Abstract
Introduction
The gastrointestinal mucosa constitutes a critical barrier where millions of microbes and environmental antigens come in close contact with the host immune system. Intestinal barrier defects have been associated with a broad range of diseases and therefore denote a new therapeutic target.
Areas covered
This review is based on an extensive literature search in PubMed of how the intestinal barrier contributes to health and as a trigger for disease. We first discuss the anatomy of the intestinal barrier and explain the available methods to evaluate its function. We then review the importance of diet and lifestyle factors on intestinal barrier function, and discuss three prototypes of chronic diseases (inflammatory bowel diseases, celiac disease and non-alcoholic fatty liver disease) that have been linked to barrier defects.
Expert Commentary
The intestinal barrier has been investigated by various methods, but correlation of results across studies is difficult, representing a major shortcoming in the field. New upcoming techniques and research on the effect of barrier-restoring therapeutics may improve our current understanding of the gut barrier, and provide a step forward towards personalised medicine.
Keywords: intestinal mucosal barrier, mucus layer, intestinal epithelial cells, intestinal permeability, tight junctions, intestinal inflammation, gut health, extra-intestinal disease
1. Introduction
The human body has multiple mucosal epithelia that form direct barriers between the environment and the internal host milieu. The gastrointestinal (GI) tract harbours one of the largest luminal interaction areas of these barriers, and plays a pivotal role in the regulation of the immune system, and hence in health [1,2]. The GI mucosa has the complex task to act as a semipermeable barrier that allows the absorption of nutrients and immune sensing, while limiting the transport of potentially harmful antigens and microorganisms. The regulation of this seemingly ‘conflicting’ task is achieved by an interplay between structural components and molecular interactions at the intestinal mucosa, which operate in a dynamic manner to maintain intestinal integrity and immune homeostasis [3]. The function of the intestinal barrier can be compromised through severe structural damage of the mucosa, or more subtle changes in the regulating components of the barrier [4].
Intestinal barrier defects have been associated with a broad range of diseases, including GI (e.g. celiac disease (CeD), inflammatory bowel disease (IBD), colon carcinoma), but also extra-intestinal disorders (e.g. chronic liver disease, type 1 diabetes, obesity). For all these diseases, it is commonly hypothesized that dysfunction of the intestinal barrier and an uncontrolled flux of antigens across the intestinal epithelium may challenge the immune system of susceptible individuals and affect the host-microbial balance, as such initiating inflammatory mechanisms in the gut or more distant organ systems [5,6].
2. Structure of the intestinal barrier
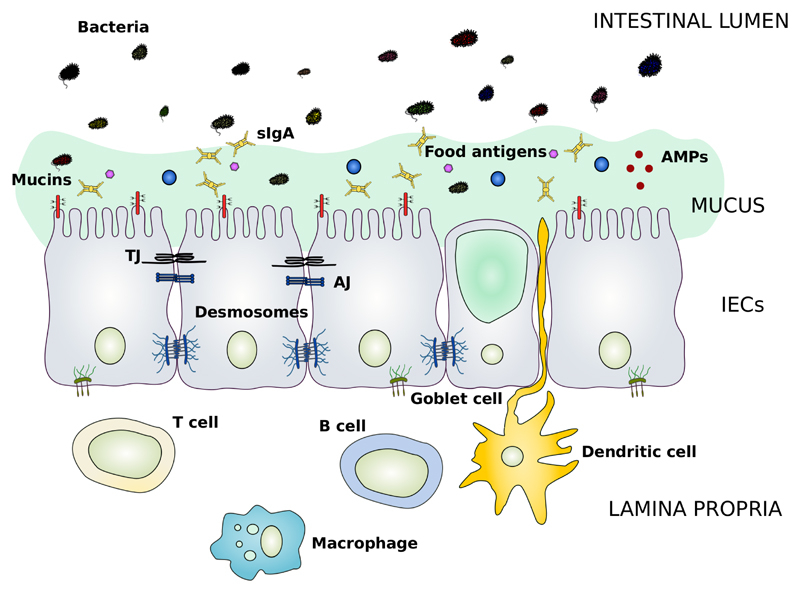
The intestinal mucosa is composed of several elements that aid in its function as a physical and immunological defence barrier. These mainly include the outer mucus layer with the commensal gut microbiota, antimicrobial proteins (AMPs) and secretory immunoglobulin A (sIgA) molecules, the central single cell layer with specialised epithelial cells, and the inner lamina propria where innate and adaptive immune cells reside such as T cells, B cells, macrophages and dendritic cells (Figure 1) [1,7].
Figure 1. Schematic representation of the main components of the intestinal barrier.
The intestinal barrier is a semipermeable structure that allows the uptake of essential nutrients and immune sensing, while being restrictive against pathogenic molecules and bacteria. Both structural and molecular components act together to fulfil this complex, but essential function of the gastrointestinal tract. The mucus layer forms a sieve-like structure overlying the intestinal epithelium. Antimicrobial peptides (AMPs) and secretory IgA molecules (sIgA) are secreted in the mucus layer as immune-sensing and regulatory proteins. The intestinal epithelial cells (IECs) form a continuous monolayer and are tightly attached to each other by junctional complexes. The tight junctions (TJs) are located at the apical side of the cells, and regulate the transport of small molecules and ions. The adherens junctions (AJs) and desmosomes provide strict cell-adhesion bonds and aid in the maintenance of the integrity of the intestinal barrier. The lamina propria contains immune cells (e.g. T cells, B cells, macrophages and dendritic cells) from the adaptive and innate immune system that take part in the immunological defence mechanisms of the intestinal barrier.
AMP, antimicrobial peptide; sIgA, secretory immunoglobulin A; IECs, intestinal epithelial cells; TJ, tight junction; AJ, adherens junction
The mucus layer is the very first line of physical defence that external molecules encounter when they arrive in the gut lumen, and which prevents bacteria from directly contacting the epithelial cells [1]. The main building blocks of the mucus layer are highly glycosylated mucin proteins that form a gel-like sieve structure overlying the intestinal epithelium [8]. In the small and large intestine, mucin 2 (MUC2) is the most abundant mucus protein secreted by goblet cells. MUC2 expression is critical in protection against disease, as Muc2 knock-out mice spontaneously develop colitis [9]. Intestinal epithelial cells (IECs) also express transmembrane mucins that remain attached to the apical surface, and form the glycocalyx together with glycolipids [8]. Remarkably, the small intestine only has one mucus gel layer whereas the colon has two layers: an outer, loose layer that allows the long-term colonisation of commensal bacteria - crucial in the colon - and an inner dense layer devoid of bacteria. Immune regulators, such as AMPs and sIgA molecules, are released in the mucus gel to reinforce the physical separation of the microbiota as a gradient from the epithelium to the lumen, and show the highest concentrations in the small intestine where the mucus layer is less dense [10]. The composition of the mucus layer can affect the microbiota in the gut, whilst the microbiota also determine the properties of the mucus gel [11].
Underneath the mucus layer, the epithelial cells are by far the strongest determinants of the physical intestinal barrier. A pool of pluripotent stem cells residing at the crypts give rise to five distinct cell types including absorptive enterocytes, goblet cells, enteroendocrine cells, Paneth cells and microfold cells [12]. These cells together form a continuous and polarised monolayer that separates the lumen from the lamina propria. Since the cell membranes are impermeable to hydrophilic solutes in the absence of specific transporters, the passage of such molecules through the IECs is highly restricted [1]. The uptake of lipophilic or large molecules is mostly dependent on diffusion and endocytosis. Transport of molecules in-between the IECs is regulated through the presence of junctional complexes. The three most important complexes are tight junctions (TJs), adherens junctions (AJs) and desmosomes [13]. TJs are the apical-most adhesive complexes that largely seal the intercellular space, and consist of transmembrane proteins (e.g. claudins, occludin), peripheral membrane proteins (e.g. zonula occludens (ZO)-1, ZO-2) and regulatory proteins. AJs are found below the TJs and are required for their assembly. Together with desmosomes, AJs provide strong adhesive bonds to maintain the integrity of the epithelium. Both TJs and AJs are linked to the peri-junctional ring of actin and myosin which permits regulation of the junctions via the cytoskeleton [1,13].
The intestinal barrier should not be regarded as a static structure, but is highly dynamic and responsive to both internal and exogenous stimuli (e.g. cytokines, bacteria, dietary factors) [1].
3. Experimental evaluation of intestinal barrier function
A number of techniques are available to evaluate the function of the intestinal barrier [14–16]. The choice for a given technique depends on the research question and is determined by the study setting and accessibility to biological material. A basic understanding of each technique is essential to appreciate the advantages and limitations (Table 1), along with the relevance for human diseases.
Table 1. Advantages and limitations of evaluation methods for intestinal barrier function.
| Advantages | Limitations | |
|---|---|---|
| Intestinal permeability | ||
| In vivo | ||
| 51Cr-EDTA | Whole intestine, easy detection, not naturally present | Limited use in humans being radio-active, single probe |
| PEGs | Whole intestine | Laborious detection with HPLC, GC-MS or LC-MS |
| Sugars | Combination in multi-sugar tests, widely used | Baseline food contamination, laborious detection with HPLC, GC-MS or LC-MS |
| Sucrose | Specific for stomach | Degraded by sucrase in the duodenum |
| Lactulose | Specific for small intestine | Degraded by intestinal bacteria |
| Mannitol, Rhamnose | Specific for small intestine | Degraded by intestinal bacteria |
| Sucralose | Resistive to bacterial degradation | Long collection time |
| Macromolecules | Correlation with food-related antigens | Limited use in humans |
| Ovalbumin, HRP, dextrans,… | Specific when used in intestinal loops, differently sized probes | Invasive when used in intestinal loops |
| Ex vivo | ||
| Ussing chambers | Region-specific, wide range of molecules, combination with cell lines possible | Need for fresh tissue, limited viability (2 hours), laborious, operator-dependent |
| In vitro | ||
| Intestinal cell lines | Long follow-up times, wide range of molecules, different test conditions | Less representative to the in vivo condition, cell culture variability |
| In situ intestinal barrier defects | ||
| Mucus & tight junctions | Mechanistic view, co-staining with bacteria possible | Does not represent functional characteristics of the barrier |
| Biomarkers | ||
| Urine | ||
| Claudin proteins | Rapid detection of tight junction loss without tissue sections/test molecules | Non-specific for gut (e.g. release from kidney epithelia) |
| FABP | Region-specific dependent on isoform, detectable in urine and blood | Only useful for acute damage |
| α-GST | Detectable in urine and blood | Non-specific, possibly only useful for acute damage |
| Blood | ||
| FABP | Region-specific dependent on isoform, detectable in urine and blood | Only useful for acute damage |
| α-GST | Detectable in urine and blood | Non-specific, possibly only useful for acute damage |
| Citrulline | Specific for enterocytes, not present in exogenous sources | Laborious detection |
| Zonulin | Specific for the small intestine, correlation with IP | Low specificity for detection with ELISA |
| Bacteria and bacterial products | Representation of effective bacterial translocation | Not all bacteria can be cultured, high false positive rate for LPS detection |
| EndoCab | Indirect representation of effective bacterial translocation | Indirect marker for bacterial translocation, correlation with IP conflicting |
| D-lactate | Easy detection | Limited data in humans |
| Faeces | ||
| Calprotectin | Stable in faeces for up to 7 days at room temperature, easily detectable | Indirect marker dependent on presence of intestinal inflammation |
51Cr-EDTA, Crohmium-labelled EDTA; PEG, polyethylene glycol; HRP, horseradish peroxidase; FABP, fatty acid-binding proteins; GST, glutathione S-transferase; EndoCab, endotoxin core antibodies; HPLC, high performance liquid chromatography; GC-MS, gas chromatography mass spectrometry; LC-MS, liquid chromatography mass spectrometry; LPS, lipopolysaccharide; IP, intestinal permeability
3.1. Intestinal permeability
Most available assays actively measure intestinal permeability, which should be interpreted in the strict sense of the term as the passage of molecules across the intestinal epithelium. These assays therefore represent only one aspect of intestinal barrier function although the terms “permeability” and “barrier function” are often used interchangeably [17]. Various marker molecules, alone or in combination, can be used to assess intestinal fluxes. Dependent on the charge and size of the molecules, distinct permeability mechanisms are evaluated. In general, the movement of molecules from the intestinal lumen to the subepithelial space can be classified in two routes: transcellular and paracellular [18]. Large antigenic molecules, lipophilic compounds and nutrients will prefer the transcellular route where molecules are transported through the IECs by endocytosis, passive diffusion or binding to specific membrane transporters. Ions (especially cations) and small hydrophilic molecules (<600 Dalton) will favour the paracellular transport pathway, and will diffuse through the intercellular spaces between adjacent IECs, with the TJs as the rate-limiting step for epithelial permeability [18,19].
3.1.1. In vivo
The most common method for in vivo assessment of intestinal permeability is the urinary recovery of administered marker molecules. Other than the detection in urine, most markers can also be retrieved in blood samples, which is often favoured in animals to avoid the need for expensive material such as metabolic cages [20]. Typical marker probes include Chromium-labelled EDTA (51Cr-EDTA), polyethylene glycols (PEG) or non-metabolizable sugars (e.g. lactulose, sucrose, rhamnose…) [16]. In clinical settings, these marker molecules are orally administered, after which they are taken up through the mucosal barrier into the circulation and eliminated by the kidneys. When intestinal barrier defects are present, a higher amount of these markers will be transported across the epithelium within a given time frame, and be detected in the circulation and the urine. The joint use of two different probes is the most optimal technique for epithelial permeability studies, since the calculation of the excretion ratio can correct for confounders such as gastric emptying, intestinal transit time and renal function [21]. The current golden standard to measure small intestinal permeability is the differential urinary excretion test with lactulose and mannitol [14,18]. Lactulose (disaccharide) is assumed to cross the barrier via the paracellular pathway in a highly restricted manner, while the smaller molecule mannitol (monosaccharide) should freely move across the intestinal epithelium via transcellular and paracellular routes. When large intestinal permeability tests are warranted, lactulose/rhamnose or mannitol cannot be used because these sugars are degraded by colonic bacteria. Alternatives are 51Cr-EDTA, PEG molecules or sucralose which remain stable throughout the GI tract. Gastroduodenal permeability testing can be performed using sucrose, a sugar that is degraded by sucrase in the duodenum [16]. Recent studies have also confirmed the feasibility and accuracy of multi-sugar tests with fractioned urine collections to simultaneously asses intestinal permeability at different locations [22]. Using animal models, permeability tests with larger molecules (e.g. ovalbumin, horseradish peroxidase, dextrans) or even specific fluorescently labelled microbes are also feasible. The recovery of these marker probes can be done in regular blood samples or, preferably, in mesenteric or portal blood using intestinal loop systems [23].
3.1.2. Ex vivo
Ussing chambers, named after the Danish biologist Hans Ussing who developed the technique using frog skin, represent an alternative but reliable assay to measure the transport of ions and molecules across fresh epithelial tissue specimens [24]. For gut permeability studies, intestinal biopsies or surgical resections from any intestinal segment of humans or animals can be used.
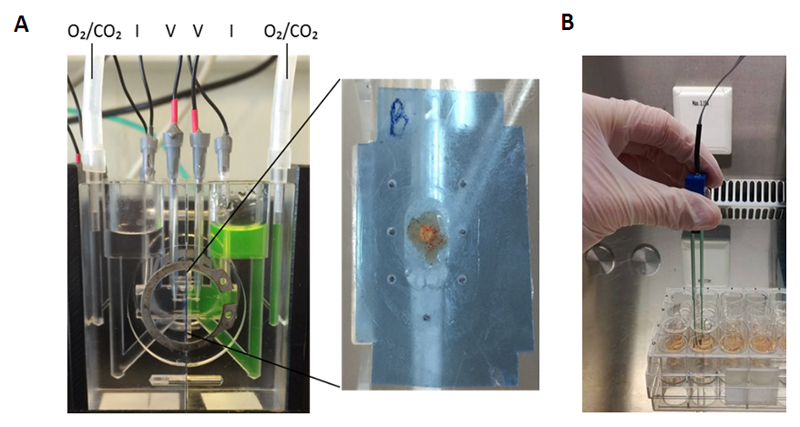
The classical set-up of an Ussing chamber is based on two halves that can separate the mucosal membrane from the basolateral side of the tissue (Figure 2A) [25]. During an experiment, each chamber-half is filled with an equal amount of Ringer solution, and is connected to a perfusion system to allow oxygenation and stirring of the buffer. Two voltage electrodes are usually inserted close to the tissue to measure the transepithelial potential difference that is generated due to active transport of ions. Two additional electrodes can inject current pulses. The total current that is needed to cancel out the spontaneous potential difference is referred to as the short-circuit current, and is considered as the sum of all ionic currents across the epithelium. Conversely, transepithelial electrical resistance (TEER) measurements are done by monitoring the voltage deflections in response to set current pulses, and is calculated based on Ohm’s law. In most experiments, marker molecules are also added to the apical chamber-half, after which basolateral samples are taken over time to assess passage across the tissue. It is important to recognise that the paracellular flux of small marker molecules will often be reflected by similar changes in TEER, but both are not necessarily related since they represent different characteristics of the barrier (non-ionic flux versus ionic conductance).
Figure 2. Ex vivo and in vitro methods to assess epithelial barrier function.
(A) Ussing chamber assay: An endosopic intestinal biopsy is placed on one half of the chamber with the mucosal side facing upwards. The tissue is gently placed in-between plastic films for mounting. After equilibration of the tissue for 30 minutes, fluorescently labelled dextrans (FD4) are added to the mucosal side of the chamber. Serosal samples are taken every 30 minutes for 2 hours to evaluate the passage through the biopsy. The transepithelial electrical resistance (TEER) is recorded every 30 minutes by the electrodes. (B) Transwell assay: Intestinal cells are grown on permeable filter supports. Medium is changed every two days after seeding the cells. TEER measurements are performed using chopstick electrodes to measure confluency of the cells.
FD4, FITC-dx4, fluorescein isothiocyanate, dextran 4000 Da; TEER, transepithelial electrical resistance
3.1.3. In vitro
Cell culture based models with immortalised intestinal cell lines (Caco-2 or HT-29) can be applied for assessment of electrical resistance and intestinal flux parameters when biological samples are not available. This approach has provided a powerful tool in drug screening and toxicity studies to measure the transport of libraries of new compounds across the intestinal barrier [26]. Cells are grown on permeable filter supports (transwells) and form polarised monolayers as seen in vivo. Most filter supports are designed to be used in culture plates with access to the apical and basolateral compartments (Figure 2B). Upon confluency, membrane supports can also be placed within the Ussing chamber set-up to evaluate vertical flux characteristics [25]. The correlation between in vitro and in vivo permeability data is highly variable and dependent on a number of confounders, including the type of molecule under investigation, the transport route and interaction effects. Larregieu et al. have reviewed the use of Caco-2 measures for drug discovery studies, and concluded that data on passively absorbed drugs correlated well with in vivo human permeability data, whereas this was poor for hydrophilic drugs [27]. Also larger dietary proteins are believed to behave in part differently in vivo as shown by Picariello and co-workers who investigated the digestion and transport of milk peptides across Caco-2 cells [28]. Although their model correctly showed the ability of selected milk-derived peptides to cross the gut epithelium, the results could not be linked to patients’ responses which highlighted that additional mechanisms can occur in vivo misjudging the actual outcomes.
3.2. In situ intestinal barrier defects
More detailed analyses of specific changes that may affect the function of the intestinal barrier can be achieved using molecular and histological approaches on intestinal tissue sections [15]. In this regard, much attention has been given to the thickness of the mucus layer and staining of mucus proteins. Also junctional gene and protein expression analyses by Western blot, qPCR or immunostaining are commonly used. These approaches provide a more mechanistic view on the intestinal barrier which is highly relevant from basic science perspectives. Without intestinal permeability data, however, it will remain hypothetical if, for example, changes in a single TJ protein will result in an increased flux of small or larger molecules, and thus are biologically relevant [14].
3.3. Non-invasive biomarkers for intestinal barrier function
The measurement of biomarkers in urine, blood or faeces provides a simple and non-invasive method to evaluate intestinal barrier function without the need for previous administration of test molecules. It is used as an alternative for interventional studies, although the sensitivity and specificity is often rather low.
3.3.1. Urinary biomarkers
In urine samples, claudin protein levels have been proposed to be suitable candidates for intestinal TJ loss. Claudins are the major components of the TJ protein complexes with a critical role in the regulation of the paracellular barrier pathway [29]. Previous studies in haemorrhagic shock, major surgery, IBD and necrotizing enterocolitis showed release of the sealing protein claudin-3 in urine [16]. Interestingly, a recent study in necrotizing enterocolitis patients also found increased urinary levels of claudin-2 [30]. Whether other TJ proteins might serve as urinary biomarkers has not been investigated yet. Alternative urinary biomarkers include fatty acid binding proteins (FABP) and glutathione s-transferases (GSTs) that correlate with intestinal epithelial cell damage and that are easily detectable using enzyme-linked immunosorbent assays (ELISA) [14–16]. FABP are abundantly expressed proteins of 14-15 kDa that aid in the transport and signalling of fatty-acids [31]. In the intestine, three isoforms are present with different specificity: liver-type FABP (L-FABP), intestinal-type FABP (I-FABP) and ileal FABP (I-FABP) (Table 2). Upon intestinal cell damage, FABP are released from the enterocytes in the systemic circulation and excreted renally. GSTs are cytosolic enzymes that are involved in the detoxification of chemicals, and are equally released when the cell membrane is damaged. The class of α-GSTs is expressed in the GI tract, but is also highly abundant in the kidneys and the liver. Although this marker has been suggested for the detection of intestinal ischemia, the unspecific distribution complicates the interpretation of the results, and diminishes its value as intestinal barrier biomarker [32].
Table 2. Distribution of intestinal fatty acid-binding proteins*.
| Most abundant expression | Other locations | |
|---|---|---|
| Intestinal-type FABP (I-FABP) | Small intestine | Liver |
| Liver-type FABP (L-FABP) | Small intestine, Liver | Pancreas, Kidney, Lung, Stomach |
| Ileal FABP (Il-FABP) | Ileum | Ovary, Adrenal gland, Stomach |
Based on Furuhashi et al. Nature Reviews Drug Discovery 2008
FABP, fatty acid-binding proteins
3.3.2. Biomarkers in serum or plasma
In addition to their presence in urine, FABP and GSTs can also be detected in the circulation. Similarly, citrulline concentrations in plasma or serum are regarded as a quantitative biomarker of enterocyte mass, and have been used in a range of diseases [33]. Citrulline is an amino acid produced by small intestinal absorptive cells from glutamine. The amino acid is not incorporated into proteins, nor is it present in food or exogenous sources making it a specific candidate as biomarker for small bowel enterocyte mass [15]. Recent studies have also proposed the serum protein zonulin as marker for intestinal permeability. Zonulin was identified as a human endogenous modulator of epithelial TJs in the small intestine, and is upregulated in several autoimmune diseases, including CeD and type 1 diabetes [34]. Increased zonulin levels correlated with in vivo increased intestinal permeability as measured using the lactulose/mannitol test in subjects with type 1 diabetes and their relatives [35].
Because disruption of the intestinal epithelial barrier is thought to increase the translocation of bacteria and bacterial-related products across the epithelium, the presence of such components in the circulation has also been proposed as intestinal barrier biomarkers [14]. The identification of living intestinal bacteria in the blood has traditionally been done using culture techniques. However, not all bacteria are able to grow in conventional cultures and their detection in blood may be hampered by rapid clearance or bacterial opsonisation [36]. An alternative is a PCR-based method to detect residues of microbial DNA in serum resulting in a higher sensitivity than the standard blood culturing methods [37]. Likewise, the major components of the outer membrane of Gram-negative bacteria, defined as lipopolysaccharides (LPS) or endotoxins, can be used as bacterial surrogates. Endotoxins are known to induce an inflammatory immune response in the bloodstream via Toll-like receptor 4 sensing [38], and their passage might be increased upon epithelial barrier dysfunction. Alternatively, human blood samples might be screened with ELISA for endotoxin core antibodies, believed to protect the host against the detrimental effects of endotoxin in the circulation. The idea is that these antibodies also serve as an indirect marker for bacterial translocation to the systemic circulation with lower free antibody levels during endotoxemia [39]. A final blood biomarker that has been tested is D-lactate. D-lactate arises from bacterial fermentation in the gut, and is prevented to enter the circulation in healthy individuals [15]. D-lactate levels increase in plasma samples because of intestinal barrier function loss [40].
Importantly, for all bacteria-related products, one should bear in mind that they are surrogates for bacterial transport, and these do not represent the effective transport mechanisms of the bacteria themselves.
3.3.3. Faecal biomarkers
Faecal biomarkers that have been proposed to reflect intestinal barrier function include inflammation-related molecules, since intestinal inflammation affects the integrity of the mucosal barrier [15,41]. The most studied marker is calprotectin. Calprotectin is a small protein in the cytoplasm of leukocytes, and is released in the lumen upon neutrophilic infiltration of the gut mucosa during inflammation. The protein is stable at room temperature for several days, and can be extracted from faeces using commercially available kits [42]. ELISA techniques are most commonly applied for quantification with good diagnostic precision. Although calprotectin has proven its efficacy as inflammation biomarker, it does not fully represent gut mucosal function, and barrier defects might occur independent of intestinal inflammation. Studies in non-affected first-degree relatives (FDR) of patients with autism or IBD have indeed shown that faecal calprotectin did not always correlate with abnormal intestinal permeability values [43,44]. Individual inflammation markers should therefore not be preferred.
3.4. Emerging assays
Recently, a few promising techniques have been introduced to evaluate intestinal barrier function, including confocal laser endomicroscopy (CLE) and primary epithelial monolayer cultures.
CLE was firstly introduced in 2004 and uses intravenous fluorescein or acriflavine as contrast agent to obtain in vivo images of the mucosal layer and cellular structures in the GI tract during endoscopy [45,46]. The technique is rapidly evolving and more options are being added, such as the use of labelled antibodies to characterise lesions on a molecular level, or marked drugs to estimate the affinity of the drug to a target organ [47]. Kiesslich and co-workers showed that the classical CLE method with fluorescein is useful and fast to determine cell shedding and loss of intestinal barrier function in humans [48]. They detected microerosions in the epithelium by observing plumes of fluorescein efflux, while white light imaging showed no epithelial damage at these sites. Parallel studies in mice demonstrated that sites with efflux of intravenous dyes (from the bowel wall into the lumen) could also be sites of inward flow (lumen to bowel wall), which is relevant for pathological conditions and confirms the utility of fluorescein-aided CLE in humans. Although special training skills are needed to perform CLE, and larger studies are still required to confirm its relevance and possibilities, this technique might become an important option for real-time evaluation of intestinal barrier function - and more - in a broad range of diseases [47].
The growth of primary IECs has been of great importance for researchers to increase the extrapolation potential of cell culture-based models. The use of primary IECs in monolayer cultures, which enable standard intestinal barrier studies, has always been challenging due to a limited viability and need for a substantial number of cells. Most studies therefore use immortalised cell lines in transwells, but these do not fully recapitulate the normal physiology of IECs given their malignant origin [26]. The group of Moon et al. was the first to describe a system to grow primary IEC monolayers from the colon of multiple genetic mouse strains in 2014, and used this method to evaluate IgA transcytosis across transwells [49]. The method was based on their previously established 3D spheroid culture system to obtain a large number of cells before seeding in transwells. One year later, the same group published an adapted method for human IECs using endoscopic biopsies as starting material [50]. Interestingly, other researchers have also worked on the development of microfluidic organs-on-chips, which enable a functional tissue context including tissue-tissue interfaces, vascular perfusion, mechanical compression and fluid flow [51]. Previous work with a human gut-on-a-chip model showed that Caco-2 cells formed functional cells from different lineages, and a high integrity barrier with better characteristics than the static transwell models [52]. Despite the power of this last method, many analytical and technical obstacles (e.g. low amount of cells for analysis, difficult to mimic spatial heterogeneity along the GI tract, need for specialized fabrication skills and in-house expertise, type of material for mass production dependent on application…) must still be overcome before organs-on-chips can be used widespread [51]. For now, primary monolayer cultures in transwells is the more accessible technique, and expected to find its place in the field of intestinal barrier research in the coming years.
4. Impact of diet and lifestyle on intestinal barrier function
Research on the role of a balanced diet and healthy lifestyle for gut homeostasis and intestinal barrier integrity has become increasingly apparent. Luminal compounds from the diet and the environment come in close contact with the intestinal epithelium, and hence form the primary stimuli that might disturb the barrier. Effects on the barrier can be either direct by inducing abrupt epithelial cell damage and intracellular signalling responses, or indirect with the intestinal microbiota as key interaction partners.
4.1. Dietary factors
Food is an important source of nutrients, but also serves as a modulator of various physiological functions in the GI tract, including intestinal barrier function [53,54]. A broad range of individual food substances have been tested in Caco-2 monolayers with divergent effects on TEER readings and epithelial flux parameters [55]. Whole extract from sweet peppers, for example, has been found to decrease TEER without cytotoxic effects when added to Caco-2 cell lines [56]. The drop in TEER was attributed to capsianoside, the active compound of sweet pepper, which induced reorganizations of the cytoskeleton in IECs. Flavonoids on the other hand are an example of plant-derived components with proven beneficial effects on the epithelial barrier [57]. Flavonoids are abundant in most vegetables, fruits, green and black tea, red wine, chocolate and coffee. The normal intake of flavonoids in the population is estimated to be below the threshold for significant beneficial effects, which would suggest the need for supplementation as an addition to the normal diet in susceptible individuals [58]. Many studies have indicated that flavonoids may protect barrier integrity by acting on the TJs, but part of the effects might also arise from modulation of the gut microbiota since flavonoid-rich cranberry extract markedly increased the proportion of Akkermansia in mice [59]. Akkermansia is a mucin-degrading bacterium that resides in the intestinal mucus layer, and its presence has been shown to be crucial for the integrity of the epithelium [60].
Lately, much interest has been given to industrial food additives which are increasingly used to improve the qualities of food, but have also been associated with intestinal barrier dysfunction and with a rising incidence of immune-related diseases [61]. Carboxymethylcellulose and polysorbate-80 are two of the most commonly applied additives in the food industry and recognizable on food labels by their E-numbers. When administered to mice at relatively low concentrations – believed to be relevant for humans when a lot of processed foods are daily consumed – they induced a reduction of mucus thickness, a higher contact of bacteria with the epithelium, an increased permeability to dextrans and low grade inflammation [62]. Remarkably, the mucus layer was not affected under germ-free conditions which indicated that the changes were not purely a direct effect of the additives on the mucus structures. Additional evidence for the influence of dietary habits comes from studies looking at the combined effect of a high fat and sugar diet, referred to as a western diet, on gut health. In the study of Martinez-Medina and colleagues, such a diet induced a shift in microbiota composition in mice, a decreased mucus layer thickness with less goblet cells and mucin expression, increased intestinal permeability with upregulation of the pore-forming claudin-2 protein, and increased inflammatory marker levels [63].
Along with nutritional compounds, vitamins, minerals and trace elements from foods have been associated with the regulation of the intestinal barrier in preclinical and clinical settings. More specifically, deficiencies of vitamin D, vitamin A and zinc have been found to compromise the epithelial barrier with an increased risk to infection and inflammation [15,64–66]. The mechanisms are not well understood, but probably involve a combination of the classical events including TJ, mucus and microbial alterations.
4.2. Alcohol
Alcohol consumption is widely accepted in our society which leads to the idea that habitual ethanol drinking does not influence our health. Studies on the effects of ethanol intake on the intestinal epithelial barrier have however shown consistent increases in intestinal permeability in different models [67]. Ethanol as well as its main metabolite, acetaldehyde, has been found to decrease small and large intestinal barrier function by direct damage to the epithelial cells, disruption of the TJs, AJs and cytoskeleton, and activation of oxidative stress responses. The microbiota also are central mediators of the effects of alcohol, since they take part in the production of acetaldehyde by bacterial metabolism. The normal microbial balances in the gut were shown to be affected by chronic alcohol abuse with an increase in Gram negative bacteria, which can further aggravate barrier function and endotoxemia [68].
4.3. Medication
Non-steroidal anti-inflammatory drugs (NSAIDs) are well-known for their damaging effects on the GI tract, and are often co-prescribed with proton pump inhibitors (PPIs) as these reduce the incidence of NSAID-induced gastroduodenal damage (e.g. peptic ulcers). However, the combination of NSAIDs and PPIs does not protect against injuries in the small and large intestine, and may even worsen the individual effects of the drugs on the mucosal barrier [69,70].
Virtually all conventional NSAIDs increase the passage of marker molecules in humans within 24 hours of ingestion, while long term use also prolongs this effect, leading to small bowel inflammation in more than 70% of patients [71]. In addition to indirect effects from cyclooxygenase inhibition, NSAIDs are thought to cause direct damage to the surface epithelium being a detergent that can interact with the phospholipid membranes of the brush border. Furthermore, NSAIDs uncouple oxidative phosphorylation in mitochondria, which leads to a reduction of intracellular ATP production, in turn resulting in dysregulation of the actin-myosin complexes that regulate the intercellular junction proteins [71,72].
Studies on the ability of PPIs to cause barrier defects have mainly focused on gastric permeability [72]. Both in Ussing chamber studies using rat gastric corpus and in humans with sucrose excretion tests, PPIs induced significant gastric leaks [73–77]. Mullin and co-workers indicated that PPI-induced leaks are probably not caused by cell death, but changes in potassium (by inhibiting the H+, K+-ATPase) and calcium homeostasis which are related to the cytoskeleton, and thus TJ permeability [75]. The inhibitory activity of PPIs on other phosphatases could also trigger de-phosphorylation of tight junctional proteins, a known event to alter intercellular barrier function. Whether these effects might also be linked to permeability changes in the lower GI tract is uncertain. Interestingly, omeprazole provoked alterations in the microbial composition of the small intestine of rats which suggests that PPI-induced barrier changes in the lower GI tract also could arise through modulation of the intestinal bacteria instead of direct toxic effects on the epithelium [69].
4.4. Smoking
The effect of tobacco smoking on gut health has been intriguing, and of particular interest in the field of IBD where smoking is one of the most important environmental risk factors with opposing effects between ulcerative colitis (UC) and Crohn’s disease (CD) [78]. In search of a molecular explanation for this divergence, the impact of smoking on intestinal permeability has been extensively investigated. Urine samples of healthy smokers contained less 51Cr-EDTA compared to non-smokers, suggesting that smoking tightens the gut [79]. Follow-up studies in healthy individuals and UC patients however failed to replicate these results [80,81]. The excretion of 51Cr-EDTA was not found to be different between smoking and non-smoking UC patients, and a permeability decrease in 6-hour urine samples from smoking and non-smoking healthy individuals was only seen after indomethacin administration. In a more recent in vitro model with Caco-2 monolayers, nicotine and its metabolites induced a decrease in epithelial gut permeability at concentrations corresponding to those reported in the blood of smokers [82]. The mechanism of action of nicotine was explained by an observed increase in expression of the TJ proteins occludin and claudin-1. Despite this model would again confirm the initial hypothesis that smoking is beneficial for the gut barrier, we should keep in mind that the in vitro system stands far away from the clinical situation [83]. An animal study with C57BL/6 mice showed that side-stream smoking induced significant changes in the gut microbiota with inhibition of the NF-KB inflammatory pathway and upregulation of claudin-3 and ZO-2 in the large intestine [84]. Conversely, Zuo and colleagues showed that exposure to mainstream cigarette smoke led to increased intestinal permeability and damaged TJs in the small bowel of male BALB/c mice with activation of the NFKB signalling pathway [85]. All changes were observed in the small intestine, while the large intestine of the mice was not affected. The most recent report on chronic smoke exposure in mice demonstrated significant changes in microbial composition and immune factors, but no differences in TJ gene expression [86]. Alternatively, altered mucin gene expression patterns in the ileum and colon of smoke-exposed mice were seen, which might be induced as protection mechanism to counteract the changes in the gut. Taken together, current findings about the impact of smoking on the gut barrier have been highly contradictory and cannot explain the divergent effect in IBD. The potential benefit of cigarette smoking on the gut barrier surely does not outweigh the well-known risks. We believe that smoking can indeed have a tightening effect on the gut barrier as shown in a simplistic model such as Caco-2 cells, but this effect can be reversed in vivo through the influence of many confounding elements, including genetic background, disease-specific stimuli, intestinal location, type of smoke exposure and interaction with immune and microbial factors.
4.5. Stress
Stress is a lifestyle factor that has been linked to deterioration of the intestinal barrier via gut-brain interactions, and is a well-known risk factor for onset and reactivation of chronic disorders. Most work on the mechanistic effects of stress on the regulation of the intestinal barrier has been done in animal models using physical and psychological stressors such as noise stress, heat stress, cold-restraint stress, crowding stress, maternal deprivation and water avoidance stress [87]. In general, the induction of mucosal barrier dysfunction is caused by activation of the corticotropic-releasing factor (CRF)-mast cell axis. Both systemic and peripheral release of CRF, together with an extensive array of chemical mediators are involved in stress responses in preclinical models with effects on three levels at the intestinal barrier: mucus layer composition, water and ion secretion, and intestinal permeability. Data on psychological stress in humans is limited. One study subjected healthy volunteers to a public speech test, which demonstrated increased small intestinal permeability values in those individuals with clear stress signals as measured by salivary cortisol [88]. The increases were CRF- and mast cell-dependent since exogenous CRF also induced increased intestinal permeability whereas a mast cell stabilizer abolished the responses. In HT-29 monolayers, CRF exposure resulted in an increased expression of claudin-2, a pore-forming TJ protein [89]. Recent work has also given attention to exercise-induced stress, believed to represent a combination of physical and psychological stress [90]. Athletes often suffer from abdominal complaints which are induced by the release of stress hormones during intense physical activity. In addition to the effects as described above, the redistribution of blood flow away from the intestines, body temperature increases, and microbial changes during intense or prolonged physical activity can further initiate the loosening of the TJs and inflammatory responses. Also in soldiers on combat-training increased permeability has been found [91]. The underlying pathways for different types of stressors seem to be fairly similar, but the magnitude of the responses can depend on the length of the stimulus, and on genetics and individual life experiences [92].
5. Intestinal barrier dysfunction in gastrointestinal diseases and beyond
Intestinal barrier defects have been associated with a wide array of human diseases, including both GI (e.g. IBD, irritable bowel syndrome, CeD, colon carcinoma, critical illness) as well as extra-intestinal disorders (e.g. type 1 diabetes, multiple sclerosis, autism, non-alcoholic fatty liver disease (NAFLD), obesity, Parkinson’s disease, depression, asthma). Because an interpretation on all of these diseases is beyond the scope of this review, we focus on three disorders that have been the subject of recent studies, and for which we have clinical and scientific data supporting a central role of the intestinal barrier in the disease pathogeneses.
5.1. Inflammatory bowel disease
IBD is a chronic relapsing inflammatory condition of the GI tract with two main entities: CD and UC. Both CD and UC are associated with multiple pathogenic features including environmental, genetic, microbial and immune factors. Although the exact mechanisms in the initial development of IBD are not fully understood, it is believed that an abnormal immune response is elicited against the intestinal microbiota in genetically predisposed individuals [93]. The intestinal barrier represents an integral part of this hypothesis being the central interaction platform between the microbiota and the host. Intestinal permeability tests in IBD patients have shown convincing evidence towards increased intestinal fluxes of molecules and changes in TJ protein expression, while also alterations in the mucus layer have been well described [12,94]. The proportion of patients with increased permeability values largely depends on the methodology of the study, the type of patients, the cut-offs used and on disease activity. Most in vivo permeability studies report the prevalence of increased gut permeability to be 40-50%, with the highest values for patients with active disease [46]. Mechanistic studies have indeed indicated that inflammatory cytokines (e.g. tumor necrosis factor (TNF)-α, the traditional therapeutic target in IBD) might be responsible for intestinal barrier defects, as these cause dysfunctions of the intestinal barrier in cultured monolayers and anti-TNF treatment normalises intestinal permeability in most CD patients [95,96]. However, other studies also propose intestinal barrier dysfunction as an early event in the disease. Suenaert et al. showed that hyperresponsiveness to indomethacin, in terms of intestinal permeability, was not suppressed by anti-TNF treatment in a subset of CD patients supporting the notion of a defective intestinal barrier independent of inflammation [97]. Also in asymptomatic CD patients, impaired intestinal permeability is found to precede clinical relapse by up to one year, although this does not exclude the possibility of subclinical inflammation in these patients [98]. Interestingly, in healthy FDR of IBD patients, which should not have inflammation, increased permeability has also been described, raising the hypothesis of genetic predisposing factors that dysregulate the intestinal barrier [12]. In one study, healthy FDR with NOD2 (sensor of bacteria) mutations had higher mucosal permeability values than wild-types [99]. The most intriguing evidence is however a case report of a girl with a strong familial history of IBD, and an increased intestinal permeability at least eight years before she was diagnosed with CD [100]. At the time of the permeability test, she was asymptomatic, and both macroscopic and microscopic CD was excluded by extensive investigations. Unfortunately, no other long-term studies of such patients have become available since then. Additional evidence for an inherited role of intestinal barrier defects comes from genome-wide association studies [101]. Herein, the intestinal barrier is found as one of the main pathways driving IBD. This was again confirmed in the recently completed trans-ancestry study that also found a new association locus for IBD involved in epithelial barrier function (OSMR) [102]. Besides human data, animal models of experimental colitis have been described with increased intestinal permeability well before any signs of disease. In interleukin-10 gene-deficient mice, pharmacological treatment with a zonulin inhibitor even attenuated the development of colitis suggesting that permeability alterations are necessary for disease development in this model and not simply an epiphenomenon [103]. Most likely, the intestinal barrier in IBD is regulated in many ways including genetic make-up, environmental triggers, and mild inflammation which might cause deterioration of the disease. The extensive data today provide sufficient evidence to consider intestinal barrier defects as more than an unrelated disease effect, and justify the attention for the intestinal barrier and research in therapeutic managing of IBD.
5.2. Celiac disease
CeD is a chronic immune-mediated enteropathy of the small intestine triggered by ingestion of dietary gluten in genetically predisposed individuals [104]. CeD is a unique and straightforward model amongst other autoimmune diseases, because the strongest genetic determinants (HLA-DQ2 or HLA-DQ8), the specific autoimmune response (tissue transglutaminase) and the triggering environmental factor (gluten) are all known [34]. It also perfectly fits within the proposed paradigm that inflammatory diseases require three conditions for disease development: an abnormality of the immune system, the presence of an inciting antigen, and the ability of the antigen to reach the immune system through a defective intestinal barrier [5]. In normal conditions, transport of the toxic gliadin fraction of gluten to the lamina propria is limited [34,105,106]. Patients with CeD are known to have an increased permeability and TJ defects, which could enable the higher interaction of gliadin peptides with the immune system [107]. In a genetic study with Dutch and British CeD patients, two genes involved as scaffolding proteins in TJ assembly (PARD3 and MAGI2) were associated with CeD [108]. These associations suggest a common aetiology through early TJ-mediated intestinal barrier impairment. Accordingly, healthy FDR of patients with CeD more often show an increased paracellular permeability than unrelated controls (20 to 30%) [109]. Recent data on a subgroup of asymptomatic, serology-negative FDR with increased permeability showed ultrastructural abnormalities and lower expression of ZO-1 and occludin in duodenal biopsies [109]. Animal studies confirm these findings as shown in Irish setter dogs that naturally develop a gluten sensitive enteropathy when reared on a normal wheat-containing diet [110]. Littermates from affected parents on a gluten-free diet exhibited increased permeability to 51Cr-EDTA when compared to unaffected dogs, and the permeability changes preceded the development of enteropathy in dogs on a normal diet. Further increases in permeability were seen in the latter with time, which indicates that secondary effects create a vicious cycle of intestinal cell damage. Indeed, luminal gluten fractions also contribute directly to altered barrier function through the release of zonulin that causes TJ disassembly and induction of pro-inflammatory signalling [34]. An interesting alternative mechanism for an increased transport of gliadin to the lamina propria during active CeD involves the formation of gliadin complexes with sIgA molecules [107]. In the normal intestine, sIgA function as protective molecules in the mucus layer. In active CeD, however, the ectopic overexpression of CD71, the sIgA receptor normally only present at the basolateral membranes, transforms sIgA into a ‘Trojan horse’ that promote the transport of gliadin peptides via transcytosis to the lamina propria.
A gluten-free diet is the first-line treatment for CeD since the recognition of gluten as the main disease culprit [104]. The diet has however been challenging and difficult to continue at the long term. Researchers are now evaluating alternative treatment options, including a TJ regulator (Larazotide Acetate) which is expected to enter phase III human clinical trials [111].
5.3. Non-alcoholic fatty liver disease
NAFLD is a spectrum of liver disease that evolves from accumulation of triglycerides (simple steatosis) through more severe infiltration with hepatic inflammation and liver cell death (non-alcoholic steatohepatitis, NASH), to fibrosis and ultimately cirrhosis. NAFLD is considered as the hepatic manifestation of the metabolic syndrome of which the prevalence is highly increasing with the sedentary and dietary lifestyle in the Western world [112].
Intestinal permeability has been found to be increased in patients with NAFLD, and is correlated with liver disease severity and disruption of the TJs [113,114]. Consistent with these data, higher endotoxin levels have been identified in the blood of NAFLD patients as an indirect measure for increased bacterial translocation [115]. Gäbele et al. went deeper into the pathogenic events in NAFLD by investigating whether intestinal barrier dysfunction could be a primary defect, and underlie the transition from steatosis to NASH and more progressive disease [116]. They found that the induction of colitis in an experimental mice model with initial mild hepatic steatosis by dextran sulfate sodium – a direct damaging agent for the intestinal barrier – enhanced hepatic inflammation and fibrosis, and increased portal LPS levels. Regarding events in humans, it was suggested that dietary habits could be involved in the initial intestinal barrier defects through direct damaging effects of food agents, or secondary dysregulations from low-grade inflammation, or by specific changes in microbial composition that affect the barrier. This latter idea has also been the basis for the proposal of pre- and probiotics in NAFLD in addition to lifestyle changes, which are often the first treatment option [117,118]. A more recent study of Luther et al. confirmed the previous associations between intestinal permeability increases and NASH, but showed that liver injury preceded the development of intestinal permeability changes in their dietary mice model, and this process was independent of hepatic TNFα [119]. Which of the two models is relevant for humans is unknown, but both confirm that NASH progression is related to intestinal permeability changes and thus key in the pathogenesis of NAFLD.
Obesity is closely interrelated with metabolic syndrome and the risk to NAFLD, and has likewise been linked to early intestinal barrier changes [120]. Amongst other non-GI disorders, type 1 diabetes is one of the best studied diseases with convincing data towards a primary role of intestinal barrier dysfunction [121]. Current evidence on central diseases such as autism, Parkinson’s disease and depression is much more limited and further studies should be awaited.
6. Expert commentary
It has become apparent that intestinal barrier defects are key players in the pathogeneses of diseases affecting the gut and beyond. We have come to realize that a Western life style has a significant impact on health, including the strength of the intestinal barrier. Although it is unlikely that lifestyle factors alone will cause disease, they could significantly increase the risk of susceptible individuals as an added trigger, and in part explain the rise of chronic diseases in the Western world.
Research on the intestinal barrier has provided interesting results, but also specific points to be worked on, and unresolved questions that must be considered. First, it is almost impossible to compare in vivo permeability results across scientific studies, since even for the same marker molecules different collection times, sampling rules (e.g. avoidance of external confounders such as NSAIDs) and experimental protocols have been used, representing a major shortcoming in the field. Biomarkers have been regarded as the easiest alternative tool to evaluate intestinal barrier features in humans, but most are indirect measures and/or lack reliable and rapid detection possibilities. Second, we should question the relevance of the paracellular pathway compared to the transcellular permeability pathway. Small molecule markers are wide spread in all kinds of models, but the use of macromolecules has been largely limited to animal models or ex vivo studies. It is thought that food-antigens and microorganisms are primarily transported through transcellular processes, which are thus mostly uncovered. Whether we should further focus on the small intestine, large intestine or both probably depends on the disease setting, but is also largely unaddressed. The small intestine has been most commonly used for in vivo settings due to practical issues, whereas the colon has been the more accessible site for studies based on biopsies and in animal models. In CeD, the duodenum is assumed to be the primary site for disease initiation and propagation, but in complex disorders such as IBD or NAFLD this is unclear. The close interaction between the microbiota and the intestinal barrier also demands more studies as this might be more important than previously thought.
In general, we should be aware that more and more extra-intestinal disorders are being associated with gut barrier defects, but not all are supported by scientific data. Nowadays, commercially available permeability tests claim to evaluate if one has a leaky gut to explain systemic symptoms and disorders like anxiety, depression and fatigue. Until there is scientific proof for the relevance and potential causal effect of barrier defects in these disorders, results from such tests should be interpreted with the necessary criticism.
7. Five-year view
Additional studies and new upcoming techniques will advance the progress of intestinal barrier research. Diseases where intestinal barrier perturbations have been established as a central mechanism in their pathogeneses through both scientific and clinical studies have also raised attention for the development of barrier-restoring therapeutics. Conventional treatments for inflammatory disorders such as steroids or anti-TNF therapy are suggested to already improve gut barrier function through reduction of inflammatory mediators. However, the evidence that barrier defects may also occur independently of inflammation argues to also look for direct barrier modulators. Examples of these could be zonulin antagonists (TJ modulator) or phosphatidylcholine (mucus stabilizer) [34,122], which both are now in phase III trials for CeD and UC respectively. Probiotics have also been proposed as barrier enhancers, a concept that is now supported by experimental studies but will need large clinical trials to prove its efficacy in chronic diseases. Not all patients might benefit from barrier-related drugs, but this would fit within the concept of personalised medicine where we need to stratify patients and treatments according to the pathways that are driving the disease. This field is still in its infancy, but is expected to receive more attention in the coming years.
Key issues.
The mucus layer and the intestinal epithelial cells represent the main determinants of the physical intestinal barrier and have a fundamental role in health.
Various methods are available to evaluate the function of the intestinal barrier, but correlation of results across studies and methodologies remains difficult.
Dietary habits and lifestyle factors have a clear impact on intestinal permeability and barrier function with known detrimental effects for a western diet, high alcohol intake, stress and certain medications. The effect of tobacco smoking remains contradictory.
Clinical and scientific data support a central role of intestinal barrier dysfunction in inflammatory bowel disease, celiac disease and non-alcoholic fatty liver disease.
Current evidence on the role of the intestinal barrier in many other extra-intestinal disorders is limited, and requires further studies.
Since conventional drugs that indirectly target the intestinal barrier are inefficient in a subset of chronic disease patients, direct barrier-modulating agents could represent a new therapeutic class.
A few promising barrier-restoring products are currently under investigation in phase III clinical trials.
References
- 1.Turner JR. Intestinal mucosal barrier function in health and disease. Nature reviews. Immunology. 2009;9(11):799–809. doi: 10.1038/nri2653. [**This is a comprehensive review about the intestinal barrier with insights on its regulation by physiological and immunological stimuli in health and disease] [DOI] [PubMed] [Google Scholar]
- 2.Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scandinavian journal of gastroenterology. 2014;49(6):681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 3.Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107(11):686–696. doi: 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- 4.Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8(4):720–730. doi: 10.1038/mi.2015.40. [DOI] [PubMed] [Google Scholar]
- 5.Meddings J. The significance of the gut barrier in disease. Gut. 2008;57(4):438–440. doi: 10.1136/gut.2007.143172. [DOI] [PubMed] [Google Scholar]
- 6.Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J Gastrointest Pathophysiol. 2012;3(1):27–43. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012;3:310. doi: 10.3389/fimmu.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelaseyed T, Bergström JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16(10):639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16(2):164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 13.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil. 2016;28(7):957–965. doi: 10.1111/nmo.12871. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2(3):61–69. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the Gut Barrier and Potential Biomarkers. Am J Physiol Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00048.2015. ajpgi.00048.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3(3):247–259. doi: 10.1038/mi.2010.5. [*This review extensively discusses the transport routes of molecules across the gut epithelium with special focus on the handling of antigens derived from food] [DOI] [PubMed] [Google Scholar]
- 19.Farre R, Vicario M. Abnormal Barrier Function in Gastrointestinal Disorders. Handbook of experimental pharmacology. 2016 doi: 10.1007/164_2016_107. [DOI] [PubMed] [Google Scholar]
- 20.Katouzian F, Sblattero D, Not T, et al. Dual sugar gut-permeability testing on blood drop in animal models. Clin Chim Acta. 2005;352(1–2):191–197. doi: 10.1016/j.cccn.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 22.van Wijck K, Verlinden TJ, van Eijk HM, et al. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr. 2013;32(2):245–251. doi: 10.1016/j.clnu.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Stappaerts J, Brouwers J, Annaert P, Augustijns P. In situ perfusion in rodents to explore intestinal drug absorption: challenges and opportunities. Int J Pharm. 2015;478(2):665–681. doi: 10.1016/j.ijpharm.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951;23(2–3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 25.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1151–1166. doi: 10.1152/ajpgi.90649.2008. [**This review gives a complete and practical overview of the Ussing chamber method with detailed descriptions and applications] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen DD, Caviedes R, Cárdenas AM, Shimahara T, Segura-Aguilar J, Caviedes PA. Cell lines as in vitro models for drug screening and toxicity studies. Drug Dev Ind Pharm. 2005;31(8):757–768. doi: 10.1080/03639040500216246. [DOI] [PubMed] [Google Scholar]
- 27.Larregieu CA, Benet LZ. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013;15(2):483–497. doi: 10.1208/s12248-013-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picariello G, Iacomino G, Mamone G, et al. Transport across Caco-2 monolayers of peptides arising from in vitro digestion of bovine milk proteins. Food Chem. 2013;139(1–4):203–212. doi: 10.1016/j.foodchem.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 29.Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61(4):431–437. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwood BP, M D, Wood DR, B S, Yuan CY, B S, et al. Urinary Claudin-2 Measurements as a Predictor of Necrotizing Enterocolitis: A Pilot Study. J Neonatal Surg. 2015;4(4):43. [PMC free article] [PubMed] [Google Scholar]
- 31.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khurana S, Corbally MT, Manning F, Armenise T, Kierce B, Kilty C. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37(11):1543–1548. doi: 10.1053/jpsu.2002.36181. [DOI] [PubMed] [Google Scholar]
- 33.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27(3):328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 35.Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 36.de Madaria E, Martínez J, Lozano B, et al. Detection and identification of bacterial DNA in serum from patients with acute pancreatitis. Gut. 2005;54(9):1293–1297. doi: 10.1136/gut.2004.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Such J, Francés R, Muñoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36(1):135–141. doi: 10.1053/jhep.2002.33715. [DOI] [PubMed] [Google Scholar]
- 38.Roger T, Froidevaux C, Le Roy D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106(7):2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penalva JC, Martínez J, Laveda R, et al. A study of intestinal permeability in relation to the inflammatory response and plasma endocab IgM levels in patients with acute pancreatitis. J Clin Gastroenterol. 2004;38(6):512–517. doi: 10.1097/01.mcg.0000129060.46654.e0. [DOI] [PubMed] [Google Scholar]
- 40.Sun XQ, Fu XB, Zhang R, et al. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World journal of gastroenterology. 2001;7(4):555–558. doi: 10.3748/wjg.v7.i4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84(3):282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 42.Ikhtaire S, Shajib MS, Reinisch W, Khan WI. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. 2016;51(5):434–446. doi: 10.1007/s00535-016-1182-4. [DOI] [PubMed] [Google Scholar]
- 43.Büning C, Geissler N, Prager M, et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;18(10):1932–1939. doi: 10.1002/ibd.22909. [DOI] [PubMed] [Google Scholar]
- 44.de Magistris L, Familiari V, Pascotto A, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 45.Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31(5):548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 46.Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/628157. 628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karstensen JG, Klausen PH, Saftoiu A, Vilmann P. Molecular confocal laser endomicroscopy: a novel technique for in vivo cellular characterization of gastrointestinal lesions. World J Gastroenterol. 2014;20(24):7794–7800. doi: 10.3748/wjg.v20.i24.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61(8):1146–1153. doi: 10.1136/gutjnl-2011-300695. [*This study shows the potential of confocal laser endomicroscopy to detect barrier defects] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7(4):818–828. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanDussen KL, Marinshaw JM, Shaikh N, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64(6):911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 52.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 2010;74(2):232–241. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- 54.De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional Keys for Intestinal Barrier Modulation. Front Immunol. 2015;6:612. doi: 10.3389/fimmu.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141(5):769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto K, Kawagishi H, Nakayama T, Shimizu M. Effect of capsianoside, a diterpene glycoside, on tight-junctional permeability. Biochimica et biophysica acta. 1997;1323(2):281–290. doi: 10.1016/s0005-2736(96)00196-4. [DOI] [PubMed] [Google Scholar]
- 57.Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Blay M, Terra X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr Res Rev. 2016;29(2):234–248. doi: 10.1017/S0954422416000159. [DOI] [PubMed] [Google Scholar]
- 58.Vogiatzoglou A, Mulligan AA, Lentjes MA, et al. Flavonoid intake in European adults (18 to 64 years) PLoS One. 2015;10(5):e0128132. doi: 10.1371/journal.pone.0128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anhê FF, Roy D, Pilon G, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 60.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14(6):479–489. doi: 10.1016/j.autrev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Medina M, Denizot J, Dreux N, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63(1):116–124. doi: 10.1136/gutjnl-2012-304119. [**This study shows the impact of a western diet on barrier function and the gut microbiota in mice] [DOI] [PubMed] [Google Scholar]
- 64.Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis. 2014;210(8):1296–1305. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 65.Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr. 2008;138(9):1664–1670. doi: 10.1093/jn/138.9.1664. [DOI] [PubMed] [Google Scholar]
- 66.Quadro L, Gamble MV, Vogel S, et al. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J Infect Dis. 2000;182(Suppl 1):S97–S102. doi: 10.1086/315920. [DOI] [PubMed] [Google Scholar]
- 67.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71(7):483–499. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 68.Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: pathogenetic role and therapeutic perspectives. World J Gastroenterol. 2014;20(44):16639–16648. doi: 10.3748/wjg.v20.i44.16639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141(4):1314–1322. doi: 10.1053/j.gastro.2011.06.075. 1322.e1311–1315. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6(11):1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol. 2009;44(Suppl 19):23–29. doi: 10.1007/s00535-008-2266-6. [DOI] [PubMed] [Google Scholar]
- 72.König J, Wells J, Cani PD, et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7(10):e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabello M, Valenzano MC, Barr M, Zurbach P, Mullin JM. Omeprazole induces gastric permeability to digoxin. Dig Dis Sci. 2010;55(5):1255–1263. doi: 10.1007/s10620-009-0851-z. [DOI] [PubMed] [Google Scholar]
- 74.Gabello M, Valenzano MC, Zurbach EP, Mullin JM. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol. 2010;16(9):1097–1103. doi: 10.3748/wjg.v16.i9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mullin JM, Valenzano MC, Whitby M, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28(11–12):1317–1325. doi: 10.1111/j.1365-2036.2008.03824.x. [DOI] [PubMed] [Google Scholar]
- 76.Murray LJ, Gabello M, Rudolph DS, et al. Transmucosal gastric leak induced by proton pump inhibitors. Dig Dis Sci. 2009;54(7):1408–1417. doi: 10.1007/s10620-008-0528-z. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins AM, McDonnell C, Breslin NP, O'Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. 2002;54(3):341–347. doi: 10.1211/0022357021778583. [DOI] [PubMed] [Google Scholar]
- 78.Thomas GA, Rhodes J, Green JT, Richardson C. Role of smoking in inflammatory bowel disease: implications for therapy. Postgrad Med J. 2000;76(895):273–279. doi: 10.1136/pmj.76.895.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prytz H, Benoni C, Tagesson C. Does smoking tighten the gut? Scand J Gastroenterol. 1989;24(9):1084–1088. doi: 10.3109/00365528909089259. [DOI] [PubMed] [Google Scholar]
- 80.Benoni C, Prytz H. Effects of smoking on the urine excretion of oral 51Cr EDTA in ulcerative colitis. Gut. 1998;42(5):656–658. doi: 10.1136/gut.42.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suenaert P, Bulteel V, Den Hond E, et al. The effects of smoking and indomethacin on small intestinal permeability. Aliment Pharmacol Ther. 2000;14(6):819–822. doi: 10.1046/j.1365-2036.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 82.McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. The effect of nicotine in vitro on the integrity of tight junctions in Caco-2 cell monolayers. Food Chem Toxicol. 2007;45(9):1593–1598. doi: 10.1016/j.fct.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Verschuere S, De Smet R, Allais L, Cuvelier CA. The effect of smoking on intestinal inflammation: what can be learned from animal models? J Crohns Colitis. 2012;6(1):1–12. doi: 10.1016/j.crohns.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Zhao JX, Hu N, Ren J, Du M, Zhu MJ. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J Gastroenterol. 2012;18(18):2180–2187. doi: 10.3748/wjg.v18.i18.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo L, Li Y, Wang H, et al. Cigarette smoking is associated with intestinal barrier dysfunction in the small intestine but not in the large intestine of mice. J Crohns Colitis. 2014;8(12):1710–1722. doi: 10.1016/j.crohns.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Allais L, Kerckhof FM, Verschuere S, et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol. 2016;18(5):1352–1363. doi: 10.1111/1462-2920.12934. [DOI] [PubMed] [Google Scholar]
- 87.Rodiño-Janeiro BK, Alonso-Cotoner C, Pigrau M, Lobo B, Vicario M, Santos J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J Neurogastroenterol Motil. 2015;21(1):33–50. doi: 10.5056/jnm14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y, Liu ZQ, Liu XY, et al. Stress-Derived Corticotropin Releasing Factor Breaches Epithelial Endotoxin Tolerance. PLoS One. 2013;8(6):e65760. doi: 10.1371/journal.pone.0065760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43. doi: 10.1186/s12970-016-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Kan EM, Lu J, et al. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther. 2013;37(8):799–809. doi: 10.1111/apt.12269. [DOI] [PubMed] [Google Scholar]
- 92.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47(6):861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 94.Johansson ME, Gustafsson JK, Holmén-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166(2):409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noth R, Stüber E, Häsler R, et al. Anti-TNF-α antibodies improve intestinal barrier function in Crohn's disease. J Crohns Colitis. 2012;6(4):464–469. doi: 10.1016/j.crohns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Suenaert P, Bulteel V, Vermeire S, Noman M, Van Assche G, Rutgeerts P. Hyperresponsiveness of the mucosal barrier in Crohn's disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis. 2005;11(7):667–673. doi: 10.1097/01.mib.0000168371.87283.4b. [DOI] [PubMed] [Google Scholar]
- 98.Vogelsang H. Do changes in intestinal permeability predict disease relapse in Crohn's disease? Inflamm Bowel Dis. 2008;14(Suppl 2):S162–163. doi: 10.1002/ibd.20617. [DOI] [PubMed] [Google Scholar]
- 99.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55(3):342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740–1744. doi: 10.1053/gast.2000.20231. [**This is an interesting case report of a girl with an increased permeability who later developed inflammatory bowel disease] [DOI] [PubMed] [Google Scholar]
- 101.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58(1):41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo NE, Theethira TG, Leffler DA. The present and the future in the diagnosis and management of celiac disease. Gastroenterol Rep (Oxf) 2015;3(1):3–11. doi: 10.1093/gastro/gou065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119(1):234–242. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 106.Matysiak-Budnik T, Candalh C, Dugave C, et al. Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology. 2003;125(3):696–707. doi: 10.1016/s0016-5085(03)01049-7. [DOI] [PubMed] [Google Scholar]
- 107.Heyman M, Abed J, Lebreton C, Cerf-Bensussan N. Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut. 2012;61(9):1355–1364. doi: 10.1136/gutjnl-2011-300327. [*This review summarises findings on the role of the intestinal barrier in celiac disease] [DOI] [PubMed] [Google Scholar]
- 108.Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57(4):463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- 109.Mishra A, Prakash S, Sreenivas V, et al. Structural and Functional Changes in the Tight Junctions of Asymptomatic and Serology-negative First-degree Relatives of Patients With Celiac Disease. J Clin Gastroenterol. 2016;50(7):551–560. doi: 10.1097/MCG.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 110.Hall EJ, Batt RM. Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut. 1991;32(7):749–753. doi: 10.1136/gut.32.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makharia GK. Current and emerging therapy for celiac disease. Frontiers in medicine. 2014;1:6. doi: 10.3389/fmed.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 113.Giorgio V, Miele L, Principessa L, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46(6):556–560. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 115.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gäbele E, Dostert K, Hofmann C, et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55(6):1391–1399. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 117.Hossain N, Kanwar P, Mohanty SR. A Comprehensive Updated Review of Pharmaceutical and Nonpharmaceutical Treatment for NAFLD. Gastroenterology research and practice. 2016;2016 doi: 10.1155/2016/7109270. 7109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vespasiani-Gentilucci U, Gallo P, Picardi A. The role of intestinal microbiota in the pathogenesis of NAFLD: starting points for intervention. Archives of Medical Science. 2016 doi: 10.5114/aoms.2016.58831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luther J, Garber JJ, Khalili H, et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1(2):222–232. doi: 10.1016/j.jcmgh.2015.01.001. [*This study provides a meta-analysis of the association between intestinal permeability and NASH, and investigates the underlying mechanism of action] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. American journal of physiology. Gastrointestinal and liver physiology. 2015;308(10):G840–851. doi: 10.1152/ajpgi.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vaarala O. Is the origin of type 1 diabetes in the gut? Immunol Cell Biol. 2012;90(3):271–276. doi: 10.1038/icb.2011.115. [DOI] [PubMed] [Google Scholar]
- 122.Stremmel W, Ehehalt R, Staffer S, et al. Mucosal protection by phosphatidylcholine. Dig Dis. 2012;30(Suppl 3):85–91. doi: 10.1159/000342729. [DOI] [PubMed] [Google Scholar]