outer hair cell shortening has been identified, and at least part of the mechanosensitive transduction channel has also been identified. Still, many questions remain about outer hair cell function and there are many gaps in our basic knowledge of hair cell structure. Hair cells should continue to attract basic researchers for many decades to come, and will no doubt repay that curiosity with many experimental insights.
Acknowledgments
Thanks to the Society for Integrative Biology for hosting the symposium from which this article derives, and thanks to Billie Swalla for her encouragement and assistance in organizing the symposium. I am also grateful to the following sponsors, without whose support the symposium could not have been held: the SICB Division of Neurobiology, Neuroethology, and Sensory Biology and the Division of Evolutionary Developmental Biology, the American Microscopical Society, The Company of Biologists, the National Science Foundation, and the National Institutes of Health. Thanks also to Art Woods and two anonymous reviewers for their constructive and helpful comments on the manuscript.
Funding
This work was supported by a grant from the National Science Foundation (IOS-1809860), a grant from the National Institutes of Health (DC017092-01), a grant from the Company of Biologists, an award from the American Microscopical Society, and by the Society for Integrative and Comparative Biology through awards from the Division for Evolutionary Developmental Biology and the Division for Neurobiology, Neuroethology and Sensory Biology.
References
- Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AFJ, Marcotti W, Kros CJ, Richardson GP.. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLos ONE 6:e19183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA.. 1985. Efferent modulation of hair cell tuning in the cochlea of the turtle. J Physiol 360:397–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R.. 1987. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol 385:207–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R.. 2006. Contribution of ionic currents to tuning in auditory hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 204–48. [Google Scholar]
- Art JJ, Wu YC, Fettiplace R.. 1995. The calcium-activated potassium channels of turtle hair cells. J Gen Physiol 105:49–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Hacohen N, Corey DP.. 1989. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci USA 86:2918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP.. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7:985–94. [DOI] [PubMed] [Google Scholar]
- Barr-Gillespie P-G. 2015. Assembly of hair bundles, an amazing problem for cell biology. Persp Cell Biol Hum Health 26:2727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Seller JR, Ahmed ZM, Griffith AJ, Friedman TB.. 2005. Myosin XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7:148–56. [DOI] [PubMed] [Google Scholar]
- Beurg M, Evans MG, Hackney CM, Fettiplace R.. 2006. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26:10992–11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R.. 2017. PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells. J Physiol 595:7039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam J-H, Ricci AJ.. 2009. Localization of inner hair cell mechanotransducer channels using high speed calcium imaging. Nat Neurosci 12:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Goldring AC, Ricci AJ, Fettiplace R.. 2016. Development and localization of reverse-polarity mechanotransducer channels in cochlear hair cells. Proc Natl Acad Sci USA 113:6767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Chen Q, Fettiplace R.. 2010. Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Tan X, Fettiplace R.. 2013. A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron 79:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Xiong W, Zhao B, Müller U, Fettiplace R.. 2015. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci USA 112:1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher SK, Warren RL.. 1978. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature 273:377–8. [DOI] [PubMed] [Google Scholar]
- Brown MC, Nuttall AL.. 1984. Efferent control of cochlear inner hair cell responses in the guinea-pig. J Physiol 354:625–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. 2006. The piezoelectric outer hair cell In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 313–47. [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y.. 1985. Evoked mechanical responses in isolated cochlear outer hair cells. Science 227:194–6. [DOI] [PubMed] [Google Scholar]
- Burighel P, Caicci F, Manni L.. 2011. Hair cells in non-vertebrate models: lower chordates and molluscs. Hear Res 273:14–24. [DOI] [PubMed] [Google Scholar]
- Caicci F, Burighel P, Manni L.. 2007. Hair cells in an ascidian (Tunicata) and their evolution in chordates. Hear Res 231:63–73. [DOI] [PubMed] [Google Scholar]
- Cho S, von Gersdorff H.. 2012. Ca2+ influx and neurotransmitter release at ribbon synapses. Cell Calcium 52:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin A, Kelley M, Manley GA, Popper AN.. 2004. Evolution of sensory hair cells In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; p. 55–94. [Google Scholar]
- Colclasure JC, Holt JR.. 2003. Transduction and adaptation in sensory hair cells of the mammalian vestibular system. Grav Space Biol Bull 16:61–70. [PubMed] [Google Scholar]
- Corey DP, Holt JR.. 2016. Are TMCs the mechanotransduction channels of vertebrate hair cells?. J Neurosci 36:10921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ.. 1979. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–7. [DOI] [PubMed] [Google Scholar]
- Corns LF, Johnson SL, Kros CJ, Marcotti W.. 2014. Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci USA 111:14918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R.. 1989. Activation and adaptation of transducer currents in turtle hair cells. J Physiol 419:405–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R.. 1991. The actions of calcium on the mechano-electrical transducer current of turtle cochlear hair cells. J Physiol 434:369–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R.. 1980. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol 306:79–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R.. 1981. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol 312:377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R.. 1985. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364:359–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. 2008. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Fakler B.. 2002. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol 3:104–11. [DOI] [PubMed] [Google Scholar]
- Dallos P, Zheng J, Cheatham MA.. 2006. Prestin and the cochlear amplifier. J Physiol 576:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K.. 2001. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet 27:103–7. [DOI] [PubMed] [Google Scholar]
- Drummond MC, Barzik M, Bird JE, Zhang D-S, Lechene CP, Corey DP, Cunningham LL, Friedman TB.. 2015. Live-cell imaging of actin dynamics revelas mechanisms of stereocilia length regulation in the inner ear. Nat Comm 6:6873.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duellman WE, Trueb L.. 1994. Biology of amphibians. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Eatock RA. 2000. Adaptation in hair cells. Ann Rev Neurosci 23:285–314. [DOI] [PubMed] [Google Scholar]
- Eatock RA, Lysakowski A.. 2006. Mammalian vestibular hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 348–442. [Google Scholar]
- Eatock RA, Rüsch A, Lysakowski A, Saeki M.. 1998. Hair cells in mammalian utricles. J Otolaryngol Head Neck Surg 119:172–81. [DOI] [PubMed] [Google Scholar]
- Farris HE, Wells GB, Ricci AJ.. 2006. Steady-state adaptation of mechanotransduction modulates the resting potential of auditory hair cells, providing an assay for endolymph [Ca2+]. J Neurosci 26:12526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R. 2016. Is TMC1 the hair cell mechanotransducer channel?. Biophys J 111:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R. 2017. Hair cell transduction, tuning and synaptic transmission in the mammalian cochlea. Compr Physiol 7:1197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA.. 1999. Mechanisms of hair cell tuning. Ann Rev Physiol 61:809–34. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Kim KX.. 2014. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev 94:951–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Ricci AJ.. 2003. Adaptation in auditory hair cells. Curr Opin Neurobiol 13:446–51. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Ricci AJ.. 2006. Mechanoelectrical transduction in auditory hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 154–203. [Google Scholar]
- Fisher JAN, Kowalik L, Hudspeth AJ.. 2011. Imaging electrical resonance in hair cells. Proc Natl Acad Sci USA 108:1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SP, Krey JF, Krystofiak ES, Cui R, Nanda S, Xu W, Kachar B, Barr-Gillespie PG, Shin J-B.. 2015. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J Neurosci 35:1999–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Hemmert W, Gummer AW.. 1999. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci USA 96:4420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW.. 1992. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci 12:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Nagai T, Evans MG.. 1988. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci 8:2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Parsons TD.. 2006. The synaptic physiology of hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 249–312. [Google Scholar]
- Fuente A. 2015. The olivocochlear system and protection from acoustic trauma: a mini literature review. Front Syst Neurosci 9:94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Hackney CM.. 2006. The structure and composition of the stereociliary bundle of vertebrate hair cells In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 20–94. [Google Scholar]
- Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM.. 2008. The dimensions and composition of stereocilia rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci 28:6342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J, Jagger D.. 2010. Cochlear supporting cells In: Fuchs PA, editor. The Oxford handbook of auditory science: the ear. New York: Oxford University Press; p. 307–28. [Google Scholar]
- Giese APJ, Tang Y-Q, Sinha GP, Bowl MR, Goldring AC, Parker A, Freeman MJ, Brown SDM, Riazuddin S, Fettiplace R, et al. 2017. CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Comm 8:43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C.. 2007. The lateral line microcosmos. Genes Dev 21:2118–30. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA.. 2000. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288:2366–8. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Grant L, Fuchs P.. 2008. Hair cell afferent synapses. Curr Opin Neurobiol 18:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Broussard DM, Büttner-Enever JA, Fukushima K, Minor LB.. 2012. The vestibular system: a sixth sense. New York: Oxford University Press. [Google Scholar]
- Goodman MB, Art JJ.. 1996. Variations in the ensemble of potassium currents underlying resonance in turtle hair cells. J Physiol 497:395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Kros CJ, Richardson GP.. 2006. The development of hair cells in the inner ear In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate hair cells. New York: Springer; p. 20–94. [Google Scholar]
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP.. 2005. Development and properties of stereocilia link types in hair cells of the mouse cochlea. J Comp Neurol 485:75–85. [DOI] [PubMed] [Google Scholar]
- Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, Dumont RA, Hintermann E, Sczaniecka A, Schwander M, et al. 2009. Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron 62:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZZ, Lovas S, Ai Y, Li Y, Beisel KW.. 2014. Prestin at year 14: progress and prospect. Hear Res 311:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu HJ, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, et al. 2010. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol 8:e1000387.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RW, Wyse GA, Anderson M.. 2016. Animal physiology, 4th ed Sunderland, MA: Sinauer Associates. [Google Scholar]
- Hille B. 2001. Ion channels in excitable membranes. 3rd ed Sunderland, MA: Sinauer Associates. [Google Scholar]
- Holt JC, Jordan PM, Lysakowski A, Shah A, Barsz K, Contini D.. 2017. Muscarinic acetylcholine receptors and M-currents underlie efferent-mediated slow excitation in calyx-bearing vestibular afferents. J Neurosci 37:1873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Kewin K, Jordan PM, Cameron P, Klapczynski M, McIntosh JM, Crooks PA, Dwoskin LP, Lysakowski A.. 2015. Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents. J Neurosci 35:3625–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Lysakowski A, Goldberg JM.. 2006. Mechanisms of efferent-mediated responses in the turtle posterior crista. J Neurosci 26:13180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Eatock RA.. 1995. Inwardly rectifying currents of saccular hair cells from the leopard frog. J Neurophysiol 73:1484–502. [DOI] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance FDW, Shah K, SHokat KM, Corey DP, Mercer JA, Gillespie PG.. 2002. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108:371–81. [DOI] [PubMed] [Google Scholar]
- Holt JR, Pan B, Koussa MA, Asai Y.. 2014. TMC function in hair cell transduction. Hear Res 311:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Jones SM, Holt JR.. 2011. HCN channels expressed in the inner ear are necessary for normal balance function. J Neurosci 31:16814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Ashmore JF.. 1986. Stiffness of sensory hair cell bundles in the sacculus of the frog. Hear Res 23:93–104. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Choe Y, Mehta AD, Martin P.. 2000. Putting ion channels to work: mechanoelectical transduction, adaptation, and amplification by hair cells. Proc Nat Acad Sci USA 97:11765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP.. 1977. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA 74:2407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Jacobs R.. 1979. Stereocilia mediate transduction in vertebrate hair cells. Proc Natl Acad Sci USA 76:1506–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean P, Lopez de la Morean D, Michanski S, Tobon LMJ, Chakrabarti R, Picher MM, Neef J, Jung SY, Gültas M, Maxeiner S, et al. 2018. The synaptic ribbon is critical for sound encoding at high rates and with temporal precision. eLife 7:e29275.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti W, Fettiplace R.. 2011. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Olt J, Cho S, von Gersdorff H, Marcotti W.. 2017. The coupling between Ca2+ channels and the exocytotic Ca2+ sensor at hair cell ribbon synapses varies tonotopically along the mature cochlea. J Neurosci 37:2471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Parks XX, Contini D, Holt JC.. 2013. A review of synaptic mechanisms of vestibular efferent signaling in turtles: extrapolation of efferent actions in mammals. J Vestib Res 23:161–75. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449:87–91. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R.. 2003. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6:832–6. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ.. 2000. KCNQ4, a K+ channel mutated in a dominant form of deafness, is expressed in the inner ear and the central auditory pathway. Proc Nat Acad Sci USA 97:4333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk ME, Meredith FL, Benke TA, Rennie KJ.. 2017. AMPA receptor-mediated rapid EPSCs in vestibular calyx afferents. J Neurophysiol 117:2312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Lewis ER, Leverenz EL, Baird RA.. 1982. Acute seismic sensitivity in the bullfrog ear. Brain Res 250:168–72. [DOI] [PubMed] [Google Scholar]
- Krey JF, Dumont RA, Wilmarth PA, David LL, Johnson KR, Barr-Gillespie PG.. 2018. ELMOD1 stimulates ARF6-GTP hydrolysis to stabilize apical structures in developing vestibular hair cells. J Neurosci 38:843–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SDM, Richardson GP, Steel KP.. 2002. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 5:41–7. [DOI] [PubMed] [Google Scholar]
- Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, Millis BA, Cui R, Nakanishi H, Fujikawa T, Kawashima Y, et al. 2015. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep 12:1606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, May BJ.. 2011. The medial olivocochlear system attenuates the developmental impact of early noise exposure. J Assoc Res Otolaryngol 12:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasurier M, Gillespie PG.. 2005. Hair-cell mechanotransduction and cochlear amplification. Neuron 48:403–15. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Hudspeth AJ.. 1995. Detection of calcium entry through mechanosensing channels localizes the site of mechanoelectrical transduction in hair cells. Proc Nat Acad Sci USA 92:10297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Disanza A, Grati MH, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B.. 2011. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol 21:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Corns LF, Goodyear RJ, Rzadzinska AK, Avraham KB, Steel KP, Richardson GP, Kros CJ.. 2016. The acquisition of mechano-electrical transducer current adaptation in auditory hair cells requires myosin VI. J Physiol 594:3667–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ.. 1999. Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA 96:14306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ, McQuone SJ.. 1995. Effects of bilateral olivocochlear lesions on pure tone discrimination in cats. Aud Neurosci 1:385–400. [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Roy P, Perrin BJ.. 2017. Stereocilia morphogenesis and maintenance through regulation of actin stability. Sem Cell Dev Biol 65:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard SS, Watson GM.. 2017. Evidence for two populations of hair bundles in the sea anemone, Nematostella vectensis. Comp Biochem Physiol A 208:14–23. [DOI] [PubMed] [Google Scholar]
- Meredith FL, Rennie KJ.. 2016. Channeling your inner ear potassium: k+ channels in vestibular hair cells. Hear Res 338:40–51. [DOI] [PubMed] [Google Scholar]
- Michalski N, Goutman JD, Auclair SM, Boutet de Monvel J, Tertrais M, Emptoz A, Parrin A, Nouaille S, Guillon M, Sachse M, et al. 2017. Otoferlin acts as a Ca2+ sensor for vesicle fusion and vesicle pool replenishment at auditory hair cell ribbon synapses. eLife 6:e31013.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Booth KT, Patni P, Cortese M, Azaiez H, Bahloul A, Kahrizi K, Labbé M, Emptoz A, Lelli A, et al. 2017. CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO J Mol Med 9:1711–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. 2008. Cadherins and mechanotransduction by hair cells. Curr Opin Cell Biol 20:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J-H, Fettiplace R.. 2012. Optimal electrical properties of outer hair cells ensure cochlear amplification. PLos ONE 7:e50572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ.. 2015. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat Comm 6:6855.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narui Y, Sotomayor M.. 2018. Tuning inner-ear tip-link affinity through alternatively spliced variants of protocadherin-15. Biochem 57:1702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y.. 2008. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci USA 105:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin F, Yoshida T, Sawamura S, Ogata G, Ota T, Higuchi T, Murakami S, Doi K, Kurachi Y, HIBINO H.. 2016. The unique electrical properties in an extracellular fluid of the mammalian cochlea: their functional roles, homeostatic properties, and pathological significance. Pflugers Arch 468:1637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. 1985. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359:189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersber JP, Fakler B.. 2000. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26:595–601. [DOI] [PubMed] [Google Scholar]
- Oliver D, Knipper M, Derst C, Fakler B.. 2003. Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels. J Neurosci 23:2141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MP, Bearden A.. 1995. Laser-feedback measurements of turtle basilar membrane using direct reflection. Hear Res 84:125–38. [DOI] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kiyoto K, Kotaro I, Yoshiyuki K, Griffith A, Holt JR.. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Holt JR.. 2015. The molecules that mediate sensory transduction in the mammalian middle ear. Curr Op Neurobiol 34:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrsic T, Reisinger E, Moser T.. 2012. Otoferlin: a multi-C2 domain protein essential for hearing. Trends Neurosci 35:671–80. [DOI] [PubMed] [Google Scholar]
- Parks XP, Contini D, Jordan PM, Holt JC.. 2017. Confirming a role for α9nAChRs and SK potassium channels in type II hair cells of the turtle posterior crista. Front Cell Neurosci 11:356.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake WT, Ling A. Jr.. 1980. Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency sensitivity. J Acoust Soc Am 67:1736–45. [DOI] [PubMed] [Google Scholar]
- Peng AW, Effertz T, Ricci AJ.. 2013. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80:960–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepermans E, Petit C.. 2015. The tip-link molecular complex of the auditory mechano-electrical transduction machinery. Hear Res 330:10–7. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, et al. 2014. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R.. 2000. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 20:7131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R.. 1998. Calcium permeation of the turtle hair cell mechanotransducer channel and its relationship to the composition of endolymph. J Physiol 506:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Gray-Keller M, Fettiplace R.. 2000. Tonotopic variations of calcium signaling in turtle auditory hair cells. J Physiol 524:423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu Y-C, Fettiplace R.. 1998. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18:8261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon F, Stach T, Caicci F, Gasparini F, Burighel P, Manni L.. 2013. Evolutionary diversification of secondary mechanoreceptor cells in tunicate. BMC Evol Biol 13:112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Wersinger E, Braude JP, Pyott SJ, Fuchs PA.. 2015. Activation of BK and SK channels by efferent synapses on outer hair cells in high-frequency regions of the rodent cochlea. J Neurosci 35:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler M-C, Bahloul A, Perfettini I, Le Gall M, Rotaing P, et al. 2006. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127:277–89. [DOI] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E.. 2011. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci 31:15092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Pangrsic T.. 2012. Molecular anatomy and physiology of exocytosis in sensory hair cells. Cell Calcium 52:327–37. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E.. 2014. Glutamatergic signaling at the vestibular hair cell calyx. J Neurosci 34:14536–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G, Richter C-P, Klinke R.. 1999. Sodium, potassium, chloride and calcium concentrations measured in pigeon perilymph and endolymph. Hear Res 129:1–6. [DOI] [PubMed] [Google Scholar]
- Scheffer DI, Zhang D-S, Shen J, Indzhykulian A, Karavitaki KD, Xu YJ, Wang Q, Lin JJ-C, Chen Z-Y, Corey DP.. 2015. XIRP2, an actin-binding protein essential for inner ear hair-cell stereocilia. Cell Rep 10:1811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Müller U.. 2010. The cell biology of hearing. J Cell Biol 190:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Zheng L, Mugnaini E, Bartles JR.. 2006. Differential expression of espin isoforms during epithelial morphogenesis, stereociliogenesis and postnatal maturation in the developing inner ear. Dev Biol 291:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-B, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, et al. 2010. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30:9683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U.. 2004. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428:950–5. [DOI] [PubMed] [Google Scholar]
- Simmons DD. 2002. Development of the inner ear efferent system across vertebrate species. J Neurobiol 53:228–50. [DOI] [PubMed] [Google Scholar]
- Smotherman N, Narins P.. 1999a. Potassium currents in auditory hair cells of the frog basilar papilla. Hear Res 132:117–30. [DOI] [PubMed] [Google Scholar]
- Smotherman N, Narins P.. 1999b. The electrical properties of auditory hair cells in the frog amphibian papilla. J Neurosci 19:5275–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman M, Narins P.. 2004. Evolution of the amphibian ear In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; p. 164–99. [Google Scholar]
- Songer JE, Eatock RA.. 2013. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33:3706–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan R, Frolenkov GI.. 2009. Fast adaptation and Ca2+ sensitivity of the mechanotransducer require myosin-XVa in inner but not outer cochlear hair cells. J Neurosci 29:4023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R.. 2000. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 20:7131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Beurg M, Hackney C, Mahendrasingam S, Fettiplace R.. 2013. Electrical tuning and transduction in short hair cells of the chicken auditory papilla. J Neurophysiol 109:2007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P-C, Watson GM.. 2014. Cadherin-23 may be dynamic in hair bundles of the model sea anemone Nematostella vectensis. PLoS ONE 9:e86084.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Bullen A, Johnson SL, Grimm-Günter E-M, Rivero F, Marcotti W, Forge A, Daudet N.. 2015. Absence of plastin 1 causes abnormal maintenance of hair cell stereocilia and a moderate form of hearing loss in mice. Hum Mol Gen 24:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturino A, Oda A, Perin P.. 2015. Hair cell-type dependent expression of basolateral ion channels shapes response dynamics in the frog utricle. Front Cell Neurosci 9:00338.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincourt JB, Jullien D, Amalric F, Girar JP.. 2003. Molecular and functional characterization of SLC26A11, a sodium-independent sulfate transporter from high endothelial venules. FASEB J 17:890–2. [DOI] [PubMed] [Google Scholar]
- Vollrath MA, Eatock RA.. 2003. Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: comparison with frog saccular hair cells. J Neurophysiol 90:2676–89. [DOI] [PubMed] [Google Scholar]
- von Bekesy G. 1960. Experiments in hearing. New York: McGraw-Hill. [Google Scholar]
- Weisstaub N, Vetter DE, Elgoyhen AB, Katz E.. 2002. The alpha9-alpha10 nicotinic receptor is permeable to and is modulated by divalent cations. Hear Res 167:122–35. [DOI] [PubMed] [Google Scholar]
- Wichmann C, Moser T.. 2015. Relating structure and function of inner hair cell ribbon synapses. Cell Tissue Res 361:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms V, Koppl C, Soffgen C, Hartmann A-M, Nothwang HG.. 2016. Molecular bases of K+ secretory cells in the inner ear: shared and distinct features between bird and mammals. Nat Sci Rep 6:34203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Art JJ, Goodman MB, Fettiplace R.. 1995. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol 63:131–58. [DOI] [PubMed] [Google Scholar]
- Wu YC, Ricci AJ, Fettiplace R.. 1999. Two components of transducer adaptation in auditory hair cells. J Neurophysiol 82:2171–81. [DOI] [PubMed] [Google Scholar]
- Wu Z, Müller U.. 2016. Molecular identity of the mechanotransduction channel in hair cells: not quiet there yet. J Neurosci 36:10927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia A, Liu X, Raphael PD, Applegate BE, Oghalai JS.. 2016. Hair cell force generation does not amplify or tune vibrations within the chick basilar papilla. Nat Comm (doi: 10.1038/ncomms13133). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner Thomas FJ, Zhao Bo, Johnson Kenneth R, Kazmierczak Piotr, Müller Ulrich.. 2012. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151:1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I-Mei, Planelles-Herrero VicenteJ, Sourigues Yannick, Moussaoui Dihia, Sirkia Helena, Kikuti Carlos, Stroebel David, Titus MargaretA, Houdusse Anne.. 2017. Myosin 7 and its adaptors link cadherins to actin. Nat Comm 8:15864.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini V, Rüttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, et al. 2011. Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS Biol 9:e1001048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdebik AA, Wangemann P, Jentsch TJ.. 2009. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology 24:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-S, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP.. 2012. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481:520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Müller U.. 2015. The elusive mechanotransduction machinery of hair cells. Curr Opin Neurobiol 34:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, Müller U.. 2014. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84:954–67. [DOI] [PMC free article] [PubMed] [Google Scholar]