Abstract
Metformin is a first-line therapeutic option for the treatment of hyperglycemia in type 2 diabetes, despite its underlying regulatory mechanisms remaining relatively unknown. Metformin lowers blood glucose levels by inhibiting hepatic glucose production (GP), originally postulated to be resultant from hepatic AMP-activated protein kinase (AMPK) activation. However, while studies have questioned the contribution of hepatic AMPK in metformin’s effect, a gut-brain-liver axis has recently been discovered to mediate intestinal nutrient- and hormonal-induced lowering of GP. Here we show that intraduodenal infusion of metformin for 50 min increases phosphorylation of duodenal AMPK and lowers GP in a model of 3 d high fat diet (HFD)-induced insulin resistance. Molecular and chemical inhibition of duodenal AMPK negates the GP-lowering effect of metformin, while a duodenal GLP-1R-PKA signaling pathway and a neuronal gut-brain-liver axis are demonstrated to be the downstream effectors. The ability of preabsorptive metformin to lower GP remains in both a 28 d HFD-induced obese and insulin resistant and a NA-STZ/HFD induced type 2 diabetic model. Finally, molecular inhibition of duodenal AMPK signaling reduces the overall acute glucose-lowering effect of a bolus treatment of metformin in diabetes. These findings unveil that metformin activates a previously unappreciated duodenal AMPK-dependent neuronal pathway to lower GP and plasma glucose levels in obesity and diabetes.
Diabetes is characterized by disrupted glucose homeostasis, resulting in hyperglycemia due partly to increased GP1. The antidiabetic biguanide, metformin, lowers plasma glucose levels by reducing GP in diabetic humans and rodents2–4. Although this GP-lowering effect of metformin has been known for decades, the underlying regulatory mechanisms responsible remain unclear. The primary target of metformin is the inhibition of mitochondrial complex I, resulting in an elevation of AMP levels5, which is postulated to activate an AMPK pathway to inhibit GP, possibly via the upstream protein liver kinase B1 (LKB1)6. The fact that metformin concentrations are highest in the liver, and metformin inhibits hepatic mitochondrial complex I5 gave rise to the first demonstration that chemical inhibition of AMPK negates the ability of metformin to inhibit GP in vitro in hepatocytes7, while hepatic knockout of LKB1 abolishes the ability of chronic oral metformin treatment to activate hepatic AMPK and lower plasma glucose levels in diabetic rodents6. However, the glucose-lowering effect of acute oral metformin is intact in mice lacking hepatic AMPK8, questioning the role of hepatic AMPK. Finally, more recent studies support the hypothesis that metformin inhibits GP through a hepatic AMPK independent mechanism, either by negating the ability of glucagon to increase hepatic cAMP levels and stimulate GP9 or through decreasing mitochondrial redox states and lowering the conversion of metabolites to glucose10. These findings collectively indicate that the underlying mechanisms responsible for the GP- and glucose-lowering effect of metformin in type 2 diabetes remain unclear.
The gastrointestinal tract has received recent attention largely due to its ability to trigger negative feedback systems to maintain glucose homeostasis11. Specifically, duodenal lipids activate a duodenal cholecystokinin (CCK)-1 receptor and gut-brain-liver-dependent neuronal network to lower GP in normal rodents, while jejunal infusions of nutrients and leptin lower GP in normal and diabetic rodents12–15. The pharmacological nature of such intestinal and neuronal sensory mechanisms, however, is completely unknown. To this end, some early evidence indicates a potential role of the gut in mediating the effects of metformin. For example, intraduodenal, compared to intraportal and intravenous, administration of metformin leads to the greatest drop of plasma glucose levels16, while chronic metformin treatment increases GLP-1 secretion and alters the microbiota profile17–19. Furthermore, AMPK is expressed in the intestine, and chronic metformin treatment increases intestinal AMPK activity20. Taking this into consideration, we hypothesize that preabsorptive metformin activates duodenal AMPK dependent mechanism, and a subsequent neuronal relay, to lower GP and plasma glucose levels in diabetes and obesity in vivo.
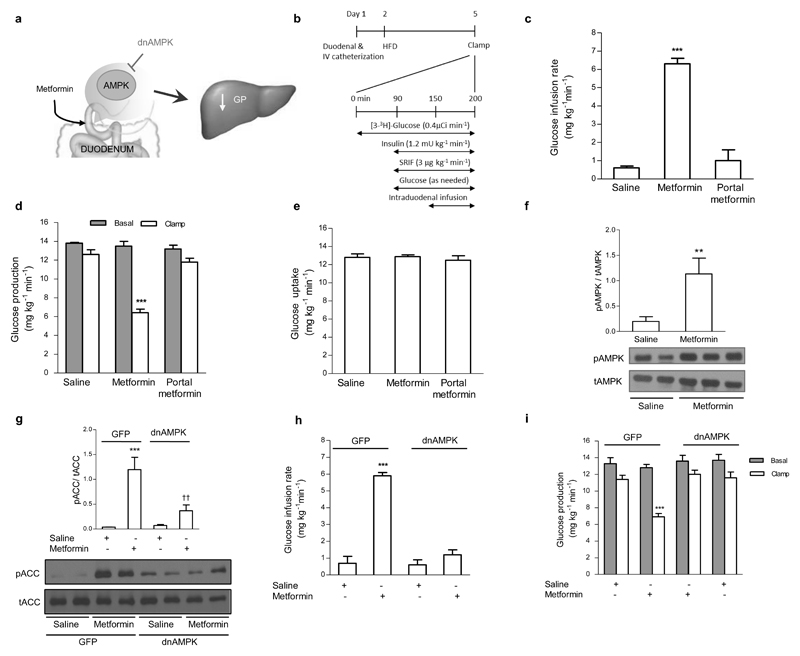
To begin addressing this hypothesis (Fig. 1a), we examined the effects of intraduodenal metformin infusion on GP in 3 d hyperphagic HFD (lard-oil enriched; Supplementary Table 1)-induced insulin resistant rats (Fig. 1b; the insulin resistance phenotype is confirmed in the co-submission). Given the fact that metformin lowers GP independently of insulin action8,9, we have chosen to assess changes in glucose metabolism using the pancreatic basal insulin-euglycemic clamp to establish our studies in a non-insulin stimulated environment. When metformin (200 mg/kg) was infused into the duodenal lumen for only 50 min, the glucose infusion rate required to maintain euglycemia was significantly increased compared to saline infusion (Fig. 1c), while GP was significantly reduced from basal compared to saline controls (Fig. 1d, Supplementary Fig. 1a). Glucose uptake (Fig. 1e) and plasma insulin and glucose levels did not change throughout the clamp (Supplementary Table 2). Importantly, a portal vein infusion of the same dose and duration of metformin failed to increase the glucose infusion rate (Fig. 1c) and lower GP (Fig. 1d, Supplementary Fig. 1a). This indicates that the observed GP-lowering effect of the 50 min intraduodenal infusion was not due to direct hepatic action, but to a preabsorptive effect of metformin localized within the duodenum.
Figure 1. Intraduodenal metformin infusion activates duodenal AMPK to lower GP in the preasbsorptive state.
(a) Schematic representation of the working hypothesis. Intraduodenal preabsorptive metformin triggers duodenal AMPK to lower hepatic glucose production. (b) Experimental procedure and pancreatic (basal insulin) euglycemic clamp protocol. (c,d,e) The glucose infusion rate (c) and rate of GP (d), and rate of glucose uptake (e) during the pancreatic (basal insulin) euglycemic in HFD-fed rats with intraduodenal saline (n=7) or metformin infusions (n=6), or portal vein metformin infusion (n=5). (f) Duodenal mucosa pAMPK protein expression normalized to tAMPK in HFD-fed rats with intraduodenal saline or metformin (**p < 0.01, calculated by unpaired t-test; n=6,6). (g) pACC protein expression normalized to tACC in HEK 293 cells infected with either GFP or dnAMPK and treated with saline or metformin for 6 hours (***p < 0.001, between saline within viral group; ††p < 0.01, between viral group within treatment; as calculated by one-way ANOVA with Tukey’s post hoc test; n=4 per treatment). (h,i) The glucose infusion rate (h) and rate of GP (i) during the pancreatic (basal insulin) euglycemic clamp in HFD-fed with either duodenal GFP or dnAMPK infection, infused with intraduodenal saline (n=5 each group) or metformin (n=7 each group). Values are shown as mean ± s.e.m. Unless noted, ***p < 0.001, versus all other groups as calculated by ANOVA with Tukey’s post hoc test. AMPK, AMP-activated protein kinase; GP, glucose production; HFD, high fat diet; SRIF, somatostatin, ACC, Acetyl-CoA carboxylase.
We next investigated whether intestinal AMPK signaling mediates this effect of metformin (Fig. 1a). First, we determined the effect of a 50 min intraduodenal metformin infusion on AMPK signaling in vivo and found that metformin increased the ratio of phosphorylated AMPK (pAMPK) – to – total AMPK (tAMPK) in the duodenal mucosa layer, compared to a saline infusion (Fig. 1f). Therefore, we next investigated the role of duodenal AMPK in the GP-lowering effect of preabsorptive metformin by using an adenovirus expressing the dominant-negative form of AMPK (Ad-dn-AMPK). We confirmed the functionality of this virus in-vitro by using HEK293 cells infected with either the Ad-dn-AMPK or GFP (Ad-GFP) adenovirus. One day after infection, cells were treated with either saline or metformin (10mM) for six hours21, and protein expression of phosphorylated acetyl-CoA carboxylase (pACC) – to - total ACC (tACC), a direct downstream target of pAMPK, was measured. Cells infected with Ad-GFP and treated with metformin exhibited an increase in pACC/tACC ratio compared to saline treatment (Fig. 1g). In contrast, the pACC/tACC ratio of Ad-dn-AMPK cells treated with metformin was not different from saline treated cells, and was significantly lower than Ad-GFP-metformin treated cells, indicating that the Ad-dn-AMPK virus abolishes the action of metformin to induce AMPK activity.
Using an identical procedure to that of our co-submission, which was shown to maximize duodenal infection without infection of the jejunum, ileum, or liver, we then injected either Ad-dn-AMPK or Ad-GFP into a 5cm ligated section of the duodenum of HFD-fed rats to suppress duodenal AMPK activity. After 20 minutes, the viral solution was flushed out and surgeries for the clamp studies were done. Three days following surgery, rats were subjected to the infusion-clamp studies. Intraduodenal infusion of metformin increased the glucose infusion rate (Fig. 1h) and reduced GP (Fig. 1i, Supplementary Fig. 1b) without affecting glucose uptake (Supplementary Fig. 1c) in Ad-GFP-injected control rats, but this effect was completely absent in Ad-dn-AMPK-injected rats. Thus, duodenal AMPK activation is required for preabsorptive metformin to lower GP.
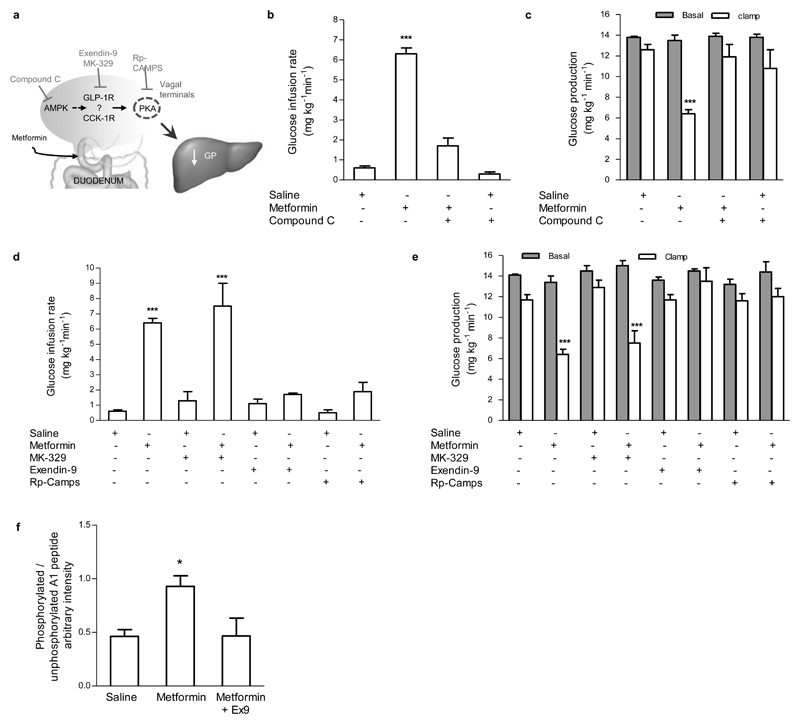
To alternatively confirm the role of duodenal AMPK, we co-administered metformin with compound C, an AMPK inhibitor (Fig. 2a). Intraduodenal compound C alone did not affect whole body glucose metabolism but fully negated the ability of metformin to increase the glucose infusion rate (Fig. 2b) or lower GP (Fig. 2c, Supplementary Fig. 2a), independent of changes in glucose uptake (Supplementary Fig. 2b). Of note, the same dose of compound C also blocked the ability of duodenal resveratrol to lower GP (co-submission).
Figure 2. A duodenal AMPK – GLP-1R – PKA signaling pathway is required for metformin to lower GP.
(a) Schematic representation of the working hypothesis. Intraduodenal metformin activates duodenal AMPK, GLP-1R, and PKA to lower GP. (b,c) The glucose infusion rate (b) and rate of GP (c) during the pancreatic (basal insulin) euglycemic clamp in HFD-fed rats with intraduodenal compound C administration alone (n=5) or in combination with metformin (n=6). (d,e) The glucose infusion rate (d) and rate of GP (e) during the pancreatic (basal insulin) euglycemic clamp in HFD-fed rats with intraduodenal MK-329, exendin-9, and Rp-CAMPS administration with saline (n=5 for each) or in combination with metformin (n=6 for each group). (f) Duodenal PKA activity quantification of clamp tissue of HFD-fed rats treated with either saline, metformin, or metformin with exendin-9 (n=5 for each group). Values are shown as mean ± s.e.m. *p < 0.05, ***p < 0.001, versus all other groups, as calculated by one-way ANOVA with Tukey’s post hoc test. AMPK, AMP-activated protein kinase; GLP-1R, glucagon-like peptide-1 receptor; PKA, protein kinase A; GP, glucose production; HFD, high fat diet.
To further delineate the downstream duodenal signaling mechanisms of the metformin-AMPK axis, we explored whether intestinal gut peptide signaling mediates the effect of metformin by co-infusing metformin with either a CCK-1 receptor antagonist, MK-329, or GLP-1 receptor (GLP-1R) antagonist, exendin-9 (Ex9) (Fig. 2a). We chose to specifically examine the roles of CCK and GLP-1, as duodenal CCK signaling triggers a gut-brain-liver axis to lower GP13, while metformin has been documented to stimulate GLP-1 release17. Interestingly, metformin still significantly increased the glucose infusion rate (Fig. 2d) and lowered GP (Fig. 2e, Supplementary Fig. 2c) when co-infused with MK-329, but coinfusion with Ex9 completely reversed the ability of metformin to increase glucose infusion rate (Fig. 2d) and lower GP (Fig. 2e, Supplementary Fig. 2c), while glucose uptake remained unchanged (Supplementary Fig 2d). Ex9 or MK-329 alone had no effect on whole body glucose metabolism (Fig. 2d,e; Supplementary Fig. 2c,d). These findings highlight the necessity of intestinal GLP-1, but not the CCK-1, signaling in preabsorptive metformin’s actions.
Given that 1) CCK lowers GP via CCK-1 receptor activation of protein kinase A (PKA) signaling on vagal afferents22, 2) GLP-1Rs are localized on vagal afferents innervating the small intestine23, and 3) beta-cell GLP-1R activation induces PKA signaling24, we examined whether metformin’s effect requires PKA signaling (Fig. 2a). While infusion of Rp-CAMPS alone did not alter the glucose infusion rate (Fig. 2d), GP (Fig. 2e, Supplementary Fig. 2c), or glucose uptake (Supplementary Fig. 2d), intraduodenal co-infusion of metformin with Rp-CAMPS completely abolished the ability of metformin to increase the glucose infusion rate (Fig. 2d) and lower GP (Fig. 2e, Supplementary Fig. 2c). We measured the PKA activity from duodenal tissues taken immediately following the clamp experiments. The A1 peptide is phosphorylated by PKA, and thus a higher ratio of phospho(P)-A1/A1 reflects a higher degree of PKA activation. Intraduodenal metformin infusion increased duodenal PKA activity, and this activation was abolished by coinfusion with Ex9 (Fig. 2f), indicating that GLP-1R activation is necessary for metformin to increase PKA. Collectively, these results demonstrate that metformin requires a duodenal AMPK-GLP-1R-PKA signaling pathway to lower GP.
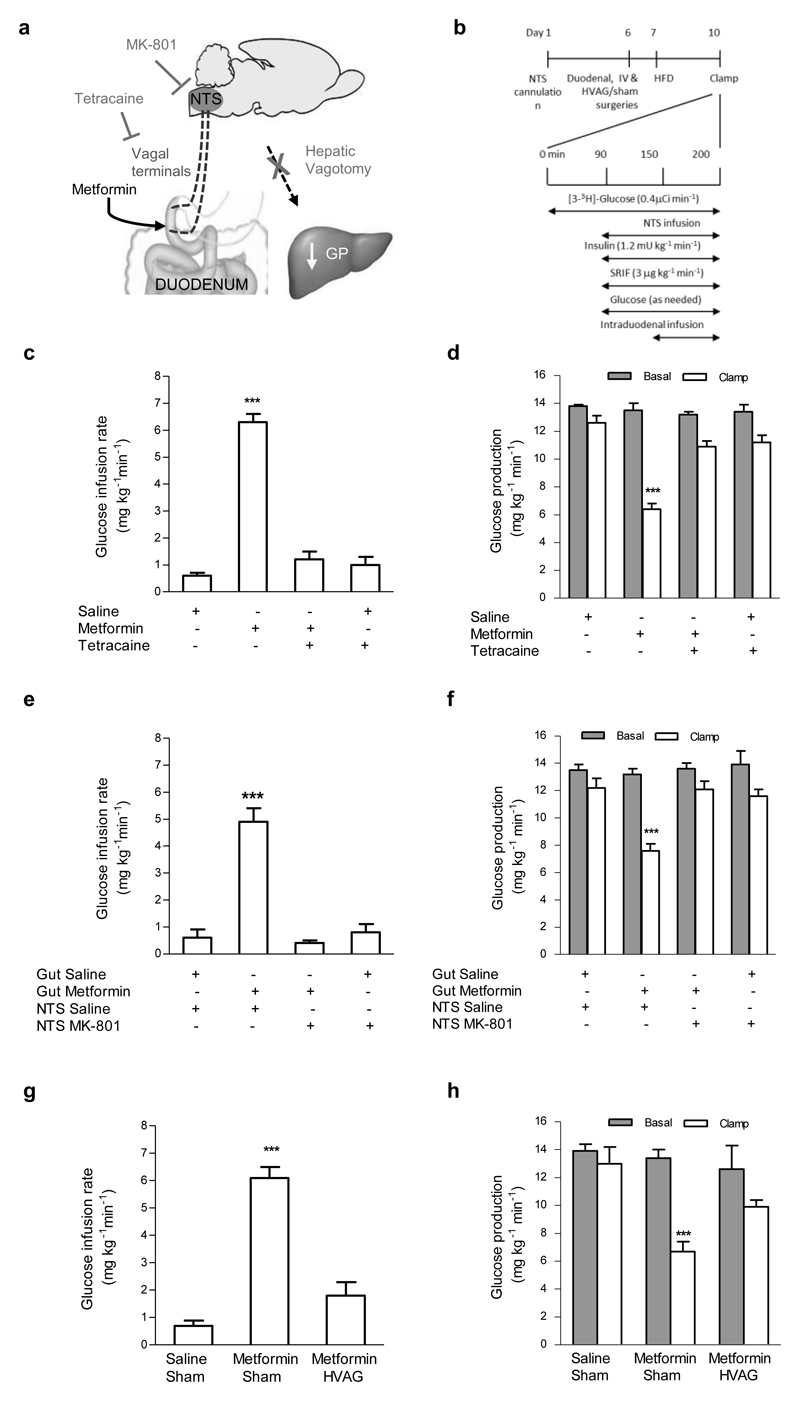
We next used three complementary techniques to examine whether a gut-brain-liver neuronal axis mediates the suppressive effect of metformin on GP in HFD-fed rats (Fig. 3a,b). We first administered the local anesthetic tetracaine, with or without metformin, into the duodenum to inhibit neurotransmission of afferent fibers innervating the gut. Tetracaine infusion alone did not affect glucose metabolism (Fig. 3c,d; Supplementary Fig. 3a,b), but fully reversed the ability of metformin to increase the glucose infusion rate (Fig. 3c) and to lower GP (Fig. 3d, Supplementary Fig. 3a). Thus, metformin signals via a neuronal network in the intestine to lower GP. Afferent vagal signaling synapses at the level of the nucleus of the solitary tract (NTS) in the hindbrain and activation of the N-methyl-D-aspartate (NMDA) receptors in the NTS mediates gut nutrient sensing to regulate GP12. Here, we found that direct infusion of the NMDA receptor inhibitor MK-801 into the NTS alone did not affect glucose metabolism (Fig. 3e,f; Supplementary Fig. 3c,d), but fully attenuated the ability of intraduodenal metformin infusion to increase the glucose infusion rate (Fig. 3e) and lower GP (Fig. 3f, Supplementary Fig. 3c) without affecting glucose uptake (Supplementary Fig. 3d). Finally, to evaluate whether this gut-hindbrain axis relays the signal generated by gut metformin to the liver to lower GP, we repeated the studies in rats that underwent hepatic vagal branch vagotomy (HVAG), effectively eliminating the neurocommunication between the brain and liver. HVAG abolished the ability of duodenal metformin to increase the glucose infusion rate (Fig. 3g) and lower GP (Fig. 3h, Supplementary Fig. 3e) compared to sham operated rats, but alone did not affect glucose metabolism. (Fig. 3g,h, Supplementary Fig. 3e,f) Together, these data illustrate that duodenal metformin activates a gut-brain-liver neuronal axis to inhibit GP.
Figure 3. A gut-brain-liver neuronal axis is required for the GP-lowering effect of metformin.
(a) Schematic representation of the working hypothesis. Duodenal metformin triggers the afferent nerve terminals in the duodenum, and signals via NMDA receptors at the level of the NTS, which signals via the hepatic vagus to lower GP. (b) Experimental procedure and pancreatic (basal insulin) euglycemic clamp protocol with NTS infusion. (c,d) The glucose infusion rate (c) and rate of GP (d) in HFD-fed rats infused with intraduodenal tetracaine alone (n=5) or in combination with metformin (n=6). (e,f) The glucose infusion rate (e) and rate of GP (f) in HFD-fed rats with intraduodenal saline or metformin and DVC saline (n=4,5) or MK-801 (n=5,6). (g,h) The glucose infusion rate (g) and rate of GP (h) in HFD-fed rats with intraduodenal metformin with either a sham surgery (n=5) or HVAG (n=6). Values are shown as mean ± s.e.m. ***p < 0.001, versus saline, as calculated by one-way ANOVA with Tukey’s post hoc test. GP, glucose production; NMDA, N-Methyl-D-aspartate; NTS, nucleus of the solitary tract; HFD, high-fat diet; SRIF, somatostatin; HVAG hepatic vagotomy.
To further determine the therapeutic potential of duodenal metformin, we additionally tested the ability of preabsorptive metformin to lower GP in both a HFD-induced obese and a type 2 diabetic rat model. First, we tested the effects of preabsorptive metformin in a chronic hyperphagic HFD-fed obese rat model (28 days; Fig. 4a), which is characterized as having increased fat mass and both hepatic and peripheral insulin resistance (see co-submission). Importantly, intraduodenal metformin was still effective in increasing the glucose infusion rate (Fig. 4b) and lowering GP (Fig. 4c, Supplementary Fig. 4a) in this obese rat model, without affecting glucose uptake (Supplementary Fig. 4b). For the type 2 diabetic model, rats were injected with nicotinamide (NA) and streptozotocin (STZ) and fed a HFD for 5-6 d, inducing mild hyperglycemia and increased GP (Supplementary Fig. 4c,d), but not insulin-deficiency, as described25 and also reported in our co-submission. In unclamped conditions, a 50 min duodenal infusion of metformin significantly reduced both plasma glucose levels (Fig. 4d) and GP in these rats (Fig. 4e), compared to a saline infusion. Thus, preabsorptive duodenal metformin is sufficient to lower plasma glucose levels and GP in obese and diabetic rats.
Figure 4. Intraduodenal infusion of metformin lowers GP in obese and diabetic rats, while the overall acute glucose-lowering effect of a bolus intragstric treatment of metformin is dependent on duodenal AMPK signaling.
(a) Experimental procedure and pancreatic (basal-insulin) euglycemic clamp protocol for 28 day HFD-fed rats. (b,c) The glucose infusion rate (b), rate of GP (c) during the pancreatic (basal insulin) euglycemic clamp in 28 day HFD-fed rats with intraduodenal saline or metformin infusion (***p < 0.001, versus saline as compared by unpaired t-test; n=6 for each group). (d,e) Plasma glucose levels (d) and the rate of GP (e) in NA-STZ/HFD induced hyperglycemic rats with intraduodenal saline or metformin (n=8 for each group). (f) Experimental procedure and gastric infusion protocol. (g,h) The plasma glucose levels (different letter denotes significant difference of p < 0.05 between groups as calculated by two-way ANOVA with Tukey’s post hoc test) (g) and the percent suppression of plasma glucose from basal levels (**p < 0.01, versus saline as compared by unpaired t-test within each timepoint) (h) in NA-STZ/HFD induced hyperglycemic rats injected with either intraduodenal GFP or dnAMPK for 5 days with a gastric bolus treatment of metformin (n=8 for each group). Values are shown as mean ± s.e.m. Unless noted, ***p < 0.001, versus saline, as calculated by one-way ANOVA with Tukey’s post hoc test. GP, glucose production; AMPK, AMP-activated protein kinase; NA, nicotinamide; STZ, streptozotocin. HFD, high fat diet.
Lastly, to assess the contribution of duodenal AMPK in the acute glucose lowering effects of a bolus treatment of metformin in an unclamped setting, we administered an intragastric bolus dose of metformin (200mg/kg) to NA-STZ/HFD type 2 diabetic rats that had received either an intraduodenal injection of Ad-dn-AMPK or Ad-GFP (as described above) (Fig. 4f). Interestingly, while metformin reduced plasma glucose levels in Ad-GFP rats after 60 minutes, it failed to do so in Ad-dn-AMPK rats. Despite metformin lowering plasma glucose levels in both groups 180 minutes after the intragastric bolus, glucose levels were still significantly higher in the Ad-dn-AMPK treated rats compared to the Ad-GFP rats (Fig. 4g). In line with this, Ad-GFP diabetic rats had an increased suppression of plasma glucose levels compared to Ad-dn-AMPK rats, starting as early as 30 minutes post gastric bolus, and importantly, was still present 180 minutes after the bolus (Fig. 4h). Thus, activation of intestinal AMPK has a significant contribution to the rapid and the overall ability of metformin to lower plasma glucose levels in diabetes.
Metformin has been the most widely prescribed treatment for type 2 diabetes for the past fifty years, yet the mechanism of action of metformin suppressing GP remains unclear. Indeed, initial investigations highlighted the ability of metformin to activate hepatic AMPK to reduce gluconeogenesis6,7, while more recently, metformin has been shown to induce a reduction in hepatic energy charge8 and antagonize glucagon-induced GP via reductions in cAMP9. Adding to, and complimenting, these described potential direct hepatic actions, we here demonstrate a novel mechanism where preasbsorptive metformin remotely (indirectly) lowers GP via activation of duodenal AMPK and a neuronal network.
Importantly, we demonstrate that our observed effect during the pancreatic clamp is preasbsorptive, as direct portal vein infusion of metformin at the same dose given in the duodenum for 50 min was unable to lower GP. This does not rule out the possibility that metformin can directly target hepatocytes to antagonize glucagon action to lower GP9, since our experimental pancreatic clamp condition is not glucagon stimulated. On the other hand, ninety minutes following the intragastric bolus of metformin during non-clamp conditions, diabetic rats with duodenal injections of Ad-GFP and Ad-dn-AMPK still exhibit reductions in plasma glucose levels, potentially from the aforementioned mechanism9. Nonetheless, it is important to note that we here discovered metformin initially (first 60 min) only reduces plasma glucose levels in Ad-GFP treated rats but not in rats with duodenal Ad-dn-AMPK. Further, this initial drop in glucose levels via duodenal AMPK activation has a significant and sustained contribution to the overall suppression of plasma glucose. These findings highlight the vital role of duodenal AMPK signaling in the overall efficacy of metformin treatment.
A recent report documented that the lipid-lowering and insulin-sensitizing effects of metformin treatment were dependent upon the ability of metformin to increase hepatic AMPK activity and subsequently phosphorylate and inhibit hepatic ACC26. Inhibition of ACC reduces the formation of malonyl-CoA and hence negates long-chain fatty acid-acyl-coenzyme A (LCFA-CoA) accumulation as well. We have previously demonstrated that an accumulation of duodenal LCFA-CoA level is required for duodenal lipids to lower GP12. Thus, it is unlikely that the ability of preabsorptive metformin to activate duodenal AMPK and lower GP that we demonstrate here is linked with duodenal LCFA-CoA sensing. In fact, consistent with this working hypothesis, while LCFA-CoA-induced reduction in GP is dependent on CCK signaling13, metformin’s action is CCK-1 receptor-independent. Instead, we demonstrate metformin signals via intestinal GLP-1R, consistent with the view that metformin can acutely and chronically increase GLP-1 levels17,19. Although it remains to be tested, it is likely that metformin acts directly on enteroendocrine L-cells localized in the duodenum27, as metformin-induced reduction of GP was attenuated with local infusion of Ex9 into the duodenum, despite metformin inducing GLP-1 release via a neuronal-hormonal reflex28. Interestingly, AICAR, an AMPK agonist, increases GLP-117,28, further supporting our data demonstrating the significance of intestinal AMPK activity in mediating GP suppression via an AMPK-GLP-1R-PKA pathway.
Similar to the importance of duodenal AMPK in mediating the beneficial effects of metformin, we have demonstrated that preabsorptive duodenal resveratrol improves insulin sensitivity via a duodenal AMPK/SIRT1-PKA dependent pathway (see co-submission). Interestingly, despite the overlap in the signaling pathways of these two treatments, such that both drugs potentially activate intestinal AMPK and SIRT1, resveratrol and metformin could target divergent downstream glucoregulatory mechanisms. For example, while metformin activates an AMPK-GLP-1R-PKA gut-brain-liver neuronal pathway to lower GP, resveratrol possibly lowers GP via a unique and separate neuronal pathway involving enhancement of hypothalamic insulin sensitivity (see co-submission).
In summary, we unveil that metformin activates a previously unappreciated duodenal AMPK-GLP-1R-PKA-dependent neuronal pathway to lower GP and plasma glucose levels in obesity and diabetes, and that activation of duodenal AMPK significantly contributes to the overall acute glucose lowering effect of metformin. Together with our discovery on preabsorptive resveratrol’s action (see co-submission), our findings lay the groundwork for the potential development of specific gut targeted treatments that could simultaneously activate intestinal energy sensor proteins, AMPK and SIRT1, to additively or synergistically lower GP and improve glycemia in diabetes and obesity.
Methods
Methods and any associated references are available in the online version of the paper.
Supplementary Material
Acknowledgements
The authors are grateful to Elena Burdett for technical assistance. This work is supported by a research grant to T.K.T.L. from the Canadian Institute of Health Research (MOP-82701). F.A.D. is a Banting Fellow. B.A.R. is supported by a CIHR Doctoral Vanier Canada scholarship. C.D.C. is supported by a Banting and Best Diabetes Centre graduate studentship. M.Z-T. is supported by a Banting and Best Diabetes Centre graduate studentship. T.K.T.L. holds the John Kitson McIvor (1915–1942) Endowed Chair in Diabetes Research and the Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
Footnotes
Author Contributions
F.A.D. conducted and designed experiments, performed data analyses and wrote the manuscript; B.A.R., C.D.C., M.Z.T., and B.M.F. assisted with experiments. G.A.R. provided the adenovirus expressing the dn-AMPK. T.K.T.L. supervised the project, designed experiments and edited the manuscript.
References
- 1.Taylor SI. Deconstructing type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 2.Hundal RS, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46:1406–1413. doi: 10.2337/diab.46.9.1406. [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 5.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RA, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madiraju AK, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 12.Wang PY, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 13.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen BA, et al. Jejunal leptin-PI3K signaling lowers glucose production. Cell Metab. 2014;19:155–161. doi: 10.1016/j.cmet.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Breen DM, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18:950–955. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]
- 16.Stepensky D, Friedman M, Raz I, Hoffman A. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metab Dispos. 2002;30:861–868. doi: 10.1124/dmd.30.8.861. [DOI] [PubMed] [Google Scholar]
- 17.Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia. 2011;54:339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 18.Shin NR, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 19.Vardarli I, Arndt E, Deacon CF, Holst JJ, Nauck MA. Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and “isoglycemic” intravenous glucose. Diabetes. 2014;63:663–674. doi: 10.2337/db13-0805. [DOI] [PubMed] [Google Scholar]
- 20.Harmel E, et al. AMPK in the small intestine in normal and pathophysiological conditions. Endocrinology. 2014;155:873–888. doi: 10.1210/en.2013-1750. [DOI] [PubMed] [Google Scholar]
- 21.He G, et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen BA, et al. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology. 2012;142:834–843 e833. doi: 10.1053/j.gastro.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 23.Richards P, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusta B, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Samuel VT, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci U S A. 2009;106:12121–12126. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullerton MD, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib AM, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulherin AJ, et al. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152:4610–4619. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.