Abstract
Objectives
Early diagnosis of childhood cancer is critical. Nevertheless, little is known about the potential role of inequality. This study aims to describe the use of primary care 2 years before a childhood cancer diagnosis and to investigate whether socioeconomic factors influence the use of consultations and diagnostic tests in primary care.
Design
A national population-based matched cohort study.
Setting and participants
This study uses observational data from four Danish nationwide registers. All children aged 0–15 diagnosed with cancer during 2008–2015 were included (n=1386). Each case was matched based on gender and age with 10 references (n=13 860).
Primary and secondary outcome measures
The primary outcome was additional rates for consultations and for invoiced diagnostic tests for children with cancer according to parental socioeconomic factors. Furthermore, we estimated the association between socioeconomic factors and frequent use of consultations, defined as at least four consultations, and the odds of receiving a diagnostic test within 3 months of diagnosis.
Results
Children with cancer from families with high income had 1.46 (95% CI 1.23 to 1.69) additional consultations 3 months before diagnosis, whereas children from families with low income had 1.85 (95% CI 1.60 to 2.11) additional consultations. The highest odds of frequent use of consultations was observed among children from low-income families (OR: 1.94, 95% CI 1.24 to 3.03). A higher odds of receiving an invoiced diagnostic test was seen for children from families with mid-educational level (OR: 1.46, 95% CI 1.09 to 1.95).
Conclusion
We found a socioeconomic gradient in the use of general practice before a childhood cancer diagnosis. This suggests that social inequalities exist in the pattern of healthcare utilisation in general practice.
Keywords: primary care, paediatric oncology, public health
Strengths and limitations of this study.
This large nationwide study is based on high-quality data from four nationwide registers.
The risk of selection bias and information bias was limited.
Matching was used to reduce potential confounding effects of age and gender.
Multiple socioeconomic variables were examined in the analysis to ensure high validity of findings.
A limitation was the lack of information on the reasons for requesting consultations and tests.
Introduction
Childhood cancer is the second most common cause of death among children in developed countries and is only outnumbered by accidents.1 Denmark has one of the highest incidence rates of childhood cancer among high-income countries, with an annual incidence rate around 14 cases per 100 000 children below 15 years of age.2 3
Diagnosis of childhood cancer is a challenging task in general practice as children with early-stage cancer often present with non-specific and vague symptoms that mimic common conditions such as viral infection.4 5 A Danish study showed that excess healthcare use, which can be seen as a proxy for symptoms of childhood cancer, occurs several months before the diagnosis is established.6 The time leading up to the cancer diagnosis is often full of worries for the involved families. Moreover, delayed diagnosis can cause longstanding effects, such as distress in the family and poor quality of life, and may negatively affect the curability and survival.5 7
Several studies have documented inequalities in the healthcare use between patients with low and high socioeconomic position (SEP).8–11 Children from families with lower SEP are more frequent in contact with the healthcare system. They more often suffer from chronic diseases, are more likely to acquire infectious diseases and have increased risk of injuries.12–14 However, the utilisation of preventive child health examinations is lower in the deprived part of the population.8 Additionally, a growing body of research shows that parental socioeconomic factors influence childhood cancer survival, even in countries with free access to high-quality healthcare.15–18
One question that arises in this context is whether socioeconomic differences influence the utilisation of primary care for childhood cancer and (if positive) to what extent. Knowledge about inequality in early diagnosis of childhood cancer is essential to ensure an optimal diagnostic route, regardless of the patient’s SEP.
The aim of this study is to describe the use of primary care 2 years before a diagnosis of childhood cancer and to investigate whether socioeconomic factors modify the use of consultations and the diagnostic tests performed in primary care.
Method
We conducted a national population-based matched cohort study using data from four nationwide Danish registers: (i) the Danish Civil Registration System, which holds basic demographic information on all Danish citizens, (ii) the Danish Cancer Register (DCR), which holds information on all cancer diagnoses in Denmark, (iii) the Danish National Health Insurance Service Register (NHSR), which holds information on all contacts to and services provided by general practice based on remuneration coding19 and (iv) Statistics Denmark, which is the central authority on Danish statistics and holds socioeconomic and demographic information on all citizens.20 The civil registration number, a unique 10-digit personal identification number assigned to every Danish citizen at birth or immigration, was used to link data at the individual level.
Setting
The Danish healthcare system is tax-financed and offers equal and universal access to healthcare for all citizens. All Danish residents have direct and free access to general practitioners (GPs), and more than 98% of all citizens are registered with a specific general practice.21 GPs act as gatekeepers to the rest of the healthcare system; they carry out initial diagnostic investigations including referrals to specialists. Specialist and hospital care is free of charge. Except for emergencies and ear–nose–throat and eye specialists, all citizens must first contact their general practice to get a referral.
Study population
All children aged 0–15 years diagnosed with an incident cancer according to the Danish version of the International Classification of Diseases (ICD-10) (C00-D48) in the period of 1 January 2008 to 31 December 2015 were identified in the DCR. All childhood cancers were divided into five diagnostic subgroups in accordance with the ICD-10 codes: leukaemia (C91-95), lymphoma (C81-85, C96), CNS tumour (C70-72, C75.1–3, D32-33, D35.2–4, D42-43, D44.3–5), bone tumour (C40-41) and other solid tumours (remaining ICD-10 cancer codes).
For each patient with childhood cancer, 10 random references were sampled and matched on date of birth and gender. Index date was the date of diagnosis for the matched cancer patient. The references had to be alive, without a history of cancer and resident in Denmark at the index date (ie, date of diagnosis) and 2 years before the index date.
The date of diagnosis in the DCR is based on the international hierarchy, that uses the dates of histological confirmation, admission to hospital and date of death. The histology date always takes precedence over any other date obtained.22
Socioeconomic factors
We used information on SEP from the calendar year before the index date in order to minimise the impact from the child’s disease on the socioeconomic indicators. SEP indicators were categorised as described in the following. Parental cohabitation status was divided into living with a partner (married/cohabitating) or living alone (divorced, widowed or never married). Household labour market affiliation was divided into employed, unemployed (unemployed, old age pension or early retirement pension, disability pension or welfare payments) and mixed (one parent employed, the other unemployed). Educational level was classified according to Unesco’s International Standard Classification of Education into three groups (low educational level: ≤10 years, medium educational level: >10 and ≤15 years, and high educational level: >15 years) and was based on the highest obtained educational level of the mother. In cases with no mother in the household, the highest obtained educational level of the father was used. Income was measured as equalised disposable household income (salaries, wages, all types of supplementary benefits and pensions) and comprised all income after taxation for the entire household adjusted for number of persons in the household.23 Income was categorised into three groups: low (1 st quartile), medium (second and third quartile) and high (fourth quartile). Number of children in the household was dichotomised as the presence of siblings (yes/no).
Primary healthcare services
The main outcomes were rates of consultations and invoiced diagnostic tests per patient performed in general practice; these data were obtained from the NHSR. Consultations included face-to-face consultations, home visits, telephone and email consultations during daytime. Planned vaccinations and preventive child health examinations were not included.
Invoiced diagnostic tests included urine tests (stick, microscopy of urine and urine culture), blood tests (C reactive protein, differential blood count, blood glucose and haemoglobin), pulmonary function tests, electrocardiography and tests for streptococcal throat infection.
Statistical analyses
We calculated the quarterly difference between rates for consultations and invoiced diagnostic tests performed for children who were later diagnosed with cancer and reference children stratified for each socioeconomic factor. In the following, this incidence rate difference (IRD) will be referred to as ‘additional rates’.
We calculated the absolute difference in ‘additional rates’ compared with the reference group for consultations and invoiced diagnostic tests. We used generalised linear models with identity link for the Poisson family. For both additional rates and absolute differences, we applied cluster robust variance estimation to account for repeated measurements for the subjects.
Logistic regression was used to estimate the association between socioeconomic variables and the odds of frequent use of consultations, frequent use was defined as having at least four consultations in the 3 months before diagnosis based on the fourth quartile. Two models were used. First, a basic model adjusted for cancer type, age and gender for each of the socioeconomic variables. Second, a model adjusted for cancer type, age, gender and all included socioeconomic variables. Similar models were used to estimate the association between SEP and the probability of receiving at least one invoiced diagnostic test during the last 3 months before diagnosis.
A p value of ≤0.05 was considered statistically significant. Analyses were performed using Stata/IC V.15.0.
Ethics
The study was approved by the Danish Data Protection Agency (j.no. 2009-41-3471). According to Danish law, approval by the National Committee on Health Research Ethics was not required as no biomedical intervention was performed and no biological material was collected.24
Patient and public involvement
Patients or public were not involved in this study.
Results
Characteristics of the study population
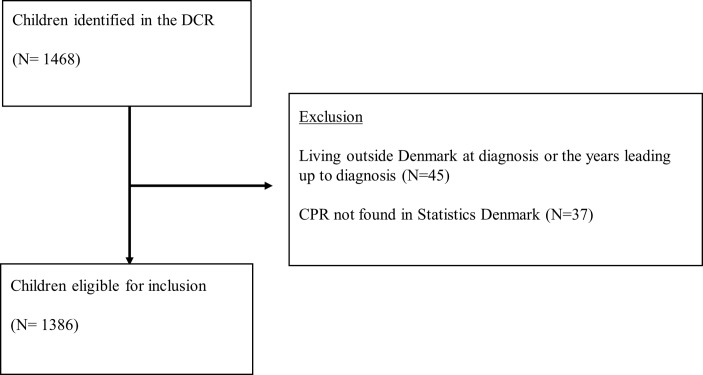
In all, 1386 eligible children with cancer and 13 860 matched references were identified (figure 1) and characteristics are shown in table 1. The proportion of children consulting general practice within 3 months before diagnosis (ie, index date) was 75.3% among cases and 37.7% among references. Invoiced diagnostic tests were performed in primary care within 3 months before diagnosis for 29.4% of cases and 6.9% of references (table 1).
Figure 1.
Flowchart of children eligible for inclusion in the study. CPR, DCR, Danish Cancer Register.
Table 1.
Characteristics of the childhood cancer cohort and the gender-matched and age-matched reference cohort
| Cases | References | |||
| n | % | n | % | |
| 1386 | 100.0 | 13 860 | 100.0 | |
| Sex | ||||
| Girls | 650 | 46.9 | 6500 | 46.9 |
| Boys | 736 | 53.1 | 7360 | 53.1 |
| Age at diagnosis (index date), years | ||||
| 10–15 | 411 | 29.7 | 4110 | 29.7 |
| 5–9 | 360 | 26.0 | 3600 | 26.0 |
| 1–4 | 475 | 34.3 | 4750 | 34.3 |
| 0 | 140 | 10.1 | 1400 | 10.1 |
| Type of cancer | ||||
| Leukaemia | 347 | 25.0 | – | – |
| Lymphoma | 170 | 12.3 | – | – |
| CNS tumour | 367 | 26.5 | – | – |
| Bone tumour | 59 | 4.3 | – | – |
| Other solid tumour | 443 | 32.0 | – | – |
| Siblings | ||||
| Yes | 1044 | 75.3 | 10.329 | 74.5 |
| No | 276 | 19.9 | 2.870 | 20.7 |
| Missing | 66 | 4.8 | 661 | 4.8 |
| Parental cohabitation status | ||||
| Living with a partner | 915 | 66.0 | 8.972 | 64.7 |
| Living alone | 393 | 28.4 | 4.136 | 29.8 |
| Missing | 78 | 5.6 | 752 | 5.4 |
| Educational level | ||||
| High (>15 years) | 547 | 39.5 | 5.587 | 40.3 |
| Medium (>10–15 years) | 531 | 38.3 | 5.267 | 38.0 |
| Low (<10 years) | 211 | 15.2 | 2.091 | 15.1 |
| Missing | 97 | 7.0 | 915 | 6.6 |
| Labour market affiliation | ||||
| Employed | 987 | 71.2 | 9.876 | 71.3 |
| Mixed | 191 | 13.8 | 1.991 | 14.4 |
| Unemployed | 130 | 9.4 | 1.241 | 9.0 |
| Missing | 78 | 5.6 | 752 | 5.4 |
| Household income | ||||
| High | 330 | 23.8 | 3.294 | 23.8 |
| Medium | 655 | 47.3 | 6.601 | 47.6 |
| Low | 334 | 24.1 | 3.297 | 23.8 |
| Missing | 67 | 4.8 | 668 | 4.8 |
| GP consultation within 3 months before diagnosis/index date | 1044 | 75.3 | 5220 | 37.7 |
| Diagnostic test performed within 3 months before diagnosis/index date | 407 | 29.4 | 960 | 6.9 |
CNS, central nervous system; GP, general practitioner.
Consultation rates before diagnosis
The consultation rates for cases and references are shown in table 2. Compared with references, a minor statistically significant increase in consultations was seen among children with cancer from 16 to 18 months before the diagnosis. A progressive increase was observed from 10 to 12 months before the diagnosis, especially during the last 3 months (IRD: 1.67; 95% CI 1.55 to 1.80) (p<0.001) (table 2).
Table 2.
Rates of consultations and invoiced diagnostic tests among cases and references
| Months before diagnosis | Rates of consultations (95% CI) |
Additional rates (95% CI) |
Rates of diagnostic tests (95% CI) |
Additional rates (95% CI) |
||
| Cases (n=1386) |
References (n=13 860) | Cases (n=1386) |
References (n=13 860) |
|||
| 1–3 | 2.43 (2.30 to 2.55) |
0.75 (0.73 to 0.76) |
1.67
(1.55 to 1.80) |
0.72 (0.64 to 0.81) |
0.12 (0.11 to 0.13) |
0.60
(0.52 to 0.69) |
| 4–6 | 1.02 (0.94 to 1.10) |
0.82 (0.80 to 0.85) |
0.20
(0.12 to 0.28) |
0.18 (0.14 to 0.23) |
0.13 (0.12 to 0.14) |
0.05
(0.01 to 0.11) |
| 7–9 | 0.99 (0.91 to 1.08) |
0.82 (0.80 to 0.84) |
0.18
(0.09 to 0.27) |
0.13 (0.11 to 0.17) |
0.12 (0.11 to 0.13) |
0.02 (−0.01 to 0.05) |
| 10–12 | 0.95 (0.87 to 1.03) |
0.83 (0.81 to 085) |
0.12
(0.04 to 0.20) |
0.13 (0.10 to 0.16) |
0.12 (0.12 to 0.13) |
0.01 (−0.02 to 0.04) |
| 13–15 | 0.90 (0.82 to 0.98) |
0.80 (0.78 to 0.83) |
0.10
(0.02 to 0.18) |
0.17 (0.13 to 0.21) |
0.12 (0.53 to 0.13) |
0.05
(0.01 to 0.08) |
| 16–18 | 0.88 (0.80 to 0.95) |
0.76 (0.74 to 0.79) |
0.11
(0.33 to 0.19) |
0.13 (0.10 to 0.16) |
0.11 (0.10 to 0.12) |
0.02 (−0.01 to 0.05) |
| 19–21 | 0.85 (0.77 to 0.92) |
0.79 (0.77 to 0.82) |
0.05 (−0.03 to 0.13) |
0.15 (0.11 to 0.18) |
0.11 (0.10 to 0.12) |
0.04 (−0.00 to 0.07) |
| 22–24 | 0.79 (0.72 to 0.85) |
0.79 (0.77 to 0.82) |
0.00 (−0.08 to 0.07) |
0.09 (0.07 to 0.11) |
0.11 (0.11 to 0.13) |
−0.03 (−0.06 to 0.00) |
| Total (1–24) |
8.82 | 6.38 |
2.43
(2.08 to 2.78) |
1.71 (1.55 to 1.87) |
0.95 (0.92 to 0.98) |
0.76 (0.64 to 0.88) |
Additional rates are the difference between consultation rates of cases and references.
Statistically significant additional rates are presented in bold.
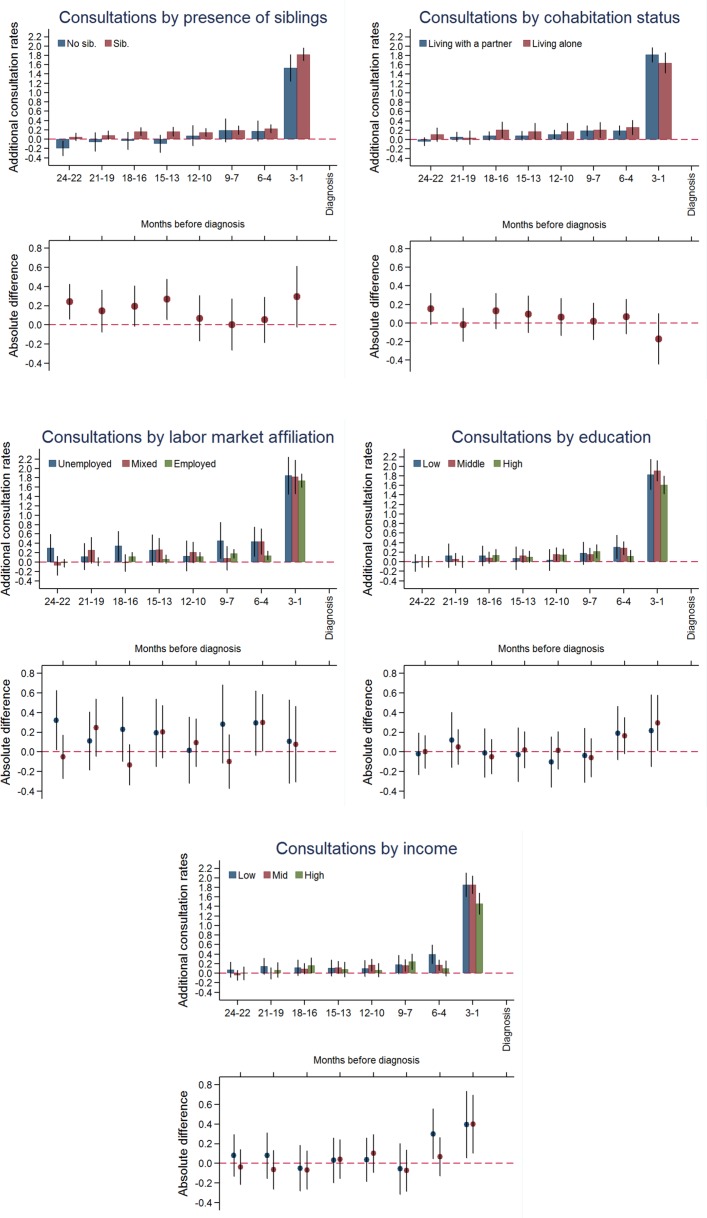
Children from families with high educational level (IRD: 1.61; 95% CI 1.43 to 1.80)) or high income (IRD: 1.46; 95% CI 1.23 to 1.69)) had lowest additional consultation rates in the last 3 months before diagnosis, whereas children from families with low educational level (IRD: 1.83; 95% CI 1.52 to 2.15)) or low income (IRD: 1.85; 95% CI 1.60 to 2.11)) had more (online supplementary table 1). No differences in additional consultation rates were observed for parental cohabitation status, having siblings or household labour market affiliation (figure 2 and online supplementary table 1).
Figure 2.
Consultation rates in general practice by socioeconomic factors. Upper part: additional consultation rates, in a 3-month intervals, for children with cancer and references 2 years before diagnosis/index date with 95% CIs. Lower part: the absolute difference in additional consultation rates with 95% CIs.
bmjopen-2018-023569supp001.pdf (190.8KB, pdf)
Odds of frequent use of consultations
Of the children with cancer, 29% were frequent users of consultations 3 months before diagnosis. The proportion was modified by income; the highest odds of frequent use of consultations was observed among children from low-income families (OR: 1.94; 95% CI 1.24 to 3.03) (table 3).
Table 3.
Odds (OR) of frequent GP attendance in the last 3 months before diagnosis
| Basic model* OR (95% CI) |
Adjusted model† OR (95% CI) |
|
| Parental cohabitation status | ||
| Living with a partner | 1.00 | 1.00 |
| Living alone | 0.90 (0.68 to 1.18) | 0.86 (0.63 to 1.16) |
| Siblings | ||
| No | 1.00 | 1.00 |
| Yes | 1.23 (0.91 to 1.69) | 1.19 (0.86 to 166) |
| Labour market affiliation | ||
| Employed | 1.00 | 1.00 |
| Mixed | 1.19 (0.84 to 1.67) | 0.99 (0.68 to 1.47) |
| Unemployed | 1.36 (0.92 to 2.02) | 1.21 (0.73 to 1.99) |
| Educational level | ||
| High | 1.00 | 1.00 |
| Medium | 1.35 (1.02 to 1.77) | 1.16 (0.87 to 1.55) |
| Low | 1.25 (0.88 to 1.79) | 0.98 (0.65 to 1.47) |
| Income | ||
| High | 1.00 | 1.00 |
| Medium | 1.76 (1.28 to 2.43) | 1.70 (1.22 to 2.37) |
| Low | 1.98 (1.38 to 2.85) | 1.94 (1.24 to 3.03) |
Statistically significant estimates are presented in bold.
*Adjusted for cancer subtype, age and gender.
†Adjusted for cancer subtype, age, gender and all socioeconomic variables.
GP, general practitioner.
A subanalysis revealed that this association was more pronounced for children with leukaemia (OR: 2.23; 95% CI 0.95 to 5.26) (online supplementary table 2) and for children from medium-level educated families (OR: 1.91; (95% CI 1.09 to 3.33) compared with children from high-level educated families. This association was not found for children with CNS or other solid tumours (online supplementary table 2).
Invoiced diagnostic tests
The rates of invoiced diagnostic tests and additional rates are shown in table 2. Children with cancer on average had 1.71 (95% CI 1.55 to 1.87) invoiced diagnostic tests performed during the 2 years before the diagnosis compared with 0.95 (95% CI 0.92 to 0.98) among the references. A progressive increase in the rates of diagnostic tests was observed in the 4–6 months before the diagnosis (table 2).
During the 3 months before the diagnosis, 29.4% of children with cancer had at least one invoiced diagnostic test performed in primary care (table 1). We found a statistically significant higher odds of receiving a diagnostic test among children from families with medium-level education (OR: 1.46 (95% CI 1.09 to 1.95)). We found no statistically significant associations between other socioeconomic variables and the odds of receiving one or more invoiced diagnostic tests (table 4).
Table 4.
Odds (OR) of receiving an invoiced diagnostic test during the last 3 months before a childhood cancer diagnosis
| Basic model* OR (95% CI) |
Adjusted model† OR (95% CI) |
|
| Parental cohabitation status | ||
| Living with a partner | 1.00 | 1.00 |
| Living alone | 0.88 (0.67 to 1.16) | 0.88 (0.66 to 1.19) |
| Siblings | ||
| No | 1.00 | 1.00 |
| Yes | 1.12 (0.81 to 1.54) | 1.06 (0.76 to 1.48) |
| Labour market affiliation | ||
| Employed | 1.00 | 1.00 |
| Mixed | 0.95 (0.66 to 1.35) | 0.86 (0.57 to 1.28) |
| Unemployed | 0.89 (0.59 to 1.37) | 0.83 (0.49 to 1.42) |
| Educational level | ||
| High | 1.00 | 1.00 |
| Medium | 1.42 (1.07 to 1.87) | 1.46 (1.09 to 1.95) |
| Low | 1.14 (0.79 to 1.65) | 1.26 (0.83 to 1.91) |
| Income | ||
| High | 1.00 | 1.00 |
| Medium | 1.12 (0.83 to 1.51) | 1.03 (0.75 to 1.41) |
| Low | 1.02 (0.71 to 1.44) | 0.98 (0.63 to 1.54) |
Statistically significant estimates are presented in bold.
*Adjusted for cancer subtype, age and gender.
†Adjusted for cancer subtype, age, gender and all socioeconomic variables.
Discussion
Principal findings
Children with cancer generally had more consultations and clinical investigations in general practice than the references. A progressive increase was seen in the 10–12 months before diagnosis, which was anticipated. However, the probability of receiving more consultations and diagnostic tests was modified by parental SEP.
Children with cancer from families with high-level education and high-level income had fewest additional consultations in the last 3 months before diagnosis. Children with cancer from households with low-level and medium-level income were thus more likely to be frequent users of consultations in the 3 months before the diagnosis compared with high-income families. This trend was more pronounced for children with leukaemia than for children with other cancer types. The odds of receiving at least one invoiced diagnostic test during the last 3 months before diagnosis was higher for children from households with medium-level education compared with high-level or low-level education.
Comparison with existing literature
The observed overall increase in the rates of both consultations and diagnostic tests is in line with previous findings.6 25 26 Previous studies have documented an association between socioeconomic factors and a prolonged diagnostic interval in childhood cancer.27–30 A prolonged interval might occur if the GP does not suspect cancer, or if the GP interprets the symptoms as something else, does not communicate or interact optimally with the child and the parents or postpones referral for specialist investigation. Our findings could indicate that some or several of these factors may be at play in parents with low education.
The GP’s intuition plays an important role in the suspicion of serious disease.31–34 A study from the UK reported that the GP–parent relationship had significant impact on the process of obtaining a paediatric leukaemia diagnosis.34 For example, the GP’s concerns and actions were partly shaped by how anxious she/he estimated the parents to be. The GP’s initial perception of a parent as being a ‘worrier’ or too sensitive could influence the way the parents’ concerns are dealt with; ‘worriers’ are generally taken less seriously. However, the importance of listening to the parents was highlighted by the GPs in one of the studies although many parents reported that the GP did not seem to take their worries seriously.34 We were able to demonstrate that children from families with lower SEP tended to see the GP more often before diagnosis. This indicates that some of these mechanisms are seen in children of parents with low income and low education. In addition, children of parents with low income might have other diseases, which may also delay the suspicion of cancer in general practice.
The communication during a consultation is a complex matter, which is influenced by numerous factors. An international review showed that patients with low SEP communicate less actively when consulting a GP and receive less information from the GP than patients with high SEP.35 This may partly explain why we observed differences in the utilisation of primary care services before a cancer diagnosis. A Danish study has shown that higher SEP of the parents, such as high education, is associated with better survival of children with cancer.36 One possible explanation raised by our study is that these children may have a delayed diagnosis.
Identifying the few children with malignant cancer disease is a major challenge in general practice, and it often includes wait-and-see strategies and very low positive predictive values for even serious symptoms of disease.37 The use of ‘safety-netting' as a strategy to manage diagnostic uncertainty is increasingly recognised as important in adult cancer diagnostics and may be even more pertinent in children.38 The term ‘safety-netting’ was introduced to general practice by Roger Neighbour who considered it a core component of the consultation. He defined safety-netting as encompassing three questions GPs might ask themselves when they make a working diagnosis; If I’m right what do I expect to happen? How will I know if I’m wrong? What would I do then? The aim is to ensure patients are monitored until their symptoms are explained.39 This may be particularly relevant if the child comes from a family with limited socioeconomic resources.
Strengths and limitations of the study
This nationwide population-based matched cohort study was based on data from several Danish national registers. Danish registers are known to be very complete and valid.40–42 A major strength was the low risk of selection bias and information bias concerning classification of diagnosis, socioeconomic factors and healthcare use. Despite the low incidence of cancer in children, we obtained sufficient data to ensure high statistical precision. This allowed us to detect small, yet clinically relevant, differences between the groups.
Our broad categorisations of, for example, income and education might have caused loss of detailed information. As these categorisations were defined a priori, some groups could have been defined too broadly and caused loss of information or introduced residual confounding. Still, we based the definitions on international standard classifications.43
A limitation of this study was the lack of information on the reasons for the requested consultations and performed tests. Potential confounding effects of age and gender were reduced by matching included cases with references. However, we cannot exclude residual confounding by other factors. For example, comorbidity or geographic factors, such as distance to GP or nearest hospital, could have influenced our results. It could be argued that geographic factors may influence the use of GP services, as there is a shortage of GPs in the more remote parts of Denmark. This might affect the accessibility and waiting time in the remote parts of Denmark, where a higher proportion of the population have lower SEP. This could potentially influence GP attendance and underestimates the effect of socioeconomic factors on utilisation of primary care. Another limitation to consider is that the date of diagnosis recorded by DCR might vary from the date of the clinical diagnosis. In some cases, the cancer may have been diagnosed clinically prior to the histopathological confirmation. However, we do not expect a systematic variation in registration according to SEP and the effect on number of consultations and diagnostic tests, if any, is therefore likely to be small.
Our study has the advantage of using multiple socioeconomic variables in the analysis. There is consensus that SEP is a complex and multifaceted aspect, which should not be considered in isolation when exploring socioeconomic inequalities in health.44–46
The generalisability of our results has certain limitations. Measuring SEP is a complex matter, and our findings may not apply to countries with different socioeconomic conditions or organisation of primary care. Yet, this challenge is seen in any study of socioeconomics and healthcare.
Conclusion and implications
This nationwide population-based cohort study shows that children who are later diagnosed with cancer tend to use primary care more often in the months before the diagnosis. We were able to demonstrate that children from families with lower SEP tended to see the GP more often before cancer diagnosis.
This study shows that despite the direct and free access to GPs and primary care, some social inequalities are seen in the healthcare utilisation and handling of these patients in general practice. These variations are likely to affect the child’s diagnostic pathway, treatment and prognosis. Our findings thus call for future research.
Supplementary Material
Acknowledgments
The authors would like to thank Kaare Rud Flarup for his indispensable assistance with the data management. They would also like to thank statistician Alina Zalounina Falborg for her help with the statistical analyses and the graphical presentation of data.
Footnotes
Contributors: CFA: collected and analysed the data; drafted the article. PV and JMA: critically revised the article multiple times. All authors conceptualised and designed the study; contributed to the interpretation of the data; approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was funded by the Danish Childhood Cancer Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The datasets analysed in the current study are stored in a secured research database and may be available upon presentation of formal approval.
References
- 1. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev 2010;36:277–85. 10.1016/j.ctrv.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer 2008;112:461–72. 10.1002/cncr.23205 [DOI] [PubMed] [Google Scholar]
- 3. van der Horst M, Winther JF, Olsen JH. Cancer incidence in the age range 0-34 years: historical and actual status in Denmark. Int J Cancer 2006;118:2816–26. 10.1002/ijc.21566 [DOI] [PubMed] [Google Scholar]
- 4. Ahrensberg JM, Hansen RP, Olesen F, et al. Presenting symptoms of children with cancer: a primary-care population-based study. Br J Gen Pract 2012;62:e458–e465. 10.3399/bjgp12X652319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon-Woods M, Findlay M, Young B, et al. Parents' accounts of obtaining a diagnosis of childhood cancer. Lancet 2001;357:670–4. 10.1016/S0140-6736(00)04130-1 [DOI] [PubMed] [Google Scholar]
- 6. Ahrensberg JM, Fenger-Grøn M, Vedsted P. Use of primary care during the year before childhood cancer diagnosis: a nationwide population-based matched comparative study. PLoS One 2013;8:e59098 10.1371/journal.pone.0059098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112(Suppl 1):S92–S107. 10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Søndergaard G, Biering-Sørensen S, Michelsen SI, et al. Non-participation in preventive child health examinations at the general practitioner in Denmark: a register-based study. Scand J Prim Health Care 2008;26:5–11. 10.1080/02813430801940877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoebel J, Rattay P, Prütz F, et al. Socioeconomic status and use of outpatient medical care: the case of Germany. PLoS One 2016;11:e0155982 10.1371/journal.pone.0155982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finnvold JE. Access to specialized health care for asthmatic children in Norway: the significance of parents' educational background and social network. Soc Sci Med 2006;63:1316–27. 10.1016/j.socscimed.2006.03.045 [DOI] [PubMed] [Google Scholar]
- 11. Stirbu I, Kunst AE, Mielck A, et al. Inequalities in utilisation of general practitioner and specialist services in 9 European countries. BMC Health Serv Res 2011;11:288 10.1186/1472-6963-11-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birken CS, Macarthur C. Socioeconomic status and injury risk in children. Paediatr Child Health 2004;9:323–5. 10.1093/pch/9.5.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lous J, Friis K, Vinding AL, et al. Social marginalization reduces use of ENT physicians in primary care. Int J Pediatr Otorhinolaryngol 2012;76:370–3. 10.1016/j.ijporl.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 14. Thrane N, Søndergaard C, Schønheyder HC, et al. Socioeconomic factors and risk of hospitalization with infectious diseases in 0- to 2-year-old Danish children. Eur J Epidemiol 2005;20:467–74. 10.1007/s10654-005-0719-2 [DOI] [PubMed] [Google Scholar]
- 15. Mogensen H, Modig K, Tettamanti G, et al. Socioeconomic differences in cancer survival among Swedish children. Br J Cancer 2016;114:118–24. 10.1038/bjc.2015.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adam M, Rueegg CS, Schmidlin K, et al. Socioeconomic disparities in childhood cancer survival in switzerland: Socioeconomic disparities in cancer survival. International Journal of Cancer 2016;138:2856–66. [DOI] [PubMed] [Google Scholar]
- 17. Syse A, Lyngstad TH, Kravdal O. Is mortality after childhood cancer dependent on social or economic resources of parents? A population-based study. Int J Cancer 2012;130:1870–8. 10.1002/ijc.26186 [DOI] [PubMed] [Google Scholar]
- 18. Erdmann F, Winther JF, Dalton SO, et al. Survival from childhood hematological malignancies in denmark: Is survival related to family characteristics?: Family traits and hematological malignancies survival. Pediatric Blood & Cancer 2016;63:1096–104. [DOI] [PubMed] [Google Scholar]
- 19. Andersen JS, Olivarius NF, Krasnik A. The Danish national health service register. Scand J Public Health 2011;39(7 Suppl):34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 20. Statistics Denmark. Privacy and cookie policy. 2017. http://www.dst.dk/en (accessed 12 Aug 2017).
- 21. Pedersen KM, Andersen JS, Søndergaard J. General practice and primary health care in Denmark. J Am Board Fam Med 2012;25(Suppl 1):S34–S38. 10.3122/jabfm.2012.02.110216 [DOI] [PubMed] [Google Scholar]
- 22. Sundhedsstyrelsen. Det moderniserede cancerregister: Metode og kvalitet, 2009. [Google Scholar]
- 23. OECD. What are equivalence scales? 2018. www.oecd.org/eco/growth/OECD-Note-EquivalenceScales.pdf
- 24. The National Committee on Health Research Ethics. Act on research ethics review of health research projects. 2017. http://www.nvk.dk/english (accessed 13 Aug 2017).
- 25. Dommett RM, Redaniel MT, Stevens MCG, et al. Features of childhood cancer in primary care: a population-based nested case–control study. Br J Cancer 2012;106:982–7. 10.1038/bjc.2011.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ansell P, Johnston T, Simpson J, et al. Brain tumor signs and symptoms: analysis of primary health care records from the UKCCS. Pediatrics 2010;125:112–9. 10.1542/peds.2009-0254 [DOI] [PubMed] [Google Scholar]
- 27. Ahrensberg JM, Olesen F, Hansen RP, et al. Childhood cancer and factors related to prolonged diagnostic intervals: a Danish population-based study. Br J Cancer 2013;108:1280–7. 10.1038/bjc.2013.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdelkhalek E, Sherief L, Kamal N, et al. Factors associated with delayed cancer diagnosis in egyptian children. Clin Med Insights Pediatr 2014;8:39 10.4137/CMPed.S16413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fajardo-Gutiérrez A, Sandoval-Mex AM, Mejía-Aranguré JM, et al. Clinical and social factors that affect the time to diagnosis of Mexican children with cancer. Med Pediatr Oncol 2002;39:25–31. 10.1002/mpo.10100 [DOI] [PubMed] [Google Scholar]
- 30. Dang-Tan T, Trottier H, Mery LS, et al. Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatr Blood Cancer 2008;51:468–74. 10.1002/pbc.21600 [DOI] [PubMed] [Google Scholar]
- 31. Hjertholm P, Moth G, Ingeman ML, et al. Predictive values of GPs' suspicion of serious disease: a population-based follow-up study. Br J Gen Pract 2014;64:e346–e353. 10.3399/bjgp14X680125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scheel BI, Ingebrigtsen SG, Thorsen T, et al. Cancer suspicion in general practice: the role of symptoms and patient characteristics, and their association with subsequent cancer. Br J Gen Pract 2013;63:e627–e635. 10.3399/bjgp13X671614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ingeman ML, Christensen MB, Bro F, et al. The Danish cancer pathway for patients with serious non-specific symptoms and signs of cancer-a cross-sectional study of patient characteristics and cancer probability. BMC Cancer 2015;15:421 10.1186/s12885-015-1424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clarke RT, Jones CH, Mitchell CD, et al. ’Shouting from the roof tops': a qualitative study of how children with leukaemia are diagnosed in primary care. BMJ Open 2014;4:e004640 10.1136/bmjopen-2013-004640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willems S, De Maesschalck S, Deveugele M, et al. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns 2005;56:139–46. 10.1016/j.pec.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 36. Simony SB, Lund LW, Erdmann F, et al. Effect of socioeconomic position on survival after childhood cancer in Denmark. Acta Oncol 2016;55:742–50. 10.3109/0284186X.2016.1144933 [DOI] [PubMed] [Google Scholar]
- 37. Dommett RM, Redaniel MT, Stevens MC, et al. Features of childhood cancer in primary care: a population-based nested case-control study. Br J Cancer 2012;106:982–7. 10.1038/bjc.2011.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicholson BD, Mant D, Bankhead C. Can safety-netting improve cancer detection in patients with vague symptoms? BMJ 2016;355:i5515 10.1136/bmj.i5515 [DOI] [PubMed] [Google Scholar]
- 39. Neighbour R. The inner consultation. 2nd edition Oxford: ed; Radcliffe Publishing, 2004. [Google Scholar]
- 40. Frank L. Epidemiology. When an entire country is a cohort. Science 2000;287:2398–9. 10.1126/science.287.5462.2398 [DOI] [PubMed] [Google Scholar]
- 41. Statens Serum Institut. Validation of the danish cancer registry and selected clinical cancer databases - english abstract. 2012. http://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/cancerregisteret (accessed 16 Aug 2017).
- 42. Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 2011;39:12–6. 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 43. Unesco. International standard classification of education. 2011. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf (Accessed 01/24, 2018).
- 44. Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 2007;99:1013. [PMC free article] [PubMed] [Google Scholar]
- 45. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA 2005;294:2879–88. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- 46. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull 2007;81-82:21–37. 10.1093/bmb/ldm001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023569supp001.pdf (190.8KB, pdf)