Abstract
Objective
To compare the glycaemic control and cardiovascular risk factor profiles of younger and older patients with type 2 diabetes. Cross-sectional analysis of data from the 2015 Australian National Diabetes Audit was undertaken.
Methods
Data were obtained from adults with type 2 diabetes presenting to Australian secondary/tertiary diabetes centres. Logistic regression examined associations with glycated haemoglobin A1c (HbA1c) >7% (53 mmol/mol) and cardiovascular risk factors.
Results
Data from 3492 patients were analysed. Mean (±SD) age was 62.9±12.5 years, mean diabetes duration 13.5±9.4 years and mean HbA1c 8.2%±1.8%. Mean HbA1c was 8.6%±2.1% and 8.0%±1.6% for the younger (<60 years) and older subgroups (≥60 years), respectively (p<0.001). The adjusted OR (aOR) of HbA1c above >7.0% was 1.5 times higher (95% CI 1.22 to 1.84) for younger patients compared with older patients after adjustment for gender, smoking, diabetes duration, renal function and body mass index. Younger patients were also more likely to have dyslipidaemia (aOR 2.02, 95% CI 1.53 to 2.68; p<0.001), be obese (aOR 1.25, 95% CI 1.05 to 1.49; p<0.001) and be current smokers (aOR 2.13 95% CI 1.64 to 2.77; p<0.001) than older patients.
Conclusions
Younger age was associated with poorer glycaemic control and adverse cardiovascular risk factor profiles. It is imperative to optimise and monitor treatment in order to improve long-term outcomes.
Keywords: general diabetes, epidemiology, general medicine (see internal medicine)
Strengths and limitations of this study.
Large dataset of patients from a nationwide survey.
Information on a broad range of variables with potential impact on glycaemic, blood pressure and lipid control.
We were unable to conduct longitudinal analyses as the data were de-identified and the cross-sectional nature of the analysis precluded investigation of causality.
Study population may largely represent a specialist referred patient group as the majority of patients were receiving care at tertiary diabetes centres.
Introduction
Driven by ageing populations, increasing obesity and decreasing physical activity, the prevalence of diabetes is expected to rise by 55% to 592 million individuals worldwide by 2035.1 Traditionally a disease of middle and older age, type 2 diabetes is increasingly diagnosed in younger patients.2 3 Diabetes and its complications contribute to 10% of Australian deaths4 and 8.4% of deaths worldwide.5
The US National Health and Nutrition Examination Survey indicated that the prevalence of type 2 diabetes has increased by 70% in people aged 20–44 years in the last three decades, making younger adults the fastest growing group of people with type 2 diabetes.6 Diabetes complications are related to duration and degree of glycaemic control,7 thus, younger people with diabetes who start their hyperglycaemic exposure at an earlier age may be at highest risk for end-organ damage. However, few studies have compared glycaemic control in younger and older patients with type 2 diabetes.8 9 Further, these studies were largely conducted within selected trial cohorts (and as such the patients examined may differ from community-based cohorts) and have reported variable findings of better glycaemic control in older patients,10 in younger patients11 or no effect of age.12
We hypothesised that there may be age-related differences in the management of patients with type 2 diabetes, which may contribute to excess cardiovascular risk in younger patients. This study investigates differences in the achieved levels and management of1 glycaemic control and2 cardiovascular risk factors between younger and older patients with type 2 diabetes.
Methods
Participants
This national, cross-sectional study examined de-identified data from the 2015 Australian National Diabetes Audit (ANDA).13 Participants were adult patients with type 2 diabetes, presenting to 1 of 49 diabetes centres nationwide. De-identified data were sourced from a range of diabetes centres located in the community/primary care (n=16) and secondary care (n=33), with patients under the care of endocrinologists, general specialists and local general practitioners. The state and territory location of participating sites is presented in online supplementary data. Information was collected regarding all consecutive patients attending a participating diabetes centre during the 1-month survey period (May or June 2015).
bmjopen-2017-020677supp001.pdf (303.5KB, pdf)
Variables
Prespecified demographic (gender, date of birth) and clinical variables (diabetes complications, comorbid conditions, blood pressure (BP), glycated haemoglobin A1c (HbA1c), body mass index (BMI), smoking status, medications) were collected for patients with type 2 diabetes. Health professionals from participating centres examined patients, reviewed medical records including pathology results and recorded the information in a standardised data collection form. All missing data, invalid entries and discrepancies were clarified with the patients’ treating centres. As per the a priori analysis plan, age at survey was calculated as date of survey (2015) minus date of birth and categorised as <60 years or ≥60 years, diabetes duration was calculated as date of survey minus date of diabetes diagnosis and categorised as <10 years or ≥10 years. Height and weight were measured to calculate BMI. Smoking status was categorised as never, previous or current. Recent pathology results (within the last 12 months) were recorded for total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TGs), HbA1c and serum creatinine; calculated estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation.14
Outcomes
The main outcome variables were HbA1c (categorised as >7.0%, 53 mmol/mol), hypertension (defined as >140 and/or 90 mm Hg), dyslipidaemia (defined as either TC >4.0 mmol/L, HDL <1.0 mmol/L, LDL >2.0 mmol/L or TG >2.0 mmol/L), obesity (defined as BMI >30 kg/m2) and smoker (categorised as never, past or current). The targets were based on the current Australian recommendations for people with diabetes as per the Australian Heart Foundation.15
Statistical analysis
Categorical variables were summarised as percentages and differences between subgroups analysed using χ2 test. Continuous variables were tested for normality to determine the most appropriate method for statistical analysis (parametric or non-parametric) and reported as means with SD or as medians with IQR. Subgroup analyses were performed using analysis of variance for normally distributed data and Mann-Whitney U tests for non-normally distributed data as appropriate. Logistic regression was used to examine factors (current age, diabetes duration, gender, smoking, calculated eGFR, BMI) associated with HbA1c, hypertension, dyslipidaemia and obesity (as the categories defined above). The selection of variables was based on identifying all measured clinical variables of known or suspected prognostic importance for the outcomes of interest and/or exhibiting a p≤0.10 on univariable analysis. All potential confounding variables were included in the multivariable models. Subgroup analyses were conducted to examine the effect of treatments (yes or no) including insulin, antihypertensive therapy and lipid-lowering therapy in patients above the glycaemic, lipid and BP targets. A prescribing gap was defined as patients who were not prescribed the relevant medications despite being above the recommended targets. A treatment gap was defined as patients who were above the recommended targets despite being on treatment. Sensitivity analyses (1) examined the effect of excluding patients with less than 2 years diabetes duration, who may have not yet had opportunity to modify treatment and achieve targets and (2) examined the effect of centre type (community/primary and secondary care) or clustering by centre. Patients were excluded from a particular analysis when data relevant to that analysis were missing, but were not excluded from other analyses where appropriate information was provided. Missing data of variables were less than 10% and not imputed. A two-sided significance level of 0.05 was considered statistically significant. All analyses were performed using Stata software V.14.2 (StataCorp).
Patient and public involvement
This research has been reviewed by the ANDA scientific advisory committee, which consists of clinical and public representatives with an interest in best practice diabetes healthcare.
Results
Overall
Data from 3492 patients (>18 years of age) were analysed. Patients from all states and territories were included (online supplementary table 1). Younger patients (<60 years) accounted for 38% (n=1328) of patients. The clinical characteristics of these patients, stratified by age, are shown in table 1. The mean (±SD) age of the whole group was 62.9±12.5 years and the mean ages of the younger and older age groups were 50.1±8.4 years and 70.7±7.0 years, respectively. Mean diabetes duration was 9.6±7.5 years for the younger age group and 15.9±9.6 years for the older age group (p<0.001). There was a higher proportion of male patients in the older (56.5%) compared with the younger age group (49.5%, p<0.001). The majority of patients (64.9%) were treated at tertiary hospitals followed by community or primary care centres (35.1%). Australian birth was reported by 68.1% of the younger age group and 62.4% of the older age group (p=0.001). Microvascular and macrovascular complications were prevalent in 35.3% and 21.6% of the younger age group and 49.3% and 43.4% of the older age group, respectively (p<0.001 for both).
Table 1.
Characteristics of study participants
| Characteristic† | Age | P values | |
| <60 years | ≥60 years | ||
| n=1328 | n=2164 | ||
| Age to 2015 (years) | 50.1 (8.4) | 70.7 (7.0) | <0.001 |
| Male | 650 (49.5) | 1208 (56.5) | <0.001 |
| Age when diabetes first diagnosed (years) | 40.6 (9.4) | 54.9 (10.6) | <0.001 |
| Diabetes duration (years) | 9.6 (7.5) | 15.9 (9.6) | <0.001 |
| HbA1c (%) | 8.6 (2.1) | 8.0 (1.6) | <0.001 |
| Cardiovascular risk factors | |||
| Systolic blood pressure (mm Hg) | 130.5 (18.1) | 134.1 (18.6) | <0.001 |
| Diastolic blood pressure (mm Hg) | 77.7 (10.5) | 72.6 (10.2) | <0.001 |
| Current smoker | 235 (20.2) | 161 (8.9) | |
| Past smoker | 350 (30.1) | 713 (39.4) | |
| Never smoker | 577 (49.7) | 936 (51.7) | |
| Total cholesterol (mmol/L) | 4.6 (1.3) | 4.0 (1.1) | <0.001 |
| LDL-cholesterol (mmol/L) | 2.4 (1.6) | 2.0 (0.9) | <0.001 |
| HDL-cholesterol (mmol/L) | 1.1 (0.4) | 1.1 (0.4) | 0.010 |
| Triglyceride (mmol/L) | 2.5 (2.4) | 2.1 (1.7) | <0.001 |
| Serum creatinine (μmol/L) | 89.5 (91.7) | 109.5 (91.3) | <0.001 |
| eGFR mL/min/1.73 m2 | 89.3 (35.9) | 65.9 (27.1) | <0.001 |
| Body mass index (kg/m2) | 34.5 (8.4) | 32.4 (6.7) | <0.001 |
| Treatments | |||
| Diet alone | 65 (4.9) | 77 (3.6) | 0.052 |
| Oral glucose lowering agents | 1050 (79.1) | 1634 (75.5) | 0.013 |
| Non-insulin injectable glucose lowering agents | 94 (7.1) | 98 (4.5) | 0.003 |
| Insulin | 769 (57.9) | 1348 (62.3) | 0.010 |
| Cardiovascular disease | |||
| Microvascular complications | 414 (35.3) | 950 (49.3) | <0.001 |
| Macrovascular complications | 247 (21.6) | 847 (43.4) | <0.001 |
Microvascular complications defined as retinopathy, nephropathy or peripheral neuropathy.
Macrovascular complications defined as either cardiovascular, cerebrovascular or peripheral vascular disease.
*Categorical variables were presented as n (%) and continuous variables as mean (SD) or median (IQR), as appropriate.
†Categorical variables were assessed with the χ2 test. Continuous variables were tested for normality, analyses were performed using analysis of variance for normally distributed data and Mann-Whitney U tests for non-normally distributed data.
eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Glycaemic control
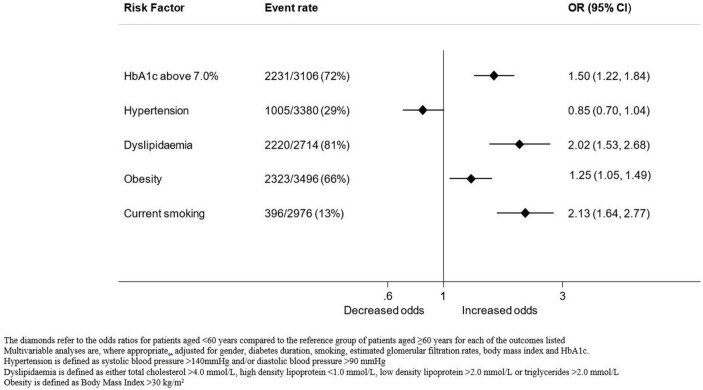
Mean HbA1c was 8.2%±1.8% for the group overall, 8.6%±2.1% and 8.0%±1.6% for the younger and older age groups, respectively (p<0.001). A greater proportion of patients in the younger age group had an HbA1c above 7.0% compared with the older age group (table 1, figure 1). On univariable analysis, age, diabetes duration, gender, smoking and BMI were all associated with an HbA1c above 7.0%. The unadjusted and adjusted ORs (95% CI) for HbA1c above 7.0% were 1.26 (1.07 to 1.49; p<0.001) and 1.50 (1.22 to 1.84; p<0.001) respectively, for younger patients compared with older patients (table 2, figure 1).
Figure 1.
Risks of adverse cardiovascular risk factor levels in patients with type 2 diabetes by age group. HbA1c, glycated haemoglobin A1c.
Table 2.
Unadjusted and adjusted odds of factors associated with suboptimal glycaemic control and adverse cardiovascular risk factor levels
| HbA1c above target (7.0%, 53 mmol/mol) |
Hypertension † | Dyslipidaemia‡ | Obesity§ | Current smoker | ||||||||||||||||
| Univariable analysis |
Multivariable analysis* |
Univariable analysis |
Multivariable analysis* |
Univariable analysis | Multivariable analysis* | Univariable analysis |
Multivariable analysis* |
Univariable analysis |
Multivariable analysis* | |||||||||||
| OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | |
| Age | ||||||||||||||||||||
| ≥60 years (ref) | ||||||||||||||||||||
| <60 years | 1.26 (1.07 to 1.49) |
0.005 | 1.50 (1.22 to 1.84) |
<0.001 | 0.81 (0.70 to 0.95) |
0.008 | 0.85 (0.70 to 1.04) |
0.119 | 2.41 (1.91 to 3.03) |
<0.001 | 2.02 (1.53 to 2.68) |
<0.001 | 1.26 (1.09 to 1.46) |
0.002 | 1.25 (1.05 to 1.49) |
0.011 | 2.60 (2.09 to 3.22) |
<0.001 | 2.13 (1.64 to 2.77) |
<0.001 |
| Duration of diabetes | ||||||||||||||||||||
| <10 years (ref) | ||||||||||||||||||||
| ≥10 years | 2.05 (1.74 to 2.40) |
<0.001 | 2.51 (2.07 to 3.03) |
<0.001 | 1.16 (0.99 to 1.35) |
0.067 | 1.03 (0.85 to 1.25) |
0.735 | 0.66 (0.53 to 0.81) |
<0.001 | 0.79 (0.60 to 1.03) |
0.087 | 1.04 (0.90 to 1.20) |
0.597 | 0.59 (0.48 to 0.73) |
<0.001 | 0.82 (0.64 to 1.06) |
0.124 | ||
| Sex | ||||||||||||||||||||
| Male (ref) | ||||||||||||||||||||
| Female | 1.18 (1.01 to 1.38) |
0.039 | 1.16 (0.97 to 1.39) |
0.100 | 1.02 (0.88 to 1.18) |
0.828 | 0.87 (0.73 to 1.04) |
0.129 | 0.76 (0.62 to 0.92) |
0.005 | 0.70 (0.55 to 0.90) |
0.005 | 1.34 (1.16 to 1.54) |
<0.001 | 1.38 (1.16 to 1.63) |
<0.001 | 0.70 (0.56 to 0.87) |
0.001 | 0.70 (0.55 to 0.89) |
0.004 |
| Smoking | ||||||||||||||||||||
| Never (ref) | ||||||||||||||||||||
| Past | 1.09 (0.9 to 1.32) |
0.368 | 0.93 (0.79 to 1.10) |
0.418 | 0.90 (0.74 to 1.09) |
0.287 | 1.10 (0.87 to 1.38) |
0.419 | 1.01 (0.77 to 1.32) |
0.947 | 1.44 (1.22 to 1.71) |
<0.001 | 1.63 (1.35 to 1.96) |
<0.001 | ||||||
| Current | 1.09 (0.84 to 1.42) |
0.512 | 0.65 (0.50 to 0.84) |
0.001 | 0.72 (0.54 to 0.96) |
0.024 | 1.73 (1.18 to 2.52) |
0.005 | 1.32 (0.87 to 1.99) |
0.187 | 0.93 (0.74 to 1.17) |
0.517 | 0.92 (0.72 to 1.18) |
0.525 | ||||||
| eGFR (mL/min/1.73 m2) (per unit) | 1.00 (0.99 to 1.00) |
0.073 | 1.00 (1.00 to 1.01) |
0.034 | 1.00 (0.99 to 1.00) |
0.001 | 1.00 (0.99 to 1.00) |
0.008 | 1.00 (1.00 to 1.01) |
0.144 | 1.00 (1.00 to 1.00) |
0.307 | 1.01 (1.01 to 1.01) |
<0.001 | 1.01 (1.00 to 1.01) |
0.001 | ||||
| BMI (kg/m2) (per unit) | 1.03 (1.02 to 1.04) |
<0.001 | 1.03 (1.02 to 1.04) |
<0.001 | 1.02 (1.01 to 1.03) |
<0.001 | 1.02 (1.01 to 1.03) |
0.001 | 1.02 (1.01 to 1.04) |
0.004 | 1.02 (1.00 to 1.03) |
0.077 | 0.98 (0.97 to 1.00) |
0.017 | 0.97 (0.95 to 0.99) |
0.001 | ||||
| HbA1c (%) (per unit) | 1.03 (0.99 to 1.07) |
0.156 | 1.18 (1.11 to 1.26) |
<0.001 | 1.14 (1.05 to 1.23) |
0.001 | 1.07 (1.03 to 1.12) |
0.001 | 1.05 (1.00 to 1.10) |
0.049 | ||||||||||
*Multivariable analyses are, where appropriate, adjusted for gender, diabetes duration, smoking, eGFRs, BMI and HbA1c.
†Hypertension is defined as systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg.
‡Dyslipidaemia is defined as either total cholesterol >4.0 mmol/L, high-density lipoprotein <1.0 mmol/L, low-density lipoprotein >2.0 mmol/L or triglycerides >2.0 mmol/L.
§Obesity is defined as BMI >30 kg/m2.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin A1c.
Glycaemic management was reported as diet only by 4%, oral agents by 77%, non-insulin injectable therapy by 5% and insulin alone or in combination with oral agents by 61% of patients. Compared with older patients, younger patients were equally likely to not be on insulin treatment despite an HbA1c >8.0%, after adjusting for gender, diabetes duration, renal function and BMI (online supplementary table 2).
Hypertension
Mean systolic BP was 130±18 mm Hg and 134±18 mm Hg for the younger and older age groups, respectively (p<0.001). A smaller proportion of patients in the younger age group were hypertensive compared with the older age group (table 1, figure 1). Younger patients were less likely to be hypertensive compared with older patients (unadjusted OR 0.81, 95% CI 0.70 to 0.95; p=0.008). However, after adjusting for gender, smoking, renal function and BMI, this effect was no longer significant (adjusted OR 0.85, 95% CI 0.70 to 1.04; p=0.119) (table 2).
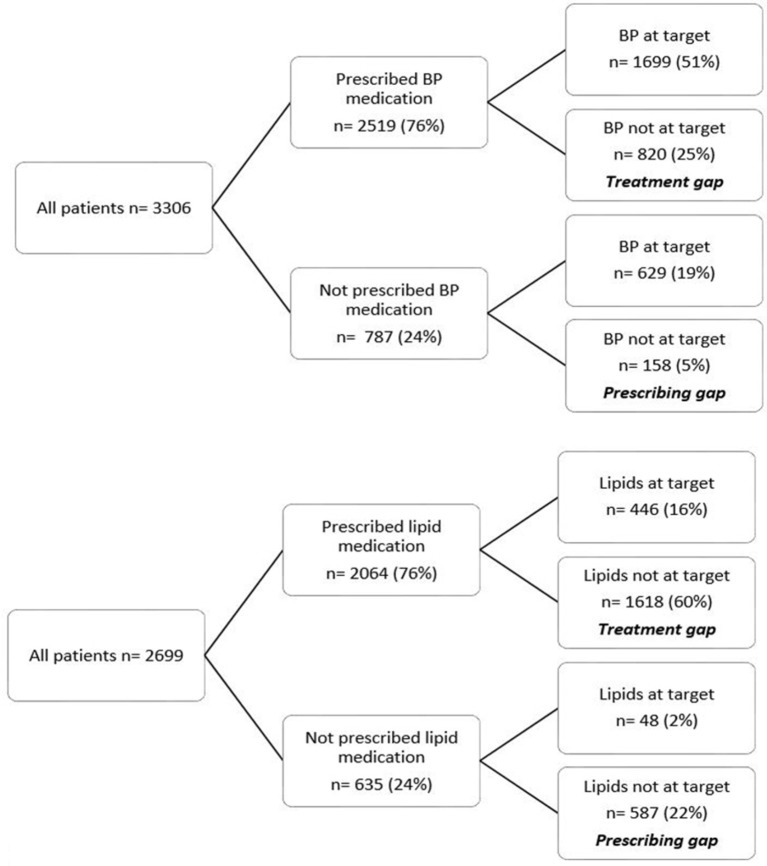
The overall study population prescribing and treatment gaps for hypertension were 5% and 25%, respectively (figure 2). Younger patients who were hypertensive were more likely to not be on BP lowering medication (prescribing gap) than older patients who were hypertensive (adjusted OR 1.84, 95% CI 1.16 to 2.92; p=0.002) (online supplementary table 2). There were no differences noted in the prescribing and treatment gaps for hypertension when male and female patients were considered separately (data not shown).
Figure 2.
Blood pressure (BP) (1) and lipid management (2) gaps in patients with type 2 diabetes.
Dyslipidaemia
The majority of patients in both age groups had abnormal lipid profiles, but a greater proportion of patients in the younger than older age group had dyslipidaemia (table 1, figure 1). On univariable analysis, age, diabetes duration, gender, smoking, BMI and HbA1c were associated with dyslipidaemia. The unadjusted and adjusted ORs (95% CI) for dyslipidaemia were 2.41 (1.91 to 3.03; p<0.001) and 2.02 (1.53 to 2.68; p<0.001), respectively, for younger patients compared with older patients (table 2).
The overall study population prescribing and treatment gaps for dyslipidaemia were 22% and 60%, respectively (figure 2). Younger patients with dyslipidaemia were more likely to not be on lipid-lowering medication (prescribing gap) than older patients with dyslipidaemia after adjustment for diabetes duration, gender, smoking, renal function and vascular disease (adjusted OR 1.48, 95% CI 1.15 to 1.90; p=0.002) (online supplementary table 2). There were no differences noted in the prescribing and treatment gaps for dyslipidaemia when male and female patients were considered separately (data not shown).
Obesity
Mean BMI was 34.5±8.4 kg/m2 and 32.4±6.7 kg/m2 for the younger and older age groups, respectively (p<0.001). A greater proportion of patients in the younger age group had a BMI in the obese category (>30 kg/m2) compared with the older age group (table 1, figure 2). On univariable analysis, age, gender and smoking were all associated with obesity. The unadjusted and adjusted ORs for obesity were 1.26 (95% CI 1.09 to 1.46; p=0.002) and 1.25 (95% CI 1.05 to 1.49; p=0.002) respectively, for younger patients compared with older (table 2).
Smoking
A greater proportion of patients in the younger age group reported being a current smoker compared with older patients (table 1, figure 1). On univariable analysis, age, diabetes duration, gender, BMI and renal function were all associated with current smoking. The unadjusted and adjusted ORs for current smoking were 2.60 (95% CI 2.09 to 3.22; p<0.001) and 2.13 (95% CI 1.64 to 2.77; p<0.001), respectively, for younger patients compared with older patients (table 2).
Sensitivity analysis
When patients with diabetes duration of 2 years or less (who may have not yet had opportunity to modify treatment practices and achieve targets) were excluded the associations were unchanged. Younger patients were still more likely to have an HbA1c over 7.0% (adjusted OR 1.59, 95% CI 1.27 to 2.00; p<0.001), dyslipidaemia (adjusted OR 1.89, 95% CI 1.41 to 2.53; p<0.001), be obese (adjusted OR 1.28, 95% CI 1.06 to 1.55; p=0.010) and smokers (adjusted OR 2.19, 95% CI 1.64 to 2.92; p<0.001) than older patients after adjusting for diabetes duration, gender, renal function, BMI and HbA1c where appropriate (online supplementary table 3). Furthermore, the associations were similar when we adjusted the models for centre type (online supplementary table 4).
Discussion
In this large national cross-sectional study of community-living patients with type 2 diabetes, we found that younger patients with significantly shorter disease duration were less likely to achieve recommended targets for glycaemic control, BP and lipids than older patients. Younger patients were also more likely to be obese and to smoke. Of patients not achieving glycaemic, BP and lipid targets, younger rather than older patients were more likely to not be on therapy after adjustment for other relevant confounders. These findings remained after exclusion of patients with more recent diabetes onset who may have been relatively new to diabetes services and not yet had opportunity to attain treatment targets.
It is not clear why younger patients demonstrate poorer glycaemic control than older patients. Some evidence suggests that early-onset type 2 diabetes may be a more aggressive phenotype than later-onset type 2 diabetes, representing a greater predisposition to beta cell failure and diagnosis at an earlier age.16 Since younger patients had higher rates of obesity compared with older patients, this may have contributed to worsening insulin resistance, and a need for greater intensification of therapy to achieve optimal glycaemic control. Longer duration of diabetes is also known to be associated with poorer glycaemic control, possibly due to progressive β-cell impairment and reduced insulin secretion,17 which in turn reduces the effectiveness of diet alone or oral agents. However, in our study the younger age group had a shorter diabetes duration than the older age group such that longer disease duration could not explain the poorer glycaemic control.
The high prevalence of poor glycaemic control and adverse cardiovascular risk factors observed in younger patients is of great concern as cardiovascular disease accounts for over half of the mortality among people with type 2 diabetes.18 19 Given the risk for cardiovascular disease doubles when hypertension is also present in people with diabetes20 and over a quarter of the patients in the younger age group had either systolic or diastolic hypertension, a review of the intensity of management is in order. This is supported by the larger prescribing and treatment gaps observed in the younger rather than older patients. In contrast, for older patients it is possible that clinicians’ concerns regarding hypotension and postural symptoms due to autonomic neuropathy may appropriately limit antihypertensive use.
Although the absolute differences in the lipid variables were not large between the younger and older age groups, it is noteworthy that among younger patients and in line with other international studies, 89% had abnormal lipids.21 High-density cholesterol levels, considered the best lipid predictor of cardiovascular disease,22 were significantly lower and TG levels significantly higher in younger patients compared with older patients suggestive of inadequate lipid management. The relative insulin deficiency seen in type 2 diabetes is known to impair the action of lipoprotein lipase, resulting in lower HDL levels and higher TG levels. However, the lower HDL and higher TG observed in younger patients cannot be attributed solely to the effect of hyperglycaemia as younger age remained independently associated with dyslipidaemia when HbA1c was included in the multivariable model. Another possible explanation is survivor effect bias whereby patients with normal lipid levels have survived longer (and into the older age group) compared with those with dyslipidaemia.
It is recognised that estimates of absolute cardiovascular risk (even for those with diabetes) are driven predominantly by age rather than modifiable risk factors.23 Indeed, in our study the majority of patients in the younger age group would have low absolute cardiovascular risk despite significant risk factor burden. The Global Burden of Disease study reported that the maximum impact in terms of healthy life years gained or disability-adjusted life years averted with cardiovascular preventive therapies would be observed between 55 and 64 years.24 However, vascular complications develop over many decades from a young age,25 well before presentation with a potentially fatal event. Additionally, younger patients have higher modifiable risk (risk factors amenable to treatment) and longer future lifetime exposure for any particular absolute risk level when compared with older people. As highlighted by our findings, a major outstanding challenge is how best to implement use of evidence-based preventive therapies in younger patients and to effectively communicate risk of future events. Among newer approaches are the concepts of heart or vascular age26 and of lifetime or modifiable risk, particularly in younger patients. This is consistent with the American College of Cardiology/American Heart Association guidelines recommending assessment of lifetime risk in younger patients in addition to the traditional absolute risk assessment.27
Other explanations for our findings include that younger patients may face more hurdles to glucose testing, regular physical activity, healthy diet and medication adherence whereas older patients may access medical care more frequently, may be more motivated to manage their medical conditions and may be more compliant with diet and medications.28–30 Further research is required to understand the barriers to better glycaemic control and cardiovascular risk profiles faced by younger patients. These data are crucial to inform strategies to assist weight reduction, lifestyle modification and escalation of glycaemic, antihypertensive and lipid-lowering therapies. Such measures would particularly benefit younger patients with type 2 diabetes, given that the incidence of macrovascular complications and mortality increases with diabetes duration7 and is reduced with management of glycaemia and cardiovascular risk factors.18 19 Good glycaemic control earlier in the course of diabetes may also be imperative, as this is demonstrated to reduce complications in the long term.31
The proportion of patients with hypertension and dyslipidaemia in our study was similar to that reported in the population-based Australian Diabetes, Obesity and Lifestyle Study (AusDiab) study. However, the proportion of patients overall with an HbA1c target ≤7.0% was greater in our study than in the AusDiab study32 and the community-based Fremantle Diabetes Study.8 In our study, younger patients had poorer glycaemic control with a mean diabetes duration approximately half that of older patients. Higher HbA1c levels have previously been independently associated with younger age.8 In contrast, the Australian general practice based NEFRON(National Evaluation of the Frequency of Renal impairment cO-existing with Non-insulin dependent diabetes mellitus) study found that younger and more obese patients with a longer duration of diabetes had poor glycaemic control.9 The differences in these studies may be due to the varying sampling frames and population characteristics.
Similar to other studies investigating gender differences in the management of type 2 diabetes, we found that female patients were more likely to report poorer glycaemic control and higher rates of obesity than males.33 However, contrary to other studies from Germany34 and Italy,35 male and female patients appeared to experience similar prescribing and treatment gaps of hypertension and dyslipidaemia in Australia. This may be due to cultural, behavioural, psychosocial and/or socioeconomic differences between these countries affecting access to healthcare and uptake of preventive measures.
A strength of this analysis is the large dataset of patients from a nationwide survey. Data were sourced from over half of the centres registered with the National Association of Diabetes centres (NADC) at the time. The participants of our study are likely to be similar to patients attending diabetes clinics throughout Australia. We obtained information on a broad range of variables with potential impact on glycaemic, BP and lipid control. Study limitations include that the majority of patients were receiving care at tertiary diabetes centres and may largely represent a specialist referred patient group. Referral bias is also possible. General practitioners may be more likely to refer younger patients while managing older patients with shorter diabetes duration. Alternatively, older patients with longer diabetes duration and interrelating comorbid conditions may also be more likely to be referred to specialist services. Another limitation was the reliance on self/healthcare worker reports as we were unable to independently verify diagnoses and treatments. This is unlikely to change the findings substantively, given previous studies have found approximately 90% of self-reported diabetes information to be valid.36 We were unable to conduct longitudinal analyses as the data were de-identified and the cross-sectional nature of the analysis precluded investigation of causality.
Conclusion
In summary, younger patients with type 2 diabetes attending diabetes centres are burdened by poorer glycaemic control and cardiovascular risk factor profiles compared with older patients. Of patients not achieving glycaemic, BP and lipid targets, younger patients were significantly more likely to not be on therapy or be above target despite treatment than older patients. Younger patients with diabetes may benefit from more targeted, evidence-based, multidisciplinary initiatives to achieve and maintain intensive glycaemic control and optimise cardiovascular risk factors. Such measures may minimise the incidence and severity of diabetes-related complications in younger patients with type 2 diabetes, thereby reducing morbidity and mortality.
Supplementary Material
Acknowledgments
We thank the participating diabetes centres for their time and generous contribution to the Australian National Diabetes Audit.
Footnotes
Contributors: NN: study design, literature review, statistical analysis, critical discussion, drafting and revision of the manuscript. AMG: statistical analysis, critical discussion, revision of the manuscript. SR: statistical analysis and interpretation of the data, revision of the manuscript. WAD: critical revision of the manuscript. JRF: critical revision of the manuscript. NW: study conception and design, revision of the manuscript. SA: study conception and design, critical revision of the manuscript. SZ: study conception and design, design of analyses, critical revision of the manuscript, supervision of the project. The authors NN, SR and SZ had full access to the data and took responsibility for the integrity of the data and accuracy of the analysis. All authors read and approved the final manuscript.
Funding: The Commonwealth Department of Health and Ageing funds the Australian National Diabetes Audit. This research has received no specific grant from any other funding agency in the public, commercial or not-for profit sectors.
Competing interests: WAD reports past participation in advisory boards and/or receiving honoraria from Novo Nordisk and Eli Lilly Australia. NW reports past participation in advisory boards and/or receiving honoraria from AstraZeneca/, Eli Lilly Australia, Merck Sharp & Dohme (Australia), Sanofi Aventis, Novo Nordisk. SA reports past participation in advisory boards and/or receiving honoraria from GlaxoSmithKline, Novartis, AstraZeneca/Bristol-Myers Squibb Australia, Eli Lilly Australia, Janssen Cilag, Merck Sharp & Dohme (Australia), Sanofi Aventis, Novo Nordisk, Servier Laboratories. SZ reports past participation in advisory boards/contract work on behalf of Monash University with AstraZeneca, Merck Sharp & Dohme (Australia) and Novo Nordisk. SZ holds a NHMRC senior research fellowship.
Patient consent: Not required.
Ethics approval: The Australian National Diabetes Audit has received approval from the Monash Health Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Application for datasets generated during and/or analysed during the current study may be considered by the corresponding author on reasonable request.
References
- 1. Guariguata L, Whiting DR, Hambleton I, et al. . Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–49. 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Rahelić D. [7TH Edition of Idf Diabetes Atlas-Call for Immediate Action]. Lijec Vjesn 2016;138:57–8. [PubMed] [Google Scholar]
- 3. Song SH, Hardisty CA. Early-onset Type 2 diabetes mellitus: an increasing phenomenon of elevated cardiovascular risk. Expert Rev Cardiovasc Ther 2008;6:315–22. 10.1586/14779072.6.3.315 [DOI] [PubMed] [Google Scholar]
- 4. Welfare AIoHa. Deaths from diabetes: Australian Institute of Health and Welfare. 2017. cited 2017http://www.aihw.gov.au/diabetes/deaths/
- 5. IDF Diabetes Atlas Group. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2011. Diabetes Res Clin Pract 2013;100:277–9. 10.1016/j.diabres.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 6. Menke A, Casagrande S, Geiss L, et al. . Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA 2015;314:1021–9. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 7. Zoungas S, Woodward M, Li Q, et al. . Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57:2465–74. 10.1007/s00125-014-3369-7 [DOI] [PubMed] [Google Scholar]
- 8. Skinner TC, Bruce DG, Davis TM, et al. . Personality traits, self-care behaviours and glycaemic control in type 2 diabetes: the Fremantle diabetes study phase II. Diabet Med 2014;31:487–92. 10.1111/dme.12339 [DOI] [PubMed] [Google Scholar]
- 9. Macisaac RJ, Jerums G, Weekes AJ, et al. . Patterns of glycaemic control in Australian primary care (NEFRON 8). Intern Med J 2009;39:512–8. 10.1111/j.1445-5994.2008.01821.x [DOI] [PubMed] [Google Scholar]
- 10. El-Kebbi IM, Cook CB, Ziemer DC, et al. . Association of younger age with poor glycemic control and obesity in urban african americans with type 2 diabetes. Arch Intern Med 2003;163:69–75. 10.1001/archinte.163.1.69 [DOI] [PubMed] [Google Scholar]
- 11. Smith NL, Heckbert SR, Bittner VA, et al. . Antidiabetic treatment trends in a cohort of elderly people with diabetes. The cardiovascular health study, 1989-1997. Diabetes Care 1999;22:736–42. 10.2337/diacare.22.5.736 [DOI] [PubMed] [Google Scholar]
- 12. Shorr RI, Franse LV, Resnick HE, et al. . Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. J Am Geriatr Soc 2000;48:264–7. 10.1111/j.1532-5415.2000.tb02644.x [DOI] [PubMed] [Google Scholar]
- 13. Do H. Australian National Diabetes Audit (ANDA): Commonwealth of Australia. 2017. cited 17 Jun 2017 http://www.health.gov.au/internet/main/publishing.nsf/content/pq-diabetes-pubs (updated 16 May 2017).
- 14. Levey AS, Bosch JP, Lewis JB, et al. . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 15. O’Callaghan CJ, Rong P, Goh MY. National guidelines for the management of absolute cardiovascular disease risk. Med J Aust 2014;200 454–6. 10.5694/mja13.11162 [DOI] [PubMed] [Google Scholar]
- 16. Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years–clinical observation from a secondary care cohort. QJM 2009;102:799–806. 10.1093/qjmed/hcp121 [DOI] [PubMed] [Google Scholar]
- 17. Saad MF, Knowler WC, Pettitt DJ, et al. . Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet 1989;1:1356–9. 10.1016/S0140-6736(89)92804-3 [DOI] [PubMed] [Google Scholar]
- 18. Cea Soriano L, Johansson S, Stefansson B, et al. . Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol 2015;14:38 10.1186/s12933-015-0204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen YY, Lin YJ, Chong E, et al. . The impact of diabetes mellitus and corresponding HbA1c levels on the future risks of cardiovascular disease and mortality: a representative cohort study in Taiwan. PLoS One 2015;10:e0123116 10.1371/journal.pone.0123116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2002;25:199–201. [DOI] [PubMed] [Google Scholar]
- 21. Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care 2001;24:1522–7. 10.2337/diacare.24.9.1522 [DOI] [PubMed] [Google Scholar]
- 22. Ingelsson E, Schaefer EJ, Contois JH, et al. . Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007;298:776–85. 10.1001/jama.298.7.776 [DOI] [PubMed] [Google Scholar]
- 23. Zoungas S, Curtis A, Tonkin A, et al. . Statins in the elderly: an answered question? Curr Opin Cardiol 2014;29:372–80. 10.1097/HCO.0000000000000082 [DOI] [PubMed] [Google Scholar]
- 24. Murray CJ, Lauer JA, Hutubessy RC, et al. . Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet 2003;361:717–25. 10.1016/S0140-6736(03)12655-4 [DOI] [PubMed] [Google Scholar]
- 25. Berenson GS, Srinivasan SR, Xu JH, et al. . Adiposity and Cardiovascular Risk Factor Variables in Childhood Are Associated With Premature Death From Coronary Heart Disease in Adults: The Bogalusa Heart Study. Am J Med Sci 2016;352:448–54. 10.1016/j.amjms.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 26. Vasan RS, Kannel WB. Strategies for cardiovascular risk assessment and prevention over the life course: progress amid imperfections. Circulation 2009;120:360–3. 10.1161/CIRCULATIONAHA.109.881995 [DOI] [PubMed] [Google Scholar]
- 27. Goff DC, Lloyd-Jones DM, Bennett G, et al. . American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adekoya N. Patients seen in emergency departments who had a prior visit within the previous 72 h-National Hospital Ambulatory Medical Care Survey, 2002. Public Health 2005;119:914–8. 10.1016/j.puhe.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 29. Bezie Y, Molina M, Hernandez N, et al. . Therapeutic compliance: a prospective analysis of various factors involved in the adherence rate in type 2 diabetes. Diabetes Metab 2006;32:611–6. 10.1016/S1262-3636(07)70316-6 [DOI] [PubMed] [Google Scholar]
- 30. Ahmad NS, Ramli A, Islahudin F, et al. . Medication adherence in patients with type 2 diabetes mellitus treated at primary health clinics in Malaysia. Patient Prefer Adherence 2013;7:525–30. 10.2147/PPA.S44698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoungas S, Chalmers J, Neal B, et al. . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392–406. 10.1056/NEJMoa1407963 [DOI] [PubMed] [Google Scholar]
- 32. Tanamas S, Magliano D, Lynch BM, et al. . AusDiab 2012. The Australian Diabetes, Obesity and Lifestyle Study. Melbourne: Baker IDI Heart and Diabetes Institute 2012. [Google Scholar]
- 33. Anand SS, Islam S, Rosengren A, et al. . Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008;29:932–40. 10.1093/eurheartj/ehn018 [DOI] [PubMed] [Google Scholar]
- 34. Gouni-Berthold I, Berthold HK, Mantzoros CS, et al. . Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care 2008;31:1389–91. 10.2337/dc08-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Penno G, Solini A, Bonora E, et al. . Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: the RIACE Italian multicentre study. J Intern Med 2013;274:176–91. 10.1111/joim.12073 [DOI] [PubMed] [Google Scholar]
- 36. Kahn LB, Marshall JA, Baxter J, et al. . Accuracy of reported family history of diabetes mellitus. Results from San Luis Valley Diabetes Study. Diabetes Care 1990;13:796–8. 10.2337/diacare.13.7.796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020677supp001.pdf (303.5KB, pdf)