Abstract
Background
The purpose of this study was to analyze the secular trends of infective endocarditis in a teaching hospital between January 1996 and December 2015.
Methods
We report on a single-center retrospective study of patients with left-side valve infective endocarditis. We performed an analysis of secular trends in the main epidemiological and etiological aspects, as well as clinical outcomes, in 5 successive 4-year periods (P1 to P5).
Results
In total, 595 episodes of infective endocarditis were included, of which 76% were community-acquired and 31.3% involved prosthetic valves. Among the cases, 70% occurred in men, and the mean age (SD) was 64.1 (14.3) years. A significant increase in older patients (age ≥70 years) between P1 (15.332%) and P5 (51.9%; P < .001) was observed. The rate of infective endocarditis on biological prostheses also increased in the prosthetic group, accounting for 30% in P1 and 67.3% in P5 (P < .001). By contrast, there were significant decreases in vascular and immunological phenomena over the study period, with decreases in the presence of moderate to severe valvular insufficiency (75.9% in P1 to 52.6% in P5; P < .001) and valvular surgery (43% in P1 vs 29.6% in P5; P = .006). Finally, overall mortality was 23.9%, and although it was highest in P1, it subsequently remained stable through P2 to P5 (38% in P1 to 20% in P5; P = .004).
Conclusions
There has been a significant increase in infective endocarditis in older patients. The decrease in moderate to severe valve regurgitation at diagnosis could explain the stable mortality despite the increase in the mean age of patients over time.
Keywords: epidemiology, etiology, infective endocarditis, secular trends, outcome
Though a relatively rare disease (5–7 cases per 100 000 people/year), infective endocarditis (IE) is an extremely serious condition due to its associated morbidity and high attributable mortality [1].
The profile of IE has varied considerably since William Osler’s original description as a subacute or chronic pathology of community-acquired infection in patients with previous rheumatic heart disease. Today, it is described as an acute acquired infection that is often health care related, occurring in elderly patients with degenerative heart disease, prostheses, or endovascular devices [2, 3]. However, epidemiological findings have not been uniform [4]. Regarding etiology, for example, while some studies have found no increase in the trend for a given pathogen [5], other authors have described a rise in coagulase-negative staphylococci (CoNS), enterococci [6], and Staphylococcus aureus [2, 4, 7].
Descriptive data about IE and knowledge of the changing spectrum of these local variations would be helpful for determining the impact of the disease and for optimizing its management. In this study, we aimed to identify and analyze secular trends in the clinical, etiological, treatment, and outcome characteristics of IE over a 20-year period in a tertiary care teaching hospital.
METHODS
Patients and Study Period
The study involved retrospective analysis of prospectively collected data from all episodes of definite or possible IE in patients hospitalized over 20 years (between January 1, 1996, and December 31, 2015). Bellvitge University Hospital is a teaching hospital with 700 beds in southern Barcelona in Catalonia, Spain. The institution has about 35 000 admissions per year, distributed in medical and surgical units, with 60 intensive care beds available. It is a referral center for adult patients from a population of more than 2 million inhabitants and covers multiple specialties, including cardiac surgery. Patients with endocarditis are usually transferred to our hospital in complicated cases and/or those requiring surgery.
The following data were collected from medical reports using a standardized protocol: demographic characteristics, comorbidities and predisposing factors, clinical characteristics, microbiological data, antibiotic treatments administered, surgeries performed, and clinical outcomes. We included only episodes of possible or definite left-side valve IE (mitral and/or aortic involvement). Those episodes with device-related infections (pacemaker and implantable defibrillators) and those with isolated right-side valve IE (as well as episodes occurring in intravenous drug users) were excluded from the study. Five 4-year periods were arbitrarily defined: 1996–1999 (P1), 2000–2003 (P2), 2004–2007 (P3), 2008–2011 (P4), and 2012–2015 (P5). Trend analyses were performed along these 5 periods to identify the main epidemiological, etiological, and therapeutic aspects, as well as the clinical outcomes, associated with IE in this region.
Definitions
The Duke criteria [8], plus the modified variant of 2000 [9], were used to diagnose definite or possible IE. Acquisition was categorized as community-acquired or non-community-acquired IE, the latter including episodes of nosocomial and health care–associated acquisition. Nosocomial IE was defined as that not present on admission, with symptom onset more than 48 hours after hospitalization and up to 6 months after discharge [10]. Health care–associated IE was defined as IE present at hospital admission or within the first 48 hours of admission in a patient with a history of receiving intravenous therapy (including chemotherapy), transfer from a specialized nursing care facility, or hemodialysis within 90 days before the index admission [11]. Early and late prosthetic IE were defined as cases occurring within or after the first year of prosthetic valve placement, respectively [12]. Cerebral embolism was defined as those symptomatic episodes of ischemic stroke (either transient ischemic attacks or hemispheric infarctions) with radiological confirmation. All patients were followed up to 1 year, during which cure was defined as the absence of relapse or mortality and mortality was defined as the 1-year case fatality rate.
Microbiological Studies
Two to 4 sets of 2 blood samples of 8–10 mL each (BACTEC Plus Aerobic and Anaerobic [BD]) were taken 30 minutes apart if IE was suspected. The samples were then processed in either a BACTEC 9240 (until May 2010) or a BACTEC-FX (since May 2010; BD Microbiology Systems) with an incubation period of 5 days. Positive blood samples were subcultured onto chocolate agar. Identification and antibiotic susceptibility testing of gram-negative bacilli and Enterococcus spp. were performed using commercial panels of the MicroScan system (Beckman Coulter, Inc). Viridans group streptococci (VGS) and other gram-positive cocci, including CoNS and Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species group bacteria, were identified using standard methods [13]. Antibiotic susceptibility was tested by microdilution using commercial Sensititre panels (TREK Diagnostic Systems Ltd.) according to Clinical and Laboratory Standards Institute (CLSI) recommendations. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and/or CLSI criteria were used to define susceptibility or resistance to antimicrobial agents [14, 15]. From November 2012 onwards, bacterial identification was performed by Matrix-Assisted Laser Desorption Ionisation (MALDI-Biotyper; Bruker Daltonics). For patients transferred from other hospitals after IE diagnosis, it is usual practice to request the shipment of the strains responsible for the infection to the microbiology laboratory to deepen studies (eg, determination of minimum inhibitory concentration, molecular studies and, in recent times, MALDI and sequencing studies). In addition, the strains responsible for endocarditis are collected and stored for future studies.
Statistical Analysis
Continuous variables were compared using the Student t test or the Mann Whitney U test, as appropriate. Categorical data were compared using the Fisher exact test or the chi-square test. Trend analyses were carried out using Cochran-Mantel-Haenszel tests for dichotomous variables and linear logistic regression for continuous data. Statistical significance was established at an α of .05, with all reported P values being 2-tailed. Analyses were performed using SPSS Software, version 15 (SPSS Inc., Chicago, IL).
Ethical Considerations
This observational study was carried out in accordance with the 1964 Declaration of Helsinki and its later amendments. To protect privacy, the information of each patient was encrypted in an electronic database. Requests for informed consent were considered unnecessary by the Clinical Research Ethics Committee because no intervention was performed and no personally identifiable information was collected.
RESULTS
Patients’ Characteristics and Clinical Presentation
In total, 595 episodes of left-side valve IE were recorded in 564 patients, with a definite diagnosis established in 473 (79.5%). Most cases affected the aortic valve or both the aortic and mitral valves (58.4%) and were community acquired (75.6%). Almost one-third of the episodes involved a prosthetic valve. The patients’ characteristics and the main clinical manifestations are summarized by period in Table 1. Most patients (70%) were men, and the mean age (SD) was 64.1 (14.3) years. Of note, there was a significant increase in the mean age in the last period (58.3 years in P1 vs 68 years in P5; P < .001). Patients older than 70 years constituted more than one-half (51.9%) in the last period. There was a significant increase in patients older than 80 years from P1 (6.3%) to P5 (20.7%; P < .001). Most patients initially presented with fever (83.7%), and more than half had moderate to severe valvular regurgitation. Neurological and immunological complications occurred in approximately 15.1% and 10.8% of patients, respectively. The median of symptom duration before diagnosis showed a nonsignificant decreasing trend (5.3 weeks in P1 vs 3.5 weeks in P5; P = .102).
Table 1.
General Characteristics and Clinical Manifestations: Global Data and Trends by Period
| Variable/Period | Overall (N = 595), No. (%) | P1 (N = 79), No. (%) | P2 (N = 113), No. (%) | P3 (N = 145), No. (%) | P4 (N = 123), No. (%) | P5 (N = 135), No. (%) | P |
|---|---|---|---|---|---|---|---|
| Male sex | 417 (70.1) | 57 (72.2) | 80 (70.8) | 99 (68.3) | 94 (76.4) | 87 (64.4) | NS |
| Mean age, y | |||||||
| Age (SD) | 64.1 (14.3) | 58.3 (14.13) | 60.7 (13.46) | 64.6 (13.76) | 66 (15.28) | 68 (13.18) | <.001 |
| ≥70 | 244 (41) | 12 (15.2) | 32 (28.3) | 66 (45.5) | 64 (52) | 70 (51.9) | <.001 |
| ≥80 | 72 (12.1) | 5 (6.3) | 6 (5.3) | 13 (9) | 20 (16.3) | 28 (20.7) | <.001 |
| Symptoms | |||||||
| Fever | 498 (83.7) | 67 (84.8) | 91 (80.5) | 121 (83.4) | 103 (83.7) | 116 (85.9) | NS |
| Median duration of symptoms (IQR), wk | 4.4 (0.7–8.1) | 5.3 (2.7–7.9) | 4.3 (1.1–7.5) | 4.9 (0.4–9.4) | 4.2 (0–8.5) | 3.5 (0.4–6.7) | .102 |
| Moderate–severe valvular regurgitation | 357 (60) | 60 (75.9) | 75 (66.4) | 84 (57.9) | 67 (54.5) | 71 (52.6) | <.001 |
| Cardiac failure | 232 (39) | 35 (44.3) | 51 (45.1) | 51 (35.2) | 38 (30.9) | 57 (42.2) | NS |
| Vascular phenomena | 226 (38) | 34 (43) | 50 (44.2) | 60 (41.4) | 45 (36.6) | 37 (27.4) | .005 |
| Major arterial embolism | 148 (24.9) | 15 (19) | 34 (30.1) | 44 (30.3) | 26 (21.1) | 29 (21.5) | NS |
| Cerebral embolism | 90 (15.1) | 6 (7.6) | 18 (15.9) | 30 (20.7) | 21 (17.1) | 15 (11.1) | NS |
| Splenic embolism | 32 (5.4) | 4 (5.1) | 10 (8.9) | 8 (5.5) | 4 (3.3) | 6 (4.4) | NS |
| Renal embolism | 11 (1.9) | 2 (2.5) | 4 (3.5) | 4 (2.8) | 0 | 1 (0.7) | NS |
| Coronary embolism | 4 (0.7) | 0 | 0 | 0 | 0 | 4 (3) | – |
| Other embolism | 11 (1.9) | 3 (3.8) | 2 (1.8) | 2 (1.4) | 1 (0.8) | 3 (2.2) | NS |
| Conjunctival hemorrhage | 42 (7.1) | 3 (3.8) | 9 (8) | 15 (10.3) | 8 (6.5) | 7 (5.2) | NS |
| Janeway lesions | 36 (6.1) | 7 (8.9) | 5 (4.4) | 16 (11) | 6 (4.9) | 2 (1.5) | .037 |
| Mycotic aneurism | 21 (3.5) | 3 (3.8) | 8 (7.1) | 2 (1.4) | 6 (4.9) | 2 (1.5) | NS |
| Immunologic phenomena | 64 (10.8) | 12 (15.2) | 22 (19.5) | 12 (8.3) | 10 (8.1) | 8 (6) | .001 |
| Roth spots | 22 (3.7) | 8 (10.1) | 5 (4.4) | 3 (2.1) | 3 (2.4) | 3 (2.2) | .011 |
| Osler nodes and cutaneous vasculitis | 26 (4.4) | 4 (5.1) | 10 (8.8) | 6 (4.1) | 6 (4.9) | 0 | NS |
| Reactive arthritis | 24 (4) | 1 (1.3) | 7 (6.2) | 10 (6.9) | 2 (1.6) | 4 (3) | NS |
| Glomerulonephritis | 10 (1.7) | 0 | 2 (1.8) | 1 (0.7) | 3 (2.4) | 4 (3) | NS |
| Valvular abscess | 79 (13.3) | 17 (21.5) | 11 (9.7) | 19 (13.1) | 16 (13) | 16 (11.9) | NS |
| Septic arthritis and spondylodiscitis | 56 (9.4) | 10 (12.7) | 12 (10.6) | 13 (9) | 6 (4.9) | 15 (11.1) | NS |
| Splenomegaly | 22 (3.7) | 2 (2.5) | 8 (7.1) | 8 (5,5) | 1 (0.8) | 3 (2.2) | NS |
Periods: P1: January 1996–December 1999; P2: January 2000–December 2003; P3: January 2004–December 2007; P4: January 2008–December 2011; P5: January 2012–December 2015.
Abbreviations: IQR, interquartile range; NS, not significant.
Acquisition Site and Microbiology
Details of the acquisition site and causative agent are shown by period in Table 2. There was a trend toward an increase in prosthetic valve IE (25.3% in P1 to 36.3% in P5; P = .198), particularly with biological valves (30% in P1 to 67.3% in P5; P < .001). Despite this, there was no increase in non-community-acquired IE.
Table 2.
Affected Valves, Microbiology and Outcomes: Global Data and Trends by Period
| Variable/Period | Overall (N = 595), No. (%) | P1 (N = 79), No. (%) | P2 (N = 113), No. (%) | P3 (N = 145), No. (%) | P4 (N = 123), No. (%) | P5 (N = 135), No. (%) | P |
|---|---|---|---|---|---|---|---|
| Non-community-acquired | 145 (24.4) | 18 (22.8) | 36 (31.9) | 31 (21.4) | 31 (25.2) | 29 (21.5) | NS |
| Affected valvea | |||||||
| Aortic | 277 (46.6) | 36 (45.6) | 47 (41.6) | 68 (46.8) | 62 (50.4) | 64 (47.4) | NS |
| Mitral | 241 (40.5) | 32 (40.5) | 56 (49.6) | 60 (41.4) | 42 (34.1) | 51 (37.7) | NS |
| Aortic–mitral | 70 (11.8) | 10 (12.7) | 9 (8) | 15 (10.3) | 19 (15.4) | 17 (12.6) | NS |
| Native valve IE | 409 (68.7) | 59 (74.7) | 79 (69.9) | 94 (64.8) | 91 (74) | 86 (63.7) | NS |
| Prosthetic valve IE | 186 (31.3) | 20 (25.3) | 34 (30.1) | 51 (35.2) | 32 (26) | 49 (36.3) | NS |
| Mechanicalb | 100 (53.7) | 13 (65) | 25 (73.5) | 36 (70.6) | 10 (31.2) | 16 (32.7) | <.001 |
| Biologicalb | 84 (45.2) | 6 (30) | 9 (26.5) | 14 (27.5) | 22 (68.8) | 33 (67.3) | <.001 |
| Earlyb | 52 (28) | 8 (40) | 8 (23.5) | 11 (21.6) | 7 (21.9) | 18 (36.7) | NS |
| Lateb | 134 (72) | 12 (60) | 26 (76.5) | 40 (78.4) | 25 (78.1) | 31 (63.2) | NS |
| Etiology | |||||||
| Viridans group streptococci | 147 (24.7) | 23 (29.1) | 22 (19.5) | 40 (27.6) | 29 (23.6) | 33 (24.4) | .05 |
| S. aureus | 110 (18.5) | 10 (12.7) | 22 (19.5) | 27 (18.6) | 26 (21.1) | 25 (18.5) | NS |
| Coagulase-negative staphylococci | 84 (14.1) | 12 (15.2) | 15 (13.3) | 16 (11) | 15 (12.2) | 26 (19.3) | NS |
| Enterococcus spp. | 83 (13.9) | 11 (13.9) | 13 (11.5) | 13 (9) | 25 (20.3) | 21 (15.6) | .186 |
| S. gallolyticus | 65 (10.9) | 4 (5.1) | 12 (10.6) | 17 (11.7) | 15 (12.2) | 17 (12.6) | .124 |
| Indication for surgery | 237 (39.8) | 39 (49.4) | 56 (49.6) | 48 (33.1) | 39 (31.7) | 55 (40.7) | .036 |
| Surgery performed | 188 (31.6) | 34 (43) | 47 (41.6) | 35 (24.1) | 32 (26) | 40 (29.6) | .006 |
| Cure | 435 (73.1) | 46 (58.2) | 81 (71.7) | 113 (77.9) | 94 (76.4) | 101 (74.8) | .02 |
| Global mortality | 142 (23.9) | 30 (38) | 30 (26.5) | 30 (20.7) | 25 (20.3) | 27 (20) | .004 |
Periods: P1: January 1996–December 1999; P2: January 2000–December 2003; P3: January 2004–December 2007; P4: January 2008–December 2011; P5: January 2012–December 2015.
Abbreviations: NS, not significant; IE, infective endocarditis.
aMural IE cases not reflected.
bThe percentages refer to the total of prosthetic valve endocarditis. Data about the type of prosthetic valve was missing in 2 cases.
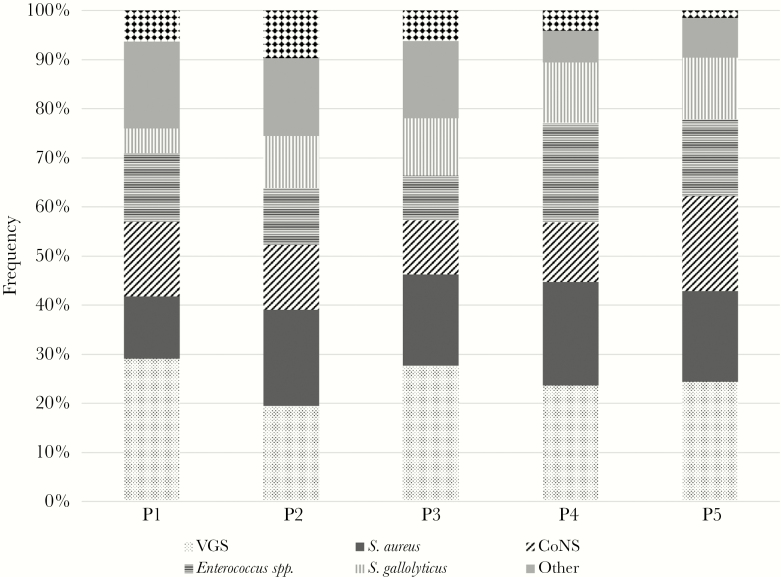
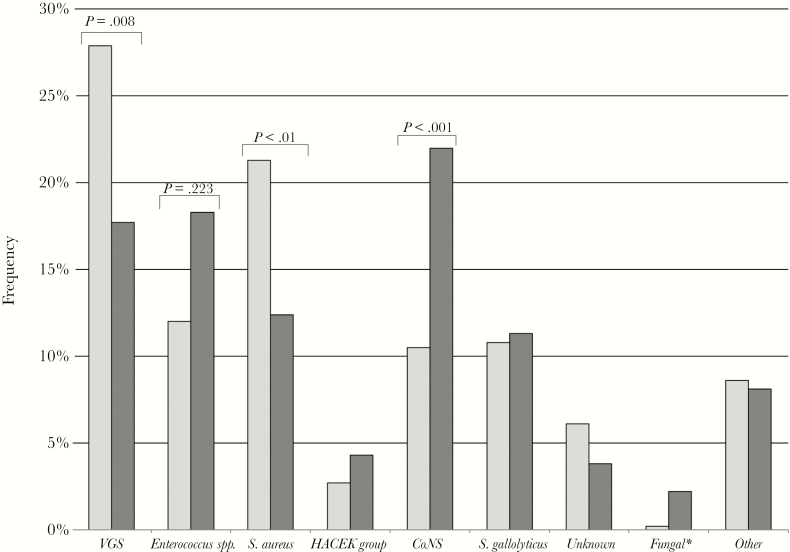
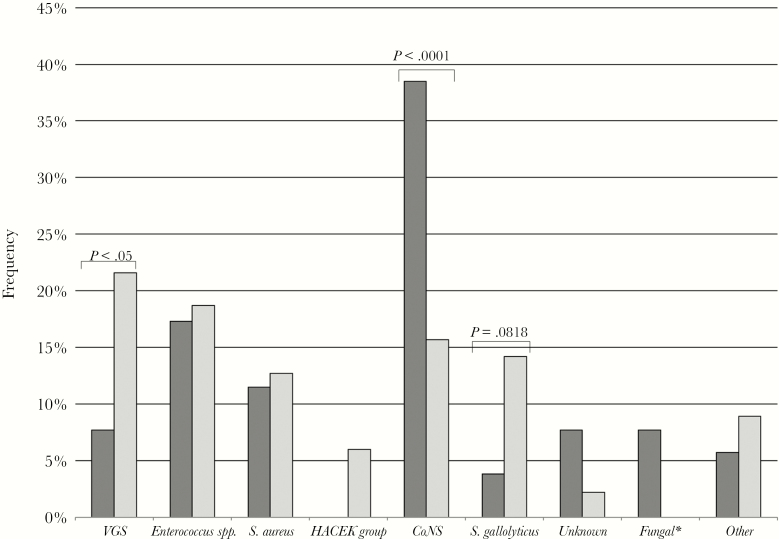
The temporal evolution by period for the different etiologies is shown in Figure 1. Overall, VGS were the most commonly isolated pathogens, followed by Staphylococcus aureus, CoNS, Enterococcus spp., and Streptococcus gallolyticus. Although the proportions remained stable over the 5 periods without any statistically significant upward or downward trend, IE caused by Enterococcus spp. and Streptococcus gallolyticus showed a slight increase in the last 2 study periods. In addition, CoNS were more frequent in early (38.5%) than in late (15.7%) prosthetic valve IE (P < .001). The etiology of late valve IE was similar to that of native IE, albeit with fewer cases due to VGS (Figures 2 and 3).
Figure 1.
Trends of causative pathogens. Abbreviations: CoNS, coagulase-negative staphylococci; VGS, viridans group streptococci.
Figure 2.
Etiology of native vs prosthetic valve infectious endocarditis. Bars indicate the frequency of each etiology in the whole study period. Dark gray: prosthetic; light gray: native. Significative differences are indicated in the figure. aThree cases caused by Candida spp. and 2 cases caused by Aspergillus spp. Abbreviations: CoNS, coagulase-negative staphylococci; HACEK group bacteria, Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; VGS, viridans group streptococci .
Figure 3.
Etiology of early vs late prosthetic valve infectious endocarditis. Bars indicate the frequency of each etiology in the whole study period. Dark gray: early; light gray: late. Significative differences are indicated in the figure. Abbreviations: CoNS, coagulase-negative staphylococci; HACEK group bacteria, Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; VGS, viridans group streptococci .
Outcomes
Data concerning valve surgery, clinical evolution, and the 1-year case fatality rate are shown by period in Table 2. Valve surgery was required in 31.6% of patients during the follow-up period, of which 20% required urgent surgery. Surgery was also indicated, but not performed, in 8.2% of the remaining episodes. Overall, there was a significant reduction in surgical interventions during the study period (43% in P1 to 29.6% in P5; P = .006).
The overall mortality of the cohort was 23.9%. It was highest in P1 (38%) but remained stable throughout the last 4 periods, reaching 20% in P5 (P = .004). Mortality was significantly higher for non-community-acquired IE (37.2% vs 19.6%; P = .001) and prosthetic valve IE (29% vs 21.5% in native valve IE; P = .046), with no difference between early and late prosthetic valve IE. Episodes with a clear indication for cardiac surgery, but in which surgery was not performed, were associated with significantly higher mortality (75.5% vs 20.2%; P < .001). Mortality rates by pathogen are shown in Supplementary Figure 1. Compared with IE caused by VGS, mortality was significantly higher for IE caused by S. aureus, methicillin-resistant S. aureus, and CoNS (a detailed description of patients with IE caused by CoNS who died is provided as a footnote in Supplementary Figure 1).
DISCUSSION
In the present study, we analyzed the details of 595 left-side valve IE episodes over a period of 20 years. During that time, we observed a shift in patient characteristics, with an increase in older patients that is consistent with other studies [6, 16, 17]. Given the aging population worldwide [18], the relative contribution of infectious diseases is bound to increase in older patients [19]. In the case of IE, however, this epidemiological variation could be related to a change in the underlying heart pathology, with rheumatic heart disease increasingly being replaced by degenerative lesions and mitral valve prolapse [5, 16, 20], which are usually observed in older people. The increased mean age could explain the increase in IE among biological prosthetic valves in our series. Unlike other cohorts, however, there was no increase in prosthetic valve, nosocomial, or health care–associated IE in our study [5, 17, 21, 22]. This may result from our decision to exclude cardiac device–related IE [23].
Several studies have reported the emergence of S. aureus as a predominant etiology of IE, with a concurrent decrease in the dominance of VGS [4, 7]. In contrast to this pattern, a population-based Spanish study noted that there had been a decline in the proportion of S. aureus over recent years [6]. In our setting, despite a nonsignificant decrease throughout the study period, the main etiology remained VGS with a stable percentage of S. aureus. This is in line with the findings of a population-based study in Olmsted County, Minnesota [5], and a recent review of IE epidemiology in Europe [24]. We detected a slight increase in the last 2 periods (without a statistically significant upward trend along the study period) of IE caused by Enterococcus spp. and S. gallolyticus, which are pathogens associated with urological manipulations, colon cancer, and polyps [25–29], and which is therefore clearly related to an aging population [30].
Clinically, over the 5 periods evaluated in our cohort, we observed a decrease in the duration of symptoms before IE was diagnosed. Although this reduction did not reach statistical significance, it could explain the significant reduction in vascular and immunological phenomena. This change in clinical presentation has been reported previously [2, 4]. We also observed a progressive and significant reduction in cardiac surgery (performed or indicated), which paralleled the significant reduction in severe valve dysfunction at the time of diagnosis. Conversely, other Western series have described increases in the indications for surgery with more severe and complicated case presentations [2, 4].
Finally, we found an initial decrease in mortality after the first period, though this remained stable at around 20% in the latter 4 periods. Again, this is consistent with most data published in the last decade, which have indicated that there have been few changes in mortality [24, 31]. Notwithstanding, a recent American cohort spanning a similar study period to ours (1998–2013) [17] showed that there were decreases in the 90-day age- and comorbidity-adjusted mortality rates. Of note, it is possible that the use of new and more sensitive diagnostic tools could have helped to improve the detection of endocarditis and its complications, and, consequently, episodes in the last periods may have been diagnosed in early stages. This stage migration phenomenon could partly explain the reductions observed in surgical interventions and in overall mortality in our cohort.
However, despite a large sample size and the long period spanned, there were 2 important limitations of our study. First, the fact that the information came from a single center means that caution is required when extrapolating the data. Second, our hospital is a referral center, which may bias our data toward more severe cases. On the other hand, although the patterns of referral did not formally change along the study period, it cannot be ruled out that transfers of care from the referring hospitals could have been optimized during the 20-year study period (for patients with more aggressive or advanced disease), which may explain the high mortality documented in the first period.
In conclusion, we found an increase in the proportion of elderly patients with IE in daily clinical practice but failed to identify any significant trends in etiology. The lower frequency of moderate to severe valve regurgitation at diagnosis could explain the stable mortality in spite of an increasing mean age during the study period.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Members of the Endocarditis Unit of Hospital de Bellvitge. Ariadna Padulles (Department of Pharmacy, Hospital Universitario de Bellvitge), Pere Cardona (Department of Neurology, Hospital Universitario de Bellvitge), Joan Peris (Hemotherapy and Hemostasis Department, Hospital Universitario de Bellvitge), Joan Guillamon (Ambulatory Care Unit, Hospital Universitario de Bellvitge), Paola Sastre (Intensive Care Unit, Hospital Universitario de Bellvitge), Ignasi Anguera (Arrhythmia Unit, Department of Cardiology, Hospital Universitario de Bellvitge), Oriol Alegre (Department of Cardiology, Hospital Universitario de Bellvitge), and Ramón Vila (Vascular Surgery Department, Hospital Universitario de Bellvitge).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mandell G, Bennet J, Dolin R, Blaser M.. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Health Sciences; 2015. [Google Scholar]
- 2. Hill EE, Herijgers P, Herregods MC, Peetermans WE. Evolving trends in infective endocarditis. Clin Microbiol Infect 2006; 12:5–12. [DOI] [PubMed] [Google Scholar]
- 3. Bor DH, Woolhandler S, Nardin R, et al. . Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One 2013; 8:e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murdoch DR, Corey GR, Hoen B, et al. ; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tleyjeh IM, Steckelberg JM, Murad HS, et al. . Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA 2005; 293:3022–8. [DOI] [PubMed] [Google Scholar]
- 6. Olmos C, Vilacosta I, Fernández-Pérez C, et al. . The evolving nature of infective endocarditis in Spain: a population-based study (2003 to 2014). J Am Coll Cardiol 2017; 70:2795–804. [DOI] [PubMed] [Google Scholar]
- 7. Fowler VG Jr, Miro JM, Hoen B, et al. ; ICE Investigators Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–21. [DOI] [PubMed] [Google Scholar]
- 8. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–9. [DOI] [PubMed] [Google Scholar]
- 9. Li JS, Sexton DJ, Mick N, et al. . Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Ami R, Giladi M, Carmeli Y, et al. . Hospital-acquired infective endocarditis: should the definition be broadened?Clin Infect Dis 2004; 38:843–50. [DOI] [PubMed] [Google Scholar]
- 11. Friedman ND, Kaye KS, Stout JE, et al. . Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 12. Piper C, Körfer R, Horstkotte D. Prosthetic valve endocarditis. Heart 2001; 85:590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray PR, Baron EJ, Jorgensen JH, et al. . Manual of Clinical Microbiology. 9th ed Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 14. CLSI. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Clin Lab Stand Inst 2013; 32:1–184. [Google Scholar]
- 15. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Available at: http://www.eucast.org/clinical_breakpoints/. Accessed January 15, 2018. [Google Scholar]
- 16. Duval X, Delahaye F, Alla F, et al. ; AEPEI Study Group Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 2012; 59:1968–76. [DOI] [PubMed] [Google Scholar]
- 17. Toyoda N, Chikwe J, Itagaki S, et al. . Trends in infective endocarditis in California and New York State, 1998-2013. JAMA 2017; 317:1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloom DE, Chatterji S, Kowal P, et al. . Macroeconomic implications of population ageing and selected policy responses. Lancet 2015; 385:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathers CD, Stevens GA, Boerma T, et al. . Causes of international increases in older age life expectancy. Lancet 2015; 385:540–8. [DOI] [PubMed] [Google Scholar]
- 20. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987; 82:681–8. [DOI] [PubMed] [Google Scholar]
- 21. Benito N, Miró JM, De Lazzari E, et al. ; International Collaboration on Endocarditis Prospective Cohort Study Investigators Health care–associated native valve endocarditis in patients with no history of injection drug use: current importance of non-nosocomial acquisition. Ann Intern Med 2009; 150:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. . A systematic review of population-based studies of infective endocarditis. Chest 2007; 132:1025–35. [DOI] [PubMed] [Google Scholar]
- 23. Cabell CH, Heidenreich PA, Chu VH, et al. . Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J 2004; 147:582–6. [DOI] [PubMed] [Google Scholar]
- 24. Slipczuk L, Codolosa JN, Davila CD, et al. . Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8:e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durante-Mangoni E, Bradley S, Selton-Suty C, et al. ; International Collaboration on Endocarditis Prospective Cohort Study Group Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168:2095–103. [DOI] [PubMed] [Google Scholar]
- 26. Cecchi E, Chirillo F, Castiglione A, et al. . Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): focus on age, intravascular devices and enterococci. Int J Cardiol 2015; 190:151–6. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis 2010; 12:164–71. [DOI] [PubMed] [Google Scholar]
- 28. Corredoira J, García-País MJ, Coira A, et al. . Differences between endocarditis caused by Streptococcus bovis and Enterococcus spp. and their association with colorectal cancer. Eur J Clin Microbiol Infect Dis 2015; 34:1657–65. [DOI] [PubMed] [Google Scholar]
- 29. Pericàs JM, Corredoira J, Moreno A, et al. . Relationship between Enterococcus faecalis infective endocarditis and colorectal neoplasm: preliminary results from a cohort of 154 patients. Rev Española Cardiol (Engl Ed) 2017; 70:451–8. [DOI] [PubMed] [Google Scholar]
- 30. Henley SJ, King JB, German RR, et al. ; Centers for Disease Control and Prevention Surveillance of screening-detected cancers (colon and rectum, breast, and cervix)—United States, 2004–2006. MMWR Surveill Summ 2010; 59:1–25. [PubMed] [Google Scholar]
- 31. Bikdeli B, Wang Y, Kim N, et al. . Trends in hospitalization rates and outcomes of endocarditis among Medicare beneficiaries. J Am Coll Cardiol 2013; 62:2217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.