Abstract
Background
A better understanding of the epidemiology and clinical features of invasive fungal infection (IFI) is integral to improving outcomes. We describe a novel case-finding methodology, reporting incidence, clinical features, and outcomes of IFI in a large US health care network.
Methods
All available records in the Intermountain Healthcare Enterprise Data Warehouse from 2006 to 2015 were queried for clinical data associated with IFI. The resulting data were overlaid in 124 different combinations to identify high-probability IFI cases. The cohort was manually reviewed, and exclusions were applied. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group definitions were adapted to categorize IFI in a broad patient population. Linear regression was used to model variation in incidence over time.
Results
A total of 3374 IFI episodes occurred in 3154 patients. The mean incidence was 27.2 cases/100 000 patients per year, and there was a mean annual increase of 0.24 cases/100 000 patients (P = .21). Candidiasis was the most common (55%). Dimorphic fungi, primarily Coccidioides spp., comprised 25.1% of cases, followed by Aspergillus spp. (8.9%). The median age was 55 years, and pediatric cases accounted for 13%; 26.1% of patients were on immunosuppression, 14.9% had autoimmunity or immunodeficiency, 13.3% had active malignancy, and 5.9% were transplant recipients. Lymphopenia preceded IFI in 22.1% of patients. Hospital admission occurred in 76.2%. The median length of stay was 16 days. All-cause mortality was 17.0% at 42 days and 28.8% at 1 year. Forty-two-day mortality was highest in Aspergillus spp. (27.5%), 20.5% for Candida, and lowest for dimorphic fungi (7.5%).
Conclusions
In this population, IFI was not uncommon, affected a broad spectrum of patients, and was associated with high crude mortality.
Keywords: Aspergillus, Candida, epidemiology, invasive fungal infection, mucormycosis
Invasive fungal infections (IFIs) have emerged in the last 3 decades as an important cause of human disease [1]. IFIs are generally distinguished from superficial mycoses based on involvement of blood and other sterile body sites or invasion into organ tissue. IFI is also referred to as serious, deep, deep-seated, disseminated, and systemic fungal infection [2]. Although superficial mycoses account for much of the overall global prevalence of fungal infection [3], IFIs are associated with disproportionately high morbidity, mortality, and economic burden [4]. Many factors have likely contributed to the emergence of IFI, including the HIV epidemic, a dramatic rise in the number of patients receiving a growing array of immunosuppressive therapies, and increasing populations with frequent nosocomial exposure and interventions. However, accurate estimation of the true burden of IFI is difficult due to variation in definitions [2] and limitations inherent to available case-finding methodologies [5, 6]. For example, diagnosis billing codes are widely accessible but inaccurate, and microbiology records only identify a fraction of infections and are often dominated by cultures representing colonization or superficial infection rather than true invasive disease. To this end, we developed a novel IFI cohort discovery technique and applied it to retrospective data in a large US integrated health network to describe the epidemiology and clinical characteristics of IFI.
METHODS
Intermountain Healthcare is an integrated health network with 22 hospitals and 180 clinics in Utah and southern Idaho serving approximately 1.5 million unique patients each year. The 2010 US census reported a population of 2 763 885 for Utah [7]. We aimed to accurately identify all IFIs by systematically querying all available records in the Intermountain Healthcare Enterprise Data Warehouse (EDW) from January 1, 2006, to December 31, 2015. To limit the cohort to only cases with invasive fungal infection, we applied the following general exclusions: superficial or mucosal infection, including dermatophytoses and onychomycoses, noninvasive allergic fungal disease, mycetoma of the lung or sinus without invasion into adjacent tissue, and organism-, test-, and specimen-specific rules to exclude likely colonization states or culture contamination. To that end, we excluded any Candida species from all specimens originating from a nonsterile mucous membrane, skin, wound, or pulmonary source, as well as Candida from noninvasive urinary specimens if less than 100 000 colony-forming units of yeast were present. We did not include Pneumocystis species in this study.
We identified, a priori, 7 candidate domains of clinical and diagnostic data that are associated with the diagnosis of IFI: (1) International Classification of Diseases, Clinical Modification (ICD-CM) codes, 9th and 10th editions, corresponding to IFI, (2) laboratory data, (3) microbiological data, (4) radiology data, (5) pathology data, (6) pharmacy data for antifungal drugs, and (7) composite data identifying immunocompromised patients at higher risk of IFI. Within each diagnostic domain, we identified specific clinical indicators of the diagnosis of IFI either in the form of structured (coded) data or unstructured (text string) data, which we extracted from pathology, microbiology, and radiology reports using natural language processing (NLP) techniques.
Laboratory data included fungal serologies for Coccidioides, Histoplasma, and Blastomyces; antigen tests: Cryptococcus from serum and cerebral spinal fluid (CSF), Histoplasma galactomannan from serum and CSF, Aspergillus galactomannan (Bio-Rad Platelia, Hercules, CA) from serum and bronchoalveolar lavage (BAL); 1,3-beta-D-glucan (Fungitell, Falmouth, MA), and Coccidioides polymerase chain reaction (PCR) assay from Mayo Medical Laboratories. We mapped microbiological results from all listed anatomical and specimen types to exclude cultures likely to represent colonization or superficial infection and used NLP and structured data techniques to identify cultures positive for fungal organisms. We identified positive radiology results using all radiographic terms describing features specific to IFI, such as “nodule,” “cavitary,” and “halo,” and used NLP to search all radiology reports during that time period for key terms and phrases. We applied a similar approach to pathology records, using NLP to search all pathology records for all boolean variations of key terms and phrases associated with IFI, such as “hyphae,” “mold,” or “angioinvasion.” We used structured data queries of pharmacy records to identify patients who had received antifungal medications but excluded topical therapies and low doses of fluconazole used to treat superficial infection. Lastly, we used a combination of ICD codes, transplant and cancer registries, and pharmacy records for immunosuppressive agents to identify patients with immunocompromising conditions including hematopoietic stem cell (HSCT) and solid organ transplant recipients, hematologic and solid tumor malignancies, HIV, other primary immunodeficiencies, and other conditions requiring immunosuppressive medications.
After electronically mining data from all patient records in the EDW for the study period, we found 157 371 positive “hits” from 34 135 unique patients (Supplementary Figure 1). We then employed a combinatorial data reduction strategy by stratifying cases into 1 of 124 possible combinations of the 7 data domains (Supplementary Table 2). We manually reviewed 20% of “hits” from each combination to identify features that were predictive of true-positive IFI and those that identified exclusions such as colonization vs invasion, etc. We then applied the exclusion criteria to the whole set and manually reviewed all remaining cases (n = 3620) for inclusion validation. By so doing, we were then able to determine the relative predictive value of each domain. Laboratory, billing code, and microbiology data had the greatest predictive value (56%, 23%, and 20% of cases, respectively, confirmed as true IFI). Conversely, pathology data were least often confirmed as true IFI (7%), usually because fungal elements were identified but invasive features were uncommon. Similarly, we were able to identify combinations that, when positive data were cumulatively present from multiple domains, were more likely to represent true-positive IFI cases (see Supplementary Table 2 for percent true-positive IFI for each combination).
Once the final cohort was validated, we rereviewed the cases to manually assign an index date of IFI onset and to classify IFI by diagnostic certainty using the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group [8]. We adapted the EORTC/MSG categories “possible,” “probable,” and “proven” to permit epidemiological categorization of a real-world population. Among immunocompromised hosts, we classified patients with mycological evidence but who did not fully meet EORTC/MSG clinical criteria as “possible.” Conversely, we also categorized as “possible” cases that fully met all clinical criteria and were treated for suspected IFI, but for whom no mycological results were identified.
For populations not explicitly included in EORTC/MSG definitions, such as the critically ill, burn victims, neonates, and others with nosocomial risk in whom IFIs frequently occur, we defined “proven” IFI on the basis of histopathological evidence confirming invasion or positive culture from a sterile body site. For “probable” disease, we considered culture results from nonsterile, invasively collected specimens that also met at least 1 of the following clinical criteria: (1) ≥2 systemic inflammatory response signs, (2) skin/soft tissue infection due to a mold, (3) peritonitis/intra-abdominal infection, (4) pulmonary infection such as cavitary pneumonia, but excluding Candida species, and fungal ball, (5) esophagitis, (6) central nervous system infection, including ventriculo-peritoneal shunt infection. Patients with direct ophthalmological exam evidence of Candida endophthalmitis but no positive cultures and those with the clinical features defined above and a positive 1,3 beta-D-Glucan or Aspergillus galactomannan test were classified as “possible” IFI. Cases where data was insufficient to categorize to the genus level were labeled “species not available.”
Demographic and other clinical data were electronically extracted for patients in the final cohort. Temporally dynamic risk factor variables were identified as the last available value before the IFI index date. Incidence was calculated based on number of cases per 100 000 unique patients cared for in the Intermountain system during a particular calendar year. Linear regression was used to model variation in incidence over time. This study was approved by the Intermountain Healthcare Institutional Review Board.
RESULTS
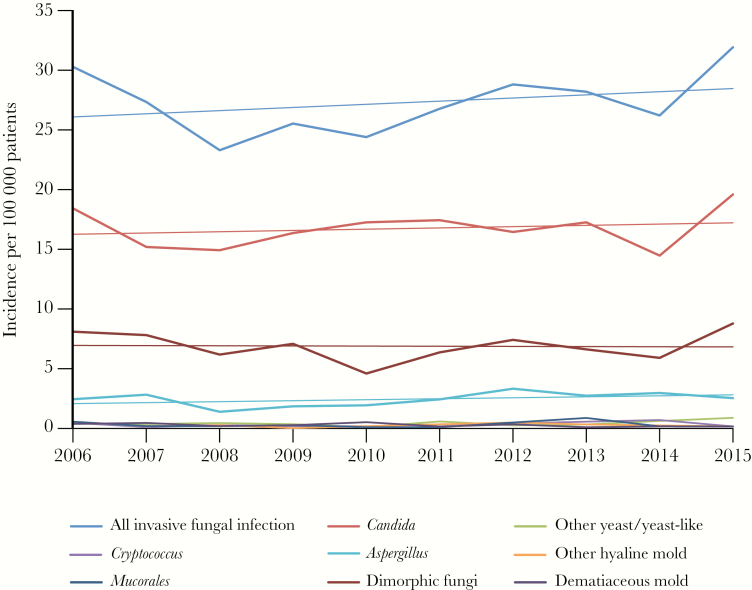
A total of 3374 IFI episodes occurred in 3154 patients (Supplementary Figure 1). The mean incidence was 27.2 cases/100 000 patients per year. Although the IFI rate varied from year to year (r2 = .09), linear regression estimated a mean annual increase of 0.24 cases/100 000 patients, or 0.9% per year (P = .21) (Table 1, Figure 1). Candida spp. were the most common (55.2%) (Table 2). Dimorphic fungi, primarily Coccidioides spp., comprised 25.2%, followed by Aspergillus spp. (8.9%) (Supplementary Figure 2). The median age (range) was 55 (0–97) years; 13.4% of cases occurred in children <18 years. Comorbidities were common, including diabetes mellitus (28.7%) and chronic pulmonary disease (44.9%). Active malignancy was present in 13.2%, autoimmune or primary immunodeficiency in 14.9%, and 5.9% of episodes occurred in transplant recipients; 26.1% of IFIs occurred in patients receiving immunosuppressive therapy, of which corticosteroids were the most common (20.8% of all IFIs). Lymphopenia (absolute lymphocyte count <500 cells/mm3) was present at the time of IFI in 22.1% of episodes. Hospital admission occurred in 76.2%; IFI occurred during intensive care unit (ICU) stay in 30.7% of cases. The median length of stay was 16 days. Crude mortality was 17.0% at 42 days and 28.8% at 1 year.
Table 1.
Incidence (per 100 000 Patients) of IFI by Category and Year
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2006–2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All IFIs | 30.2 | 27.2 | 23.2 | 25.4 | 24.3 | 26.7 | 28.7 | 28.1 | 26.1 | 31.8 | 27.2 |
| Candida | 18.3 | 15.1 | 14.9 | 16.3 | 17.2 | 17.4 | 16.4 | 17.2 | 14.4 | 19.5 | 15.0 |
| Other yeast/yeast-like | 0.4 | 0.3 | 0.4 | 0.3 | 0.1 | 0.6 | 0.2 | 0.3 | 0.6 | 0.9 | 0.4 |
| Cryptococcus | 0.4 | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 | 0.4 | 0.5 | 0.7 | 0.1 | 0.3 |
| Aspergillus | 2.4 | 2.8 | 1.4 | 1.8 | 1.9 | 2.4 | 3.3 | 2.7 | 2.9 | 2.5 | 2.4 |
| Other hyaline mold | 0.4 | 0.2 | 0.3 | 0.0 | 0.2 | 0.3 | 0.5 | 0.3 | 0.2 | 0.1 | 0.2 |
| Mucorales | 0.5 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.9 | 0.2 | 0.1 | 0.3 |
| Dimorphic fungi | 8.1 | 7.8 | 6.1 | 7.0 | 4.6 | 6.3 | 7.4 | 6.6 | 5.9 | 8.7 | 6.9 |
| Dematiaceous mold | 0.3 | 0.4 | 0.2 | 0.3 | 0.5 | 0 | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 |
Abbreviation: IFI, invasive fungal infection.
Figure 1.
Incidence of invasive fungal infection (IFI) by category (per 100000 patients).
Table 2.
Demographic and Clinical Data
| All Cases | Candida | Other Yeast | Cryptococcus | Aspergillus | Other Hyaline Mold | Mucorales | Dimorphic Fungi | Species Not Available | Dematiaceous Mold | |
|---|---|---|---|---|---|---|---|---|---|---|
| All, No. | 3374 | 1862 (55.2) | 51 (1.5) | 40 (1.2) | 301 (8.9) | 33 (1.0) | 36 (1.1) | 849 (25.2) | 222 (6.6) | 28 (0.8) |
| Demographics | ||||||||||
| Age, y | 55 (34–68) | 55 (34–69) | 50 (26–68) | 53.5 (39–64) | 51 (23–62) | 48 (21–62) | 49 (27–61) | 59 (40–71) | 52.5 (26–64) | 41 (25–53) |
| Pediatric | 451 (13.4) | 257 (13.8) | 10 (19.6) | 4 (10) | 65 (21.6) | 6 (18.2) | 7 (19.4) | 54 (6.4) | 47 (21.2) | 6 (21.4) |
| Male | 1735 (51.4) | 911 (48.9) | 27 (52.9) | 32 (80) | 170 (56.5) | 15 (45.5) | 26 (72.2) | 456 (53.7) | 120 (54.1) | 11 (39.3) |
| Charlson Comorbidity Score | 3 (1–6) | 4 (1–7) | 4 (2–7) | 4 (1–7) | 4 (2–7) | 4 (1–6) | 4 (2–6) | 2 (1–5) | 4 (2–7) | 3 (1–4.5) |
| Chronic pulmonary disease | 1515 (44.9) | 832 (44.7) | 26 (50.9) | 18 (45) | 139 (46.2) | 14 (42.4) | 13 (36.1) | 373 (43.9) | 105 (47.3) | 10 (37) |
| Renal insufficiency | 764 (22.6) | 494 (26.5) | 12 (23.5) | 14 (35) | 69 (22.9) | 3 (9.0) | 8 (22.2) | 117 (13.8) | 61 (27.5) | 2 (7.1) |
| Congestive heart failure | 777 (23) | 476 (25.6) | 20 (39.2) | 5 (12.5) | 79 (26.2) | 8 (24.2) | 4 (11.1) | 140 (16.5) | 52 (23.4) | 3 (10.7) |
| Hepatic disease | 991 (29.4) | 625 (33.6) | 21 (41.2) | 10 (25) | 94 (31.2) | 12 (36.4) | 11 (30.6) | 189 (22.3) | 52 (23.4) | 8 (28.6) |
| Rheumatologic | 278 (8.2) | 154 (8.3) | 4 (7.8) | 1 (2.5) | 20 (6.6) | 4 (12.1) | 1 (2.8) | 68 (8) | 22 (9.9) | 5 (17.9) |
| Diabetes mellitus | 969 (28.7) | 616 (33.1) | 11 (21.6) | 13 (32.5) | 63 (20.9) | 7 (21.2) | 13 (36.1) | 195 (23) | 54 (24.3) | 4 (14.3) |
| Neurological disease | 615 (18.2) | 386 (20.7) | 7 (13.7) | 7 (17.5) | 45 (15) | 6 (18.2) | 5 (13.9) | 122 (14.4) | 42 (18.9) | 4 (14.3) |
| History of malignancy | 1072 (31.8) | 538 (28.9) | 19 (37.3) | 14 (35) | 178 (59.1) | 13 (39.4) | 17 (47.2) | 181 (21.3) | 124 (55.9) | 10 (35.7) |
| Hematological malignancy | 207 (6.1) | 45 (2.4) | 6 (11.8) | 2 (5.0 ) | 96 (31.9) | 4 (12.1) | 7 (19.4) | 7 (0.8) | 54 (24.3) | 2 (7.1) |
| Hematopoietic stem cell transplant | 123 (3.6) | 15 (0.8) | 3 (5.9) | 1 (2.5) | 77 (25.6) | 2 (6.1) | 4 (11.1) | 4 (0.5) | 27 (12.1) | 1 (3.6) |
| Allogeneic HSCT | 107 (3.2) | 13 (0.7) | 2 (3.9) | 0 (0) | 72 (23.9) | 2 (6.1) | 4 (11.1) | 3 (0.4) | 20 (9.0) | 1 (3.6) |
| Autologous HSCT | 16 (0.5) | 2 (0.1) | 1 (2.0) | 1 (2.5) | 5 (1.7) | 0 (0) | 0 (0) | 1 (0.1) | 7 (3.2) | 0 (0) |
| Solid organ transplant | 79 (2.3) | 49 (2.6) | 3 (5.9) | 2 (5.0) | 9 (3) | 1 (3.0) | 2 (5.6) | 2 (0.2) | 13 (5.9) | 1 (3.6) |
| Solid tumor malignancy | 240 (7.1) | 195 (10.5) | 4 (7.8) | 5 (12.5) | 18 (6.0) | 2 (6.1) | 2 (5.6) | 34 (4.0) | 10 (4.5) | 3 (10.7) |
| Other immunocompromised | 502 (14.9) | 282 (15.1) | 9 (17.6) | 10 (19.2) | 53 (17.6) | 8 (24.2) | 3 (8.3) | 94 (11.1) | 48 (21.6) | 8 (28.6) |
| Immunosuppressive medications | 879 (26.1) | 459 (24.7) | 14 (27.5) | 9 (22.5) | 182 (60.5) | 13 (39.4) | 22 (61.1) | 71 (8.4) | 126 (56.8) | 10 (35.7) |
| Corticosteroids | 701 (20.8) | 394 (21.2) | 11 (21.6) | 8 (20) | 140 (46.5) | 13 (39.4) | 15 (41.7) | 54 (6.4) | 87 (39.2) | 6 (21.4) |
| Calcineurin inhibitors | 202 (6) | 65 (3.5) | 3 (5.9) | 4 (10.0) | 85 (28.2) | 4 (12.1) | 6 (16.7) | 8 (0.9) | 34 (15.3) | 1 (3.6) |
| Antimetabolite | 203 (6) | 61 (3.3) | 5 (9.8) | 1 (2.5) | 68 (22.6) | 5 (15.2) | 6 (16.7) | 5 (0.6) | 53 (23.9) | 4 (14.3) |
| Mycophenolate mofetil | 36 (1.1) | 13 (0.7) | 0 (0) | 1 (2.5) | 13 (4.3) | 1 (3.0) | 0 (0) | 2 (0.2) | 6 (2.7) | 0 (0) |
| Chemotherapy | 220 (6.5) | 81 (4.4) | 5 (9.8) | 0 (0) | 70 (23.3) | 7 (21.2) | 8 (22.2) | 7 (0.8) | 44 (19.8) | 4 (14.3) |
| Monoclonal anti–B cell | 55 (1.6) | 15 (0.8) | 2 (3.9) | 1 (2.5) | 21 (7) | 2 (6.1) | 2 (5.6) | 3 (0.4) | 12 (5.4) | 1 (3.6) |
| T-cell depletion | 36 (1.1) | 16 (0.9) | 1 (2.0) | 1 (2.5) | 14 (4.7) | 0 (0) | 3 (8.3) | 0 (0) | 3 (1.4) | 0 (0) |
| Anti-TNF | 21 (0.7) | 12 (0.6) | 1 (2.0) | 0 (0) | 2 (0.6) | 1 (3.0) | 0 (0) | 6 (0.7) | 0 (0) | 0 (0) |
| Labs | ||||||||||
| Lymphopenia, absolute lymphocyte <500 cells/mm3 | 746 (22.1) | 436 (23.4) | 12 (23.5) | 3 (7.5) | 153 (50.8) | 10 (30.3) | 14 (38.9) | 45 (5.3) | 97 (43.7) | 4 (14.3) |
| Neutropenia, absolute neutrophil <500 cells/mm3 | 245 (7.3) | 88 (4.7) | 6 (11.8) | 1 (2.5) | 89 (29.6) | 6 (18.2) | 10 (27.8) | 7 (0.8) | 49 (22.1) | 3 (10.7) |
| Immunoglobulin G <500 mg/dL | 71 (2.1) | 11 (0.6) | 0 (0) | 0 (0) | 29 (9.6) | 0 (0) | 3 (8.3) | 1 (0.1) | 29 (13.1) | 0 (0) |
| Outcomes | ||||||||||
| IFI-related admission | 2571 (76.2) | 1631 (87.6) | 46 (90.2) | 32 (80) | 257 (85.4) | 25 (75.8) | 33 (91.7) | 404 (47.6) | 178 (80.2) | 19 (67.9) |
| Length of stay, d | 16 (7–30) | 17 (8–31) | 16 (9–38.3) | 16 (9–22) | 25 (13–46) | 33 (15.5–60) | 26 (12–44) | 7 (4–13) | 20 (9–35) | 15 (6.3–29.3) |
| 42-d all-cause mortality | 574 (17) | 384 (20.6) | 10 (19.6) | 9 (22.5) | 83 (27.6) | 2 (6.1) | 10 (27.8) | 63 (7.4) | 34 (15.3) | 3 (10.7) |
| 1-y all-cause mortality | 971 (28.8) | 610 (32.8) | 16 (31.4) | 13 (32.5) | 147 (48.8) | 11 (33.3) | 15 (41.7) | 109 (12.8) | 76 (34.2) | 4 (14.3) |
Data are presented as median (IQR) or No. (%).
Abbreviations: HSCT, hematopoietic stem cell transplant; IFI, invasive fungal infection; TNF, tumor necrosis factor.
Candida
A total of 1862 cases of Candida IFI were identified. The mean incidence was 15.0 cases/100 000 patients per year (yearly range, 14.9–19.5; mean increase, 0.10 cases/100 000/y; r2 = .04; P = not significant [NS]). Candida albicans was the most common (60.6%), followed by C. glabrata (24.4%) (Supplementary Table 1). The proportion of Candida IFI caused by non-albicans species varied between 31.4% and 42.9%, with a 0.2% increase per year (r2 = .018; P = NS) (Supplementary Figure 3). Most (95.5%) cases were classified as proven (Table 3), with bloodstream (45%) and intra-abdominal (39.6%) sites being the most common. Comorbidities were common (median Charlson score, 4; Interquartile interval (IQI), 1–7) (Table 2). Corticosteroid use (21.2%) was common, as was lymphopenia at the time of IFI (23.4%); 51.7% of cases occurred in patients with central venous catheters, 36.9% in patients admitted to the ICU, and 12.5% in patients on total parenteral nutrition. Forty-five percent (41.5%) of patients had undergone abdominal surgery in the preceding 60 days, and 75% of cases had antibiotic exposure in the prior 2 months. Fluconazole (61.9%) and echinocandins (41.7%) were the most common antifungals (Table 4). Hospitalization occurred in 87.6% of cases, with a median length of stay (LOS) of 17 days. Crude mortality was 20.6% at 42 days and 32.8% at 1 year. Mortality for candidemia was much higher (26.6% and 39.6%, respectively) than for intra-abdominal site of infection without bloodstream involvement (5.8% at 42 days and 9.9% at 1 year).
Table 3.
IFI by Site of Infection and Classification of Diagnostic Certainty
| All Cases | Candida | Other Yeast | Cryptococcus | Aspergillus | Other Hyaline Mold | Mucorales | Dimorphic Fungi | Species Not Available | Dematiaceous Mold | |
|---|---|---|---|---|---|---|---|---|---|---|
| All, No. | 3374 | 1862 (55.2) | 51 (1.5) | 40 (1.2) | 301 (8.9) | 33 (1.0) | 36 (1.1) | 849 (25.2) | 222 (6.6) | 28 (0.8) |
| Proven | 2245 (66.5) | 1779 (95.5) | 41 (80.4) | 37 (92.5) | 113 (37.5) | 23 (69.7) | 31 (86.1) | 214 (25.2) | 50 (22.3) | 21 (75.0) |
| Probable | 853 (25.3) | 81 (4.4) | 9 (17.6) | 3 (7.5) | 150 (49.8) | 9 (27.3) | 3 (8.3) | 559 (65.8) | 50 (22.5) | 4 (14.3) |
| Possible | 245 (7.3) | 2 (0.11) | 1 (2.0) | 0 (0) | 38 (12.6) | 1 (3.0) | 2 (5.6) | 76 (9) | 122 (55.0) | 3 (10.7) |
| Site | ||||||||||
| Blood | 1192 (35.3) | 838 (45) | 28 (54.9) | 14 (35.0) | 3 (1.0) | 6 (18.2) | 6 (16.7) | 46 (5.4) | 0 (0) | 3 (10.7) |
| Bone/joint | 70 (2.1) | 48 (2.6) | 0 (0) | 2 (5) | 5 (1.7) | 2 (6.1) | 4 (11.1) | 5 (0.6) | 1 (0.5) | 0 (0) |
| Cardiovascular | 102 (3) | 87 (4.7) | 0 (0) | 1 (2.5) | 8 (2.7) | 1 (3.0) | 0 (0) | 5 (0.6) | 0 (0) | 0 (0) |
| CNS | 50 (1.5) | 10 (0.5) | 1 (2.0) | 16 (40) | 12 (4) | 3 (9.1) | 2 (5.6) | 11 (1.3) | 0 (0) | 0 (0) |
| Sinus/orbital | 73 (2.2) | 23 (1.2) | 3 (5.9) | 0 (0) | 21 (7) | 9 (27.2) | 14 (38.9) | 4 (0.5) | 4 (1.8) | 6 (21.4) |
| Hepatobiliary | 75 (2.2) | 67 (3.6) | 1 (2.0) | 1 (2.5) | 2 (0.7) | 0 (0) | 1 (2.8) | 2 (0.2) | 3 (1.4) | 0 (0) |
| Intra-abdominal | 793 (23.5) | 737 (39.6) | 11 (21.6) | 6 (15) | 10 (3.3) | 1 (3.0) | 5 (13.9) | 6 (0.7) | 27 (12.2) | 5 (17.9) |
| Lung | 546 (16.2) | 9 (5) | 10 (19.6) | 12 (30) | 251 (83.4) | 8 (24.2) | 10 (27.8) | 179 (21.1) | 19 (8.6) | 6 (21.4) |
| Renal | 43 (1.3) | 18 (1) | 0 (0) | 2 (5) | 4 (1.3) | 0 (0) | 2 (5.6) | 21 (2.5) | 1 (0.5) | 0 (0) |
| Skin | 22 (0.7) | 6 (0.3) | 0 (0) | 0 (0) | 5 (1.7) | 4 (13.3) | 4 (11.1) | 4 (0.5) | 2 (0.9) | 1 (3.6) |
| Serological/antigen | 1073 (31.8) | 269 (14.4) | 5 (9.8) | 24 (12.5) | 62 (60.0) | 16 (48.5) | 18 (50) | 737 (86.8) | 178 (80.2) | 14 (50.0) |
Data are presented as No. (%).
Abbreviations: CNS, central nervous system; IFI, invasive fungal infection.
Table 4.
Antifungal Use, by IFI Category
| All Cases | Candida | Other Yeast | Cryptococcus | Aspergillus | Other Hyaline Mold | Mucorales | Dimorphic Fungi | Dematiaceous Mold | Species Not Available | |
|---|---|---|---|---|---|---|---|---|---|---|
| All, No. | 3374 | 1862 (55.2) | 51 (1.5) | 40 (1.2) | 301 (8.9) | 33 (1.0) | 36 (1.1) | 849 (25.2) | 28 (0.8) | 222 (6.6) |
| Antifungal | ||||||||||
| Amphotericin B | 341 (10.1) | 144 (7.7) | 10 (19.6) | 20 (50) | 90 (29.9) | 8 (24.2) | 24 (66.7) | 31 (3.7) | 7 (25.0) | 29 (13.1) |
| Anidulafungin | 32 (0.9) | 20 (1.1) | 1 (2.0) | 0 (0) | 11 (3.7) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 3 (1.4) |
| Caspofungin | 642 (19) | 460 (24.7) | 10 (19.6) | 4 (10) | 113 (37.5) | 7 (21.2) | 13 (36.1) | 12 (1.4) | 6 (21.4) | 42 (18.9) |
| Micafungin | 474 (14) | 296 (15.9) | 19 (37.3) | 5 (12.5) | 83 (27.6) | 7 (21.2) | 9 (25) | 18 (2.1) | 2 (7.1) | 59 (26.6) |
| Fluconazole | 1515 (44.9) | 1152 (61.9) | 19 (37.3) | 23 (57.5) | — | 6 (18.2) | — | 194 (22.9) | 3 (10.7) | 73 (32.9) |
| Isavuconazonium | 8 (0.2) | 0 (0) | 1 (2.0) | 0 (0) | 4 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (1.8) |
| Itraconazole | 22 (0.7) | 2 (0.1) | 0 (0) | 0 (0) | 4 (1.3) | 0 (0) | 0 (0) | 12 (1.4) | 0 (0) | 4 (1.8) |
| Posaconazole | 86 (2.5) | 18 (1) | 6 (11.8) | 0 (0) | 34 (11.3) | 3 (9.1) | 12 (33.3) | 4 (0.5) | 3 (10.7) | 17 (7.7) |
| Voriconazole | 307 (9.1) | 88 (4.7) | 8 (15.7) | 5 (12.5) | 151 (50.2) | 15 (45.5) | — | 11 (1.3) | 7 (25.0) | 36 (16.2) |
Data are presented as No. (%).
Abbreviation: IFI, invasive fungal infection.
Other Yeast/Yeast-Like Organisms
Non-Candida yeast and yeast-like organisms comprised 1.5% of the cohort (Table 1). Incidence was stable over time (Table 1, Figure 1). Saccharomyces, Rhodotorula, and Trichosporon spp. were the most common in this group (Supplementary Table 1). Most IFIs in this group were proven (80.8%), with blood (53.8%), intra-abdominal (21.2%), and lung (19.2%) being the most common sites of infection (Table 3). Fifty percent of patients had chronic pulmonary disease, and 11.5% were transplant recipients. Nine cases (17.3%) occurred while on antifungal prophylaxis and represented breakthrough. Echinocandins were the most frequently used (Table 4). Crude mortality rates at 42 days and 1 year were 19.2% and 30.8%, respectively.
Cryptococcus
Forty cases of cryptococcal IFI were identified (1.2% of the total cohort); incidence was stable over time (Figure 1). Most cases were disseminated (82.5%), 40% presented with meningitis, and 30% involved the lung (Table 3). Eighty percent (80%) of episodes occurred in men. Five cases (12.5%) occurred in patients with HIV/AIDS, 7.5% in transplant recipients, and 7.5% in solid tumor malignancy (Table 2). Hepatic disease was present in 25% of cases. Amphotericin B (50%) and fluconazole (57.5%) were commonly prescribed (Table 4). Crude mortality was 22.5% at 42 days and 32.5% at 1 year.
Aspergillus
Invasive aspergillosis (IA) comprised 8.9% of cases (Table 2). Incidence was stable over time, with a mean of 2.4 cases/100 000 patients per year (Table 1). Proven cases comprised 37.5%; 49.8% were probable and 12.6% possible (Table 3). Lung was the predominant site of infection (83.4%). In cases where a species was identified, A. fumigatus was the most common (Supplementary Table 2.). Sixty-five cases (21.6%) occurred in pediatric patients (Table 2). Most cases occurred in patients on immunosuppressive medications (60.5%), those with hematological malignancy (31.9%), and recipients of HSCT (25.6%). Among HSCT recipients, IA occurred at a median (range) of 116 (34–235) days post-transplant, and complicated graft-vs-host disease occurred in 62.3% of cases. Acute myeloid leukemia was the most common underlying malignancy. Lymphopenia (50.8%) and neutropenia (absolute neutrophil count <500 cells/mm3; 29.6%) frequently preceded onset; 42.2% of IA occurred in patients in the ICU. Treatment regimens most commonly included voriconazole (50.2%), amphotericin B (29.9%), and caspofungin (37.5%) (Table 4). Crude mortality was 27.6% at 42 days and 48.8% at 1 year.
Other Hyaline Molds
Hyaline molds other than Aspergillus comprised less than 1% of the cohort (Table 2). Fusarium and Pseudallescheria/Scedosporium spp. were the most common organisms (Supplementary Table 2). The mean incidence was 0.2 cases per 100 000 patients per year (Table 1). Lymphopenia was present in 30% of cases, and 36.7% were on corticosteroids. The most common sites of infection were sinus (30%), lung (26.7%), and bloodstream (20%) (Table 3). Voriconazole (46.7%) and amphotericin B (26.7%) were the most common antifungal agents (Table 4). LOS was the longest in this group (33 days; IQI, 15.5–60.0 days). Forty-two-day crude mortality was only 6.7%, but 1-year mortality was 30%.
Mucorales
Mucorales spp. accounted for 1.1% of cases (Table 2). The mean incidence was 0.3 cases/100 000 patients per year (Table 1). Male gender was disproportionate (72.2%). Diabetes mellitus was present in 36.1%, hematological malignancy in 19.4%, and HSCT in 11.1%; 61.1% of patients were on immunosuppressive medications, of which corticosteroids were predominant (41.7%). Lymphopenia or neutropenia directly preceded diagnosis in 38.9% and 27.8% of cases, respectively. Sinus (38.9%) and lung (27.8%) were the most frequent sites of infection. Breakthrough infection despite prophylaxis (most often with an echinocandin) occurred in 33.3% of cases. Amphotericin B (66.6%), caspofungin (36.1%), and posaconazole (33.3%) were the most prescribed therapies. Forty-two-day and 1-year crude mortality in this group were 27.8% and 41.7%, respectively.
Dimorphic Fungi
Eight hundred forty-nine cases of IFI (25.2%) due to dimorphic fungus were identified (Table 2). The vast majority were Coccidioides spp. (Supplementary Table 2). The mean annual incidence was 6.9 per 100 000 (Table 1). The affected population was comparatively healthy (median Charlson score, 2; IQI, 1–5). Very few cases occurred in immunocompromised populations, and only 8.4% of patients were on immunosuppressive therapy. Less than half (47.6%) of cases resulted in hospital admission. Mortality rates were 7.4% at 42 days and 12.8% at 1 year.
Dematiaceous Mold
Compared with other classes of fungi, dematiaceous molds comprised a smaller proportion of all IFIs (0.8%). The mean annual incidence was 0.2/100 000 (Table 1). Many (29.6%) of these cases occurred in patients with nonmalignant immunocompromising conditions such as connective tissue and inflammatory bowel disease (Table 2). Forty-two-day and 1-year crude mortality rates were 11.1% and 14.8%, respectively.
Species Not Available
Two hundred twenty-four cases of IFI were identified by clinical data for which identification was not possible on the organism level (Table 2). Many of these were identified by compatible syndrome and positive fungal biomarkers in high-risk groups such as those with hematological malignancy (24.6%), transplant recipients (18.3%), and those receiving immunosuppressant therapy (57.1%) or critical care (38.4%). All-cause mortality rates at 42 days and 1 year were 15.2% and 34.8% in this group.
DISCUSSION
These data comprise one of the largest cohorts of IFI to date and shed further light on the epidemiology and impact of this disease state in the western region of the United States. The novel case finding methodology developed for this study addresses the limitations of population, point prevalence, and laboratory-based strategies commonly used in IFI epidemiology [6]. This hybrid approach combines the granularity of hospital-based point prevalence surveillance and population surveys that extrapolate incidence from census data using laboratory testing, with billing codes as the numerator. By integrating data from additional clinical sources, including pathology, radiology, and microbiology reports via NLP technology, we were able to electronically survey a large population while maintaining resolution at the syndrome and patient levels. This multidimensional approach may serve as a framework for cohort discovery in the future, overcoming a historical impediment to better research on IFI.
As with all IFI studies, local geography, climate, and demographics significantly influence results. Similar data from other geographic locations in the United States would be a useful comparator. Overall incidence of IFI in this cohort was modestly higher than, but generally consistent with, population-based estimates from France (20.3 per 100 000) [9] and the United Kingdom (14.1/100 000) [10]. The higher rate in our cohort may be attributable to a more comprehensive case-finding strategy. We observed a 1% annual increase in incidence over the study period, similar to a contemporary study from France [11], suggesting that the rapid emergence of IFI observed in the previous 2 decades [1] may have stabilized.
The demographics of this cohort identify an IFI at-risk population with significant burden of comorbid illness, immunosuppression, and health care exposure with well-established risk factors for IFI. Although not designed to evaluate relative risk of factors, we were interested by the number of patients in this study in whom absolute lymphopenia preceded IFI onset. This likely trends with the use of immunosuppressive agents but may warrant additional investigation. The high overall crude mortality observed highlights both the impact of IFI and the propensity for poor outcomes in the patient population most likely to be affected.
Despite the expected variation due to differences in geography and climate, the distribution of organisms was generally similar to that observed in other studies [1, 9–12]. Incidence of Candida IFI in our cohort was near the median reported in other studies (ranging between 1.4 and 29 cases per 100 000) [9, 10, 12–15]. Incidence of Candida IFI was also stable over time, in keeping with recent reports [15, 16]. Although the proportion of non-albicans species overall was generally stable, increased rates at the end of the study period merit further study to determine if this represents a trend. Observed Cryptococcus incidence was nearly identical to other reports (ranging from 0.1 to 0.3 cases/100 000) [9–13]. Although few epidemiological data are available for non-Candida yeast/yeast-like organisms, these results support a possible increasing trend in these infections [1], which often present as breakthrough despite prophylaxis in heavily immunosuppressed populations [17–19]. The mean incidence of IA in this cohort (2.4) closely mirrored reports from other cohorts (median, 2.0 per 100 000) [1, 9–13]. Although the majority of IA occurred in predictable immunocompromised populations, we did note an increase in the number of cases in ICU patients, similar to recent reports from Europe [20, 21]. In keeping with other reports [1], other hyaline molds comprised a small proportion of cases with similar demographics as Aspergillus but with mortality more similar to Candida IFI. Another rare group, dematiaceous molds, was often identified by culture data but infrequently met criteria for invasive infection. Incidence was slightly higher than in previous reports [1], and comorbidities and mortality were much lower in this population. Lastly, dimorphic fungi were the third most common group in this cohort, with Coccidioides spp. being overwhelmingly predominant. Incidence was several-fold lower than what has been reported in known endemic regions [22] but greater than expected for our local geography. Further investigation of this observation is warranted to determine which areas within our cohort catchment areas may be considered endemic.
This study has several limitations. Despite extensive manual validation, electronic data extraction methodology is likely to result in some inaccuracies. This was especially true with respect to outpatient data and site of infection. As with all epidemiological studies, differences in definitions and case-finding methods, geography, climate, and patient case mix limit accurate comparisons. We also did not include Pneumocystis jiroveci infection during the planning phases of this study but now acknowledge that it has been included in most recent surveys sponsored by the Global Action Fund for Fungal Infection [3]; we plan to include these data in future studies.
CONCLUSIONS
In this large epidemiological survey in a US integrated health network, IFI was relatively common, affecting multiple, distinct at-risk groups and patients with frequent health care exposure and high comorbid burden. Observed incidence increased by roughly 1% per year over the last 10 years but did not achieve statistical significance. We observed that IFI was associated with high crude mortality and should remain a focus for further research and intervention.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. B.W., J.F., and S.R. contributed to the study design. B.W., J.F., S.R., S.K., and B.G. contributed to data acquisition. B.W. performed the statistical analysis and interpretation of the data. All authors contributed to the development of this manuscript.
Financial support. This work was supported by funding from Astellas Pharma Global Development, Inc.
Potential conflicts of interest. B.W., J.F., S.K., B.G., and S.R. received research support from Astellas. J.S. is an employee of Astellas Pharma Global Development. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1–53. [DOI] [PubMed] [Google Scholar]
- 2. Hobson RP. The global epidemiology of invasive Candida infections—is the tide turning?J Hosp Infect 2003; 55:159–68; quiz 233. [DOI] [PubMed] [Google Scholar]
- 3. Denning DW. Global action fund for fungal infections Available at: https://www.gaffi.org. Accessed 12 December 2017.
- 4. Drgona L, Khachatryan A, Stephens J, et al. . Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis 2014; 33:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enoch DA, Yang H, Aliyu SH, Micallef C. The changing epidemiology of invasive fungal infections. Methods Mol Biol 2017; 1508:17–65. [DOI] [PubMed] [Google Scholar]
- 6. Park BJ, Chiller TM, Brandt ME, Warnock DW. Epidemiology of systemic fungal diseases: an overview. In: Kauffman CA. Essentials of Clinical Mycology. Vol. 13. New York: Springer, 2011:553. [Google Scholar]
- 7.U.S. Census Bureau. 2010 Census of Population (Report No. P94-171). Washington, DC: U.S. Government Printing Office, 2011. [Google Scholar]
- 8. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy And Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangneux JP, Bougnoux ME, Hennequin C, et al. . An estimation of burden of serious fungal infections in France. J Mycol Med 2016; 26:385–90. [DOI] [PubMed] [Google Scholar]
- 10. Pegorie M, Denning DW, Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect 2017; 74:60–71. [DOI] [PubMed] [Google Scholar]
- 11. Bitar D, Lortholary O, Le Strat Y, et al. . Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis 2014; 20:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamagni TL, Evans BG, Shigematsu M, Johnson EM. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9). Epidemiol Infect 2001; 126:397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dufresne SF, Cole DC, Denning DW, Sheppard DC. Serious fungal infections in Canada. Eur J Clin Microbiol Infect Dis 2017; 36:987–92. [DOI] [PubMed] [Google Scholar]
- 14. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cleveland AA, Harrison LH, Farley MM, et al. . Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One 2015; 10:e0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magill SS, Edwards JR, Bamberg W, et al. . Multistate point-prevalence survey of health care-associated infections. N Eng J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bretagne S, Renaudat C, Desnos-Ollivier M, et al. . Predisposing factors and outcome of uncommon yeast species-related fungaemia based on an exhaustive surveillance programme (2002–14). J Antimicrob Chemother 2017; 72:1784–93. [DOI] [PubMed] [Google Scholar]
- 18. Chan TS, Gill H, Hwang YY, et al. . Breakthrough invasive fungal diseases during echinocandin treatment in high-risk hospitalized hematologic patients. Ann Hematol 2014; 93:493–8. [DOI] [PubMed] [Google Scholar]
- 19. Corzo-León DE, Satlin MJ, Soave R, et al. . Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses 2015; 58:325–36. [DOI] [PubMed] [Google Scholar]
- 20. Montagna MT, Caggiano G, Lovero G, et al. . Epidemiology of invasive fungal infections in the intensive care unit: results of a multicenter Italian survey (AURORA Project). Infection 2013; 41:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tortorano AM, Dho G, Prigitano A, et al. . Invasive fungal infections in the intensive care unit: a multicentre, prospective, observational study in Italy (2006–2008). Mycoses 2012; 55:73–9. [DOI] [PubMed] [Google Scholar]
- 22. Galgiani JN, Ampel NM, Blair JE, et al. ; Infectious Diseases Society of America Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.