Abstract
Objectives
We have previously reported that F8-IL4, a fusion protein consisting of the F8 antibody specific to the alternatively-spliced EDA domain of fibronectin and of murine interleukin-4, cures mice with established arthritis, when used in combination with dexamethasone. The goal of this study was to assess whether other therapeutic agents, besides dexamethasone, could induce cures in combination with F8-IL4 and to elucidate which leukocytes are most affected by the pharmacological treatment.
Methods
We performed therapy experiments in mice with collagen-induced arthritis, using intravenous administrations of F8-IL4 in combination with (i) dexamethasone, (ii) methotrexate, (iii) murine CTLA4 fused to the Fc portion of murine IgG2a, as well as monoclonal antibodies to (iv) murine IL17A or (v) the p40 subunit of murine IL12/IL23. Histology and immunohistochemistry for the identification of the various leukocytes was performed on the paws of mice euthanized at different therapy time points.
Results
Only the use of F8-IL4 in combination with dexamethasone induced complete remissions, while all other combinations did not lead to cures. The light microscopical evaluation of paws with arthritis revealed a predominant infiltration of neutrophils, which substantially decreased 24 hours after treatment with F8-IL4 and dexamethasone.
Conclusions
The combination of F8-IL4 with dexamethasone promotes a rapid anti-arthritic action by potently inhibiting neutrophil activity. A fully human analogue of F8-IL4 may find clinical utility for the treatment of neutrophil-driven chronic inflammatory conditions.
Keywords: collagen-induced arthritis, interleukin-4, immunocytokines, EDA domain of fibronectin, Type II interleukin-4 receptor, neutrophils
Introduction
The advent of antibody-based products, capable of neutralizing TNF or other cytokine mediators of inflammation, has revolutionized the treatment of patients with rheumatoid arthritis (RA) and other chronic inflammatory conditions.[1] Unfortunately, however, a substantial portion of RA patients do not respond to treatments with various lines of biologics.[2, 3] Furthermore, even for those patients who benefit from treatment with biopharmaceuticals (e.g., TNF blockers, IL6 receptor blockers, anti-CD20 antibodies and/or fusion proteins of CTLA4), the disease remains incurable and can lead to progressive disability with the destruction of cartilage and bone, systemic complications such as cardiovascular or pulmonary diseases and a higher risk for lymphoma and early death.[4] Thus, there is a need for further improvement in the treatment and management of RA patients.
Mice with collagen-induced arthritis (CIA) have often been used as an animal model to test novel therapeutic modalities[5, 6], thus facilitating subsequent clinical translation activities. In this model, blockade of murine TNF inhibits disease progression, but does not cure the disease.[5, 7–10] Similarly, methotrexate[11, 12], mouse analogues of abatacept[13, 14], as well as monoclonal antibodies to murine interleukin-17A (IL17A) (serving as mimics for the biopharmaceuticals secukinumab and ixekizumab)[15, 16] or the p40 subunit of murine IL12/IL23 (serving as mimics for ustekinumab)[17], have shown activity in the CIA model but no induction of complete and durable remissions.
The antibody-based pharmacodelivery of immunomodulatory cytokines has been proposed as an alternative avenue for the treatment of chronic inflammatory conditions and our laboratory has produced and evaluated immunocytokines based on several immunomodulatory payloads.[18, 19] So far, the only product that could cure mice with established CIA was F8-IL4, a fusion protein consisting of the F8 antibody (which recognizes the alternatively-spliced extra domain A (EDA) of fibronectin) and murine IL4, when used in combination with dexamethasone.[20, 21] Interestingly, when used as single agents, neither dexamethasone nor F8-IL4 were able to induce complete remissions. The objective of this study was to discern whether (i) methotrexate, (ii) murine CTLA4 fused to the Fc portion of murine IgG2a or monoclonal antibodies to (iii) murine IL17A or (iv) the p40 subunit of murine IL12/IL23 could also induce cures in the CIA model in combination with F8-IL4, as they correspond to pharmaceutical agents that are often used for the treatment of patients with chronic inflammatory conditions. Additionally, the goal was to determine the cell populations most affected by pharmacological treatments that cured mice with established CIA.
Materials and Methods
Cell lines, proteins and animals
CHO-S cells (Invitrogen, Buchs, Switzerland) were cultured in suspension in PowerCHO-2 CD (Lonza, Verviers, Belgium) supplemented with 4 mM ultraglutamine (Lonza, Verviers, Belgium) in shaking incubators. The cloning and production of F8-IL4 has been described previously.[22] Male DBA/1J mice were purchased from Janvier (St. Berthevin, France).
Mouse model of collagen-induced arthritis
Eight-week old male DBA/1J mice were immunized with 0.05 mL emulsion of bovine type II collagen emulsified in Complete Freud’s Adjuvant (Hooke Laboratories, Massachusetts, United States) by subcutaneous (s.c.) injection at the base of the tail. After three weeks, a 0.04 mL booster injection of bovine type II collagen in Complete Freud’s Adjuvant was given. From here on mice were inspected daily and monitored for signs of disease using a clinical scoring system assigning a score to each paw (0 = normal; 1 = one toe inflamed and swollen; 2 = more than one toe, but not entire paw, inflamed and swollen or mild swelling of entire paw; 3 = entire paw inflamed and swollen; 4 = very inflamed and swollen paw). A maximum total score of 16 can be reached for each animal. Additionally, daily measurements of the swelling of affected paws were performed under isoflurane anesthesia using a caliper and the paw thickness was expressed as the mean of all four paws of each animal. Animals were enrolled in a therapy group on the day of arthritis onset with arthritic scores ranging from 1 – 6. All experiments were performed under a project license granted by the cantonal veterinary office (05/2014) in agreement with Swiss regulations.
Combination therapy
Mice with a new arthritic score of 1 – 6 were randomly assigned to receive either phosphate buffered saline (PBS) (n=8; control group) or a combination of F8-IL4 and methotrexate (MTX) (Methotrexate Pfizer®, Zürich, Switzerland) (n=8), murine CTLA4 fused to the Fc portion of murine IgG2a (muCTLA4-Fc) (C4483, Sigma-Aldrich®, Missouri, United States) (n=8), a monoclonal antibody to murine interleukin-17A (anti-IL17A) (clone 17F3, BioXCell, New Hampshire, United States) (n=8), a monoclonal antibody to the p40 subunit of murine interleukin-12/interleukin-23 (anti-IL12/IL23) (clone C17.8, BioXCell, New Hampshire, United States) (n=7) or dexamethasone (DXM) (Dexamethason Helvepharm, Zentiva, Frauenfeld, Switzerland) (n=7). On days 1, 4 and 7 the control group received 100 μL PBS intravenously (i.v.) and the treatment groups 100 μg of F8-IL4 i.v. with either 50 μg MTX (approx. 2.5 mg/kg) intraperitoneally (i.p.), 100 μg muCTLA4-Fc i.p., 100 μg anti-IL17A i.p. or 100 μg anti-IL12/IL23 i.p.. The clones, dosages and route of administration were chosen on the basis of previous reports, which had shown efficacy in murine animal models.[12–14, 16, 17, 23] Mice in the combination group with DXM received 100 μg of F8-IL4 i.v. on days 1 and 4 and 100 μg DXM i.p. until the day of sacrifice (the mice were all sacrificed before day 7 for histological analyses). Of all mice the arthritic scores, weights and thicknesses of inflamed paws were monitored daily in a blinded fashion. Mice were sacrificed on day 5 (PBS), day 8 (MTX), day 9 (muCTLA4-Fc) and day 21 (anti-IL12/IL23) due to reaching termination criteria based on arthritic score and weight loss in accordance with local regulations. Mice from the DXM group were sacrificed concomitantly at various time points and mice from the anti-IL17A group were sacrificed on day 28 as this is the maximum amount of time our license allows us to keep mice after onset and treatment of arthritis if termination criteria are not reached.
Histological and immunohistochemical examinations
Immediately after euthanasia, paws from selected animals (controls on day 1 (n=2) and day 5/6 (n=1) after onset of the disease; F8-IL4 combination group with DXM at 6 h (n=1), 12 h (n=1), 24 h (n=2) and 72 h (n=1) after the first F8-IL4 treatment and at 24 h (n=1) and 48 h (n=1) after the second F8-IL4 treatment) were removed and fixed in 4% paraformaldehyde in PBS (pH 7.4) for approximately 48 h, then decalcified in EDTA (Biosystems, Muttenz, Switzerland) for 14 days. Decalcified paws were trimmed (sagittal hemisections, comprising the phalanges to the radius and tibia, respectively) and routinely paraffin wax embedded.[24] Serial section (3-5 μm) were prepared and stained with hematoxylin-eosin (HE) for the histological examination, or used for immunohistochemical staining.
Immunohistochemistry was performed to identify neutrophils and neutrophil extracellular traps (NETs), macrophages, T cells and B cells, using the horseradish peroxidase (HRP) and the avidin biotin complex (ABC) method. The following primary antibodies were applied: rat anti-mouse Ly6G (neutrophil marker; clone 1A8, Biolegend, California, United States), rabbit anti-Iba-1 (macrophage marker; antigen: AIF1; Wako Chemicals; Zurich, Switzerland), mouse anti-human CD3 (T cell marker; clone F7.2.38, Agilent Technologies, Basel, Switzerland), rat anti-mouse CD45R (B cell marker; clone B220, BD Biosciences, Allschwil, Switzerland) and rabbit anti-histone H3 (citrulline R2 + R8 + R17; NET marker, abcam; Cambridge, United Kingdom). Briefly, after deparaffination, sections underwent antigen retrieval in citrate buffer (pH 6.0, 20 min at 98°C; for Ly6G, Iba-1 and CD45R) and EDTA buffer (pH 9.0, 20 min at 98°C; for CD3), followed by blocking of endogenous peroxidase (peroxidase block, S2023, Dako, Baar, Switzerland) for 10 min at room temperature (RT). Slides were then incubated with the primary antibodies (diluted in dilution buffer, Dako, Baar, Switzerland) for a) CD3 and Iba-1 (60 min at RT), followed by a 30 min incubation at RT with the secondary antibody (Envision mouse and rabbit, respectively, Dako, Baar, Switzerland) in an autostainer (Dako, Baar, Switzerland), and b) Ly6G (60 min at RT) and CD45R (overnight at 4°C), followed by rabbit anti-rat IgG and the ABC kit (both 30 min at RT; Ventana, Tucson, United States). Staining for histone H3 was undertaken with an autostainer (Discovery XT, Ventana, Tucson, United States), using citrate buffer, dilution buffer and detection kits provided by the manufacturer. The antibody reaction was visualized with 3,3'-diaminobenzidin and sections counterstained with hemalaun.
Sections of a mouse spleen served as positive controls for the leukocyte markers. For negative controls, the primary antibody was omitted.
All examinations were undertaken by a veterinary pathologist (AK) who was blinded to the treatment of the animals and the arthritis scores when assessing the histological and immunohistochemical specimens.
Results
Combination therapy studies in mice with collagen-induced arthritis
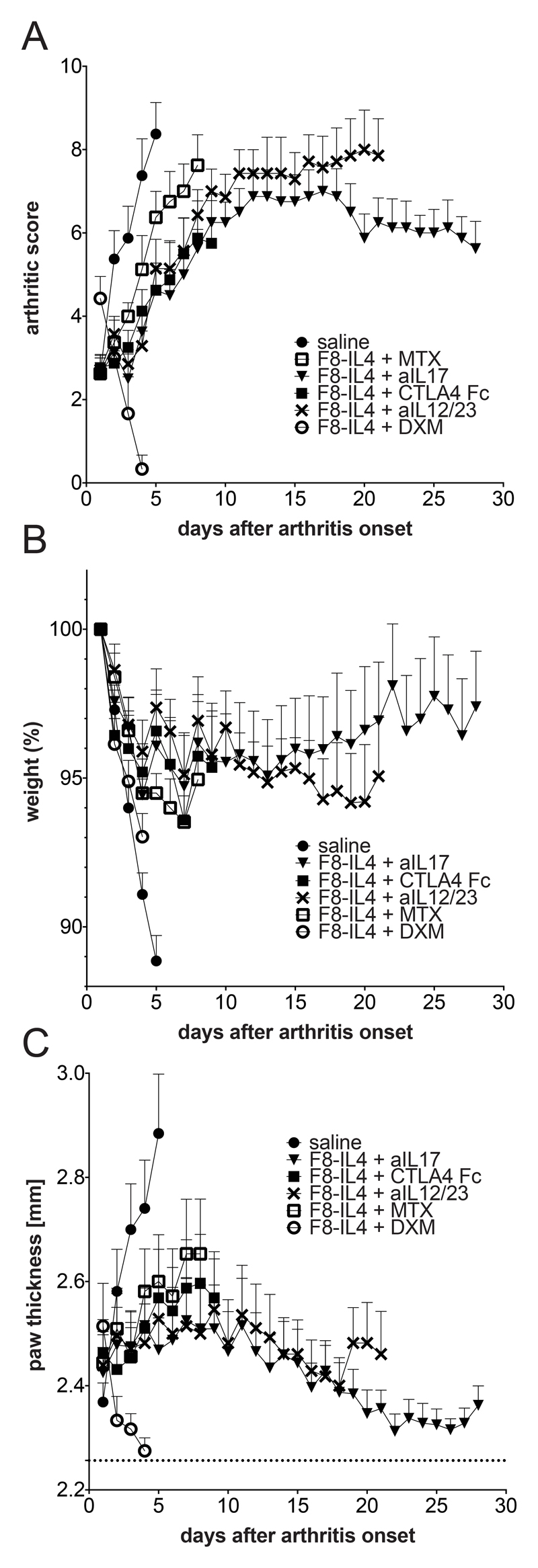
The therapeutic activity of combination treatments of F8-IL4 with (i) methotrexate, (ii) murine CTLA4 fused to the Fc portion of murine IgG2a (an analogue of abatacept), as well as monoclonal antibodies to (iii) murine IL17A and (iv) the p40 subunit of murine IL12/IL23 was assessed in the collagen-induced model of arthritis in male DBA/1J mice. The combination therapy of F8-IL4 with dexamethasone as well as the therapeutic use of the single agents has been reported previously [20, 21]. The combination therapy was performed again to generate specimens for histological analysis and characterization of the inflammatory process. As previously described, the combined use of dexamethasone and F8-IL4 led to a rapid reduction of arthritic score and paw thickness (Figure 1). In contrast, none of the other combination modalities led to complete regressions of the disease. Among those regimens, the combination of F8-IL4 with the anti-IL17A antibody yielded the best results with a stabilization of the arthritic score and a decrease in paw thickness, but much more slowly and to a lesser extent compared to F8-IL4 plus dexamethasone (Figure 1). All other therapeutic combination groups had to be sacrificed before the pre-defined endpoint at day 28, as the termination criteria were reached.
Figure 1. Combination therapy experiments of F8-IL4 with five different therapeutic agents in mice with collagen-induced arthritis.
All therapeutics were administered on day 1, 4 and 7 after arthritis onset except dexamethasone which was given daily during treatment course until sacrifice. Therapeutic efficacy was evaluated daily using the (A) arthritic score expressed as the sum of the scores of all 4 paws, (B) weight given as percentage of the starting weight on day of enrolment and (C) paw swelling depicted as the mean of the thickness of all four paws, with the dashed line at 2.26 mm indicating baseline paw thickness of mice without disease (n= 7 – 8; mean and SEM).
In situ analysis of the extent and composition of the inflammatory process in the paws in the course of CIA with and without pharmacological intervention
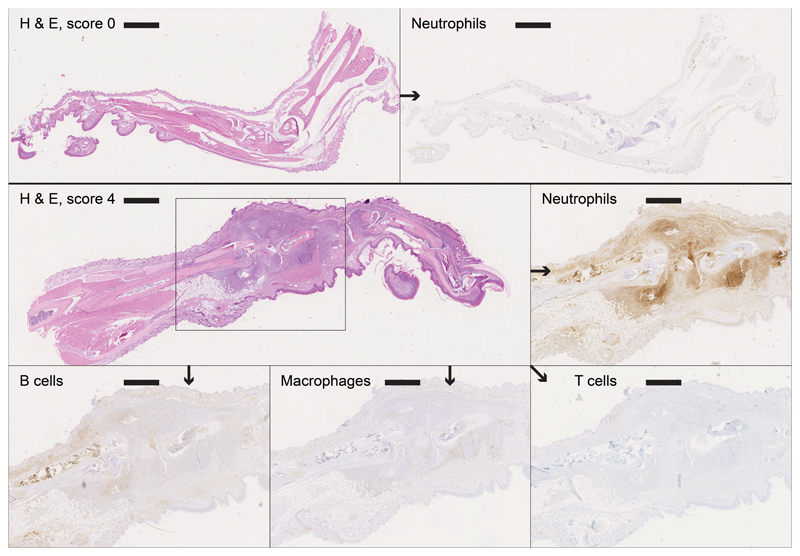
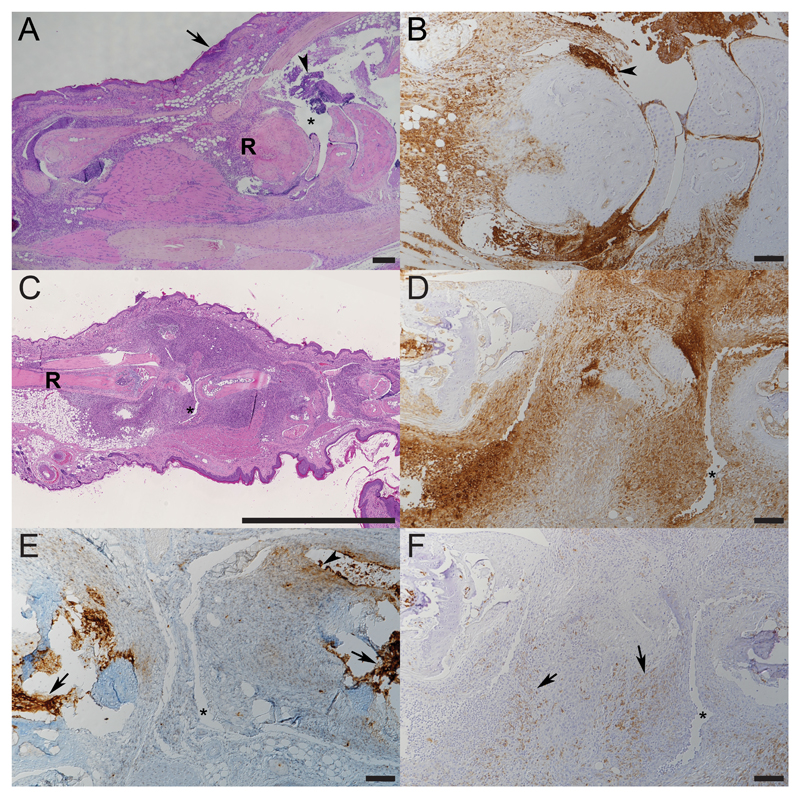
A detailed histological and immunohistochemical examination was undertaken on selected paws of control CIA animals (scores 0-4) to assess the time course of the inflammatory processes and on animals treated with F8-IL4 in combination with dexamethasone. Representative findings are shown in Figures 2, 3 and 4. Score 0 paws did not exhibit any pathological changes, and no inflammatory cell infiltration. In affected paws a variably intense (increasing with higher scores) neutrophil (Ly6G+)-dominated infiltration (with abundant degenerate neutrophils and extensive NET deposition) was observed around tendons and along the long bones, extending into the subcutis and focally, the epidermis, between skeletal muscle bundles and into the joint spaces. The inflammatory infiltrate also contained a moderate proportion of macrophages (Iba-1+) and small numbers of individual T cells (CD3+), whereas B cells (CD45R+) were almost entirely absent (Figure 2 and 3).
Figure 2. Histological analysis of untreated paws with and without collagen-induced arthritis.
Top: Right hindleg (score 0) with no signs of neutrophil infiltration; left: HE, bar = 1609 μm; right: neutrophil staining, bar = 1555 μm.
Bottom: Left foreleg (score 4) with an infiltrate showing an intense neutrophil domination, a moderate proportion of macrophages, small numbers of individual T cells and virtually no B cells; HE, bar = 1017 μm; neutrophil staining, bar = 695 μm; B cell staining, bar = 652 μm, macrophage staining, bar = 698 μm and T cell staining, bar = 657 μm.
Figure 3. Histological analysis of untreated paws with collagen-induced arthritis at different time points.
A, B. Left foreleg of a mouse 24 h post onset of clinical signs (score 3). R - radial bone; arrowhead: accumulation of degenerate neutrophils, arrow: epidermis. C-F. Left foreleg of a mouse 5 days post onset of clinical signs (score 4). R - radius, * - joint space. E. Neutrophils in the infiltrate are found to express NETs (arrowheads). F. Macrophages can be seen in moderate numbers (arrows). A, C: HE stain; B, D-F: HRP (Iba-1, histone H3) and ABC (Ly6G) method, hemalaun counterstain. Bars = 200 μm (A), 100 μm (B, D-F). 2.5 mm (C).
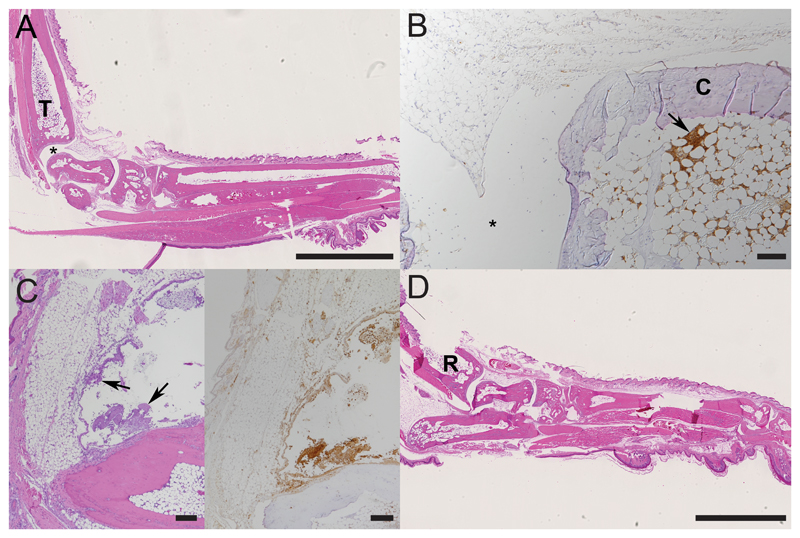
Figure 4. Histological findings in paws with collagen-induced arthritis after one dose of treatment.
A, B. Right hindleg of a mouse 12h post treatment (score 0; initial score 2) with neutrophils only found in bone marrow, T - tibial bone; * joint space; C – cartilage. C. Left hindleg of a mouse 24h post treatment (score 0; initial score 2). D. Right foreleg of a mouse 72h post treatment (score 0; initial score 3), R - radial bone. B, C: Arrows: accumulations of viable and degenerate neutrophils. A, C (left), D: HE stain; B, C (right): staining for neutrophils (Ly6G+); ABC method, hemalaun counterstain. Bars = 2.5 mm (A, D) and 100 μm (B, C).
Already after the first administration of F8-IL4 plus dexamethasone, but consistently after the second administration, the inflammatory processes had either entirely resolved or had decreased to a mild or moderate inflammatory infiltration around the joints or along the long bones (Figure 4 and Supplementary Figures 1 and 2). This was still neutrophil dominated (Figure 4C), with few macrophages, rare T cells and no B cells. The histological findings were in concomitance with a drop of arthritic score.
Treatment with F8-IL4 in combination with dexamethasone induced a substantial reduction of the inflammatory processes in CIA, which was evident shortly after the first administration of the combination therapy.
Discussion
All investigated combination partners chosen in this study corresponded to pharmaceutical agents that are often used for the treatment of patients with chronic inflammatory conditions. As previously reported, only the combination of F8-IL4 with dexamethasone was curative in mice with CIA, leading to paws that were grossly indistinguishable from the ones of healthy animals, which highlights the synergistic benefit achieved by the two compounds. Whereas all other combination partners were given three times in a week, dexamethasone was administered daily, as corticosteroid treatment has previously been shown to be beneficial when applied for multiple consecutive days.[25–27] The duration of dosing could have influenced the ability to achieve durable remissions with the combination of dexamethasone and F8-IL4, however, the dosing regimen for all therapeutics was chosen based on reports, in which positive effects of the agents had been observed in the CIA model.[15, 17, 28, 29] Furthermore, dexamethasone alone does not cure mice and additionally, it is well tolerated in a daily dosing setting, whilst this does not apply to all other agents.[20]
Our immunohistochemical analysis revealed that neutrophils were by far the dominant leukocytes, and a large proportion of these was found to be degenerate, with strong release of neutrophil extracellular traps (NETs) that are known to contribute to the pathogenesis of RA and induce further damage due to the NETosis-associated release of, among others, neutrophil enzymes.[30–32] As we knew from previous experiments that macroscopically a clinical reduction of arthritis score could already be observed 24 h after the first injection of F8-IL4 and dexamethasone, we decided to focus on the earliest phase of treatment, to be able to observe the initial changes in cell populations. Besides the time points presented in Figures 2 - 4, which already show the treatment effect on neutrophils, an additional three time points were studied (6 h, 96 h and 120 h after the first injection of F8-IL4), demonstrating the same rapid action of the treatment regimen (Supplementary Figure 2). After treatment with F8-IL4 plus dexamethasone the composition of the infiltrate and did not change substantially, however, it decreased rapidly in its extent and in the majority of paws led to an arthritis score of 0. This confirms that the recruitment of neutrophils drives the inflammation in CIA and accordingly, the combination of F8-IL4 and dexamethasone effectively dampens the process. In previously published work regulatory T cells were thought to be the main cell type influenced by the treatment of F8-IL4 plus dexamethasone.[21] However, the aforementioned report also noted reduction in neutrophil counts after therapy. Additionally, IL4 has recently been shown to potently inhibit neutrophil activity, expansion and migration by antagonizing granulocyte colony-stimulating factor (G-CSF) and chemokine receptor-mediated signals in a process which crucially depends on the Type II IL4 receptor on neutrophils.[33, 34]
The observation that a targeted delivery of IL4 to the site of inflammation potently reduces the extent of neutrophil recruitment and thereby also the damaging effect of their enzymes, which are released with NETs and with their degeneration, suggests that a fully human analogue of F8-IL4 may be useful for the treatment of diseases characterized by extensive neutrophil infiltration and activity. Previous histological analyses of human rheumatoid arthritis showed that various types of infiltrates can be observed in inflamed joints. In some patients an abundant perivascular T cell infiltration is observed, while in other subjects macrophages and granulocytes predominate.[35–37] The synovial fluid of patients with rheumatoid arthritis contains 10-100 times more leukocytes compared to healthy individuals. In patients with chronic rheumatoid arthritis, more than 60% of the leukocytes in the synovial fluid are neutrophils.[37] Further inflammatory conditions characterized by an abundant neutrophil infiltration and activity include ankylosing spondylitis[38, 39], ulcerative colitis[40] and a set of autoimmune diseases such as small vessel vasculitis, Behçet’s disease and systemic lupus erythematosus.[41, 42] There have been attempts to predict clinical response to infliximab based on the presence of synovial lymphocyte aggregates.[43] However, in contrast to this study, a clear correlation between the type of cellular infiltrate and the response to therapy has so far not been reported.[43]
The results presented in this article provide a rationale for the clinical development of a fully human analogue of F8-IL4, in combination with dexamethasone, for the treatment of patients with neutrophil-driven inflammatory conditions.
Supplementary Material
24 hours after treatment of arthritic mice a reduction in arthritic score and much lower neutrophil densities were observed (C, D) than in paws of untreated mice (A, B). A: Untreated arthritic paw (score 3), bar = 786 μm, magnified section bar = 336 μm. B: Untreated arthritic paw (score 4), bar = 1136 μm, magnified section bar = 342 μm. C, D: Treated arthritic paws with score reduction from 3 to 2. C: bar = 1273 μm, magnified section bar = 308 μm. D: bar = 962 μm, magnified section bar = 304 μm. A-D: Staining for neutrophils (Ly6G+); ABC method, hemalaun counterstain.
Mice treated with the combination therapy of F8-IL4 and DXM until sacrifice were euthanized 6 h, 12 h, 24 h, 72 h, 96 h, 120 h after the first injection if F8-IL4. Whereas at 6 h after injection a large infiltrate of neutrophils can still be observed, at later time points the inflammatory process has almost completely resolved. Staining for neutrophils (Ly6G+); ABC method, hemalaun counterstain; 6 h bar = 1140 μm, 12 h bar = 1613 μm, 24 h bar = 1580 μm, 72 bar = 1344 μm h, 96 h bar = 1301 μm, 120 bar = 1475 μm.
Key messages.
F8-IL4 combined with dexamethasone cures mice with established CIA.
Other commonly used therapeutics did not substantially potentiate F8-IL4 and the combinations were not curative.
IL4 is a potent inhibitor of neutrophil activity and F8-IL4 holds promise for the treatment of conditions with neutrophil-driven pathology.
Acknowledgments
Financial support from ETH Zürich, the Swiss National Science Foundation [Project Nr. 310030B_163479/], the Swiss Federal Commission for Technology and Innovation (KTI) [Project Nr. 17072.1] and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program [grant agreement No 607603] is gratefully acknowledged.
We thank Dr. Mattia Matasci for an aliquot of F8-murine IL4. We are also grateful to the laboratory staff of the Laboratory for Animal Model Pathology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, for skillful technical support.
Funding
This work was supported by ETH Zürich, the Swiss National Science Foundation [Project Nr. 310030B_163479/1]; the Swiss Federal Commission for Technology and Innovation (KTI) [Project Nr. 17072.1] and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program [grant agreement No 607603].
Abbreviations
- anti-IL12/IL23
monoclonal antibody to the p40 subunit of murine interleukin-12/interleukin-23
- anti-IL17A
monoclonal antibody to murine interleukin-17A
- CD3
cluster of differentiation 3
- CD20
cluster of differentiation 20
- CD45R
cluster of differentiation 45 restricted
- CHO cells
Chinese hamster ovary cells
- CIA
collagen-induced arthritis
- CTLA4
cytotoxic T-lymphocyte-associated Protein 4
- DXM
dexamethasone
- EDA
extra domain A
- Fc
fragment crystallisable
- G-CSF
granulocyte colony-stimulating factor
- IgG
Immunoglobulin G
- IL4
interleukin-4
- IL6
interleukin-6
- i.p.
intraperitoneal
- i.v.
intravenous
- Ly-6G
Lymphocyte antigen 6 complex locus G6D
- MTX
methotrexate
- muCTLA4-Fc
murine CTLA4 fused to the Fc portion of murine IgG2a
- PBS
phosphate buffered saline
- RA
rheumatoid arthritis
- s.c.
subcutaneous
- TNF
tumor necrosis factor
Footnotes
Conflict of interest statement
DN is a cofounder and shareholder of Philogen SpA (Siena, Italy), the company that owns the F8 antibody and developed Dekavil (F8-huIL10). The experiments of this article have been co-financed by Philochem AG (Otelfingen, Switzerland), a fully-owned company of the Philogen group, in the frame of a collaborative Swiss Federal KTI MedTech Project with ETH (Kommission für Technologie und Innovation). All other authors declare that they have no conflicts of interest.
References
- 1.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 2.Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl 1):S1. doi: 10.1186/ar2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S, Vital EM, Horton S, et al. Abatacept or tocilizumab after rituximab in rheumatoid arthritis? An exploratory study suggests non-response to rituximab is associated with persistently high IL-6 and better clinical response to IL-6 blocking therapy. Ann Rheum Dis. 2014;73(5):909–912. doi: 10.1136/annrheumdis-2013-204417. [DOI] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 5.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malfait AM, Williams RO, Malik AS, Maini RN, Feldmann M. Chronic relapsing homologous collagen-induced arthritis in DBA/1 mice as a model for testing disease-modifying and remission-inducing therapies. Arthritis Rheum. 2001;44(5):1215–1224. doi: 10.1002/1529-0131(200105)44:5<1215::AID-ANR206>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Joosten LA, Helsen MM, Saxne T, et al. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163(9):5049–5055. [PubMed] [Google Scholar]
- 8.Doll F, Schwager K, Hemmerle T, Neri D. Murine analogues of etanercept and of F8-IL10 inhibit the progression of collagen-induced arthritis in the mouse. Arthritis Res Ther. 2013;15(5):R138. doi: 10.1186/ar4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwerina J, Hayer S, Tohidast-Akrad M, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50(1):277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- 10.Williams RO, Marinova-Mutafchieva L, Feldmann M, Maini RN. Evaluation of TNF-alpha and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-alpha/anti-CD4 therapy. J Immunol. 2000;165(12):7240–7245. doi: 10.4049/jimmunol.165.12.7240. [DOI] [PubMed] [Google Scholar]
- 11.Neurath MF, Hildner K, Becker C, et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol. 1999;115(1):42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang B, Wang QT, Song SS, et al. Combined use of etanercept and MTX restores CD4(+)/CD8(+) ratio and Tregs in spleen and thymus in collagen-induced arthritis. Inflamm Res. 2012;61(11):1229–1239. doi: 10.1007/s00011-012-0520-0. [DOI] [PubMed] [Google Scholar]
- 13.Ko HJ, Cho ML, Lee SY, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34(2):111–120. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26(10):2320–2328. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 15.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 16.Bai F, Tian H, Niu Z, et al. Chimeric anti-IL-17 full-length monoclonal antibody is a novel potential candidate for the treatment of rheumatoid arthritis. Int J Mol Med. 2014;33(3):711–721. doi: 10.3892/ijmm.2013.1611. [DOI] [PubMed] [Google Scholar]
- 17.Butler DM, Malfait AM, Maini RN, Brennan FM, Feldmann M. Anti-IL-12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol. 1999;29(7):2205–2212. doi: 10.1002/(SICI)1521-4141(199907)29:07<2205::AID-IMMU2205>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Bootz F, Neri D. Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today. 2016;21(1):180–189. doi: 10.1016/j.drudis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmerle T, Doll F, Neri D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A. 2014;111(33):12008–12012. doi: 10.1073/pnas.1402783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawalkowska JZ, Hemmerle T, Pretto F, et al. Targeted IL-4 therapy synergizes with dexamethasone to induce a state of tolerance by promoting Treg cells and macrophages in mice with arthritis. Eur J Immunol. 2016;46(5):1246–1257. doi: 10.1002/eji.201546221. [DOI] [PubMed] [Google Scholar]
- 22.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134(2):467–477. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 23.Hemmerle T, Zgraggen S, Matasci M, et al. Antibody-mediated delivery of interleukin 4 to the neo-vasculature reduces chronic skin inflammation. J Dermatol Sci. 2014;76(2):96–103. doi: 10.1016/j.jdermsci.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Caplazi P, Baca M, Barck K, et al. Mouse Models of Rheumatoid Arthritis. Vet Pathol. 2015;52(5):819–826. doi: 10.1177/0300985815588612. [DOI] [PubMed] [Google Scholar]
- 25.Joosten LA, Helsen MM, Saxne T, et al. Synergistic protection against cartilage destruction by low dose prednisolone and interleukin-10 in established murine collagen arthritis. Inflamm Res. 1999;48(1):48–55. doi: 10.1007/s000110050396. [DOI] [PubMed] [Google Scholar]
- 26.Joosten LA, Lubberts E, Helsen MM, et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1(1):81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang I, Lee WW, Lee Y. Modulation of collagen-induced arthritis by IL-4 and dexamethasone: the synergistic effect of IL-4 and dexamethasone on the resolution of CIA. Immunopharmacology. 2000;49(3):317–324. doi: 10.1016/s0162-3109(00)00248-4. [DOI] [PubMed] [Google Scholar]
- 28.Jansen DT, el Bannoudi H, Arens R, et al. Abatacept decreases disease activity in a absence of CD4(+) T cells in a collagen-induced arthritis model. Arthritis Res Ther. 2015;17:220. doi: 10.1186/s13075-015-0731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwager K, Kaspar M, Bootz F, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11(5):R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado-Rizo V, Martinez-Guzman MA, Iniguez-Gutierrez L, et al. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pires RH, Felix SB, Delcea M. The architecture of neutrophil extracellular traps investigated by atomic force microscopy. Nanoscale. 2016;8(29):14193–14202. doi: 10.1039/c6nr03416k. [DOI] [PubMed] [Google Scholar]
- 33.Woytschak J, Keller N, Krieg C, et al. Type 2 Interleukin-4 Receptor Signaling in Neutrophils Antagonizes Their Expansion and Migration during Infection and Inflammation. Immunity. 2016;45(1):172–184. doi: 10.1016/j.immuni.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(10):593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 35.Yanni G, Whelan A, Feighery C, Bresnihan B. Analysis of cell populations in rheumatoid arthritis synovial tissues. Semin Arthritis Rheum. 1992;21(6):393–399. doi: 10.1016/0049-0172(92)90040-k. [DOI] [PubMed] [Google Scholar]
- 36.Lindblad S, Klareskog L, Hedfors E, Forsum U, Sundstrom C. Phenotypic characterization of synovial tissue cells in situ in different types of synovitis. Arthritis Rheum. 1983;26(11):1321–1332. doi: 10.1002/art.1780261104. [DOI] [PubMed] [Google Scholar]
- 37.Konttinen YT, Bergroth V, Nordstrom D, et al. Cellular immunohistopathology of acute, subacute, and chronic synovitis in rheumatoid arthritis. Ann Rheum Dis. 1985;44(8):549–555. doi: 10.1136/ard.44.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paramarta JE, Baeten D. Spondyloarthritis: from unifying concepts to improved treatment. Rheumatology. 2014;53(9):1547–1559. doi: 10.1093/rheumatology/ket407. [DOI] [PubMed] [Google Scholar]
- 39.Ambarus C, Yeremenko N, Tak PP, Baeten D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Current Opinion in Rheumatology. 2012;24(4):351–358. doi: 10.1097/BOR.0b013e3283534df4. [DOI] [PubMed] [Google Scholar]
- 40.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15(5):219. doi: 10.1186/ar4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciccarelli F, De Martinis M, Ginaldi L. An update on autoinflammatory diseases. Curr Med Chem. 2014;21(3):261–269. doi: 10.2174/09298673113206660303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klaasen R, Thurlings RM, Wijbrandts CA, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 2009;60(11):3217–3224. doi: 10.1002/art.24913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
24 hours after treatment of arthritic mice a reduction in arthritic score and much lower neutrophil densities were observed (C, D) than in paws of untreated mice (A, B). A: Untreated arthritic paw (score 3), bar = 786 μm, magnified section bar = 336 μm. B: Untreated arthritic paw (score 4), bar = 1136 μm, magnified section bar = 342 μm. C, D: Treated arthritic paws with score reduction from 3 to 2. C: bar = 1273 μm, magnified section bar = 308 μm. D: bar = 962 μm, magnified section bar = 304 μm. A-D: Staining for neutrophils (Ly6G+); ABC method, hemalaun counterstain.
Mice treated with the combination therapy of F8-IL4 and DXM until sacrifice were euthanized 6 h, 12 h, 24 h, 72 h, 96 h, 120 h after the first injection if F8-IL4. Whereas at 6 h after injection a large infiltrate of neutrophils can still be observed, at later time points the inflammatory process has almost completely resolved. Staining for neutrophils (Ly6G+); ABC method, hemalaun counterstain; 6 h bar = 1140 μm, 12 h bar = 1613 μm, 24 h bar = 1580 μm, 72 bar = 1344 μm h, 96 h bar = 1301 μm, 120 bar = 1475 μm.