Abstract
Doxorubicin as a chemotherapeutic drug is widely used for the treatment of patients with cancer. However, clinical use of this drug is hampered by its cardiotoxicity, which is manifested as electrocardiographic abnormalities, arrhythmias, irreversible degenerative cardiomyopathy and congestive heart failure. The precise mechanisms underlying the cardiotoxicity of doxorubicin are not clear, but impairment of calcium homeostasis, generation of iron complexes, production of oxygen radicals, mitochondrial dysfunction and cell membrane damage have been suggested as potential etiologic factors. Compounds that can neutralize the toxic effect of doxorubicin on cardiac cells without reducing the drug’s antitumor activity are needed. In recent years, numerous studies have shown that herbal medicines and bioactive phytochemicals can serve as effective add-on therapies to reduce the cardiotoxic effects of doxorubicin. This review describes different phytochemicals and herbal products that have been shown to counterbalance doxorubicin-induced cardiotoxicity.
Keywords: adriamycin, cardiotoxicity, chemotherapy, phytochemicals
1. Introduction
Doxorubicin is used for the treatment of patients with different types of cancer, but the cardiotoxicity of this drug is a major limitation for its clinical application [1]. Doxorubicin- induced cardiotoxicity is manifested as electrocardiographic changes, irreversible degenerative cardiomyopathy, arrhythmias and congestive heart failure [2, 3]. Different factors are involved in doxorubicin-induced cardiotoxicity. One of the important mechanisms is the generation of free radicals which cause lipid peroxidation, reduction of sulfhydryl groups and depletion of antioxidant enzymes. In addition, doxorubicin causes apoptosis and DNA damage in cardiac cells [4]. Owing to the role of free radicals in doxorubicin-induced cardiotoxicity, antioxidant compounds can be of potential therapeutic value.
Animals and isolated cardiomyocytes are widely used as models for the investigation of doxorubicin-induced toxic effects. The H9c2 cell line is applied for the investigation of the protective effects of compounds against doxorubicin-induced toxicity [5–7]. In vitro studies have shown that doxorubicin induces hypertrophy in adult H9c2 cells [8, 9]. Beta blockers have been shown to improve left ventricular function by exerting an antioxidant activity [10, 11]. Dexrazoxane has also been reported to decrease the cardiotoxicity of doxorubicin, but this compound can reduce the chemotherapeutic activity of doxorubicin [12]. Drugs that are commonly used to reduce doxorubicin-induced cardiotoxicity can have adverse effects; therefore, replacing these drugs with antioxidant compounds that have fewer side effects and are less expensive, such as herbal medicines, would be better [13, 14]. In this review, we describe medicinal herbs and some active phytochemicals that have been reported to exert protective effects against doxorubicin-induced cardiotoxicity in vitro and in vivo.
2. Reduction of Doxorubicin-induced Toxicity by Using Medicinal Herbs in vitro
H9c2 is an appropriate cell line for the investigation of doxorubicin-induced cardiotoxicity. This cell line is able to differentiate skeletal or cardiac muscle phenotypes [15] (Table 1). R. turkestanicum Janisch is found in Asia and northeastern of Iran. In traditional medicine, the roots of this plant are applied for the treatment of patients with diabetes, hypertension and cancer [16]. Recent studies have shown that the rheum species contain antioxidant compounds. The antioxidant compounds, such as rhapontigenin and rhaponticin, that have been isolated from R. undulatum scavenge free radicals, hydrogen peroxide and 1,1-diphenyl- 2-picrylhydrazyl [17]. Membrane lipids and DNA are important targets for reactive oxygen species (ROS). The above-mentioned compounds reduce damage to lipids and DNA via their antioxidant properties. Doxorubicin reduces the viability of cells via generation of (ROS) and peroxidation of lipids. Hosseini et al. showed that R. turkestanicum decreased doxorubicin toxicity in H9c2 cells by attenuation of ROS production, lipid peroxidation and apoptosis. This protective effect may be related to the presence of antioxidant compounds [18].
Table 1.
Cardioprotective effect of herbal medicine against doxorubicin in in vitro studies
| Herbal medicine | Model of study | Protocol | Results | Ref. |
|---|---|---|---|---|
| R. turkestanicum | In vitro/H9c2 | Pretreatment with extract for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼lipidperoxidation ▼ROS ▼Apoptosis |
18 |
| Nigella sativa with Glycyrrhiza glabra and Zingiber officinale | In vitro/H9c2 | Pretreated with extracts for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼lipidperoxidation ▼ROS ▼Apoptosis |
26 |
| Ginkgolide B | In vitro/H9c2 | Cell were pretreated with GB for 30 min; then, incubated with DOX for 48 h | ▼Intracellular calcium ▼ROS ▼Apoptosis ▼Activation of AKT |
28 |
| C. spinose | In vitro/H9c2 | Pretreatment with extract for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼lipidperoxidation ▼ROS ▼Apoptosis |
36 |
| L. serriola | In vitro/H9c2 | Pretreatment with extract for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼lipidperoxidation ▼ROS ▼Apoptosis ▼Bax/bcl2 and cas3 |
38 |
| H. sabdariffa | In vitro/H9c2 | Pretreatment with extract for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼lipidperoxidation ▼ROS ▼Apoptosis |
42 |
| Paeoniflorin | In vitro/H9c2 | Pretreatment with for 2 h; then, incubated by using 5-μM DOX for 24 h | ▼ROS ▼Cas3 ▼NOX2, NOX4 |
43 |
| Ginkgo biloba extract 761 | Primary cultured neonatal rat cardiomyocytes | Treated with doxorubicin (1 μM) and EGb761(25 μg/mL) | ▼p53 mRNA expression ▼Apoptosis Improvement of mitochondrial membrane potential |
46 |
| C. maxima | In vitro/H9c2 | Pretreated with extract (10, 100, and 1000 μg/mL) for 30 min; then, DOX was added (0.1μM) | ▲GST ▲GSH Reduction of ROS |
48 |
Reactive oxygen species (ROS), Glutathione (GSH), Glutathione s-transferase (GST)
N. sativa, G. glabra and Z. officinale are applied in different industries. N. sativa is used as a preservative in food [19]. N. sativa has been found to decrease the cardiotoxic effect of lead via reduction of oxidative stress, the levels of pro-inflammatory cytokines and cardiac damage [20]. This plant has also been found to reduce cyclosporine-A-induced cardiotoxicity [21]. Z. officinale has different pharmacological uses such as the treatment of patients with cardiovascular disease. The beneficial effects of this plant on the cardiovascular system are related to its active compounds, such as gingerols [22]. G. glabra possesses nephroprotective [23], hypoglycemic [24] and hypocholesterolemic [25] properties. The combination of the foregoing three herbs (NGZ) was used against doxorubicin toxicity in H9c2 cells. Results showed that the combination of herbs increased cell viability and decreased lipid peroxidation. NGZ has been found to have a much higher protective effect than each plant alone because it reduces oxidative stress and inhibits apoptosis [26].
Ginkgolide B is a terpenoid obtained from Ginkgo biloba leaves. This terpenoid compound has antioxidant effects and reduces oxidative stress in different tissues [27]. This compound has been found to decrease doxorubicin toxicity in H9c2 cells via attenuation of ROS and intracellular calcium levels and elevation of Akt phosphorylation [28].
C. spinosa is applied as an anti-inflammatory [29], antibacterial [30], antioxidative [31], anti-diabetic[32], anti-hepatotoxic [33] and anti-proliferative [34] agent. Studies have shown that C. spinose contains antioxidant compounds, such as flavonoids, quercetin and kaempferol glycosides [35]. C. spinose has been found to decrease doxorubicin-induced cardiotoxicity in H9c2 cells via improvement of the antioxidant capacity and reduction of apoptosis [36].
Lactuca serriola (L. serriola, Compositae) has different names, such as Kahu, jagged lettuce, prickly lettuce, and Khas [37]. It is found in Atlantic areas, the Himalayas, Siberia, Iran, Pakistan and India [37]. In traditional medicine, different properties have been reported for L. serriola, such as antitussive, sedative, expectorant, vasorelaxant, purgative, antiseptic, diuretic and antispasmodic properties [37]. Phytochemical studies have shown that L.serriola contains lactucin, lactucone, lactucic acids, lactucopicrin, oxalic acid and sesquiterpenes [37].
Pharmacological effects of this plant include anti-inflammatory, analgesic and antioxidant activities, which are related to the high content of phenolic ingredients. This plant also reduces oxidative stress via scavenging of free radicals [37]. L. serriola has been reported to decrease lipid peroxidation and ROS production, as well as the levels of bax/bcl2 and caspase3 in H9c2 cells [38].
Hibiscus Sabdariffa (H. sabdariffa) grows in the south of Iran. Studies have shown that this plant has therapeutic effects against atherosclerosis [39], cardiovascular disease [39], hypertension and liver diseases [40, 41]. Different compounds, such as gossypitrin, anthocyanins, flavonol glycoside, myricetin, sabdaretin, quercetin, hibiscetrin, luteolin, luteolin glycoside, chlorogenic acid, flavonoids (gossypetin, hibiscetin and their respective glycosides) and protocatechuic acid, are found in this plant [40]. H9c2 cells were pretreated with H. sabdariffa extract and then incubated with doxorubicin. The extract reduced doxorubicin toxicity in H9c2 cells via decreasing the oxidative stress and apoptosis [42].
Paeoniflorin is an active ingredient that is isolated from Paeonia lactiflora and Salvinia molesta. Doxorubicin elevated apoptosis in cardiac cells via expression of caspase 3, ROS generation and upregulation of NADPH Oxidase (NOX2 and NOX4) expression. Pretreatment of H9c2 cells with paeoniflorin reduced toxicity via reduction of ROS and attenuation of NOX2, NOX4 and NOX activities [43].
Ginkgo biloba extract 761 is obtained from Ginkgo biloba extract and has different properties, such as anti-angiogenic and anti-platelet properties [44]. It also reduces cardiotoxicity after ischemia-reperfusion via antioxidant mechanisms [45]. In addition, it decreases doxorubicin toxicity in the heart via downregulation of p53, modulation of the bax/bcl2 ratio, improvement of the mitochondrial function, and free radical reduction [46].
Citrus maxima (C. maxima) belongs to the citrus family and is found in areas, such as Thailand, China, Japan and India. The juices of C. maxima contain antioxidant compounds that scavenge free radicals [47]. This herb reduces doxorubicin cardiotoxicity via decreasing oxidative stress, elevating the GSH level and enhancing the activities of antioxidant enzymes. This protection is related to the antioxidant properties of this herb [48].
Baicalein is a natural flavonoid compound found in Scutellaria baicalensis Georgi root. It has antioxidant properties and mitigates oxidative damage [49]. Baicalein has protective effects against ischemia/reperfusion injury and contractile dysfunction by scavenging mitochondrial ROS [49]. Chang and co-workers reported that baicalein lowered cardiotoxicity by decreasing mitochondrial oxidant injury and Jun N-terminal kinase (JNK) activation [50]. Co-treatment of chick cardiomyocytes with baicalein (25 μM) and doxorubicin for 24 h showed that baicalein reduced doxorubicin toxicity by decreasing ROS generation and phosphorylation of JNK. As a result, cell death was attenuated in the presence of baicalein [50].
Aspalathus linearis contains different polyphenolic compounds such as aspalathin (ASP) that has pharmacological properties, including antioxidant, anti-inflammatory and anti- apoptotic properties [51–53]. Co-treatment of H9c2 cells with doxorubicin and aspalathin (0.2 μM) for 5 days attenuated apoptosis via reduction of the bax/bcl2 ratio. Also, ASP enhanced autophagy via decreasing nucleoporin p62 through induction of adenosine monophosphate- activated protein kinase (AMPK) [52].
3. Reduction of Doxorubicin Ttoxicity by Using Medicinal Herbs in vivo
Doxorubicin-induced heart damage in rats is manifested as decreased left ventricular systolic and diastolic pressures, ejection fraction, fractional shortening, and contractility index as demonstrated by echocardiography, electrocardiography, and hemodynamic parameters relative to control animals (Table 2).
Table 2.
Cardioprotective effect of herbal medicine against doxorubicin in in vivo studies
| Herbal medicine | Model of study | Protocol | Results | Ref. |
|---|---|---|---|---|
| Ellagic acid | rat | DOX injected at a dose of 3.75 mg/kg at weeks 2, 3, 4, and 5. Extract was administered (100 and 200 mg/kg, orally) for 6 weeks. | ▲SOD ▲CAT ▲GSH ▼MDA ▼CK-MB ▼LDH Reduction morphological changes |
(58) |
| G. lucidum | rat | Extract was administered (500 and 1,000 mg/kg orally) 1 h before the doxorubicin (6 mg/kg) injection. | ▲GSH ▲CAT, ▲SOD and GPx ▼MDA ▼CK |
(62) |
| C. hystrix | rat | Animals received extract (500 and 1000 mg/kg, p.o.) for 11 days, Dox (4.67 mg/kg, i.p.) was administered on the 1st and the 6th days. | Improved histopathological changes in the heart and liver. Did not reduce AST and ALT | (63) |
| P. granatum | rat | Extract was administered (5 mL/kg) for 18 days and Dox was injected (10 mg/kg). | ▲GSH ▼QT ▼LDH ▼CK-MB Histopathological changes showed low protection against Dox |
(67) |
| Grape seed | rat | DOX (2 mg/kg/48 h, for 12 days) and GSE (100 mg/kg/24 h, for 16 days) | Improved ventricular function, structural changes and ECG | (74) |
| S. torvum | rat | DOX (67.75 mg/kg, i.v., 2 days), S. torvum extract (100 and 300 mg/kg, p.o.) | Decreased the changes in the ECG; ▼CK-MB ▼LDH ▲SOD ▲CAT Histopathological studies showed cellular infiltration. |
(80) |
| P. biglobosa | rat | Animals received extract (25 – 100 mg/kg/day) for 14 days and DOX (15 mg/kg) on the 13th day. | ▼CK-MB ▼LDH ▲SOD ▲CAT |
(81) |
| S. nigrum | rat | Received S. nigrum 1 g/kg/day p.o. daily and DOX at a dose of 20 mg/kg i.p. | ▼CK-MB ▼LDH ▲SOD ▲CAT |
(88) |
| Green tea | rat | Extract (100, 200 and 400 mg/kg, p.o.) s administered for 30 days. DOX (20 mg/kg) was administered on the 29th day. | ▲GPX ▲GST ▲GR ▲SOD ▲CAT |
(91) |
| W. somnifera | rat | Extract (300 mg/kg) was administered for 14 days and DOX (10 mg/kg) as a single dose. | ▲SOD ▲CAT ▲Bcl2 Decreasing histopathological changes |
(97) |
| C. longa | rat | Extract (200 mg/kg) was administered for 7 days and DOX (15 mg/kg) as a single dose. | ▼CK-MB ▼LDH ▼MDA ▼Cardiac calcium ▲SOD |
(111) |
| Crocin | rat | DOX (2 mg/kg/12 days), and animals received DOX (20 and 40 mg/kg/24 h for 20 days). | Reduced DOX-induced heart damage, structural changes in the myocardium and ventricular function. | (118) |
| Gingko biloba | mice | Received extract (100 mg/kg) for 4 weeks and DOX (4 mg/kg, cumulative dose 16mg/kg) | Reduced mortality, ascites, and myocardial lipid peroxidation; normalization of antioxidant enzymes; reversal of ECG changes | (120) |
| P. niruri | rat | Aqueous extract was administered (200 mg/kg) for 2 weeks and DOX was injected (2.5 mg/kg i.p.) to make 15 mg/kg. | ▲SOD, CAT and GSH ▼MDA |
(125) |
| Saffron | rabbit | The isolated heart rabbit were perfused with 30-μM DOX and 10 μg/mL of saffron. | ▼ROS ▼MDA Improvement of myocardial function |
(139) |
| G. uralensis | mice | The extract was administered (100 mg/kg) for 8 days and DOX (20mg/kg) once. | ▼CK-MB ▼LDH ▲GSH |
(145) |
| C. aronia | rat | DOX was injected (2.5 mg/kg) every 2 days for 14 days. Animals received 200 mg/kg of aqueous extract for 14 days. | ▼Myofibrils, infiltration of mononuclear cells, fibrosis and vacuolation ▼Oxidative stress ▼Lipidperoxidation ▼BNP |
(147) |
| A. sativum | rat | Garlic extract (250 mg/kg) for 27 days and a single dose of DOX (25 mg/kg) | ▼MDA ▼CPK ▼LDH |
(152) |
| Z. officinale | rat | DOX was injected 2.5 mg/kg (cumulative dose, 15mg/kg) and animals were received extract (200mg/kg) for 6 weeks | ▼MDA Improvement of ECG |
(160) |
| C. asiatica | rat | Extract was given orally (200 mg/kg) for 3 weeks. DOX was injected 2.5 mg/kg (cumulative dose, 15mg/kg) | ▲SOD, CAT, GPx, GST ▼LDH and CPK |
(165) |
| T. arjuna | rat | Dox 20 mg/kg, single dose, extract was given (0.42 mg/kg, 0.85 mg/kg, 1.7 mg/kg, 3.4 mg/kg and 6.8 mg/kg) for 6 days/week for 4 weeks. | ▼CK-MB ▼MDA Improvement of morphological changes |
(169) |
| L. barbarum | rat | DOX (5 mg/kg) three times, i.e. at 7, 14 and 21 days. The extract was given 25mg/kg for 3 weeks | Normalization of antioxidative enzymes and serum AST and CK, improvement of arrhythmias | (172) |
Catalase (CAT), malondialdehyde (MDA), super oxide dismutase (SOD), glutathion (GSH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione s-transferase (GST), B-type natriuretic peptide (BNP), lactate dehydrogenase (LDH), creatinine phosphokinase (CPK), creatinine kinase-MB (CK-MB), creatinine kinase (CK), electrocardiogram (ECG), doxorubicin (DOX).
Ellagic acid is a polyphenolic compound found in nuts and berries; it has different properties in biological systems. The pharmacological properties of this compound include antioxidant, antiapoptotic, chemopreventive, cardioprotective, anti-inflammatory, anti- cataractogenic, gastroprotective, ulcer healing, antifibrotic, antidiabetic, hypolipidemic, anti- atherosclerotic, and estrogenic/anti-estrogenic properties [54–57]. Doxorubicin reduced the level of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione, and elevated the level of malondialdehyde (MDA) in rats. Ellagic acid treatment at doses of 100 mg/kg and 200 mg/kg for 6 weeks reduced the cardiotoxic effects of doxorubicin in animals [58].
Ganoderma lucidum (G. lucidum) grows in the South of India and has different properties, such as anti-inflammatory, antitumor, nephroprotective and anti-nociceptive properties [59–61]. Doxorubicin was reported to enhance serum creatine kinase (CK) activity and lipid peroxidation in cardiac tissue, but G. lucidum extract at doses of 500 and 1000 mg/kg elevated the levels of glutathion (GSH) and the activities of SOD, glutathione peroxidase (GPx) and catalase (CAT). The CK activity, ROS generation, and lipid peroxidation were all decreased following G. lucidum administration [62].
Citrus hystrix (C. hystrix) contains high levels of flavonoids, such as naringenin and hesperidin which have antioxidant properties [61]. The ethanolic extract of C. hystrix peels reduced doxorubicin toxicity in cardiac tissue, which was related to the presence of antioxidant compounds in the extract [63].
Pomegranate (Punica granatum L.) is applied for different purposes. The peel of P. granatum has beneficial properties, such as antibacterial [64], immunomodulatory [65] and antioxidant [66] properties. Pomegranate extract decreased cardiac histopathological changes. The extract elevated the levels of antioxidant enzymes such as SOD and GSH and reduced the levels of lactate dehydrogenase (LDH), creatinine kinase-MB (CK-MB), and MDA [67].
Grape seed extract (GSE) contains high levels of antioxidant compounds which cause different pharmacological effects [68]. It improves liver function [69], arrhythmias, and lipid profiles [70, 71]. In vivo and in vitro studies have reported that this extract scavenges free radicals and reduces lipid peroxidation [72]. Isolated pro-anthocyanidins from grape seed scavenge free radicals, such as superoxide anions and hydroxyl radicals, in a way similar to β-carotene and vitamins C and E [73]. The GSE improved ventricular function and reduced histopathological modifications and antioxidant contents in the heart, but it did not decrease the antitumor effect of doxorubicin. GSE treatment has been shown to attenuate markedly doxorubicin-induced toxicity and structural changes of myocardium and to improve ventricular function. Additionally, GSE did not intervene with the antitumor effect of doxorubicin [74].
Solanum Torvum (S. torvum) is known as Turkey berry. It has pharmacological activities, such as antiviral [75], immunomodulatory [76], antioxidant [77], cardioprotective and anti- platelet [78] activities. These observed activities are related to the presence of antioxidant compounds, such as flavonoids which are able to scavenge free radicals [79]. S. torvum decreases doxorubicin toxicity via reductions of electrocardiogram (ECG) changes and CK- MB and LDH levels, as well as elevations of the levels of antioxidant enzymes, such as SOD and CAT [80].
In traditional medicine, Parkia biglobosa (P. biglobosa (Jacq.)) is applied for the treatment of arterial hypertension, amoebiasis, abscesses, burns, coughs, zoster, and bronchitis [81]. Its antioxidant effects have been previously reported [82]. Phytochemical studies have shown that this herb contains total flavonoids, tannins, saponins and cardiac glycosides. The extract decreases lipid peroxidation and the levels of CKMB and LDH, but increases the levels of cardiac glutathione, GSH, and SOD in rats treated with doxorubicin [81].
Solanum nigrum (S. nigrum) belongs to the Solanaceae family. In folk medicine, it is applied to treat patients suffering from inflammation, pain, enteric diseases and fever [83, 84]. The pharmacological effects include antioxidant, cardioprotective, anti-seizure, anti-cancer and anti-inflammatory effects [84–87]. The extract attenuates the levels of LDH, CKMB, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In addition, following doxorubicin toxicity, S. nigrum extract decreases histopathological changes and lipid peroxidation and increases the activities of antioxidant enzymes [88].
Green tea is consumed in Asia without causing side effects [89]. It contains a high level of epigallocatechin-3-gallate (EGCG), which has beneficial effects for the treatment of patients with different diseases [90]. Green tea also increases the levels of glutathione peroxidase, SOD, CAT, and glutathione s-transferase (GST) in rats experiencing doxorubicin-induced cardiotoxicity [91].
Withania somnifera (W. somnifera) belongs to the Solanaceae family and is used in folk medicine [92]. Withanolides are active compounds in the roots and the leaves of this plant [92, 93]. This plant has several pharmacological effects, including antitumor, immunomodulatory and hepatoprotective effects [3,9]. The extract has cardioprotective effects against toxic agents, such as strophanthin-K [95]. The administration of this extract has been shown to decrease myelosuppression and urotoxicity [96] in mice challenged with anti-neoplastic drugs. The animals that were pretreated with the extract showed lower degeneration in heart muscle fibers. The extract has also been shown to prevent elevation of oxidative stress following doxorubicin treatment and to increase the contents of antioxidants such as SOD and CAT. The extract also attenuates apoptosis via enhancement of Bcl2 expression [97].
Curcuma longa (Curcuma longa) is a known plant in traditional and modern medicine because of its numerous pharmacological effects, such as its antioxidant, anticancer, immunomodulatory, hepatoprotective and anti-inflammatory effects [98–110]. Moreover, curcumin metabolites, such as tetrahydrocurcumin, are active and inhibit oxidation of the membranes of erythrocytes. Ethanolic or water extract of C. longa at a dose of 200 mg/kg administered orally reduced mortality in rats that had been administered doxorubicin. It decreased the CK-MB, LDH, MDA, serum nitric oxide and cardiac calcium levels while increasing the levels of GSH and cardiac ascorbic acid. The protective effect of this herb is linked to the presence of polyphenolic compounds [111].
Crocin is a carotenoid found in Crocus sativus and has pharmacological effects, such as anti-hyperlipidemic, anti-inflammatory, antidepressant, antitumor, antioxidant and anti- atherosclerotic effects [112, 113]. Crocin also plays a role in the treatment of patients with conditions such as depression [114], anxiety [115], memory dysfunction [116] and Alzheimer’s disease [117]. Crocin reduces cardiac histopathological changes, ventricular pressure, ejection fraction, and systolic and diastolic pressures following doxorubicin administration without affecting the antitumor activity of doxorubicin [118].
Ginkgo biloba is a known plant owing to its antibiotic and anti-fungal properties [119]. People in China use the leaves of this herb as a medicine. G. biloba possesses antioxidant activity, scavenges free radicals, and reduces cyclosporine-induced hepatotoxicity. G. biloba reduces doxorubicin toxicity in cardiac cells via reducing ascites and lipid peroxidation, improves the levels of antioxidant enzymes, and reduces changes in the ECG [120].
Phyllantus niruri (P. niruri) has different pharmacological effects, including hepatoprotective, wound healing [121], antitumor [122], antioxidant, and anti-amnesic [123] effects. The antioxidant activity of this plant is related to the presence of hypophyllanthin, phyllanthin, niruriflavone, gallic acid and ellagic acid [124]. The protective effects of this herb against the cardiotoxicity of doxorubicin have been reported to be exerted via a reduction in lipid peroxidation and increases in the levels of antioxidant enzymes such as glutathione, SOD and CAT [125]. The observed effects may be related to the antioxidant properties of the plant.
Saffron (C. sativus) is known for its medicinal properties [126] such as its anticonvulsant [127–129], antioxidant [129–131], antitumor [132] and antidepressant [114, 133–136] properties. This plant also alleviates memory problems [137]. Doxorubicin induces ROS production and lipid peroxidation; it also decreases ventricular pressure, the heart rate, coronary flow, the levels of antioxidant enzymes, and changes in the myocardial structure. Treatment of animals with saffron reduced doxorubicin toxicity, decreased toxic indices, and improved cardiac function [138]. During ischemia-reperfusion, doxorubicin leads to toxicity while administration of saffron in the beginning of reperfusion prevents the p38MAPK and AKT/mTOR/4EBP1 pathways. Nevertheless, the antioxidant and the antiapoptotic effects of saffron decrease doxorubicin toxicity in cardiac tissue under ischemia-reperfusion [139].
Glycyrrhiza uralensis (G. uralensis, Radix Glycyrrhizae) is used in tobacco, dietary supplements, foods, beverages and candies [140]. It has also pharmacological effects, such as antiulcer, anti-inflammatory and anti-carcinogenic effects [141–142]. Glycyrrhizin is the active compound of G. uralensis which is used as an antidote for the alkaloid urethane, carbon tetrachloride, benzene, and saponin poisoning [143]. Studies have also reported other effects, such as anti-genotoxic and hepatoprotective effects for glycyrrhizin [144]. A recent study showed that G. uralensis decreased the levels of LDH and CK-MB and improved the activities of antioxidant enzymes, as well as the heart’s morphology, without changing the antitumor activity of doxorubicin [145].
Crataegus aronia syn. Azarolus (L) (C. aronia) grows in the mountains of the Mediterranean region and is consumed in traditional medicine as a remedy for diabetes, cancer, hyperlipidemia and cardiovascular diseases [146]. Treatment with C. aronia leads to amelioration of cardiac oxidative stress, improvement of antioxidant activity, normalization of B-type natriuretic peptide (BNP) levels and reduction of lipid peroxidation. These effects are probably related to the antioxidant activity of C. aronia [147].
Aged garlic (Allium sativum or garlic) is used in the food and drug industries in different forms, such as tablets, dried raw plant parts, and fresh, boiled and cooked products [148]. The pharmacological effects of garlic include hepatoprotective, anti-mutagenic, immunomodulatory, antioxidant and anti-carcinogenic effects [149–151]. Administration of aged garlic extract following doxorubicin treatment reduces the LDH and creatinine phosphokinase (CPK) activities and the MDA content and elevates the total antioxidant content in cardiac tissue. Aged garlic extract also reduces edema, congestion and vascular dilation, as well as histopathological changes induced by doxorubicin [152].
Gallic acid is a polyphenol found in most fruits and vegetables [153]. Gallic acid is obtained from hydrolysis of gallotannins. It scavenges free radicals and has antioxidant properties [154]. Recent studies have reported antiviral, anti-inflammatory, antibacterial and anticancer effects for this polyphenol [155–157]. Cardioprotective effects of gallic acid have been shown against isoproterenol and linden, following induction of diabetes [97]. Gallic acid decreases ROS generation and the MDA level, but increases the levels of CAT, SOD, GSH and alleviates histopathological changes and ECG modifications [158].
Zingiber officinale (Z. officinale) belongs to the Zingeberaceae family and is commonly known as ginger. Numerous pharmacological effects, such as anti-microbial, anti- proliferative, anti-inflammatory, antioxidant, neuro-protective and hepatoprotective effects, have been reported for ginger. The cardioprotective effect of Z. officinale is related to the presence of active compounds, such as gingerols [6], shogaols, methyl isogingerol [6] and paradol [159]. The ethanolic extract of ginger reduces the cardiotoxicity of doxorubicin via reductions of oxidative stress, lipid peroxidation, and ECG changes, increases in the levels of antioxidant enzymes, and decreases in histopathological alterations [160].
Centella asiatica (C. asiatica L.) is a herbal medicine that has been suggested for the treatment of patients with a memory deficit [161], hypertension, atherosclerosis and cancer [162, 163]. The active compounds of this plant include triterpenes, asiatic acid and asiaticoside [164]. Its extract has been reported to reduce ROS production, lipid peroxidation, and the levels of LDH, CPK, ALT and AST while elevating the levels of antioxidant enzymes, such as SOD, CAT, GPx and GST [165].
Terminalia arjuna (T. arjuna) is used in traditional medicine to treat patients with different types of cardiac problems. Studies have reported that the aqueous extract of this herb increases cardiac contraction [166] and exerts anti-anginal effects via a reduction in the number of anginal episodes [167]. Also, the alcoholic extract of the plant reduces the blood pressure and the heart rate in animals [168]. Moreover, T. arjuna has protective effects against doxorubicin-induced cardiotoxicity via decreasing the CKMB level, reducing morphological changes and lipid peroxidation, and increasing the levels of antioxidant enzymes [169].
The fruits of Lycium barbarum (L. barbarum) are known to possess antisenility, anti- inflammation and antipyretic effects. Antioxidant activity, scavenging of superoxide anions and prevention of superoxide production are other properties of L. barbarum [170, 171]. This herb decreases doxorubicin toxicity in the heart via a reduction of oxidative stress and a normalization of antioxidant enzymes [172].
4. Conclusion
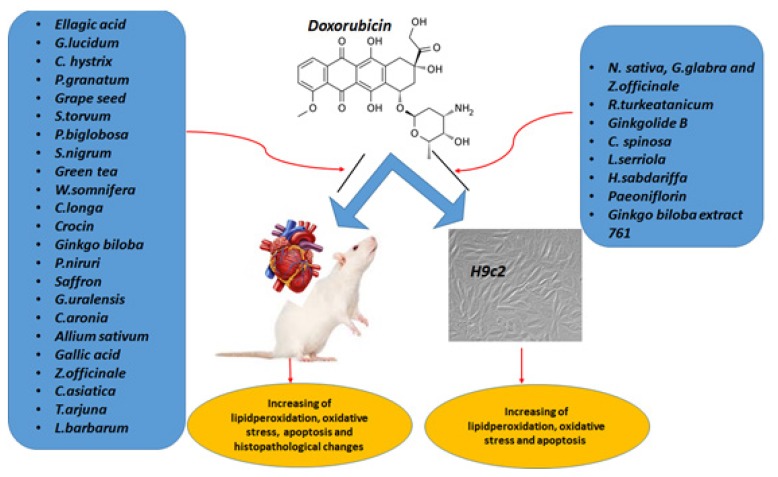
As detailed above, doxorubicin-induced toxicity is an important challenge for successful chemotherapy. For that reason, the introduction of adjuvants capable of decreasing this toxicity without impairing the drug’s anti-tumor efficacy is of particular interest. For this purpose, several plant extracts and phytochemicals have been tested and shown to attenuate the cardiotoxicity of doxorubicin (Figure I). According to mechanistic investigations, antioxidant effects may be the main mechanism behind the cardioprotective action of phytochemicals; mitigation of inflammation and apoptosis are other mechanisms that have been repeatedly reported as well.
Figure 1.
Different herbal products and phytochemicals with protective activity against doxorubicin-induced cardiotoxicity.
While the reported protective activities of phytochemicals and medicinal plants against doxorubicin-induced cardiotoxicity certainly hold promise for future developments, most of the available evidence emanates from preclinical studies. This highlights the necessity for confirmatory studies in clinical practice. However, before proof-of-concept clinical trials are carried out, it is imperative to ensure sufficient bioavailability of herbal products, particularly those that are known to have low water solubility and to cause extensive metabolism. Therefore, an elaboration of tailored pharmaceutical delivery systems might be needed to optimize bioavailability and to ensure the clinical efficacy of cardioprotective phytochemicals.
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Danesi R, Fogli S, Gennari A, Conte P, Del Tacca M. Pharmacokinetic- pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet. 2002;41(6):431–44. doi: 10.2165/00003088-200241060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wouters KA, Kremer L, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131(5):561–78. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 3.Lenneman AJ, Wang L, Wigger M, Frangoul H, Harrell FE, Silverstein C, et al. Heart transplant survival outcomes for adriamycin-dilated cardiomyopathy. Am J Cardiol. 2013;111(4):609–12. doi: 10.1016/j.amjcard.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–25. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Chua CC, Liu X, Gao J, Hamdy RC, Chua BH. Multiple actions of pifithrin-α on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol-Heart Circ Physiol. 2006;290(6):H2606–H13. doi: 10.1152/ajpheart.01138.2005. [DOI] [PubMed] [Google Scholar]
- 6.L’Ecuyer T, Sanjeev S, Thomas R, Novak R, Das L, Campbell W, et al. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol. 2006;291(3):H1273–H80. doi: 10.1152/ajpheart.00738.2005. [DOI] [PubMed] [Google Scholar]
- 7.Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Bauer JA. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol. 2005;96(1):80–7. doi: 10.1111/j.1742-7843.2005.pto960112.x. [DOI] [PubMed] [Google Scholar]
- 8.Merten KE, Jiang Y, Feng W, Kang YJ. Calcineurin activation is not necessary for Doxorubicin-induced hypertrophy in H9c2 embryonic rat cardiac cells: involvement of the phosphoinositide 3-kinase-Akt pathway. J Pharmacol Exp Ther. 2006;319(2):934–40. doi: 10.1124/jpet.106.108845. [DOI] [PubMed] [Google Scholar]
- 9.Lushnikova E, Klinnikova M, Molodykh O, Nepomnyashchikh L. Morphological manifestations of heart remodeling in anthracycline-induced dilated cardiomyopathy. Bull Exp Biol Med. 2004;138(6):607–12. doi: 10.1007/s10517-005-0138-0. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, et al. Carvedilol- mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol. 2004;200(2):159–68. doi: 10.1016/j.taap.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37(4):837–46. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–32. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 13.Nazish J, Shoukat A. Cardioprotective and antilipidemic potential of Cyperus rotundus in chemically induced cardiotoxicity. Int J Agric Biol. 2012;14(6):989–92. [Google Scholar]
- 14.Hina S, Rehman K, Dogar Z-u-H, Jahan N, Hameed M, Khan ZI, et al. Cardioprotective effect of gemmotherapeutically treated Withania somnifera against chemically induced myocardial injury. Pak J Bot. 2010;42(2):1487–99. [Google Scholar]
- 15.Branco AF, Sampaio SF, Moreira AC, Holy J, Wallace KB, Baldeiras I, et al. Differentiation-dependent doxorubicin toxicity on H9c2 cardiomyoblasts. Cardiovasc Toxicol. 2012;12(4):326–40. doi: 10.1007/s12012-012-9177-8. [DOI] [PubMed] [Google Scholar]
- 16.Dorsey JF, Kao GD. Aloe (-emodin) for cancer? More than just a comforting salve. Cancer Biol Ther. 2007;6(1):89–90. doi: 10.4161/cbt.6.1.3845. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Kang KA, Piao MJ, Lee KH, Jang HS, Park MJ, et al. Rhapontigenin from Rheum undulatum protects against oxidative-stress-induced cell damage through antioxidant activity. J Toxicol Environ Health A. 2007;70(13):1155–66. doi: 10.1080/15287390701252766. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini A, Rajabian A. Protective effect of Rheum turkestanikum root against doxorubicin-induced toxicity in H9c2 cells. Rev Bras de Farmacogn. 2016;26(3):347–51. doi: 10.1016/j.bjp.2016.02.004. [DOI] [Google Scholar]
- 19.Goreja W. Black seed: nature’s miracle remedy. Karger Publishers; 2003. [Google Scholar]
- 20.Ahmed MA, Hassanein KM. Cardio protective effects of Nigella sativa oil on lead induced cardio toxicity: Anti inflammatory and antioxidant mechanism. J Physiol Pathophysiol. 2013;4(5):72–80. doi: 10.5897/JPAP2013.0083. [DOI] [Google Scholar]
- 21.Ebru U, Burak U, Yusuf S, Reyhan B, Arif K, Faruk TH, et al. Cardioprotective effects of Nigella sativa oil on cyclosporine A-induced cardiotoxicity in rats. Basic Clin Pharmacol Toxicol. 2008;103(6):574–80. doi: 10.1111/j.1742-7843.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 22.Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardio. 2009;131(3):408–9. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 23.Bafna P, Balaraman R. Antioxidant activity of DHC-1, an herbal formulation, in experimentally-induced cardiac and renal damage. Phytothe Res. 2005;19(3):216–21. doi: 10.1002/ptr.1659. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa K, Kishida H, Arai N, Nishiyama T, Mae T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK-Ay mice. Biol Pharm Bull. 2004;27(11):1775–8. doi: 10.1248/bpb.27.1775. [DOI] [PubMed] [Google Scholar]
- 25.Lim WYA, Chia YY, Liong SY, Ton SH, Kadir KA, Husain SNAS. Lipoprotein lipase expression, serum lipid and tissue lipid deposition in orally-administered glycyrrhizic acid-treated rats. Lipids Health Dis. 2009;8(1):31. doi: 10.1186/1476-511X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini A, Shafiee-Nick R, Mousavi SH. Combination of Nigella sativa with Glycyrrhiza glabra and Zingiber officinale augments their protective effects on doxorubicin-induced toxicity in h9c2 cells. Iran J Basic Med Sci. 2014;17(12):993. [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud F, Abul H, Onadeko B, Khadadah M, Haines D, Morgan G. In vitro effects of Ginkgolide B on lymphocyte activation in atopic asthma: comparison with cyclosporin A. Jpn J Pharmacol. 2001;83(3):241–5. doi: 10.1254/jjp.83.241. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Chen T, Zhao D, Zheng J, Liu Z. Ginkgolide B exerts cardioprotective properties against doxorubicin-Induced cardiotoxicity by regulating reactive oxygen species, akt and calcium signaling pathways in vitro and in vivo. PloS one. 2016;11(12):e0168219. doi: 10.1371/journal.pone.0168219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham SVPI, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res. 2011;42(8):658–68. doi: 10.1016/j.arcmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Boga C, Forlani L, Calienni R, Hindley T, Hochkoeppler A, Tozzi S, et al. On the antibacterial activity of roots of Capparis spinosa L. Nat Prod Res. 2011;25(4):417–21. doi: 10.1080/14786419.2010.487189. [DOI] [PubMed] [Google Scholar]
- 31.Siracusa L, Kulisic-Bilusic T, Politeo O, Krause I, Dejanovic B, Ruberto G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J Agric Food Chem. 2011;59(23):12453–9. doi: 10.1021/jf203096q. [DOI] [PubMed] [Google Scholar]
- 32.Huseini HF, Hasani-Rnjbar S, Nayebi N, Heshmat R, Sigaroodi FK, Ahvazi M, et al. Capparis spinosa L.(Caper) fruit extract in treatment of type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2013;21(5):447–52. doi: 10.1016/j.ctim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Aghel N, Rashidi I, Mombeini A. Hepatoprotective activity of Capparis spinosa root bark against CCl4 induced hepatic damage in mice. Iran J Pharm Res. 2010:285–90. [Google Scholar]
- 34.Wu J-H, Chang F-R, Hayashi K-i, Shiraki H, Liaw C-C, Nakanishi Y, et al. Antitumor agents. Part 218: Cappamensin A, a new in vitro anticancer principle, from Capparis sikkimensis. Bioorganic Med Chem lett. 2003;13(13):2223–5. doi: 10.1016/S0960-894X(03)00379-2. [DOI] [PubMed] [Google Scholar]
- 35.Argentieri M, Macchia F, Papadia P, Fanizzi FP, Avato P. Bioactive compounds from Capparis spinosa subsp. Rupestris. Indust Crops Prod. 2012;36(1):65–9. doi: 10.1016/j.indcrop.2011.08.007. [DOI] [Google Scholar]
- 36.Mousavi SH, Hosseini A, Bakhtiari E, Rakhshandeh H. Capparis spinosa reduces Doxorubicin-induced cardio-toxicity in cardiomyoblast cells. Avicenna J Phytomed. 2016;6(5):488. [PMC free article] [PubMed] [Google Scholar]
- 37.Janbaz KH, Latif MF, Saqib F, Imran I, Zia-Ul-Haq M, De Feo V. Pharmacological effects of Lactuca serriola L. in experimental model of gastrointestinal, respiratory, and vascular ailments. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/304394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosseini A, Mahdian D. PROTECTIVE EFFECT OF LACTUCA SERRIOLA ON DOXORUBICIN-INDUCED TOXICITY IN H9C2 CELLS. Acta Pol Pharm. 2016;73(3):659–66. [PubMed] [Google Scholar]
- 39.Chen CC, Chou FP, Ho YC, Lin WL, Wang CP, Kao ES, et al. Inhibitory effects of Hibiscus Sabdariffa L extract on low-density lipoprotein oxidation and anti– hyperlipidemia in fructose-fed and cholesterol-fed rats. J Sci Food Agric. 2004;84(15):1989–96. doi: 10.1002/jsfa.1872. [DOI] [Google Scholar]
- 40.Ali BH, Wabel NA, Blunden G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: a review. Phytother Res. 2005;19(5):369–75. doi: 10.1002/ptr.1628. [DOI] [PubMed] [Google Scholar]
- 41.Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertension. 2015;33(6):1119–27. doi: 10.1097/HJH.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 42.Hossein A, Bakhtiari E, Mousavi SH. Protective Effect of Hibiscus Sabdariffa on Doxorubicin-induced Cytotoxicity in H9c2 Cardiomyoblast Cells (Spring 2017) Iran J Pharm Res. 2017 [PMC free article] [PubMed] [Google Scholar]
- 43.Li J-Z, Yu S-Y, Wu J-H, Shao Q-R, Dong X-M. Paeoniflorin protects myocardial cell from doxorubicin-induced apoptosis through inhibition of NADPH oxidase. Can J Physiol Pharmacol. 2012;90(12):1569–75. doi: 10.1139/y2012-140. [DOI] [PubMed] [Google Scholar]
- 44.Lu G, Wu Y, Mak YT, Wai SM, Feng ZT, Rudd JA, et al. Molecular evidence of the neuroprotective effect of Ginkgo biloba (EGb761) using bax/bcl-2 ratio after brain ischemia in senescence-accelerated mice, strain prone-8. Brain Res. 2006;1090(1):23–8. doi: 10.1016/j.brainres.2006.02.138. [DOI] [PubMed] [Google Scholar]
- 45.Trumbeckaite S, Bernatoniene J, Majiene D, Jakštas V, Savickas A, Toleikis A. Effect of Ginkgo biloba extract on the rat heart mitochondrial function. J Ethnopharmacol. 2007;111(3):512–6. doi: 10.1016/j.jep.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Liu T-J, Yeh Y-C, Ting C-T, Lee W-L, Wang L-C, Lee H-W, et al. Ginkgo biloba extract 761 reduces doxorubicin-induced apoptotic damage in rat hearts and neonatal cardiomyocytes. Cardiovasc Res. 2008;80(2):227–35. doi: 10.1093/cvr/cvn192. [DOI] [PubMed] [Google Scholar]
- 47.Haruenkit SPR. Investigation of limonoids, flavanones, total polyphenol content and antioxidant activity in seven thai pummelo cultivars. Kasetsart J. 2009;43:458–66. [Google Scholar]
- 48.Chularojmontri L, Gerdprasert O, Wattanapitayakul SK. Pummelo protects doxorubicin- induced cardiac cell death by reducing oxidative stress, modifying glutathione transferase expression, and preventing cellular senescence. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/254835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang WT, Shao ZH, Yin JJ, Mehendale S, Wang CZ, Qin Y, et al. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol. 2007;566(1–3):58–66. doi: 10.1016/j.ejphar.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang WT, Li J, Haung HH, Liu H, Han M, Ramachandran S, et al. Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. J Cell Biochem. 2011;112(10):2873–81. doi: 10.1002/jcb.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson R, Shabalala S, Louw J, Kappo AP, Muller CJF. Aspalathin Reverts Doxorubicin-Induced Cardiotoxicity through Increased Autophagy and Decreased Expression of p53/mTOR/62 Signaling. Molecules. 2017;22(10) doi: 10.3390/molecules22101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson R, Dludla P, Joubert E, February F, Mazibuko S, Ghoor S, et al. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol Nut Food Res. 2016;60(4):922–34. doi: 10.1002/mnfr.201500656. [DOI] [PubMed] [Google Scholar]
- 53.Snijman PW, Joubert E, Ferreira D, Li XC, Ding Y, Green IR, et al. Antioxidant activity of the dihydrochalcones Aspalathin and Nothofagin and their corresponding flavones in relation to other Rooibos ( Aspalathus linearis ) Flavonoids, Epigallocatechin Gallate, and Trolox. J Agric Food Chem. 2009;57(15):6678–84. doi: 10.1021/jf901417k. [DOI] [PubMed] [Google Scholar]
- 54.Priyadarsini KI, Khopde SM, Kumar SS, Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J Agric Food Chem. 2002;50(7):2200–6. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- 55.Türk G, Ateşşahin A, Sönmez M, Çeribaşi AO, Yüce A. Improvement of cisplatin- induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril. 2008;89(5):1474–81. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 56.Corbett S, Daniel J, Drayton R, Field M, Steinhardt R, Garrett N. Evaluation of the anti- inflammatory effects of ellagic acid. J Perianesth Nurs. 2010;25(4):214–20. doi: 10.1016/j.jopan.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Kannan MM, Quine SD. Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. Eur J Pharmacol. 2011;659(1):45–52. doi: 10.1016/j.ejphar.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 58.Warpe VS, Mali VR, Arulmozhi S, Bodhankar SL, Mahadik KR. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in wistar rats. J Acute Med. 2015;5(1):1–8. doi: 10.1016/j.jacme.2015.02.003. [DOI] [Google Scholar]
- 59.Sheena N, Ajith T, Janardhanan K. Anti-inflammatory and anti-nociceptive activities of Ganoderma lucidum occurring in South India. Pharm Biol. 2003;41(4):301–4. doi: 10.1076/phbi.41.4.301.15677. [DOI] [PubMed] [Google Scholar]
- 60.Jones S, Janardhanan KK. Antioxidant and antitumor activity of Ganoderma lucidum (Curt.: Fr.) P. Karst.—Reishi (Aphyllophoromycetideae) from South India. Int J Med Mushrooms. 2000;2(3) doi: 10.1615/IntJMedMushr.v2.i3.20. [DOI] [Google Scholar]
- 61.Han X, Gao S, Cheng Y, Sun Y, Liu W, Tang L, et al. Protective effect of naringenin-7- O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci Trends. 2012;6(1):19–25. doi: 10.5582/bst.2012.v6.1.19. [DOI] [PubMed] [Google Scholar]
- 62.Sheena N, Ajith T, Janardhanan K. Protective effect of methanolic extract of Ganoderma lucidum P. Karst. Reishi from South India against doxorubicin-induced cardiotoxicity in rats. Orient Pharm Exp Med. 2005;5(1):62–8. doi: 10.3742/OPEM.2005.5.1.062. [DOI] [Google Scholar]
- 63.Putri H, Nagadi S, Larasati YA, Wulandari N, Hermawan A. Cardioprotective and hepatoprotective effects of Citrus hystrix peels extract on rats model. Asian Pac J Trop Biomed. 2013;3(5):371–5. doi: 10.1016/S2221-1691(13)60079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prashanth D, Asha M, Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001;72(2):171–3. doi: 10.1016/S0367-326X(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 65.Ross RG, Selvasubramanian S, Jayasundar S. Immunomodulatory activity of Punica granatum in rabbits—a preliminary study. J Ethnopharmacol. 2001;78(1):85–7. doi: 10.1016/S0378-8741(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 66.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50(17):4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 67.Hassanpour Fard M, Ghule AE, Bodhankar SL, Dikshit M. Cardioprotective effect of whole fruit extract of pomegranate on doxorubicin-induced toxicity in rat. Pharm Biol. 2011;49(4):377–82. doi: 10.3109/13880209.2010.517758. [DOI] [PubMed] [Google Scholar]
- 68.Xia E-Q, Deng G-F, Guo Y-J, Li H-B. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–46. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khoshbaten M, Aliasgarzadeh A, Masnadi K, Farhang S, Tarzamani MK, Babaei H, et al. Grape seed extract to improve liver function in patients with nonalcoholic fatty liver change. Saudi J Gastroenterol. 2010;16(3):194. doi: 10.4103/1319-3767.65197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Najafi M, Vaez H, Zahednezhad F, Samadzadeh M, Babaei H. Study the effects of hydroalcoholic extract of grape seed (Vitis vinifera) on infarct size and cardiac arrhythmias in ischemic-reperfused isolated rat heart. Pharmaceut Sci. 2011;16:187–94. [Google Scholar]
- 71.Babaei H, Aliasgarzadeh A, Poorabdollahi P. Effect of supplementation with grape seed extract (vitis vinifera) on serum lipid profiles in patient with type 2 diabetes. Iran J Endocrin Metab. 2013;15(1):59–66. [Google Scholar]
- 72.Bagchi D, Swaroop A, Preuss HG, Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat Res. 2014;768:69–73. doi: 10.1016/j.mrfmmm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicol. 2000;148(2):187–97. doi: 10.1016/S0300-483X(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 74.Razmaraii N, Babaei H, Nayebi AM, Assadnassab G, Helan JA, Azarmi Y. Cardioprotective Effect of Grape Seed Extract on Chronic Doxorubicin-Induced Cardiac Toxicity in Wistar Rats. Adv Pharm Bull. 2016;6(3):423. doi: 10.15171/apb.2016.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arthan D, Svasti J, Kittakoop P, Pittayakhachonwut D, Tanticharoen M, Thebtaranonth Y. Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochem. 2002;59(4):459–63. doi: 10.1016/S0031-9422(01)00417-4. [DOI] [PubMed] [Google Scholar]
- 76.Israf D, Lajis NH, Somchit M, Sulaiman M. Enhancement of ovalbumin-specific IgA responses via oral boosting with antigen co-administered with an aqueous Solanum torvum extract. Life Sci. 2004;75(4):397–406. doi: 10.1016/j.lfs.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 77.Sivapriya M, Leela S. Isolation and purification of a novel antioxidant protein from the water extract of Sundakai (Solanum torvum) seeds. Food Chem. 2007;104(2):510–7. doi: 10.1016/j.foodchem.2006.11.060. [DOI] [Google Scholar]
- 78.Nguelefack TB, Feumebo CB, Ateufack G, Watcho P, Tatsimo S, Atsamo AD, et al. Anti-ulcerogenic properties of the aqueous and methanol extracts from the leaves of Solanum torvum Swartz (Solanaceae) in rats. J Ethnopharmacol. 2008;119(1):135–40. doi: 10.1016/j.jep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Václavíková R, Kondrová E, Ehrlichová M, Boumendjel A, Kovář J, Stopka P, et al. The effect of flavonoid derivatives on doxorubicin transport and metabolism. Bioorganic Med Chem. 2008;16(4):2034–42. doi: 10.1016/j.bmc.2007.10.093. [DOI] [PubMed] [Google Scholar]
- 80.Kamble S, Mohan M, Kasture S. Protective effect of Solanum torvum on doxorubicin-induced cardiactoxicity in rats. Pharmacologyonline. 2009;2:1192–204. [Google Scholar]
- 81.Komolafe K, Akinmoladun A, Olaleye T. Methanolic leaf extract of Parkia biglobosa protects against doxorubicin-induced cardiotoxicity in rats. Int J Appl Res Nat Prod. 2013;6(3):39–47. [Google Scholar]
- 82.Millogo-Kone H, Guissou I, Nacoulma O, Traore A. Comparative study of leaf and stem bark extracts of Parkia biglobosa against enterobacteria. Afr J Tradit Complement Altern Med. 2008;5(3):238–43. doi: 10.4314/ajtcam.v5i3.31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atanu F, Ebiloma U, Ajayi E. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol Mol Biol Rev. 2011;6(1):1–8. [Google Scholar]
- 84.Ravi V, Saleem TM, Patel S, Raamamurthy J, Gauthaman K. Anti-inflammatory effect of methanolic extract of Solanum nigrum Linn berries. Int J Appl Res Nat Prod. 2009;2(2):33–6. [Google Scholar]
- 85.Patel S, Gheewala N, Suthar A, Shah A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int J Pharm Pharm Sci. 2009;1(1):38–46. [Google Scholar]
- 86.Kumar V, Sharma S, Modi PK. Exploration of hepatoprotective activity of aqueous extract of Solanum nigrum-an experimental study. Int J Pharm Sci Res. 2013;4(1):464. [Google Scholar]
- 87.Nitish B, Pratim MP, Abhinit K, Atul T, Tasneem A, Uzzaman KM. Evaluation of cardio protective Activity of Methanolic Extract Of Solanum Nigrum Linn. in Rats. Int J Drug Dev Res. 2011 [Google Scholar]
- 88.Varshney P, Vishwakarma P, Sharma M, Saini M, Bhatt S, Singh G, et al. Cardioprotective effect of Solanum nigrum against doxorubicin induced cardiotoxicity-an experimental study. Int J Basic Clin Pharmacol. 2016;5(3):748–53. doi: 10.18203/2319-2003.ijbcp20161513. [DOI] [Google Scholar]
- 89.Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139(1):58–62. doi: 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]
- 90.Zheng J, Lee HCM, bin Sattar MM, Huang Y, Bian J-S. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. Eur J Pharmacol. 2011;652(1):82–8. doi: 10.1016/j.ejphar.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 91.Khan G, Haque SE, Anwer T, Ahsan MN, Safhi MM, Alam M. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol Pharm. 2014;71(5):861–8. [PubMed] [Google Scholar]
- 92.Mishra L-C, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5(4):334–46. [PubMed] [Google Scholar]
- 93.Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71(1):23–43. doi: 10.1016/S0378-8741(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 94.Jahanbakhsh SP, Manteghi AA, Emami SA, Mahyari S, Gholampour B, Mohammadpour AH, et al. Evaluation of the efficacy of Withania somnifera (Ashwagandha) root extract in patients with obsessive-compulsive disorder: A randomized double-blind placebo- controlled trial. Complement Ther Med. 2016;27:25–9. doi: 10.1016/j.ctim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 95.Dhuley JN. RETRACTED: adaptogenic and cardioprotective action of ashwagandha in rats and frogs. J Ethnopharmacol. 2000;70(1):57–63. doi: 10.1016/S0378-8741(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 96.Davis L, Kuttan G. Effect of Withania somnifera on cyclophosphamide-induced urotoxicity. Cancer lett. 2000;148(1):9–17. doi: 10.1016/S0304-3835(99)00252-9. [DOI] [PubMed] [Google Scholar]
- 97.Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24(1):63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 98.Fryer RA, Galustian C, Dalgelish AG. Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol. 2009;4(2):102–12. doi: 10.2174/157488409788185007. [DOI] [PubMed] [Google Scholar]
- 99.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–7. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Ramsewak R, DeWitt D, Nair M. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I–III from Curcuma longa. Phytomed. 2000;7(4):303–8. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 101.Ganjali S, Blesso CN, Banach M, Pirro M, Majeed M, Sahebkar A. Effects of curcumin on HDL functionality. Pharmacol Res. 2017;119:208–18. doi: 10.1016/j.phrs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 102.Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, et al. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. TheScientificWorldJournal. 2014;2014:898361. doi: 10.1155/2014/898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, et al. Curcumin: A new candidate for melanoma therapy? Int J Cancer. 2016;139(8):1683–95. doi: 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- 104.Panahi Y, Alishiri GH, Parvin S, Sahebkar A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J Diet Suppl. 2016;13(2):209–20. doi: 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- 105.Panahi Y, Ghanei M, Bashiri S, Hajihashemi A, Sahebkar A. Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res. 2015;65(11):567–73. doi: 10.1055/s-0034-1389986. [DOI] [PubMed] [Google Scholar]
- 106.Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015;34(6):1101–8. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendia LE, Sahebkar A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J Cardiovasc Pharmacol. 2016;68(3):223–9. doi: 10.1097/FJC.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 108.Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, et al. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo- controlled Trial. Phytother Res : PTR. 2016;30(9):1540–8. doi: 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- 109.Sahebkar A. Curcuminoids for the management of hypertriglyceridaemia. Nat Rev Cardiol. 2014;11(2):123. doi: 10.1038/nrcardio.2013.140-c1. [DOI] [PubMed] [Google Scholar]
- 110.Sahebkar A, Cicero AFG, Simental-Mendia LE, Aggarwal BB, Gupta SC. Curcumin downregulates human tumor necrosis factor-alpha levels: A systematic review and meta- analysis ofrandomized controlled trials. Pharmacol Res. 2016;107:234–42. doi: 10.1016/j.phrs.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 111.El-Sayed EM, El-azeem ASA, Afify AA, Shabana MH, Ahmed HH. Cardioprotective effects of Curcuma longa L. extracts against doxorubicin-induced cardiotoxicity in rats. J Med Plants Res. 2011;5(17):4049–58. [Google Scholar]
- 112.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 113.Jam IN, Sahebkar AH, Eslami S, Mokhber N, Nosrati M, Khademi M, et al. The effects of crocin on the symptoms of depression in subjects with metabolic syndrome. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2017;26(6):925–30. doi: 10.17219/acem/62891. [DOI] [PubMed] [Google Scholar]
- 114.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. I International Symposium on Saffron Biology and Biotechnology 650; 2003. [Google Scholar]
- 115.Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis P, Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomed. 2008;15(12):1135–9. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667(1):222–9. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Khalili M, Hamzeh F. Effects of active constituents of Crocus sativus L., crocin on streptozocin-induced model of sporadic Alzheimer’s disease in male rats. Iran Biomed J. 2010;14(1–2):59. [PMC free article] [PubMed] [Google Scholar]
- 118.Razmaraii N, Babaei H, Nayebi AM, Assadnassab G, Helan JA, Azarmi Y. Crocin treatment prevents doxorubicin-induced cardiotoxicity in rats. Life Sci. 2016;157:145–51. doi: 10.1016/j.lfs.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 119.Goodman J, Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophysic Res Communications. 1977;77(2):797–803. doi: 10.1016/S0006-291X(77)80048-X. [DOI] [PubMed] [Google Scholar]
- 120.Naidu M, Kumar KV, Mohan IK, Sundaram C, Singh S. Protective effect of Gingko biloba extract against doxorubicin-induced cardiotoxicity in mice. 2002 [PubMed] [Google Scholar]
- 121.Devi V, Shanbhag TV, Bairy K, Rao N, Shenoy S. Effect of Phyllanthus niruri on wound healing in rats. Indian J Physiol Pharmacol. 2004;49(4):487–90. [PubMed] [Google Scholar]
- 122.Islam A, Selvan T, Mazumder U, Gupta M, Ghosal S. Antitumour effect of phyllanthin and hypophyllanthin from Phyllanthus amarus against Ehrlich ascites carcinoma in mice. Pharmacologyonline. 2008;2:796–807. [Google Scholar]
- 123.Joshi H, Parle M. Pharmacological evidences for antiamnesic potentials of Phyllanthus amarus in mice. Afr J Biomed Res. 2007;10(2) [Google Scholar]
- 124.Sharma A, Singh RT, Handa SS. Estimation of phyllanthin and hypophyllanthin by high performance liquid chromatography in Phyllanthus amarus. Phytochem Analysis. 1993;4(5):226–9. doi: 10.1002/pca.2800040507. [DOI] [Google Scholar]
- 125.Thippeswamy A, Shirodkar A, Koti B, Sadiq AJ, Praveen D, Swamy AV, et al. Protective role of Phyllantus niruri extract in doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 2011;43(1):31. doi: 10.4103/0253-7613.75663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mohajeri SA, Hosseinzadeh H, Keyhanfar F, Aghamohammadian J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J Sep Sci. 2010;33(15):2302–9. doi: 10.1002/jssc.201000183. [DOI] [PubMed] [Google Scholar]
- 127.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8(3):394–9. [PubMed] [Google Scholar]
- 128.Hosseinzadeh H, Sadeghnia H. Protective effect of safranal on pentylenetetrazol- induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomed. 2007;14(4):256–62. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 129.Mousavi SH, Tayarani N, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30(2):185–91. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α-tocopherol. Neurosci lett. 2004;362(1):61–4. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 131.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L.(Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6(3):343–50. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molnar J, Szabo D, Pusztai R, Mucsi I, Berek L, Ocsovszki I, et al. Membrane associated antitumor effects of crocine-, ginsenoside-and cannabinoid derivates. Anticancer Res. 2000;20(2A):861–7. [PubMed] [Google Scholar]
- 133.Moshiri E, Basti AA, Noorbala A-A, Jamshidi A-H, Abbasi SH, Akhondzadeh S. Crocus sativus L.(petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomed. 2006;13(9):607–11. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 134.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi A-H, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816] BMC Complement Alternat Med. 2004;4(1):12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Javadi B, Sahebkar A, Emami SA. A survey on saffron in major islamic traditional medicine books. Iran J Basic Med Sci. 2013;16(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 136.Shafiee M, Arekhi S, Omranzadeh A, Sahebkar A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J Affect Disord. 2017;227:330–7. doi: 10.1016/j.jad.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 137.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26(3):381–6. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 138.Chahine N, Hanna J, Makhlouf H, Duca L, Martiny L, Chahine R. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm Biol. 2013;51(12):1564–71. doi: 10.3109/13880209.2013.802812. [DOI] [PubMed] [Google Scholar]
- 139.Chahine N, Makhlouf H, Duca L, Martiny L, Chahine R. Cardioprotective effect of saffron extracts against acute doxorubicin toxicity in isolated rabbit hearts submitted to ischemia-reperfusion injury. Z Naturforsch C. 2014;69(11–12):459–70. doi: 10.5560/znc.2014-0124. [DOI] [PubMed] [Google Scholar]
- 140.Lin S-P, Tsai S-Y, Hou Y-C, Chao P-DL. Glycyrrhizin and licorice significantly affect the pharmacokinetics of methotrexate in rats. J Agric Food Chem. 2009;57(5):1854–9. doi: 10.1021/jf8029918. [DOI] [PubMed] [Google Scholar]
- 141.Sheela M, Ramakrishna M, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol. 2006;6(3):494–8. doi: 10.1016/j.intimp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Kim J-Y, Park SJ, Yun K-J, Cho Y-W, Park H-J, Lee K-T. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur J Pharmacol. 2008;584(1):175–84. doi: 10.1016/j.ejphar.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 143.Agarwal R, Wang ZY, Mukhtar H. Inhibition of mouse skin tumor-initiating activity of DMBA by chronic oral feeding of glycyrrhizin in drinking water. 1991 doi: 10.1080/01635589109514126. [DOI] [PubMed] [Google Scholar]
- 144.Isbrucker R, Burdock G. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46(3):167–92. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Yang Y, Yu L, Wang Y, Liu L, Fan X. Cardioprotective effects of Glycyrrhiza uralensis extract against doxorubicin-induced toxicity. Int J Toxicol. 2011;30(2):181–9. doi: 10.1177/1091581810393033. [DOI] [PubMed] [Google Scholar]
- 146.Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73(1):221–32. doi: 10.1016/S0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 147.Shatoor AS, Ahmed MAAS. Cardioprotective effect of Crataegus aronia syn. Azarolus (L) Aqueous Extract Against Doxorubicin-Induced Cardiotoxicity and Heart Failure in Wistar Rats. J Basic Appl Sci Res. 2014;4:102–14. [Google Scholar]
- 148.Gorinstein S, Leontowicz H, Leontowicz M, Drzewiecki J, Najman K, Katrich E, et al. Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metabolism in cholesterol-fed rats. Life Sci. 2006;78(6):655–63. doi: 10.1016/j.lfs.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 149.Al-Numair KS. Hypocholesteremic and antioxidant effects of garlic (Allium sativum L.) extract in rats fed high cholesterol diet. Pak J Nutr. 2009;8(2):161–6. doi: 10.3923/pjn.2009.161.166. [DOI] [Google Scholar]
- 150.Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001;131(3):977S–9S. doi: 10.1093/jn/131.3.977S. [DOI] [PubMed] [Google Scholar]
- 151.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinolo Invest. 2015;38(11):1147–57. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 152.Alkreathy H, Damanhouri ZA, Ahmed N, Slevin M, Ali SS, Osman A-MM. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;48(3):951–6. doi: 10.1016/j.fct.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y-X, Korth M. Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circulation Res. 1995;76(4):645–53. doi: 10.1161/01.RES.76.4.645. [DOI] [PubMed] [Google Scholar]
- 154.Okuda T, Yoshida T, Hatano T. Hydrolyzable tannins and related polyphenols Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Springer; 1995. pp. 1–117. [DOI] [PubMed] [Google Scholar]
- 155.Singal P, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanisms and modulation. Mol Cell Biochem. 2000;207(1):77–86. doi: 10.1023/A:1007094214460. [DOI] [PubMed] [Google Scholar]
- 156.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 157.Rather SA, Saravanan N. Protective effect of gallic acid on immobilization induced stress in encephalon and myocardium of male albino Wistar rats. Int J Nutr, Pharmacol, Neurol Dis. 2013;3(3):269. doi: 10.4103/2231-0738.114854. [DOI] [Google Scholar]
- 158.Kulkarni J, Swamy AV. Cardioprotective effect of gallic acid against doxorubicin- induced myocardial toxicity in albino rats. Indian J Health Sci Biomed Res. 2015;8(1):28. doi: 10.4103/2349-5006.158219. [DOI] [Google Scholar]
- 159.Banerjee S, Mullick H, Banerjee J, Ghosh A. Zingiber officinale:‘a natural gold’. Int J Pharmaceutical Bio-Sci. 2011;2:283–94. [Google Scholar]
- 160.Gala AAA. Protective effect of Zingiber officinale (ginger) on doxorubicin induced oxidative cardiotoxicity in rats. Life Sci J. 2013 [Google Scholar]
- 161.Kumar MV, Gupta Y. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol. 2002;79(2):253–60. doi: 10.1016/S0378-8741(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 162.Incandela L, Belcaro G, Nicolaides A, Cesarone M. Modification of the echogenicity of femoral plaques after treatment with total triterpenic fraction of Centalla asiatica: A prospective, randomized, placebo-controlled trial. Angiology. 2001;52:S69. [PubMed] [Google Scholar]
- 163.De Sanctis M, Belcaro G, Incandela L, Cesarone M. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: A clincial, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology. 2001;52:S55. doi: 10.1177/000331970105202S11. [DOI] [PubMed] [Google Scholar]
- 164.Inamdar P, Yeole R, Ghogare A, De Souza N. Determination of biologically active constituents in Centella asiatica. J Chromatogr A. 1996;742(1–2):127–30. doi: 10.1016/0021-9673(96)00237-3. [DOI] [Google Scholar]
- 165.Gnanapragasam A, Ebenezar KK, Sathish V, Govindaraju P, Devaki T. Protective effect of Centella asiatica on antioxidant tissue defense system against adriamycin induced cardiomyopathy in rats. Life Sci. 2004;76(5):585–97. doi: 10.1016/j.lfs.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 166.Radhakrishnan R, Wadsworth R, Gray A. Terminalia arjuna, an Ayurvedic cardiotonic, increases contractile force of rat isolated atria. Phytother Res. 1993;7(3):266–8. doi: 10.1002/ptr.2650070314. [DOI] [Google Scholar]
- 167.Bharani A, Ganguli A, Mathur L, Jamra Y, Raman P. Efficacy of Terminalia arjuna in chronic stable angina: a double-blind, placebo-controlled, crossover study comparing Terminalia arjuna with isosorbide mononitrate. Indian Heart J. 2002;54(2):170–5. [PubMed] [Google Scholar]
- 168.Singh N, Kapur K, Singh S, Shanker K, Sinha J, Kohli R. Mechanism of cardiovascular action of Terminalia arjuna. Planta Med. 1982;45(06):102–4. doi: 10.1055/s-2007-971255. [DOI] [PubMed] [Google Scholar]
- 169.Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, et al. Protective effects of Terminalia arjuna against Doxorubicin-induced cardiotoxicity. J Ethnopharmacol. 2008;117(1):123–9. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 170.Wu SJ, Ng LT, Lin CC. Antioxidant activities of some common ingredients of traditional chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother Res. 2004;18(12):1008–12. doi: 10.1002/ptr.1617. [DOI] [PubMed] [Google Scholar]
- 171.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76(2):137–49. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 172.Xin YF, Zhou GL, Deng ZY, Chen YX, Wu YG, Xu PS, et al. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother Res. 2007;21(11):1020–4. doi: 10.1002/ptr.2186. [DOI] [PubMed] [Google Scholar]