Abstract
Objective
Test effectiveness of an educational intervention for general practitioners (GPs) on quality of life and depression outcomes for patients.
Design
Double-blind, cluster randomised controlled trial.
Setting
General practices in Australia between 2007 and 2010.
Participants
General practices were randomly allocated to the waitlist (n=37) or intervention (n=66) group, in a ratio of 1:2. A total of 2030 (1478 intervention; 552 waitlist) community-dwelling participants aged 75 years or older were recruited via 168 GPs (113 intervention; 55 waitlist).
Interventions
A practice-based academic detailing intervention led by a peer educator that included: (1) training in use of the GP assessment of cognition dementia screening instrument; (2) training in diagnosis and management based on Royal Australian College of General Practitioners Dementia Guidelines; (3) addressing GPs’ barriers to dementia diagnosis; and (4) a business case outlining a cost-effective dementia assessment approach.
Outcome measures
Primary outcome measures were patient quality of life and depression; secondary outcome measures were: (1) sensitivity and specificity of GP identification of dementia; (2) referral to medical specialists and/or support services; (3) patient satisfaction with care; and (4) carer quality of life, depression and satisfaction with care.
Results
The educational intervention had no significant effect on patient quality of life or depression scores after 12 months. There were however improvements in secondary outcome measures including sensitivity of GP judgement of dementia (p=0.002; OR 6.0, 95% CI 1.92 to 18.73), satisfaction with GP communication for all patients (p=0.024; mean difference 2.1, 95% CI 0.27 to 3.93) and for patients with dementia (p=0.007; mean difference 7.44, 95% CI 2.02 to 12.86) and enablement of carers (p=0.0185; mean difference 24.77, 95% CI 4.15 to 45.40).
Conclusion
Practice-based academic detailing did not improve patient quality of life or depression scores but did improve detection of dementia in primary care and patient satisfaction with GP communication.
Trial registration number
ACTRN12607000117415; Pre-results.
Keywords: geriatric medicine, dementia, primary care
Strengths and limitations of this study.
Individual and contemporaneous home assessments were completed for each participant, rather than relying on administrative data such as general practitioner (GP) records.
The educational intervention was specifically designed to address a number of identified barriers to GP identification and management of dementia and was also personalised to each GP.
Evaluation measures included detection and management of dementia and patient and carer outcomes, thus capturing the last (and essential) translational output from the intervention.
Findings relating to carers must be interpreted with caution due to their relatively high (and differential) loss to follow-up.
GP learning was not directly measured, and the adherence to dementia guidelines was assessed by self-reporting of dementia related tests and referrals by GPs.
Introduction
Dementia is a complex and variable condition that affects cognition, behaviour and the person’s ability to perform everyday tasks. The number of people living with dementia worldwide is currently estimated at 46.8 million. This number is expected to double by 2030 and almost triple by 2050, due to the increasing longevity of the world population.1 As the number of people living with dementia increases, a shift from a specialist-led approach to a primary care-based model would enable improved diagnosis and dementia care pathways to be implemented in an affordable manner.2 Primary care is ‘more local, more holistic and personalised, and more comprehensive, integrated and continuous’ than secondary care,2 and thus well placed to provide dementia identification and management and integration across primary health and social care services. This accords with the WHO identifying integrated care for the elderly at the centre of a new initiative.3 Primary care physicians can attain similar outcomes to specialists when given responsibility for dementia care,4 with further improvements attainable given appropriate training, mentoring and resources.
Timely diagnosis and management of dementia is desired by many patients with dementia and their carers5–7 to improve their access to interventions and support at the most appropriate time.8 Diagnostic disclosure of memory problems is associated with better physical and environmental quality of life (QoL) in people with dementia9 and is not associated with poorer health-related QoL.10 A timely diagnosis may help people with dementia, and their carers, understand and cope with the challenging symptoms of dementia, fulfil short-term goals and facilitate planning for the future while they are still competent to do so.11 12 Referral can be made for social support services and specialist treatments, including anti-Alzheimer’s medications that may slow the course of cognitive decline.2 4
General practitioners (GPs) fail to identify about 50% of mild dementia cases in the community13–15 and demonstrate gaps in recorded diagnostic processes against guidelines.16 A number of barriers to diagnosis of dementia can be attributed to patient, carer, GP and systemic factors.17 18 The gradual decline in functional ability in the early stages of dementia can be attributed to ‘normal’ ageing by persons with the condition or those close to them and by their GPs.19–21 The stigma associated with dementia may delay help seeking.22 Only one in five people who mention memory problems to their GP have dementia,15 so the GP may choose to observe such a patient, rather than proceeding early to what may be perceived as an expensive and alarming diagnostic assessment. Other GP-related barriers to early diagnosis include lack of knowledge12 and/or confidence,19 20 23–26 the reality that dementia diagnosis is difficult due to slow and fluctuating onset and overlap of symptoms with other diseases, lack of a definitive diagnostic test25 and the perception of dementia diagnosis as a specialist domain.27 No medication exists that will effectively reverse or halt the progress of these disorders, and GPs may not conceptualise social and system support for ongoing cognitive decline as therapeutic, and this nihilism may also hinder management.28 29
GPs’ detection and management of dementia have been addressed in several educational interventions with varying success.18 30–34 Large seminar-based interventions have limited effectiveness28; however, educational interventions that incorporated active small-group learning tasks have improved detection of dementia.32 33 The most effective educational strategies in the general practice setting incorporate an academic detailing approach35 36 that presents evidence-based content in an engaging and clinically relevant manner while allowing flexibility to address the needs and concerns of individual practitioners.34 37
The objective of this study was to determine the effectiveness of an educational intervention that included an academic detailing visit to each practice using model cases to illustrate case identification and management and designed to address individual GP needs. The barriers to GP diagnosis and management of dementia addressed were: the limited time available for consultation, attitudinal factors and lack of relevant knowledge. Further discussion with the GP elicited and addressed any additional barriers. Primary outcomes were patient focused (QoL and depression scores); secondary outcome measures included GP and carer factors.
Methods
Study design
This study (the Ageing in General Practice trial) was a cluster randomised trial with a 12-month follow-up. A parallel design was employed. Practices with participating GPs were randomly allocated in a ratio of 2:1 to either an intervention or waitlist group. Intervention practices (n=66) received a dementia-related educational peer outreach visit and completed two patient audits with feedback. Waitlist practices (n=37) completed two audits without feedback and were mailed the then-current Royal Australian College of General Practitioners (RACGP) Dementia Guidelines at 12 months.38 The rationale and study design have been reported in detail previously.39
Participants
Practices eligible for inclusion in the study were located within 30 km of each urban study site headquarters (Sydney, Newcastle, Melbourne and Adelaide) or from the rural study site of Bendigo or its surrounding towns; had community dwelling patients aged ≥75 years; and used a computerised patient database. GPs that had been involved in development of the project were excluded. The cluster randomisation has been described elsewhere.39 Briefly, a list of all eligible practices was compiled and sent to an independent party, the Centre for Epidemiology and Biostatistics at the University of Newcastle (CCEB), for randomisation. CCEB provided the approach order for the practices. A project nurse or GP visited each practice to explain the project and recruit GPs prior to allocation of the practice to intervention or waitlist. Practices were stratified by site and by size of practice as either standard or large (>5 GPs working in the practice) and then allocated to intervention or waitlist in a ratio of 2:1 in randomly rotated blocks of 3 and 6. Of the 2800 GPs approached, 168 (6%) entered the study. This sample was representative based on comparison with demographics of all active recognised GPs in Australia.40
GPs sent letters of invitation to all patients who met the inclusion criteria, inviting them to participate in the study. Those who agreed to participate responded by returning a consent form to the local study site. Patients eligible for inclusion in the study were aged ≥75 years, had visited their GP within the last 24 months and were able to speak and understand English. The exclusion criteria were Parkinson’s disease, multiple sclerosis, motor neuron disease, central nervous system inflammation, psychotic symptoms prior to recruitment, developmental disability, progressive malignancy or substance abuse, too sick to complete the study or resident of aged care facility at entry to the study.
Carers of patients were eligible for the study if they had been identified as a carer or support person by a patient scoring <80 on the revised Cambridge Cognitive Examination (CAMCOG-R),41 had prior consent from the patient with dementia for his or her carer to participate and were able to speak and understand English. Eligible carers were provided with an information pack and a letter of invitation. Those who agreed to participate responded by returning a consent form to the local study site.
Patient and public involvement
Local reference groups at the Newcastle and Sydney sites included representatives of Alzheimer’s Australia (now Dementia Australia) to provide patients’ and carers’ perspectives in best practice management of dementia. The reference groups (that also included members of local divisions of general practice, geriatricians and practice nurses) provided stakeholder input into the study protocol, requested wording changes to proposed information and consent forms, considered the RACGP dementia guidelines and adapted them for local use, for example, developed a list of local services for patients with dementia.
Acceptability of the interview process and dementia screening were measured. Participants were asked to complete a short survey, which was returned to the local study site in a reply-paid envelope to avoid bias due to the presence of the nurse or practice staff.
Results of the interview process were provided to patients via their GP to ensure that they were understood and discussed as appropriate.
Intervention
The intervention in this study consisted of an educational academic detailing session conducted at each GP’s surgery by a trained peer medical or nurse educator. GPs completed an audit of their patients prior to the education in order to obtain a baseline measure of their dementia diagnosis rates and management practices. The educational session that followed included: (1) instruction in the use of the General Practitioner assessment of Cognition (GPCOG) dementia screening instrument42; (2) an interactive presentation on dementia diagnosis, diagnostic workup and management based on the RACGP Dementia Guidelines38; (3) an exploration of the GP’s perceived barriers to dementia diagnosis; and (4) a business case outlining the cost recovery potential of dementia assessment in terms of the Australian government’s Medicare Benefits Schedule. The systemic issue of lack of time in the GP consultation was addressed by training the GPs in the use of a brief screening instrument and by discussing potential methods of obtaining assistance from the practice nurse. Case studies were used to illustrate appropriate management including behavioural, environmental and pharmacological strategies; when to refer to support services (eg, aged care assessment team, memory assessment unit, Alzheimer’s Australia, Meals on Wheels and respite care), solicitor or specialists; and carer health. Intervention GPs were provided with a full copy of the RACGP Dementia Guidelines, as well as an A4-sized summary poster at the conclusion of the academic detailing visit. A second audit was held at 12 months in order to determine the effect of the educational intervention on outcome measures while allowing sufficient time for the GP to have seen the patient several times. Following the second audit, GPs were provided with the results of the comprehensive nurse assessments conducted at baseline and 12 months and offered an opportunity for self-reflection and discussion with their academic detailer.
Waitlist GPs completed two audits (baseline and 12 months) of their patients. Waitlist GPs were mailed a written summary of their patient’s home assessment and the RACGP Dementia Guidelines after completion of the 12-month audit.
Data collection
At baseline, waitlist and intervention GPs received a list of their participating patients to audit. This audit task required GPs to provide their clinical judgement in relation to each patient’s dementia status using one of four options: no dementia, possible dementia, probable dementia and definite dementia. GPs completed a supplementary audit for any patients with possible, probable or definite dementia to gather data on memory-related tests and investigations performed (ie, paper-and-pencil test for cognition or depression, pathology and radiology) and referrals to services (including care services, memory assessment services and the Aged care Assessment Team and medical specialists). Differential diagnosis and identification of reversible causes were also requested. This audit was repeated at 12 months. Although GPs were aware that there were intervention and waitlist groups, they were not informed of their group allocation; both groups participated in the audit.
Patient and carer assessments were conducted at their home by a research nurse at baseline and 12 months. Information was collected from patients and carers relating to their personal and social circumstances including socioeconomic status using the Index of Relative Social Advantage and Disadvantage,43 QoL, depression and satisfaction with GP care. The cognitive function of patients was assessed using the GPCOG and CAMCOG-R. All nurses were trained in administration of each instrument and adhered to a standardised interview protocol to minimise interviewer bias. The specific patient characteristics collected and the instruments and criteria used have been described previously.9 15 If requested by the GP, the nurse also conducted a ‘75+Health Assessment’, an item that can be rebated under the Australian Medicare system. These data were not used by the study but were returned to the GP for his or her use. Research nurses and patients were blinded to the group allocation for the entire study.
At the completion of the baseline assessment, each patient received a letter prepared by the project manager, directing them to obtain an appointment with his or her GP. Patients in the waitlist group were seen by their GP to review their 75+Health Assessment only. For patients in the intervention group, the GP followed up on the 75+Health Assessment, readministered the GPCOG and provided care in the light of their recent education. Results of the GP-administered GPCOG were forwarded to the local study site headquarters. GPs were not informed of the outcome of the research nurse assessment until after the 12-month audit in order to determine the effectiveness of the GP education process on GP diagnosis and management of patients over the 12-month study period. Following their GP visit, patients and carers in the intervention group were asked to complete a short satisfaction survey regarding the use of the GPCOG by their GP. The survey was returned immediately to administrative staff at the GP surgery or to the study team via a reply-paid envelope.
Study outcomes
The outcome measures (collected at baseline and 12 months) used to examine the effect of the educational intervention were:
Primary outcomes
Secondary outcomes
Sensitivity and specificity of GP identification of dementia compared with the CAMCOG-R, a brief neuropsychological test battery from The Cambridge Examination for Mental Disorders of the Elderly.41 A cut-off point of 79/80 differentiates between those having dementia and those not having dementia with 93% sensitivity and 87% specificity.46 For the purposes of this study, a CAMCOG-R score of less than 80 was used as an indicator of dementia.
The number of GP reported test types (pathology, pencil-and-paper and imaging) and referrals (specialist and care support services) related to dementia.
WHOQoL-BREF scores for carers.
Beck Depression Inventory47 scores for carers (higher total scores over 13 indicative of more severe depressive symptoms).
General Practice Assessment Questionnaire Version 2 (GPAQ)48 scores for patients and carers. The GPAQ domains used in this study were related to GP communication (eight questions) and patient enablement (three questions related to patients’ ability to understand and cope with their illness or problem) following consultation with their GP. Mean domain scores were transformed into a percentage of the maximum possible score, with higher scores indicative of higher satisfaction or enablement.
GP identification of differential diagnoses.
GP identification of reversible causes of dementia (eg, depression, vitamin B12 deficiency, hypothyroidism and adverse drug reaction).
Acceptability of memory screening using the GPCOG.
Specialist and care services accessed.
Due to the low reporting of differential diagnoses or treatment of reversible causes of dementia by GPs at baseline and 12 months, the effect of the intervention on these secondary outcome measures39 could not be evaluated. Likewise, the dementia management practices of GPs during the study period, such as referral to specialists or care support services, and the services actually accessed by participants were not effectively captured. The effect of the intervention on these secondary outcomes related to management of dementia could therefore not be determined.
Sample size
We calculated that a total sample size of 150 dementia patients would have 90% power to detect a mean difference of 7.0 between waitlist and intervention in the change in prescore and postscore on any of the four domain scales of the WHOQOL-BREF with a type I error rate of 0.05. The SD used was 18.5, and the overall correlation between prescore and postscore was assumed to be 0.7. The study would also have 90% power to detect a mean difference of 0.9 on the 15-point GDS, assuming an SD of 2.442 and using the same set of assumptions for alpha and correlations.
The estimated sample size was adjusted for correlation due to clustering of patients within GP practices. Clustering within GP practices was discounted, as each GP was expected to have very few patients in the study. Assuming an intraclass correlation coefficient of 0.05 and an average cluster size of five patients per practice, the design effect is 1.2 and a total of 180 patients would be required.
An allocation of two patients in the intervention group for each patient in the control group also allowed comparisons within the intervention group related to the benefits and acceptability of a screening or case-finding approach to dementia diagnosis (to be reported separately). Therefore, 68 patients in the waitlist control group and 135 patients in the intervention group were required.
We allowed for a 15% drop out over 12 months in this elderly patient group. Thus, based on a dementia prevalence of approximately 10% in Australians aged over 75 years, we aimed to recruit a total of 2400 participants.
Statistical analyses
Sensitivity of the GP’s diagnosis was calculated as the percentage of patients that the GP correctly classified as having dementia. Specificity was calculated as the percentage of patients that the GP correctly classified as not having dementia. The difference in sensitivity of GP diagnosis of dementia between the waitlist and intervention groups was tested by fitting a generalised estimating equation (GEE) model (specifically a logistic regression) to the population with CAMCOG <80. The outcome in the model was whether the GP’s diagnosis at the 12-month audit agreed with the classification given by the CAMCOG at 12 months. The predictor variable was group (intervention or waitlist), and the clustering variable was GP practice. Site was included as a categorical covariate. A similar model was used to test the difference in specificity between the groups by fitting a logistic regression GEE to the population with CAMCOG ≥80 at baseline.
For all other outcome measures, the average score was compared between intervention and waitlist groups using a linear regression GEE. The predictor variable of interest was group, and the clustering variable was GP practice. Site was included as a categorical covariate. Baseline scores were also included as a predictor.
Results
Characteristics of participants
Of 2030 community-dwelling participants aged 75 years or older recruited via 168 GPs (table 1), 43 in the waitlist and 124 in the intervention group had dementia diagnosed as per the CAMCOG. The baseline characteristics and outcome measures for GP, patient and carer participants are shown in tables 1 and 2.
Table 1.
Characteristics of GPs, patients and carers in the waitlist and intervention groups at baseline
| Class or mean (SD) | Waitlist | Intervention | |
| Patient characteristics | (n=552) | (n=1478) | |
| Gender, n (%)* | Male | 259 (47) | 671 (45) |
| Female | 293 (53) | 805 (55) | |
| Age (years) | Mean (SD) | 81.2 (4.4) | 81.3 (4.2) |
| IRSAD | Mean (SD) | 6.7 (2.5) | 7.1 (2.5) |
| CAMCOG score | Mean (SD) | 90.6 (7.8) | 90.0 (8.2) |
| CAMCOG diagnosis, n (%) | Impaired | 43 (7.8) | 124 (8.4) |
| Not impaired | 505 (92) | 1352 (92) | |
| Carer characteristics | (n=21) | (n=90) | |
| Gender, n (%) | Male | 6 (29) | 25 (28) |
| Female | 15 (71) | 65 (72) | |
| Age (years) | Mean (SD) | 70.3 (12.8) | 73.0 (16.3) |
| IRSAD | Mean (SD) | 6.9 (2.2) | 6.5 (2.5) |
| GP characteristics | (n=55) | (n=113) | |
| Gender, n (%)* | Male | 28 (58) | 63 (58) |
| Female | 20 (42) | 46 (42) | |
| Age (years) | Mean (SD) | 51.5 (9.9) | 50.4 (8.5) |
| Practice size, n (%) | Solo | 10 (22) | 17 (16) |
| 2–4 GPs | 12 (27) | 38 (36) | |
| More than 5 GPs | 23 (51) | 52 (49) | |
| Number of patients in study | Mean (SD) | 10.0 (6.6) | 13.1 (11.6) |
*Gender was not disclosed by all patient or GP participants.
CAMCOG, Cambridge Cognitive Examination; GP, general practitioner; IRSAD, Index of Relative Social Advantage and Disadvantage.
Table 2.
Baseline outcome measures of patients, dementia patients and their carers in the waitlist and intervention groups
| Waitlist Mean (SD) |
Intervention Mean (SD) |
|
| Patient measures | (n=552) | (n=1478) |
| GDS | 1.9 (1.9) | 2.1 (2.1) |
| WHOQoL-BREF | ||
| Physical | 70.4 (15.3) | 69.5 (15.0) |
| Psychological | 72.1 (12.9) | 70.8 (12.6) |
| Social | 79.7 (13.6) | 78.8 (13.2) |
| Environmental | 81.4 (10.8) | 80.6 (11.4) |
| GPAQ | ||
| Communication | 81.4 (15.5) | 80.5 (14.4) |
| Enablement | 67.7 (31.8) | 66.0 (32.1) |
| GP management of dementia patients* | (n=63) | (n=192) |
| Number of tests per patient (0—3) | 0.79 | 1.13 |
| Number of referrals per patient (0—2) | 0.24 | 0.31 |
| Dementia patient measures† | (n=43) | (n=124) |
| Accessed memory services (% yes) | 6 (14) | 13 (12) |
| GDS | 3.5 (3.2) | 3.3 (2.8) |
| WHOQoL- | ||
| Physical | 66.45 (15.94) | 63.80 (14.98) |
| Psychological | 67.44 (13.71) | 64.80 (12.56) |
| Social | 73.58 (12.90) | 76.65 (12.69) |
| Environmental | 76.04 (10.57) | 74.60 (14.01) |
| GPAQ | ||
| Communication | 74.4 (14.2) | 75.8 (15.1) |
| Enablement | 57.0 (33.7) | 60.2 (34.6) |
| Carer measures | (n=21) | (n=90) |
| Beck Depression Inventory | 10.3 (8.6) | 8.5 (6.3) |
| WHOQoL-BREF | ||
| Physical | 69.6 (16.7) | 70.2 (13.8) |
| Psychological | 68.4 (14.4) | 69.0 (10.9) |
| Social | 76.0 (19.2) | 77.1 (12.2) |
| Environmental | 77.5 (12.5) | 77.2 (11.6) |
| GPAQ | ||
| Communication | 67.9 (21.1) | 78.4 (16.8) |
| Enablement | 50.0 (35.8) | 61.2 (36.4) |
*Participants with GP audit of possible, probable or definite dementia.
†Participants with CAMCOG <80.
CAMCOG, Cambridge Cognitive Examination; GDS, Geriatric Depression Scale; GP, general practitioner; GPAQ, General Practice Assessment Questionnaire.
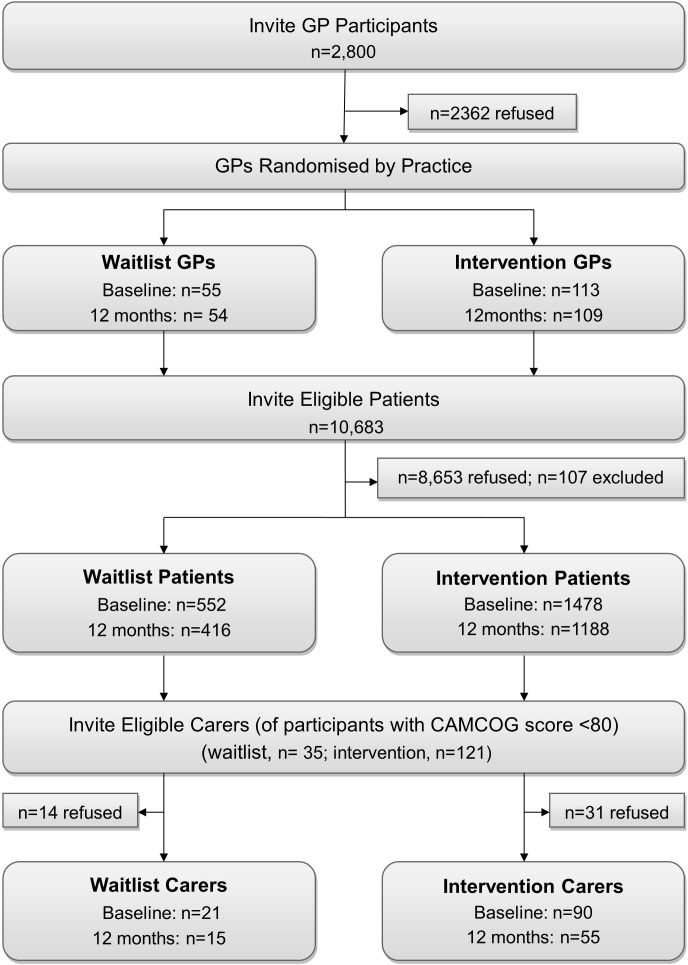
The 12-month assessment was completed by 97% of GPs (98% waitlist; 96% intervention), 79% of patients (75% waitlist; 80% intervention) and 63% of carers (71% waitlist; 61% intervention) who entered the study (figure 1).
Figure 1.
Flow of participants through the study. Baseline patient interviews were conducted from May 2007 to November 2009; 12-month interviews were conducted from August 2008 to December 2010. CAMCOG, Cambridge Cognitive Examination; GP, general practitioner.
Primary outcome measures
Outcome measures were examined for all patients and separately for patients with CAMCOG-R dementia. In both populations, there was no significant difference in depression (all patients: p=0.683; patients with dementia: p=0.333) or QoL domain scores (all patients: p=0.488 (physical), p=0.318 (psychological), p=0.959 (social), p=0.627 (environmental); patients with dementia: p=0.186 (physical), p=0.133 (psychological), p=0.730 (social), p=0.766 (environmental)) for the waitlist and intervention groups at 12 months (table 3).
Table 3.
Twelve-month outcome measures of patients, dementia patients and their carers in the waitlist and intervention groups
| Waitlist Mean (SD) |
Intervention Mean (SD) |
Intervention versus waitlist Difference at 12 months adjusted for baseline and site Mean (95% CI) or OR (95% CI) |
Adjusted p value (GEE) | |
| Patient measures | (n=416*) | (n=1188*) | ||
| GDS | 2.1 (2.2) | 2.1 (2.0) | −0.04 (−0.22 to 0.14) | 0.6832 |
| WHOQoL – BREF: | ||||
| Physical | 68.7 (15.6) | 68.8 (14.9) | 0.45 (−0.83 to 1.73) | 0.4880 |
| Psychological | 71.5 (12.9) | 71.0 (12.3) | 0.67 (−0.65 to 2) | 0.3183 |
| Social | 77.7 (12.9) | 77.1 (12.2) | −0.04 (−1.5 to 1.42) | 0.9593 |
| Environmental | 81.1 (10.8) | 79.9 (11.6) | −0.35 (−1.77 to 1.07) | 0.6272 |
| GPAQ | ||||
| Communication | 78.3 (16.4) | 79.7 (15.0) | 2.1 (0.27 to 3.93) | 0.0242 |
| Enablement | 63.4 (34.9) | 64.3 (32.5) | 1.23 (−3.71 to 6.18) | 0.6248 |
| GP management of dementia patients* | (n=44) | (n=166) | ||
| Number of tests per patient (0—3) | 0.64 | 0.55 | OR: 1.01 (0.52 to 1.97) | 0.9729 |
| Number of referrals per patient (0—2) | 0.16 | 0.18 | OR: 1.50 (0.55 to 4.10) | 0.4285 |
| Dementia patient measures† | (n=34) | (n=82) | ||
| Accessed memory services (% yes) | 3 (11%) | 9 (13%) | OR: 2.15 (0.32 to 14.49) | 0.4333 |
| GDS | 3.1 (2.9) | 2.7 (2.0) | −0.41 (−1.24 to 0.42) | 0.3335 |
| WHOQoL-BREF | ||||
| Physical | 65.4 (16.0) | 66.0 (12.6) | 2.55 (−1.23 to 6.34) | 0.1864 |
| Psychological | 66.4 (15.0) | 65.2 (12.6) | 2.63 (−0.8 to 6.07) | 0.1334 |
| Social | 73.6 (13.2) | 75.0 (11.6) | 0.79 (−3.7 to 5.29) | 0.7297 |
| Environmental | 77.1 (13.6) | 75.6 (12.2) | 0.54 (−3.01 to 4.09) | 0.7660 |
| GPAQ | ||||
| Communication | 72.6 (18.6) | 78.6 (15.3) | 7.44 (2.02 to 12.86) | 0.0072 |
| Enablement | 55.8 (36.1) | 63.9 (33.3) | 5.65 (−8.68 to 19.98) | 0.4395 |
| Carer measures | (n=15) | (n=55) | ||
| Beck Depression Inventory | 8.2 (6.2) | 7.3 (4.4) | −2.67 (−6.93 to 1.59) | 0.2195 |
| WHOQoL-BREF: | ||||
| Physical | 76.3 (10.4) | 69.8 (12.3) | −5.15 (−13.02 to 2.72) | 0.1995 |
| Psychological | 71.4 (7.6) | 69.0 (11.3) | 1.58 (−2.56 to 5.71) | 0.4556 |
| Social | 73.8 (16.0) | 73.3 (13.5) | 5.88 (−2.89 to 14.66) | 0.1889 |
| Environmental | 81.0 (6.5) | 77.6 (11.1) | −1.53 (−5.77 to 2.70) | 0.4786 |
| GPAQ | ||||
| Communication | 75.0 (13.2) | 77.1 (19.0) | 1.91 (−8.02 to 11.85) | 0.7060 |
| Enablement | 42.9 (33.8) | 64.1 (37.1) | 24.77 (4.15 to 45.40) | 0.0185 |
*Participants with GP audit of possible, probable or definite dementia at 12 months.
†Participants with CAMCOG <80 at 12 months.
CAMCOG, Cambridge Cognitive Examination; GDS, Geriatric Depression Scale; GEE, generalised estimating equation; GP, general practitioner; GPAQ, General Practice Assessment Questionnaire.
Secondary outcome measures
Detection of dementia
The percentage of patients with CAMCOG-R dementia who were correctly identified by the GP (as having possible, probable or definite dementia) was similar in the waitlist (43%) and intervention (45%) groups at baseline (table 4). At 12 months following a single educational visit in the intervention group and prior to feedback on the baseline audit, there was an increase (to 65%) in the percentage of patients who were correctly identified as having dementia in the intervention group but a decrease (to 29%) in the waitlist group (table 4). After adjusting for baseline values, the sensitivity of GP judgement of dementia was significantly higher in the intervention than the waitlist group at 12 months (p=0.002; OR 6.0, 95% CI 1.92 to 18.73). This means that GPs who had received training in the value of diagnosing dementia and in the use of a screening instrument were more likely to detect dementia than GPs who did not receive the training.
Table 4.
Sensitivity and specificity of the GP audit (compared with CAMCOG-R, a standardised instrument used to measure the extent of dementia) in the waitlist and intervention groups at baseline and 12 months
| Baseline | 12 months | Intervention versus waitlist difference at 12 months* OR (95% CI) |
Adjusted p value (GEE) | |||
| Waitlist (n=548) |
Intervention (n=1476) |
Waitlist (n=415) | Intervention (n=1187) | |||
| Sensitivity, n (%)† | 18 (43) | 55 (45) | 7 (29) | 47 (65) | 6.00 (1.92 to 18.73) | 0.0020 |
| Specificity, n (%)‡ | 429 (91) | 1196 (90) | 272 (88) | 844 (88) | 0.79 (0.38 to 1.65) | 0.5298 |
*Adjusted for baseline and site.
†Patients with CAMCOG-R score <80 that were judged by GP as having possible, probable or definite dementia.
‡Patients with CAMCOG-R score ≥80 that were judged not to have dementia by their GP.
CAMCOG-R, revised Cambridge Cognitive Examination; GEE, generalised estimating equation; GP, general practitioner.
Approximately 90% of patients without CAMCOG-R dementia were correctly identified by waitlist and intervention GPs at baseline and 12 months. That is, the specificity of GP judgement of dementia was approximately 90% at baseline and 88% at 12 months in both the waitlist and intervention groups (table 4). The lack of any significant difference in specificity between the groups at 12 months (p=0.530) indicates that the higher sensitivity in the intervention group was not at a significant cost to specificity. The number of diagnostic assessment test types (pencil-and-paper, pathology and radiology) and referrals (specialist and services) per patient was recorded at baseline (table 2) and 12 months (table 3) for those patients with a GP judgement of dementia. There was no difference between the intervention and waitlist group in the number of tests or referrals per patient at baseline (tests: p=0.05; referrals: p=0.53) or 12 months (tests: p=0.973; referrals: p=0.429).
Satisfaction with care
Satisfaction with GP communication was higher in the intervention group compared with control at 12 months for all patients (p=0.024; mean difference 2.1, 95% CI 0.27 to 3.93) and for patients with CAMCOG-R dementia (p=0.007; mean difference 7.44, 95% CI 2.02 to 12.86).
Of the 245 patients in the intervention group who returned their survey on acceptability of the GPCOG screening test administered by their GP, 68.4% liked the examination and a further 30.3% were neutral; 78.3% felt reassured that the GP had checked their memory and concentration, while less than 1% felt irritated or very irritated by the examination.
Secondary outcome measures for carers of patients with CAMCOG-R dementia
Carer outcomes measures at 12 months (adjusted for baseline and site) are presented in table 3. There was no significant difference in depression or QoL domain scores for the waitlist and intervention groups at 12 months (table 3). Carers in the intervention group had a higher GPAQ enablement score (p=0.019; mean difference 24.77, 95% CI 4.15 to 45.40) at 12 months.
Discussion
Principal findings
This study examined the effects of a dementia-related educational intervention for GPs. The primary outcome measures related to QoL and depression scores for patients and carers were not affected by the intervention. There were, however, significant findings for several secondary outcome measures, including a significant improvement in the identification of patients with dementia by GPs in the intervention compared with the waitlist group. The higher sensitivity of GP clinical judgement of dementia in the intervention group was not at a significant cost to specificity, which remained similar in the two groups. Satisfaction with GP communication was higher at follow-up in the intervention group compared with the waitlist group for all patients and specifically for those with dementia. Carer satisfaction with GP communication was not significantly different between the groups at 12 months; however, carers of people with dementia in the intervention group reported higher enablement (ie, better ability to understand and cope with their situation). We found no difference in GP management of dementia between intervention and control groups based on the number of tests and referrals to specialists or care services. It may be that the intervention had a stronger emphasis on identification of dementia than on these aspects of management due to the time spent addressing attitudinal barriers to dementia identification.
Comparison with other studies
Considering how little is known about the trajectory of QoL across the stages of dementia or its responsiveness to change49; it was an optimistic choice of primary outcome measure for a GP-based educational intervention. QoL is a complex multidimensional construct, with no strong common or unique predictors identified across the stages of dementia (reviewed by ref 50). The other primary outcome measure for this study, patient depression score, is consistently but moderately associated with decreased QoL in dementia, especially in the early stage50 but also difficult to address in people with dementia, with both psychological and pharmacological approaches to treatment having mixed and marginal effects (reviewed by ref 51). Although improvement in patient-related outcomes such as QoL and depression scores may be the ultimate goal, and are certainly important outcome measures, their responsiveness to changes in GP in diagnosis and management may be slow and dependent on the effectiveness of downstream support services. Most other GP-based interventions for dementia have used behaviour, performance and practice of the health professional as the primary study outcomes.30–34
The improvement of dementia detection compared with waitlist by GPs following our practice-based educational intervention is consistent with previous studies using a small-group workshop, decision support software or an interactive seminar approach.32 33 The GPCOG proved to be an effective element of the intervention.52 Adherence to management guidelines was not improved by any of these interventions but was improved in a study that combined the educational intervention with appointment of dementia care managers.53 Despite the lack of change in adherence to management guidelines in our study, in terms of test ordering and referrals, the improvement in satisfaction with GP communication and/or enablement in patients and carers in the intervention compared with the waitlist suggest some other changes in GP management of dementia patients not measured here. An external audit process conducted by independent clinical research staff may be more effective at capturing dementia-related management during GP clinical encounters than the GP self-report by audit process used in this study.
Satisfaction with GP communication encompasses a number of factors (including provision of adequate time, exploring patients’ needs, listening, explaining, giving information and sharing decisions) and is a strong predictor of overall satisfaction with primary care.54 Effective GP–patient communication can potentially have a significant impact on patients’ QoL; it is positively associated with psychological QoL in people with dementia and with physical, psychological, social and environmental QoL in elderly patients without dementia.9 Despite the higher satisfaction with GP communication found in this study, there was no concomitant difference in QoL measures for patients or carers at 12 months as a result of the intervention. Importantly though, the improvement in the rate of dementia identification in the intervention group compared with the waitlist did not result in a decline in any of the QoL domains, a concern expressed previously by both carers and GPs.55–57
The improvement in enablement scores for carers of people with dementia in the intervention, compared with the waitlist group, at 12 months indicates carers’ increased capacity and confidence with respect to treatment and self-management. Since enablement is related more to the communication and empathy characteristics of the GP than to the fulfilment of patient or carer expectation regarding service outcomes,54 58 there may have been some change in the GP management of patients with dementia that was not captured by monitoring rates of tests and referrals. Unfortunately, the improvement in enablement scores was not accompanied by any difference in QoL scores for carers. This is consistent with the literature; there is little evidence that support-based interventions for caregivers of people with dementia are effective.59–62
Strengths and limitations
This study is strengthened by the use of individual and contemporaneous home assessment of each participant, meaning that the project assessed current dementia status using a standardised instrument (CAMCOG-R) rather than relying on administrative data such as GP records, commonly used in GP research. The educational intervention used activities that had proved effective in previous research; was specifically designed to address a number of identified barriers to GP identification and management of dementia; and was personalised to each GP. An additional strength is that the effect of the intervention was assessed on detection and management of dementia and on patient and carer outcomes, thus capturing the last (and essential) translational output from the intervention.
While retention of GPs in the study was excellent, a limitation was the 6% higher than expected drop-out rate for patients and the relatively high (and differential) loss to follow-up of carers. The results must, therefore, be interpreted with caution. The observed improvement in GP identification of dementia in the intervention group compared with waitlist did not lead to differences in management in terms of referrals to medical specialists or care services. Since GP learning was not directly measured, and the adherence to dementia guidelines was assessed by self-reporting of dementia-related tests and care and specialist referrals by GPs, it is possible that some may have improved their practice but did not record it.63
The relatively short follow-up period for this study is also a limitation. An educational intervention combined with structured care management resulted in a reduction in the decline of health-related QoL for patients with dementia after 18 months but not 12 months53 suggesting that improvements to QoL measures may manifest slowly.
Implications for clinicians and policymakers
This trial illustrates that a simple academic detailing intervention, though more expensive than large group teaching, can produce significant improvements in GP dementia identification and that these can translate into improved communication with consumers and enablement for carers of people with dementia. Given the huge impact that dementia will have on health services in the future, and the benefits to both the individual and the health system from timely diagnosis and carer enablement, this is an important finding for both clinicians and policy makers.
Future research and conclusion
This trial raises a number of questions for future research. One concerns the best way to improve GP management of dementia according to guidelines. Dementia management is complex and ranges from diagnostic assessment through to a primary care team approach to those living with dementia in the community and on to management in residential aged care. Further research on how best to do this, and also how best to teach it, is urgently needed. Another question concerns the long-term effects of better identification of dementia; further longitudinal studies in primary care are needed for this. Funders should consider longitudinal studies of dementia in primary care that capture the experience of consumers and carers from prior to diagnosis, as in this study, and throughout their journey to explore the complex interactions between personal, health system and broader community factors on the dementia pathway.
Supplementary Material
Acknowledgments
The authors acknowledge the invaluable contributions of Professor Charles Bridges-Webb, the patient, the public and professional advisors for the study, and the GP, patient and carer participants who made this study possible.
Footnotes
Contributors: All authors had full access to data (including statistical reports and tables) and take responsibility for the integrity of the data and accuracy of the data analysis. DP conceived and developed this study, drafted the manuscript, had overall management of the project and is guarantor. KM assisted in study design, data management and statistical analysis and drafted the manuscript. NS assisted in study design and managed operations at the Adelaide site. JG assisted in study design and managed operations at the Melbourne site. JM assisted in study design. PD assisted in study design and managed operations at the Bendigo site. PM assisted in study design and drafted the manuscript. NP and GH developed the educational intervention and assisted in study design. SG assisted in study design and provided project management and drafted the manuscript. NW performed the statistical analyses. HB assisted in study design and managed operations at the Sydney site. All authors read and approved the final manuscript.
Funding: This project was funded by the Australian National Health and Medical Research Council (Grant ID #351220 and #510745), Beyond Blue: The National Depression Initiative and the Australian Government Department of Health and Ageing (Project # ITA195/0506). The authors analysed results and prepared this manuscript independently of the funding body.
Disclaimer: The funding body did not have a role in the study design, the collection, analysis and interpretation of data or the writing of the article and the decision to submit it for publication.
Competing interests: DP and HB have sat on advisory boards for Pfizer, Novartis, Janssen, Lundbeck and Nutricia and been speakers sponsored by Pfizer, Novartis (HB only) and Janssen (HB only). HB has been an investigator on projects funded by Pfizer, Novartis, Janssen, Lundbeck, Lilly and Sanofi and acted as a consultant for Merck and Baxter.
Patient consent: Not required.
Ethics approval: Ethics approval was sought and granted initially from the Newcastle University Human Research Ethics Committee (Approval No. H-151-1205), and following this, from the appropriate Ethics Committees at each site. All participants gave written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Prince M, Wimo A, Guerchet M, et al. World azheimer report 2015 - the global impact of dementia: an anlysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International, 2015:84. [Google Scholar]
- 2. Prince M, Comas-Herrera A, Knapp M, et al. World alzheimer report 2016 - improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London: Alzheimer’s Disease International, 2016:131. [Google Scholar]
- 3. World Health Organization. Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva: World Health Organization, 2017:46. [PubMed] [Google Scholar]
- 4. Meeuwsen EJ, Melis RJ, Van Der Aa GC, et al. Effectiveness of dementia follow-up care by memory clinics or general practitioners: randomised controlled trial. BMJ 2012;344:e3086 10.1136/bmj.e3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pinner G, Bouman WP. Attitudes of patients with mild dementia and their carers towards disclosure of the diagnosis. Int Psychogeriatr 2003;15:279–88. 10.1017/S1041610203009530 [DOI] [PubMed] [Google Scholar]
- 6. Jha A, Tabet N, Orrell M. To tell or not to tell-comparison of older patients' reaction to their diagnosis of dementia and depression. Int J Geriatr Psychiatry 2001;16:879–85. 10.1002/gps.412 [DOI] [PubMed] [Google Scholar]
- 7. Byszewski AM, Molnar FJ, Aminzadeh F, et al. Dementia diagnosis disclosure: a study of patient and caregiver perspectives. Alzheimer Dis Assoc Disord 2007;21:107–14. 10.1097/WAD.0b013e318065c481 [DOI] [PubMed] [Google Scholar]
- 8. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 9. Mate KE, Pond CD, Magin PJ, et al. Diagnosis and disclosure of a memory problem is associated with quality of life in community based older Australians with dementia. Int Psychogeriatr 2012;24:1962–71. 10.1017/S1041610212001111 [DOI] [PubMed] [Google Scholar]
- 10. Hurt CS, Banerjee S, Tunnard C, et al. Insight, cognition and quality of life in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2010;81:331–6. 10.1136/jnnp.2009.184598 [DOI] [PubMed] [Google Scholar]
- 11. Bamford C, Lamont S, Eccles M, et al. Disclosing a diagnosis of dementia: a systematic review. Int J Geriatr Psychiatry 2004;19:151–69. 10.1002/gps.1050 [DOI] [PubMed] [Google Scholar]
- 12. Phillips J, Pond D, Shell A. No time like the present: the importance of a timely dementia diagnosis. Sydney: Alzheimer’s Australia, 2010:31. [Google Scholar]
- 13. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med 2000;160:2964–8. 10.1001/archinte.160.19.2964 [DOI] [PubMed] [Google Scholar]
- 14. Pentzek M, Wollny A, Wiese B, et al. Apart from nihilism and stigma: what influences general practitioners' accuracy in identifying incident dementia? Am J Geriatr Psychiatry 2009;17:965–75. 10.1097/JGP.0b013e3181b2075e [DOI] [PubMed] [Google Scholar]
- 15. Pond CD, Mate KE, Phillips J, et al. Predictors of agreement between general practitioner detection of dementia and the revised Cambridge Cognitive Assessment (CAMCOG-R). Int Psychogeriatr 2013;25:1639–47. 10.1017/S1041610213000884 [DOI] [PubMed] [Google Scholar]
- 16. Wilcock J, Iliffe S, Turner S, et al. Concordance with clinical practice guidelines for dementia in general practice. Aging Ment Health 2009;13:155–61. 10.1080/13607860802636206 [DOI] [PubMed] [Google Scholar]
- 17. Phillips J, Pond D, Goode S. Timely diagnosis of dementia: can we do better. Sydney: Alzheimer’s Australia, 2011:43. [Google Scholar]
- 18. Koch T, Iliffe S. Dementia diagnosis and management: a narrative review of changing practice. British Journal of General Practice 2011;61:e513–25. 10.3399/bjgp11X588493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahill S, Clark M, Walsh C, et al. Dementia in primary care: the first survey of Irish general practitioners. Int J Geriatr Psychiatry 2006;21:319–24. 10.1002/gps.1464 [DOI] [PubMed] [Google Scholar]
- 20. van Hout HP, Vernooij-Dassen MJ, Stalman WA. Diagnosing dementia with confidence by GPs. Fam Pract 2007;24:616–21. 10.1093/fampra/cmm046 [DOI] [PubMed] [Google Scholar]
- 21. Boise L, Camicioli R, Morgan DL, et al. Diagnosing dementia: perspectives of primary care physicians. Gerontologist 1999;39:457–64. 10.1093/geront/39.4.457 [DOI] [PubMed] [Google Scholar]
- 22. Phillipson L, Magee C, Jones S, et al. Exploring dementia and stigma beliefs. A pilot study of Australian adults aged 40 to 65 yrs. Sydney: Alzheimer’s Australia, 2012:15. [Google Scholar]
- 23. Turner S, Iliffe S, Downs M, et al. General practitioners' knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing 2004;33:461–7. 10.1093/ageing/afh140 [DOI] [PubMed] [Google Scholar]
- 24. Turner S, Iliffe S, Downs M, Bryans M, et al. Decision support software for dementia diagnosis and management in primary care: relevance and potential. Aging Ment Health 2003;7:28–33. 10.1080/1360789021000058148 [DOI] [PubMed] [Google Scholar]
- 25. Koch T, Iliffe S. EVIDEM-ED project. Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a systematic review. BMC Fam Pract 2010;11:52 10.1186/1471-2296-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iliffe S, Wilcock J. The identification of barriers to the recognition of, and response to, dementia in primary care using a modified focus group approach. Dementia 2005;4:73–85. 10.1177/1471301205049191 [DOI] [Google Scholar]
- 27. Kaduszkiewicz H, Wiese B, van den Bussche H. Self-reported competence, attitude and approach of physicians towards patients with dementia in ambulatory care: results of a postal survey. BMC Health Serv Res 2008;8:54 10.1186/1472-6963-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koch T, Iliffe S. Dementia diagnosis and management: a narrative review of changing practice. Br J Gen Pract 2011;61:e513–25. 10.3399/bjgp11X588493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livingston G, Baio G, Sommerlad A, et al. Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial. PLoS Med 2017;14:e1002252 10.1371/journal.pmed.1002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perry M, Drašković I, Lucassen P, et al. Effects of educational interventions on primary dementia care: a systematic review. Int J Geriatr Psychiatry 2011;26:1–11. 10.1002/gps.2479 [DOI] [PubMed] [Google Scholar]
- 31. Mukadam N, Cooper C, Kherani N, et al. A systematic review of interventions to detect dementia or cognitive impairment. Int J Geriatr Psychiatry 2015;30:32–45. 10.1002/gps.4184 [DOI] [PubMed] [Google Scholar]
- 32. Downs M, Turner S, Bryans M, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomised controlled study. BMJ 2006;332:692–6. 10.1136/bmj.332.7543.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rondeau V, Allain H, Bakchine S, et al. General practice-based intervention for suspecting and detecting dementia in France: A cluster randomized controlled trial. Dementia 2008;7:433–50. [Google Scholar]
- 34. Wilcock J, Iliffe S, Griffin M, et al. Tailored educational intervention for primary care to improve the management of dementia: the EVIDEM-ED cluster randomized controlled trial. Trials 2013;14:397 10.1186/1745-6215-14-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avorn J. Academic detailing: "Marketing" the best evidence to clinicians. JAMA 2017;317:361–2. 10.1001/jama.2016.16036 [DOI] [PubMed] [Google Scholar]
- 36. Lawson G, Basch CH, Zybert P, et al. Acceptability of physician directed academic detailing to increase colorectal cancer screening: an application of the RESPECT Approach. Health Promot Perspect 2015;5:169–75. 10.15171/hpp.2015.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Figueiras A, Sastre I, Gestal-Otero JJ. Effectiveness of educational interventions on the improvement of drug prescription in primary care: a critical literature review. J Eval Clin Pract 2001;7:223–41. 10.1046/j.1365-2753.2001.00234.x [DOI] [PubMed] [Google Scholar]
- 38. Bridges-Webb C, Wolk J. Care of patients with dementia in general practice guidelines. Sydney: The Royal Australian College of General Practitioners and NSW Health, 2003. [Google Scholar]
- 39. Pond CD, Brodaty H, Stocks NP, et al. Ageing in general practice (AGP) trial: a cluster randomised trial to examine the effectiveness of peer education on GP diagnostic assessment and management of dementia. BMC Fam Pract 2012;13:12 10.1186/1471-2296-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2009–10. General practice series no. 27. Cat. no. GEP 27. Canberra: Australian Institute of Health and Welfare, 2010:199. [Google Scholar]
- 41. Roth M, Huppert FA, Mountjoy CQ, et al. CAMDEX-R: the Cambridge examination for mental disorders of the elderly - revised. Cambridge: Cambridge University Press, 1998:108. [Google Scholar]
- 42. Brodaty H, Kemp NM, Low LF. Characteristics of the GPCOG, a screening tool for cognitive impairment. Int J Geriatr Psychiatry 2004;19:870–4. 10.1002/gps.1167 [DOI] [PubMed] [Google Scholar]
- 43. Australian Bureau of Statistics. Census of population and housing: socio-economic indexes for areas (SEIFA), Australia (ABS Cat No. 2033.0.55.001). Canberra: Australian Bureau of Statistics, 2006. [Google Scholar]
- 44. World Health Organization. Division of mental health and prevention of substance abuse. WHOQOL: measuring quality of life. Geneva: World Health Organization, 1997:10. [Google Scholar]
- 45. Sheikh JI, Yesavage JA, Scale GD. (GDS): Recent evidence and development of a shorter version Brink TL, Clinical Gerontology: A Guide to Assessment. New York: Haworth Press, 1986:165–73. [Google Scholar]
- 46. Huppert FA, Jorm AF, Brayne C, et al. Psychometric properties of the CAMCOG and its efficacy in the diagnosis of dementia. Aging, Neuropsychology, and Cognition 1996;3:201–14. 10.1080/13825589608256624 [DOI] [Google Scholar]
- 47. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 48. Mead N, Bower P, Roland M. The general practice assessment questionnaire (GPAQ) - development and psychometric characteristics. BMC Fam Pract 2008;9:13 10.1186/1471-2296-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Banerjee S, Samsi K, Petrie CD, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 2009;24:15–24. 10.1002/gps.2090 [DOI] [PubMed] [Google Scholar]
- 50. Jing W, Willis R, Feng Z. Factors influencing quality of life of elderly people with dementia and care implications: A systematic review. Arch Gerontol Geriatr 2016;66:23–41. 10.1016/j.archger.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 51. Ford AH, Almeida OP. Management of depression in patients with dementia: is pharmacological treatment justified? Drugs Aging 2017;34:89–95. 10.1007/s40266-016-0434-6 [DOI] [PubMed] [Google Scholar]
- 52. Brodaty H, Connors MH, Loy C, et al. Screening for dementia in primary care: a comparison of the GPCOG and the MMSE. Dement Geriatr Cogn Disord 2016;42:323–30. 10.1159/000450992 [DOI] [PubMed] [Google Scholar]
- 53. Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med 2006;145:713–26. [DOI] [PubMed] [Google Scholar]
- 54. Paddison CA, Abel GA, Roland MO, et al. Drivers of overall satisfaction with primary care: evidence from the English General Practice Patient Survey. Health Expect 2015;18:1081–92. 10.1111/hex.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Phillips J, Pond CD, Paterson NE, et al. Difficulties in disclosing the diagnosis of dementia: a qualitative study in general practice. British Journal of General Practice 2012;62:e546–53. 10.3399/bjgp12X653598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maguire CP, Kirby M, Coen R, et al. Family members' attitudes toward telling the patient with Alzheimer’s disease their diagnosis. BMJ 1996;313:529–30. 10.1136/bmj.313.7056.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holroyd S, Turnbull Q, Wolf AM. What are patients and their families told about the diagnosis of dementia? Results of a family survey. Int J Geriatr Psychiatry 2002;17:218–21. 10.1002/gps.552 [DOI] [PubMed] [Google Scholar]
- 58. Mercer SW, Jani BD, Maxwell M, et al. Patient enablement requires physician empathy: a cross-sectional study of general practice consultations in areas of high and low socioeconomic deprivation in Scotland. BMC Fam Pract 2012;13:6 10.1186/1471-2296-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Argimon JM, Limon E, Vila J, et al. Health-related quality of life in carers of patients with dementia. Fam Pract 2004;21:454–7. 10.1093/fampra/cmh418 [DOI] [PubMed] [Google Scholar]
- 60. Zhang S, Edwards H, Yates P, et al. Self-efficacy partially mediates between social support and health-related quality of life in family caregivers for dementia patients in Shanghai. Dement Geriatr Cogn Disord 2014;37:34–44. 10.1159/000351865 [DOI] [PubMed] [Google Scholar]
- 61. Thompson CA, Spilsbury K, Hall J, et al. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr 2007;7:18 10.1186/1471-2318-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ 2013;347:f6276 10.1136/bmj.f6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mercer SW, Watt GC. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med 2007;5:503–10. 10.1370/afm.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.