ABSTRACT
Studies in temperate fishes provide evidence that cardiac mitochondrial function and the capacity to fuel cardiac work contribute to thermal tolerance. Here, we tested the hypothesis that decreased cardiac aerobic metabolic capacity contributes to the lower thermal tolerance of the haemoglobinless Antarctic icefish, Chaenocephalus aceratus, compared with that of the red-blooded Antarctic species, Notothenia coriiceps. Maximal activities of citrate synthase (CS) and lactate dehydrogenase (LDH), respiration rates of isolated mitochondria, adenylate levels and changes in mitochondrial protein expression were quantified from hearts of animals held at ambient temperature or exposed to their critical thermal maximum (CTmax). Compared with C. aceratus, activity of CS, ATP concentration and energy charge were higher in hearts of N. coriiceps at ambient temperature and CTmax. While state 3 mitochondrial respiration rates were not impaired by exposure to CTmax in either species, state 4 rates, indicative of proton leakage, increased following exposure to CTmax in C. aceratus but not N. coriiceps. The interactive effect of temperature and species resulted in an increase in antioxidants and aerobic metabolic enzymes in N. coriiceps but not in C. aceratus. Together, our results support the hypothesis that the lower aerobic metabolic capacity of C. aceratus hearts contributes to its low thermal tolerance.
KEY WORDS: Mitochondria, Aerobic metabolism, Cardiac muscle, Antarctic fish
Summary: Hearts of icefishes have a lower capacity to maintain ATP levels through aerobic metabolic pathways compared with those of red-blooded species, which may constrain cardiac performance and lower thermal tolerance.
INTRODUCTION
The Western Antarctic Peninsula region is warming at an unprecedented rate (Cheng et al., 2017; Clarke et al., 2007; King, 1994; Meredith and King, 2005; Turner et al., 2014). While the impacts of warming on phytoplankton, invertebrates and penguin populations have been documented (Atkinson et al., 2004; Ducklow et al., 2007; Trivelpiece et al., 2011), the consequences for the fish fauna are largely unknown. The extreme stenothermy of Antarctic fishes was first described 50 years ago for several species of notothenioids that displayed an upper incipient lethal temperature of only ∼6°C (Somero and DeVries, 1967). More recent studies have demonstrated that some, but not all, Antarctic fishes have a limited capacity to adjust certain aspects of their physiology (e.g. aerobic metabolic capacity and heart rate) in response to acclimation 6–7°C above ambient temperature (−1.8°C) (Egginton and Campbell, 2016; Franklin et al., 2007; Jayasundara et al., 2013; Sandersfeld et al., 2015; Seebacher et al., 2005). However, it is unknown whether any of these malleable traits constrain thermal tolerance. The capacity of the Antarctic fish fauna to persist as the climate warms will ultimately depend upon the phenotypic plasticity and adaptive capacity of key physiological traits limiting thermal tolerance (Chevin et al., 2010; Seebacher et al., 2015). Therefore, the identification of those traits is paramount to predicting the potential for notothenioids to survive in a warming world.
Haemoglobinless Antarctic channichthyid icefishes have a lower critical thermal maximum (CTmax) than red-blooded notothenioid fishes inhabiting the Western Antarctic Peninsula region (Beers and Sidell, 2011). While it is tempting to attribute this difference in thermal tolerance to the approximately 90% lower arterial oxygen-carrying capacity of icefishes compared with red-blooded fishes, supplemental oxygen does not increase CTmax, indicating that oxygen does not limit upper thermal tolerance as measured by CTmax (Devor et al., 2016), as has also been shown for temperate fishes (Ekström et al., 2016; Wang et al., 2014). Based on studies of temperate fishes and invertebrates, cardiac performance appears to influence thermal tolerance (Farrell, 2009; Stillman and Somero, 2000). Consistent with this, recent studies (Joyce et al. 2018; S. E. Egginton, M. Axelsson, E. L. Crockett, K. M. O’Brien and A. P. Farrell, unpublished) show that maximum cardiac performance is also limited at warm temperatures in notothenioids.
Although the molecular mechanism(s) underlying cardiac failure in fishes at elevated temperature has not been fully elucidated, mitochondrial function is a strong candidate (Hilton et al., 2010; Iftikar and Hickey, 2013; Iftikar et al., 2014; Leo et al., 2017). Studies of both temperate and tropical fishes have shown that mitochondrial function becomes impaired at temperatures less than that of heart failure (THF) (Iftikar and Hickey, 2013; Iftikar et al., 2014). In a temperate species, Notolabrus celidotus, the efficiency of ATP production declines at assay temperatures lower than THF as a result of a greater increase in the rate of proton leak compared with flux through the electron transport system (ETS) as temperature increases (Iftikar and Hickey, 2013). Exposure of fishes to acute heat stress also increases mitochondrial membrane leakiness in N. celidotus (Iftikar and Hickey, 2013), in its congener Notolabrus fucicola and in the tropical Thalassoma lunare (Iftikar et al., 2014). Similarly, cardiac mitochondria from Arctic polar cod (Boreogadus saida) display an apparent decrease in the efficiency of ATP synthesis and activity of cytochrome c oxidase, and an increase in mitochondrial membrane leakiness at temperatures lower than those for the more thermally tolerant Atlantic cod (Gadus morhua) (Leo et al., 2017). The capacity of mitochondria to synthesize ATP is dependent not only on the integrity of the inner mitochondrial membrane and activity of ETS complexes but also on the activity of the Krebs cycle, which provides reducing equivalents (NADH and FADH2) to the ETS. In hearts of the European perch (Perca fluviatilis), the activity of citrate synthase (CS) is more thermally sensitive than components of the ETS, declining in activity at temperatures lower than the CTmax, potentially limiting cardiac performance and hence thermal tolerance (Ekström et al., 2017).
In this study, we tested the hypothesis that mitochondrial function and the capacity to synthesize ATP through aerobic metabolic pathways in ventricular myocardium contribute to a lower thermal tolerance of the icefish Chaenocephalus aceratus compared with the red-blooded nototheniid Notothenia coriiceps. We selected these two species because their thermal tolerance has been well characterized and differs by 3.2°C, and some aspects of their cardiac metabolism have been described (Beers and Sidell, 2011; Devor et al., 2016; Mueller et al., 2012; O'Brien et al., 2014; Urschel and O'Brien, 2009). We measured cardiac mitochondrial function, adenylate and lactate levels, and maximal activities of CS and lactate dehydrogenase (LDH) in hearts of fishes either held at ambient temperature or following exposure to their CTmax. The CTmax protocol is commonly used to assess thermal tolerance in organisms because it can be measured in a large number of animals within a short period of time (Beitinger et al., 2000; Lutterschmidt and Hutchinson, 1997). We also quantified changes in mitochondrial protein expression in response to CTmax using mass spectrometry. The results show that N. coriiceps hearts have a higher aerobic metabolic capacity compared with those of C. aceratus at ambient temperature and CTmax, which was correlated with higher ATP levels and energy charge at both temperatures. While state 3 respiration rates in cardiac mitochondria were not impaired in either species following exposure to CTmax, proton leakage (state 4 rate) increased in C. aceratus but not N. coriiceps. Unlike C. aceratus hearts, N. coriiceps hearts increased expression of several mitochondrial proteins in response to CTmax, including aerobic metabolic enzymes and antioxidants. Together, these adjustments may contribute to the superior cardiac performance of N. coriiceps at elevated temperature and greater maximal sustainable temperature.
MATERIALS AND METHODS
Animal collection
Animals were collected in April–June 2015 off the southwestern shore of Low Island (63°30′S, 62°42′W) and in Dallmann Bay (64°08′S, 62°40′W). Specimens of C. aceratus (Lönnberg 1906) (1540±448 g, mean±s.d.) were collected using otter trawls deployed from the ARSV Laurence M. Gould, whereas N. coriiceps Richardson 1844 (1157±449 g) were collected using both otter trawls and baited fish traps. Animals were held in flow-through seawater tanks on board the Laurence M. Gould and transferred to aquaria at the US Antarctic research station, Palmer Station, where they were held in circulating seawater tanks maintained at 0±1.0°C. Notothenia coriiceps were fed once every 2–3 days a diet of chopped fish; icefish do not feed in captivity so these animals were used within ∼10–14 days of capture. All procedures were approved by the University of Alaska, Fairbanks Institutional Animal Care Committee (570217-9).
Measuring CTmax
CTmax was measured as the temperature at which cardiac function failed during warming or animals displayed loss of righting response as described previously (Devor et al., 2016). For measuring CTmax as loss of cardiac function, fish were anaesthetized in MS222 (1:7500) dissolved in seawater and placed dorsal-side up on a thoracic cradle. Gills were irrigated with MS222 (1:10,000) dissolved in seawater. Electrocardiogram (ECG) recording electrodes were inserted through the opercular septum at the base of the left fourth gill arch using a hypodermic needle, ensuring that the pericardial cavity was not compromised. Bipolar ECG signals were recorded using a bioamplifier (ML 136) interfaced with a digital recording system and LabChart v7 (PowerLab, ADInstruments, Colorado Springs, CO, USA) (Fig. S1). Fish were allowed to recover for 24–48 h (according to recovery of maximal heart rate variability determined from ECG) before CTmax was measured by increasing the temperature at a rate of 3.6°C h−1, as described previously (Devor et al., 2016). Animals were held in a cradle to minimize movement; aquarium lights were turned off for the duration of the experiments and animals were observed under red light. Routine (minimum) heart rates of fish in the cradle were similar to those obtained from unrestrained fish with implantable heart rate data loggers (Campbell et al., 2008, 2007). Temperature data loggers (HOBO, Onset Computer, Pocasset, MA, USA) were deployed in each tank to continuously record water temperature. Once cardiac function failed, animals were immediately removed from the cradle and killed by a sharp blow to the head followed by spinal transection. Heart ventricles were rapidly excised and blood cleared from the ventricular lumen by rinsing in ice-cold notothenoid Ringer's solution (260 mmol l−1 NaCl, 2.5 mmol l−1 MgCl2, 5.0 mmol l−1 KCl, 2.5 mmol l−1 NaHCO3, 5.0 mmol l−1 NaH2PO4, pH 8.0). Fresh tissue was used for isolating mitochondria. Enzyme activity was measured using tissues flash frozen in liquid nitrogen. Adenylate and lactate levels were measured in freeze-clamped heart ventricles. All frozen tissues were stored at −70 to −80°C.
Mitochondrial function
Heart ventricles were minced into 1–2 mm blocks on an ice-cold stage and then gently homogenized with 6 passes of a pestle in a Tenbroeck homogenizer in 8 volumes of isolation buffer (0.1 mol l−1 sucrose, 140 mmol l−1 KCl, 10 mmol l−1 EDTA, 5 mmol l−1 MgCl2, 20 mmol l−1 Hepes, 0.5% fatty acid-free BSA, pH 7.3 at 4°C, 435 mOsm) as described in Urschel and O'Brien (2009). The homogenate was then centrifuged at 1400 g for 5 min at 4°C. The supernatant was collected and centrifuged at 9000 g for 10 min at 4°C. The resulting pellet was gently resuspended in 1 ml of isolation buffer and then diluted to 10 ml with isolation buffer and centrifuged at 1400 g for 5 min at 4°C. The supernatant was then decanted and centrifuged at 11,000 g for 10 min at 4°C. The mitochondrial pellet was gently resuspended in assay buffer (173 mmol l−1 sucrose, 135 mmol l−1 KCl, 5 mmol l−1 KH2PO4, 20 mmol l−1 Hepes, 0.5% BSA, pH 7.3 at 4°C, 435 mOsm). Mitochondria were frozen in liquid nitrogen and stored at −70 to −80°C until used to measure ATP synthase and succinate dehydrogenase activities or were used immediately to measure mitochondrial respiration rate. In some cases, hearts from two N. coriiceps were pooled for mitochondrial isolation. Protein concentration was determined using the Bradford assay (Bradford, 1976).
Mitochondria used to quantify protein expression by mass spectrometry were isolated as described in Urschel and O'Brien (2009), except that the isolation buffer lacked BSA, and when resuspended the second time the isolation buffer was supplemented with one tablet of Complete Mini Protease Inhibitor (Roche Diagnostics, Indianapolis, IN, USA) per 10 ml buffer. The final mitochondrial pellet was resuspended in RIPA buffer (50 mmol l−1 Tris-HCl, 150 mmol l−1 NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol l−1 EDTA, 1 mmol l−1 PMSF, pH 7.4 at 4°C), frozen in liquid nitrogen and stored at −70 to −80°C.
Mitochondrial respiration rates were measured at 2 and 10°C in duplicate (when possible) using an SI130 microcathode oxygen electrode with a SI782 dual channel meter and RC300 respiration cell (all from Strathkelvin, North Lanarkshire, UK) cooled with a circulating water bath. Electrodes were calibrated daily at each temperature; pH of the assay buffer was adjusted to 7.4 for 2 and 10°C. Respiration rates were measured in 1 ml of assay buffer with 25–40 µl of mitochondrial protein for measurements at 2°C (average of 400 µg) and 15–30 µl for measurements at 10°C (average of 330 µg). All rates were monitored for 3 min. The Krebs cycle was initiated by addition of 1 mmol l−1 malate and 5 mmol l−1 pyruvate to measure state 2 respiration rates; 0.5 mmol l−1 of ADP was then added to measure state 3 respiration rates. Following depletion of ADP, state 4 respiration rates were monitored. A second aliquot of ADP was added to measure state 3 and 4 rates a second time. Then, 1 µg ml−1 of oligomycin was added to inhibit ATP synthase, followed by 10 µmol l−1 FCCP to measure maximal rate of respiration (ETS). Next, 12.5 µmol l−1 of rotentone, 15 mmol l−1 malonate and 10 µmol l−1 antimycin was added to inhibit complexes I, II and III, respectively; 0.5 mmol l−1 TMPD and 2 mmol l−1 ascorbate was then added to measure the activity of cytochrome c oxidase (COX) (Fig. S2). N=4–6 for measurements at 2°C, and N=3–6 for measurements at 10°C. Protein concentration was determined using the Bradford assay (Bradford, 1976). All biochemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) for these assays and measurements of enzyme activity (described below).
Enzyme assays
The maximal activity of all enzymes was measured at 5±0.5°C using a PerkinElmer Lambda 35 spectrophotometer (Waltham, MA, USA) equipped with a circulating water bath.
Maximal activity of ATP synthase (EC 3.6.3.14) was quantified in isolated mitochondria as described previously (Galli et al., 2013). Mitochondria were frozen and thawed at least once prior to measuring activity. The final reaction mixture contained 20 mmol l−1 Hepes, 5 mmol l−1 MgCl2, 100 mmol l−1 KCl, 1 mmol l−1 KCN, 2.5 mmol l−1 PEP, 0.2 mmol l−1 NADH, 10 U ml−1 PK and 15 U ml−1 LDH, adjusted to pH 7.8 at 5°C. ATP was added at a final concentration of 2.5 mmol l−1 and the mixture was incubated for 6 min to allow conversion of any residual ADP to ATP. Then, 100 mg of mitochondrial protein was added to initiate the reaction. Absorbance at 340 nm was monitored for 5 min. Samples containing 2.5 µmol l−1 oligomycin to determine background rate of oxidation of NADH were run in parallel. All measurements were made in duplicate with n=6–8.
Succinate dehydrogenase (SDH; EC 1.3.5.1) was measured in mitochondria isolated as described above and thawed at least once prior to measuring activity as described by Janssen et al. (2007) and Barrientos et al. (2009). The final reaction mixture contained 80 mmol l−1 K2HPO4, 1 g l−1 BSA, 2 mmol l−1 EDTA, 0.2 mmol l−1 ATP, 80 μl 2,6-dichlorophenolindophenol (DCPIP), 10 mmol l−1 succinate, 50 μmol l−1 decylubiquinone, 0.3 mmol l−1 KCN, 1 μmol l−1 antimycin A and 3 μmol l−1 rotenone, pH 7.8 at 5°C. The mixture was allowed to incubate for 10 min after addition of 600 μg of mitochondrial protein and succinate. Decylubiquinone was then added to initiate the reaction and absorbance at 600 nm was measured for 5 min. Background activity was measured by adding 10 mmol l−1 malonate and monitoring absorbance for an additional 5 min. All measurements were made in duplicate with n=5–6.
For assays of CS (EC 2.3.3.1) and LDH (EC 1.1.1.27), heart ventricles were homogenized (10% w/v) on ice using a Tenbroek ground glass homogenizer in an ice-cold buffer solution of 40 mmol l−1 Hepes, 1 mmol l−1 EDTA, 2 mmol l−1 MgCl2 and 2 mmol l−1 dithiothreitol (DTT) adjusted to a pH of 7.8 at 1°C. Maximal activity of CS was measured using a slight variation of a protocol originally described by Srere et al. (1963), following the reduction of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) at 412 nm. The final reaction mixture contained 75 mmol l−1 Tris, 0.25 mmol l−1 DTNB, 0.4 mmol l−1 acetyl CoA and 0.5 mmol l−1 oxaloacetate, adjusted to pH 8.0 at 5°C; 10 μl of tissue homogenate was added and background activity measured for 5 min. The reaction was then initiated by the addition of oxaloacetate and activity was monitored for 5 min. All measurements were made in duplicate with n=6–7.
Maximal activity of LDH was measured using a method described by Hansen and Sidell (1983). The final reaction mixture contained 50 mmol l−1 imidazole, 0.8 mmol l−1 KCN, 0.2 mmol l−1 NADH and 2 mmol l−1 pyruvate, adjusted to pH 7.7 at 1°C. The oxidation of NADH was monitored at 340 nm for 3 min in the absence of pyruvate. The reaction was then initiated by addition of pyruvate and the change in absorbance at 340 nm monitored for 3 min. All measurements were made in duplicate with n=6.
Quantification of adenylates
Adenylates were quantified by HPLC using methods previously described (Manfredi et al., 2002). AMP was purchased from Fluka (Mexico City, Mexico) and ADP and ATP were purchased from Sigma-Aldrich.
Approximately 25 mg of ventricle tissue was homogenized in 1 ml ice-cold 6% TCA using a Teledyne Tekmar Tissumizer (Tekmar, Cincinnati, OH, USA). Protein concentration was determined using the Bradford assay (Bradford, 1976). The homogenate was centrifuged for 10 min at 12,000 g at 4°C and then adjusted to a pH of 7.0–8.0 with 4 mol l−1 KOH. The supernatant was filtered by centrifuging for 15 min at 10,000 g at 4°C in Costar Spin-X® Centrifuge tube 0.22 µm cellulose acetate filter columns (Corning, Corning, NY, USA). Samples were stored on ice until they were injected into the HPLC system. Each sample was run in duplicate with n=7–10.
HPLC was performed using a 1525 HPLC Binary Pump equipped with an In-Line Degasser AF and a 2996 Photodiode Array Detector (Waters Corporation, Milford, MA, USA). Separation was achieved using a reverse-phase XSelect HSS T3 3.5 µm 4.6×150 mm column with an XSelect HSS T3 3.5 µm VanGuard cartridge and holder. Two buffers were used with a constant flow rate of 1 ml min−1. Buffer A contained 0.3 mmol l−1 tetrabutylammonium hydrogen sulfate (TBA-SO4) and 20 mmol l−1 Na2HPO4 adjusted to pH 5.0 with 2 mol l−1 H3PO4. Buffer B contained 0.3 mmol l−1 TBA-SO4, 200 mmol l−1 Na2HPO4 and 10% (v/v) acetonitrile, adjusted to pH 5.0 with 2 mol l−1 H3PO4. Each run lasted 30 min with 100% buffer A from 0 to 5 min, 100% buffer A to 100% buffer B from 5 to 20 min, 100% buffer B to 100% buffer A from 20 to 22.5 min, and 100% buffer A from 22.5 to 30 min; 50 µl of the sample or standard was injected into the HPLC system and absorbance was monitored at 254 nm. Analytes were identified by comparing retention times with standards.
Concentrations of adenylates were determined using standard curves of each adenylate between 0 and 100 µmol l−1 run in duplicate, with peak area and height determined at 254 nm. A line of best fit was established for each analyte with R2≥0.96, and used to calculate the amount of analyte in each sample. Energy charge was calculated as follows (Atkinson and Walton, 1967):
 |
Quantification of lactate
Heart ventricles were homogenized on ice in 0.5 mol l−1 Tris (pH 8.3) using a Tekmar Tissuemizer (Tekmar). Homogenates were centrifuged for 30 min at 16,100 g at 4°C. Supernatants were collected and a 300–500 μl aliquot was centrifuged in an Amicon Ultra filtration unit (0.5 ml, 10K ultracel) at 14,000 g for 45 min at 4°C. Filtrates were stored at −80°C until use.
Lactate levels were measured as described previously (Devor et al., 2016). The assay buffer contained 5 mmol l−1 NAD+ and 1:50 dilution stock of WST-1 cell proliferation reagent (Roche Diagnostics). Filtrates were diluted 1:2 in 0.5 mol l−1 Tris (pH 8.3) and 100 µl of filtrate was added to individual wells of a microplate; 30 µl of assay buffer was then added to each well. Absorption at 440 nm was measured in kinetic mode for 3 min using a SpectraMax Plus 384 plate reader (Molecular Devices, Sunnyvale, CA, USA) and the software SoftMax Pro 6.3 to obtain an initial time point (time=0). Then, 20 µl of lactate dehydrogenase solution (0.083 U μl−1) was added to each well. The absorbance of WST-1 was measured at 440 nm after 60 min, by which time absorbance had reached a maximum value. To determine lactate levels, initial absorbance values were subtracted from those obtained at 60 min. The final concentration of lactate was determined from a standard curve of sodium-l-lactate (Sigma-Aldrich) with nine concentrations between 0 and 1000 µmol l−1, measured on each plate in triplicate. Samples were measured in triplicate with n=7–9.
Proteomics
Samples were digested and desalted according to Lundby and Olsen (2011) (n=4–5). Protein concentration was determined using a bicinchoninic acid (BCA) assay (Thermo-Fisher Scientific, San Jose, CA, USA). Proteins were reduced and alkylated and subjected to methanol–chloroform precipitation prior to digestion with endoproteinase Lys-C (Wako, Richmond, VA, USA) at an enzyme to protein ratio of 1:50. The samples were then further digested with trypsin at an enzyme to protein ratio of 1:100 (Promega, Madison, WI, USA) and desalted and concentrated using C18 Sep-Pak Cartridges (Waters Corporation).
Peptides were mass tagged using a TMT isobaric label kit (cat. no. 90061, Thermo-Fisher Scientific), following the manufacturer's protocol and then combined. Methods for quantifying differences in protein expression using multiplexed quantification with isobaric tags are based on McAlister et al. (2014).
Pooled TMT-labelled peptides were subjected to basic pH reversed-phase fractionation on an Ultimate 3000 HPLC (Thermo-Fisher Scientific) using an integrated fraction collector. Elution was performed with a 10 min gradient of 0–20% solvent B followed by a 50 min gradient of solvent B from 20% to 45% (solvent A: 5.0% acetonitrile, 10 mmol l−1 ammonium bicarbonate, pH 8.0; solvent B: 90.0% acetonitrile, 10 mmol l−1 ammonium bicarbonate, pH 8.0) on a Zorbax 300 Extend-C18 column (Agilent) at a flow rate of 0.4 ml min−1. A total of 24 fractions were collected at 37 s intervals in a looping fashion for 60 min and then combined. Peptide elution was monitored at a wavelength of 220 nm using a Dionex Ultimate 3000 variable wavelength detector (Thermo-Fisher Scientific). Each fraction was centrifuged to near dryness and desalted using C18 Sep-Pak Cartridges followed by centrifugation to near dryness and reconstituted with 20 µl of 5% acetonitrile and 0.1% formic acid.
Peptide fractions were separated using an UltiMate 3000 RSLCnano system (Thermo-Fisher Scientific) on a self-packed UChrom C18 column (100 μm×35 cm). Elution was performed using a 90 min gradient of solvent B from 2% to 27% (solvent A: 0.1% formic acid; solvent B: acetonitrile, 0.1% formic acid) at 50°C using a digital Pico View nanospray source (New Objectives, Woburn, MA, USA) that was modified with a custom-built column heater and an ABIRD background suppressor (ESI Source Solutions, Woburn, MA, USA). Briefly, the self-packed column tapered tip was pulled with a laser micropipette puller P-2000 (Sutter Instrument Co., Novato, CA, USA) to an approximate i.d. of 10 µm. The column was then packed with 1–2 cm of 5 µm Magic C18 (Michrom Bioresources, Auburn, CA, USA), followed by 35 cm of 1.8 μm UChrom C18 (120A) at 9000 psi using a nano LC column packing kit (nanoLCMS, Gold River, CA, USA).
Mass spectral analysis was performed using an Orbitrap Fusion mass spectrometer (Thermo-Fisher Scientific). TMT analysis was performed using an MS3 multi-notch approach (McAlister et al., 2014). The MS1 precursor selection range was from 400 to 1400 m/z at a resolution of 120k and an automatic gain control (AGC) target of 2.0×105 with a maximum injection time of 100 ms. Quadrupole isolation at 0.7 Th for MS2 analysis using CID fragmentation in the linear ion trap with a collision energy of 35%. The AGC was set to 4.0×103 with a maximum injection time of 150 ms. The instrument was set in a top speed data-dependent mode with a most intense precursor priority. Dynamic exclusion was set to an exclusion duration of 60 s with a 10 ppm tolerance. MS2 fragment ions were then captured in the MS3 precursor population. These MS3 precursors were isolated within a 2.5 Da window and subjected to high energy collision-induced dissociation with a collision energy of 55%. The ions were then detected in the Orbitrap at a resolution of 60,000 with an AGC of 5.0×104 and a maximum injection time of 150 ms.
Peptides were analysed using Sequest (Thermo-Fisher Scientific) to validate protein identification and provide quantification using the ratio of TMT reporter ions within the isobarically labelled peptides. Peptides were identified by searching perciform sequences available from Uniprot (www.uniprot.org).
Statistical analyses
Significant differences in enzyme activity, and adenylate and lactate levels between species and in response to temperature treatment were determined using a two-way ANOVA followed by a post hoc Tukey's test. Differences in mitochondrial function between species, assay temperature and temperature treatments were determined using a three-way ANOVA followed by a post hoc Holm–Sidak test. Significance for all tests was set at P<0.05. Data were log transformed as needed to meet assumptions of normality, determined using a Shapiro–Wilks test, and equal variance. Data were analysed with Sigma Plot 11.0 (Systat Software, San Jose, CA, USA) and JMP 7 (SAS Institute, Cary, NC, USA). Values are presented as means±s.e.m.
Protein abundance levels were first normalized using the maximum values of pooled samples for across-species comparison. Abundance levels were normalized by dividing by the sample-specific normalization factor, following the TMT Thermo-Fisher Scientific protocol (Thermo Proteome Discoverer User Guide, software version 2.1). The 630 proteins identified in all species were extracted for further study, and a 2-way ANOVA performed following log2-transformation using the Protein∼Species×Temperature linear model to include interaction effects. A false discovery rate correction for multiple comparisons was applied to reduce the likelihood of false positives (Benjamini and Hochberg, 1995). A Tukey's post hoc test was performed on any protein with a statistically significant species effect (adjusted P<0.05) to identify changes. Similarly, a Tukey's post hoc test was performed on any protein with a statistically significant Species*Temperature effect (adjusted P<0.05).
RESULTS
CTmax is higher in red-blooded N. coriiceps
The CTmax, measured as the temperature at which hearts failed, was higher in N. coriiceps (16.31±0.46°C; n=6) than in C. aceratus (12.55±0.70°C; n=8) (P<0.05). Because of the smaller heart size of N. coriiceps compared with that of C. aceratus, additional samples were needed to complete all biochemical measurements and so CTmax was measured in additional N. coriiceps as the loss of the righting response (Devor et al., 2016), which was not significantly different from the CTmax measured as the temperature at which hearts failed (15.92±0.22°C; n=14).
Mitochondrial function is affected by exposure to CTmax
State 2–4 respiration rates, ETS rate and COX activity were all higher in cardiac mitochondria when measured at 10°C versus 2°C for both N. coriiceps and C. aceratus (Fig. 1). The Q10 ranged between 2.4 for COX activity and 4.0 for state 3 rate of respiration (average of both species). State 2 respiration rate was not significantly different between the two species (F=3.338, P=0.078) and was unaffected by exposure to CTmax (F=0.447, P=0.495) (Fig. 1A). State 3 respiration rate was also equivalent between species (F=0.245, P=0.624). Exposure to CTmax increased state 3 respiration rate (F=0.017, P<0.001; Fig. 1B), which was largely driven by the increase in state 3 rate in C. aceratus by 1.1- and 1.3-fold when measured at 2 and 10°C, respectively (P=0.013) (Fig. 1B). When measured at 2°C, state 4 respiration rate, which approximates the rate of proton leakage, was lower in C. aceratus than in N. coriiceps at ambient temperature (P=0.004), and increased in C. aceratus following exposure to CTmax (P=0.018) but not in N. coriiceps (Fig. 1C).
Fig. 1.
Mitochondrial function in Nototheniacoriiceps and Chaenocephalusaceratus held at ambient temperature and exposed to CTmax. State 2 (A), state 3 (B), state 4 (C) and uncoupled respiration rate (electron transport system, ETS; D), and the activity of cytochrome c oxidase (COX; E) were measured at assay temperatures of 2°C (n=4–6) and 10°C (n=3–6). The ratio of COX activity to state 3 activity was also calculated (F). Values are means±s.e.m. *Significant difference between species; ‡significant difference in response to exposure to CTmax within a species (P<0.05).
Uncoupled rates of mitochondrial respiration (ETS) were 1.25-fold higher at 2°C and 1.18-fold higher at 10°C in mitochondria of N. coriiceps versus C. aceratus (F=7.068, P=0.013) and were higher in animals at CTmax than at ambient temperature (F=8.527, P=0.007; Fig. 1D) as the result of an increase in C. aceratus (P=0.018).
COX activity was on average 1.8-fold higher in mitochondria from N. coriiceps versus C. aceratus (F=51.453, P<0.001), but was not affected by exposure to CTmax (F=0.593, P=0.447; Fig. 1E). The ratio of COX to state 3 respiration was also higher in N. coriiceps than in C. aceratus (F=65.542, P<0.001) but did not change with exposure to CTmax in either species (F=1.798, P=0.190; Fig. 1F).
The respiratory control ratio (RCR), quantified as the ratio between state 3 and state 4 rates of respiration, ranged between 16.1±3.4 (N. coriiceps at 2°C) and 39.1±7.5 (C. aceratus at 2°C). The RCR did not differ between the two species (F=1.850, P=0.185), nor was there an effect of assay temperature (F=0.124, P=0.727) or exposure to CTmax (F=0.105, P=0.748). There was, however, an effect of the interaction between species and temperature so that the RCR was higher in C. aceratus than N. coriiceps at ambient temperature when measured at 2°C (P=0.047, F=4.319).
Maximal activity of ATP synthase did not differ among species, nor did it change in response to exposure to CTmax (F=2.634, P=0.118 for species; F=0.866, P=0.362 for temperature; and F=0.0711, P=0.792 for interaction between species and temperature; Fig. 2A). Activities ranged between 0.090±0.010 µmol min−1 mg−1 in C. aceratus at ambient temperature and 0.116±0.012 µmol min−1 mg−1 in N. coriiceps at CTmax.
Fig. 2.
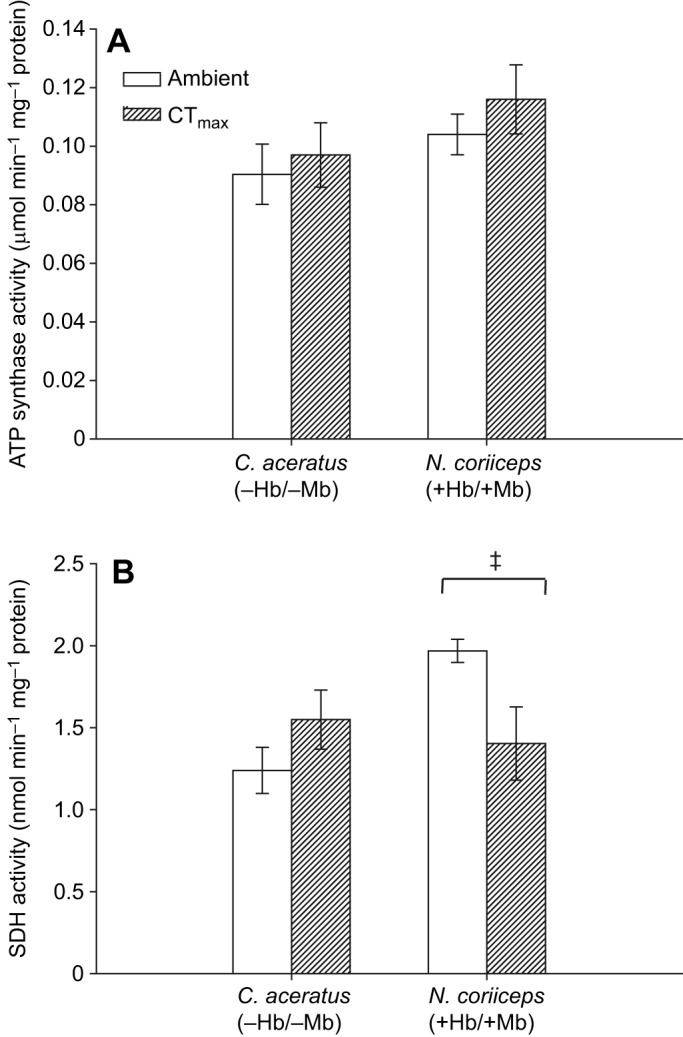
Maximal activity of ATP synthase and succinate dehydrogenase in mitochondria isolated from N. coriiceps and C. aceratus hearts held at ambient temperature and exposed to CTmax. (A) ATP synthase; (B) succinate dehydrogenase (SDH). Maximal enzyme activity was measured at 5±0.5°C (n=6–8 for ATP synthase; n=5–6 for SDH). Values are means±s.e.m. Hb, haemoglobin; Mb, myoglobin. ‡Significant difference in response to exposure to CTmax within a species (P<0.05).
Maximal SDH activity did not significantly differ between species (F=3.312, P=0.085) or with exposure to CTmax (F=0.639, P=0.435; Fig. 2B). There was, however, a significant interaction between species and temperature (F=7.489, P=0.014). Activity was significantly higher in N. coriiceps (1.97±0.07 nmol min−1 mg−1) than in C. aceratus (1.24±0.14 nmol min−1 mg−1) at ambient temperature and significantly declined in N. coriiceps in response to CTmax (1.40±0.22 nmol min−1 mg−1) (Fig. 2B; P<0.05).
Aerobic metabolic potential is higher in N. coriiceps hearts and anaerobic metabolic capacity is higher in C. aceratus hearts
Maximal activity of CS was, on average, 1.5-fold higher in N. coriiceps hearts than in C. aceratus hearts (F=2.394, P<0.001; Fig. 3A), and increased in response to exposure to CTmax (F=4.360, P=0.049; Fig. 3A). In contrast, maximal activity of LDH was 1.6-fold higher in C. aceratus hearts than in N. coriiceps hearts (F=39.400, P<0.001; Fig. 3B), and there was no effect of exposure to CTmax (F=1.980, P=0.175; Fig. 3B). Although the mean body mass of C. aceratus was larger than that of N. coriiceps used for these assays, body mass was not significantly correlated with either CS or LDH activity in either species (P<0.05), as has been found for the activity of these enzymes in glycolytic muscles of teleosts (Somero and Childress, 1980).
Fig. 3.
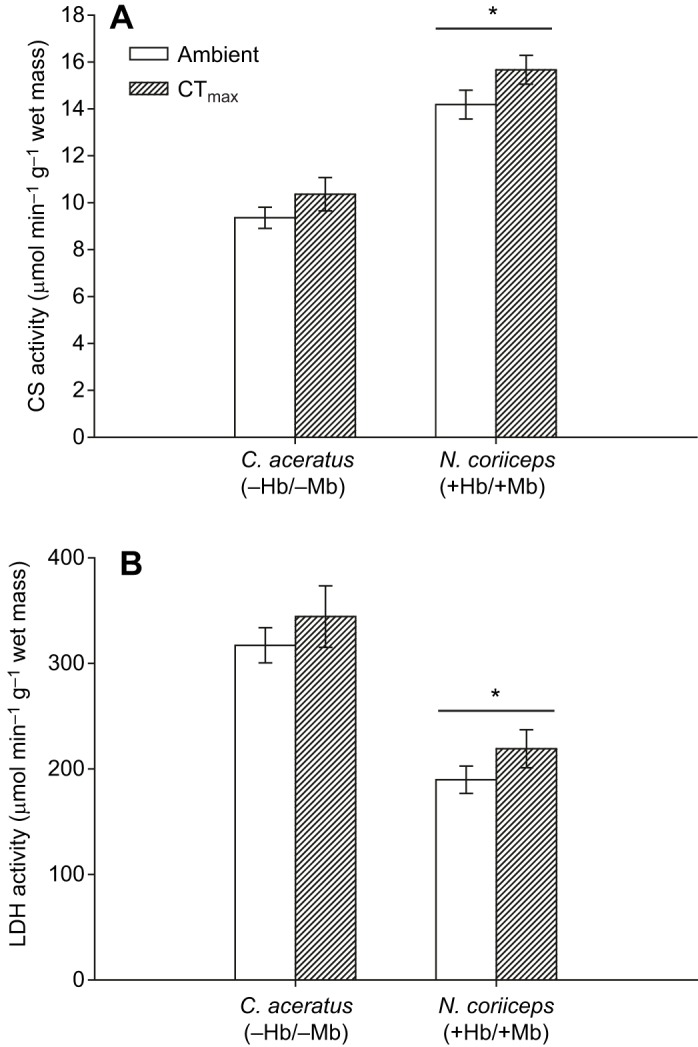
Maximal activity of metabolic enzymes in heart ventricles of notothenioids. Maximal activity of citrate synthase (CS; A) and lactate dehydrogenase (LDH; B) was measured in heart ventricles at 5±0.5°C (n=6–7 for CS; n=6 for LDH). Values are means±s.e.m. *Significant difference between species (P<0.05).
Cardiac ATP levels decline in response to CTmax in both species but are always higher in N. coriiceps hearts than in C. aceratus hearts
ATP levels and energy charge were significantly higher in N. coriiceps hearts than in C. aceratus hearts (F=24.058 for ATP, F=48.334 for energy charge, P<0.001 for both; Table 1). At ambient temperature, ATP concentration was 1.7-fold higher, and ADP and AMP levels were 1.2- and 17-fold lower, respectively, in N. coriiceps hearts compared with C. aceratus hearts (P<0.001 for ATP and AMP, P=0.027 for ADP; Table 1). As a result, energy charge was 1.2-fold higher in N. coriiceps hearts than in C. aceratus hearts.
Table 1.
Adenylate and lactate levels in heart ventricles of notothenioids at ambient temperature (0±1°C) and critical thermal maximum (CTmax)
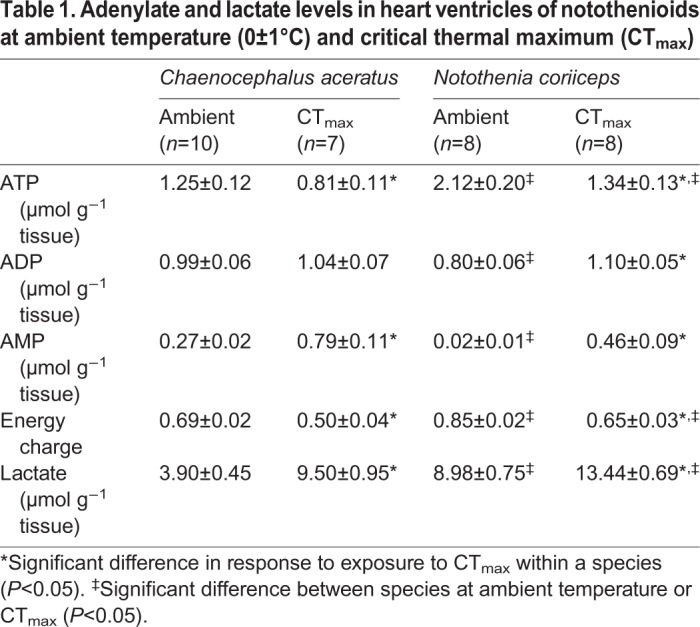
In response to exposure to CTmax, ATP levels decreased (F=18.249, P<0.001) and AMP levels increased (F=77.071, P<0.001) in hearts of both N. coriiceps and C. aceratus, resulting in a decrease in energy charge of both species (F=70.614; P<0.001; Table 1). Despite this decline, hearts of N. coriiceps maintained a 1.7-fold higher level of ATP at CTmax compared with those of C. aceratus (P=0.018; Table 1). Energy charge decreased to a similar extent in C. aceratus and N. coriiceps hearts in response to CTmax (1.4- and 1.3-fold, respectively) but remained higher in N. coriiceps hearts (Table 1).
Lactate levels were significantly higher in hearts of N. coriiceps compared with those of C. aceratus (F=41.097, P<0.001) and increased in both species in response to CTmax (F=51.281, P<0.001, Table 1), but to a lesser extent in hearts of N. coriiceps (1.5-fold), compared with those of C. aceratus (2.4-fold).
The interaction between temperature and species affects mitochondrial protein expression in N. coriiceps hearts
While there were many differences in protein expression between the two species, there was no significant effect of temperature alone on mitochondrial protein expression. However, there were many peptides that differed in expression as a result of the temperature × species interaction; 96 of these peptides differed in response to exposure to CTmax in cardiac mitochondria of N. coriiceps, and one in C. aceratus (P<0.05). Many of these peptides were not identifiable, including the one in C. aceratus. Of the 96 peptides in N. coriiceps, 63 were not identifiable and many were peptides from the same protein, resulting in 24 unique proteins identified in N. coriiceps (P<0.05; Table 2). Twenty proteins involved in aerobic metabolism, antioxidant defence and protein homeostasis increased in response to exposure to CTmax, whereas levels of three cytoskeletal proteins declined (P<0.05). The fold increases in protein expression ranged between 1.38 (α-subunit of ATP synthase) and 2.32 (subunit of Tim13).
Table 2.
Mitochondrial proteins that change significantly as a result of the interaction between temperature and species in N. coriiceps

DISCUSSION
Results from this study suggest that the lower thermal tolerance of the icefish C. aceratus versus N. coriiceps may be due to the lower aerobic metabolic potential of its myocardium, limiting the capacity to fuel cardiac work.
Mitochondrial function may impair ATP supply at elevated temperature in hearts of icefishes
Cardiac mitochondria of notothenioids are exceptionally well coupled, as reflected by their high RCR values, which are ∼2- to 5-fold higher than those of temperate fishes (Iftikar et al., 2014; Rodnick et al., 2014). Prior in vitro studies have shown that cardiac mitochondrial proton leak is 10-fold higher in rainbow trout at 15°C (Leary et al., 2003) compared with that in N. coriiceps measured at 2°C, and mitochondria from icefishes are even more tightly coupled than those of red-blooded notothenioids (Mueller et al., 2011). Additionally, proton leak is temperature sensitive and increases between assay temperatures of 2 and 10°C in cardiac mitochondria of both C. aceratus and N. coriiceps with a Q10≥20 (Mueller et al., 2011). Consistent with this, we found an increase in state 4 respiration rate, though not state 2, in response to an increase in assay temperature in both species.
Exposure to CTmax also increased the rate of proton leak (as measured by state 4 respiration rate) in cardiac mitochondria of C. aceratus but not in N. coriiceps. The increase in proton leak in the icefish is partially offset by an increase in state 3 respiration rate. However, because the thermal sensitivity is greater for state 4 than state 3 respiration in C. aceratus, the capacity to fuel cardiac work may be more impaired in C. aceratus than in N. coriiceps at elevated temperatures. When rates of respiration were compared between ambient animals measured at 2°C and those exposed to CTmax and measured at 10°C to approximate in vivo temperature conditions, state 3 rate increased 2.8-fold between 2 and 10°C in C. aceratus, whereas state 4 rate increased 4.6-fold. In contrast, in N. coriiceps, state 3 rate increase 2.3-fold and state 4 rate only 1.5-fold. The loss of efficiency of mitochondrial ATP production in hearts of C. aceratus may be compensated in part, by its greater anaerobic metabolic capacity. LDH activity was significantly higher in C. aceratus hearts than in N. coriiceps hearts and there was a greater increase in lactate levels in C. aceratus (2.4-fold) than in N. coriiceps (1.5-fold) following exposure to CTmax.
It is important to note that state 2 rate, indicative of proton leak in the absence of ATP, is not significantly different between C. aceratus and N. coriiceps. The higher rate of proton leak in the presence of ATP (state 4) may be attributable to the lack of mitochondrial creatine kinase (CK) in C. aceratus hearts (O'Brien et al., 2014). In contrast, both the sarcomeric and ubiquitous mitochondrial CK isoforms are abundant in N. coriiceps hearts (O'Brien et al., 2014). Mitochondrial CK is important for maintaining low concentrations of ATP in mitochondria, maintaining flux through the respiratory chain, decreasing membrane potential and minimizing the formation of reactive oxygen species (Wallimann et al., 1992).
Both state 3 and ETS (uncoupled) rates of mitochondrial respiration increase following exposure to CTmax and may be attributable to post-translational modifications such as acetylation and/or phosphorylation of ETC proteins (Papa et al., 2008; Rardin et al., 2013). This is in contrast to studies in temperate fishes (e.g. Iftikar and Hickey, 2013; Iftikar et al., 2014; Iftikar et al., 2015), where acute temperature stress causes a decline in mitochondrial function. For example, New Zealand wrasses exposed to THF show a decrease in ETS activity, COX and/or complex I of cardiac mitochondria (Iftikar and Hickey, 2013; Iftikar et al., 2014). Interestingly, the greater resilience of notothenioid mitochondria to temperature stress compared with these temperate fishes is also reflected in their greater thermal scope, calculated as the difference between CTmax (or THF) and habitat (or acclimation) temperature, of ∼13°C for icefishes and ∼17°C for red-blooded notothenioids but only 5.8–9.5°C for the wrasses (Iftikar et al., 2014). An increase in assay temperature also did not elicit a decline in mitochondrial function. Prior studies have shown the Arrhenius break temperature (ABT) of state 3 respiration rate for notothenioids ranges between 28.7°C (C. aceratus) and 31.4°C (Gobionotothen gibberifrons) (Urschel and O'Brien, 2009), which, although markedly higher than their habitat temperature, falls within the range of predicted ABTs of several marine organisms from differing thermal habitats (Weinstein and Somero, 1998).
Despite similar rates of state 3 respiration between N. coriiceps and C. aceratus, the activity of COX was 1.8-to 1.9-fold higher per mg mitochondrial protein in cardiac mitochondria of N. coriiceps, resulting in a higher ratio of COX activity to state 3 respiration rate (1.4 in C. aceratus versus 2.5 in N. coriiceps at ambient temperature). Excess COX activity relative to state 3 respiration rate is characteristic of highly aerobic tissues such as the mammalian heart (Brand et al., 2003; Porter et al., 1996), and oxidative muscle of the Arctic charr, Salvelinus alpinus (Benard et al., 2006). Excess COX activity corresponds with a low flux control coefficient (Blier and Lemieux, 2001), which allows COX activity to be inhibited to a large extent without affecting mitochondrial respiration rates (Gnaiger et al., 1998). This may afford protection against the deleterious effects of mitochondrial DNA mutations that accumulate during ageing and could decrease COX activity (Letellier et al., 1993). Excess COX activity in cardiac muscle of the intertidal New Zealand triplefin fish Bellapiscis medius compared with that of the subtidal triplefin species Forsterygion varium and Forsterygion malcomi, is associated with their greater hypoxia tolerance (Hilton et al., 2010). Excess COX activity also permits an increase in flux through the respiratory chain when demand warrants, such as during exposure to elevated temperature (Payne and Chinnery, 2015), which may contribute to the greater cardiac performance of N. coriiceps versus C. aceratus.
Cardiac aerobic metabolic capacity is higher in N. coriiceps than in C. aceratus
While there were no differences in state 3 or ETS rates between N. coriiceps and C. aceratus, maximal activity of CS (per g tissue) was nearly 2-fold higher in N. coriiceps hearts than in C. aceratus hearts, suggesting that the capacity to provide reducing equivalents (NADH and FADH2) to mitochondria may be limiting cardiac performance at elevated temperature in C. aceratus. Similarly, a recent study of cardiac metabolism in the European perch, Perca fluviatilis, revealed that thermal sensitivity of CS was greater than that of the mitochondrial respiratory chain complexes, with maximal activity of CS declining at 32.5°C, close to the CTmax (29.8–33.0°C), and that of complexes I and I and III combined declining above CTmax, at 36°C (Ekström et al., 2017).
The higher aerobic metabolic capacity (as measured by CS activity) of N. coriiceps hearts is consistent with their higher levels of ATP and energy charge. While cardiac ATP levels declined in hearts of both N. coriiceps and C. aceratus in response to exposure to CTmax, levels were always higher in N. coriiceps hearts, despite the longer duration of warming (4 versus 3 h) and higher CTmax (16 versus 13°C). The greater activity of LDH in C. aceratus hearts suggests that the icefish may rely on anaerobic metabolic pathways to fuel the work of the heart more so than N. coriiceps, yet most teleosts are unable to sustain cardiac work by anaerobic metabolic pathways, with an estimated 2% of ATP regenerated through anaerobic metabolic pathways in teleost hearts under normoxic conditions (Driedzic, 1983; Sidell et al., 1987). The higher lactate levels in N. coriiceps hearts probably reflect their greater cardiac work at ambient temperature and with warming versus C. aceratus hearts. Alternatively, the higher LDH activity and lower lactate levels in hearts of C. aceratus may reflect their usage of lactate as an aerobic fuel. The activity of CS increased in response to acute warming, supporting the supposition that the activity of the Krebs cycle is crucial to maintaining cardiac performance and ATP levels at elevated temperature. Similarly, CS activity increased in hearts of rainbow trout shifted from 10°C to 16°C within 1 day of acclimation (Pichaud et al., 2017).
The greater activity of CS (and higher energy charge) in N. coriiceps hearts compared with C. aceratus hearts is supported by high levels of the intracellular oxygen-binding protein myoglobin (Mb), which is absent in C. aceratus hearts (Moylan and Sidell, 2000; O'Brien et al., 2018; Sidell et al., 1997). In vitro studies have shown that hearts of icefishes that express Mb are capable of maintaining cardiac output at higher afterload pressures compared with those lacking Mb (Acierno et al., 1997), and we have found that Chionodraco rastrospinosus, whose heart expresses Mb, achieves higher heart rates in situ compared with C. aceratus (S. E. Egginton, M. Axelsson, E. L. Crockett, K. M. O'Brien and A. P. Farrell, unpublished). While high mitochondrial densities in hearts of icefishes that lack Mb may enhance oxygen diffusion rates between the ventricular lumen and mitochondria (O'Brien and Sidell, 2000), the lower aerobic metabolic capacity of –Mb icefish hearts suggest compensation may be incomplete.
Changes in mitochondrial protein expression may sustain cardiac performance
The interaction between temperature and species significantly affected mitochondrial protein expression, primarily in hearts of N. coriiceps where the expression of 20 proteins increased significantly in response to exposure to CTmax. This may reflect species-specific differences in the thermal sensitivity of protein expression or differences in the duration of warming. The 3–4°C higher CTmax of N. coriiceps resulted in a warming duration of ∼4 h compared with only 3 h for C. aceratus. The majority of proteins that increased in expression were those involved in energy homeostasis, protection against oxidative stress and protein homeostasis. Only one other study to date has quantified changes in protein expression in response to warming in fish hearts (Jayasundara et al., 2015), with warm acclimation of the goby, Gillichthys mirabilis, resulting in changes in protein levels within the same categories as N. coriiceps. Several enzymes of the Krebs cycle increased in cardiac mitochondria of N. coriiceps in response to warming, including CS, malate dehydrogenase and isocitrate dehydrogenase. Consistent with this, maximal activity of CS also increased in response to exposure to CTmax. The α and β subunits of ATP synthase, comprising the F1 region of the enzyme (the catalytic head), also increased in response to warming in N. coriiceps, and perhaps with extended warming, levels of these ATP synthase subunits would increase sufficiently to increase activity of the enzyme.
Exposure to elevated temperature increases oxidative stress in fishes (e.g. Almroth et al., 2015; Banh et al., 2016; Cheng et al., 2015; Jeffries et al., 2014). Prior work in our lab has shown that levels of oxidized proteins and lipids increase in C. aceratus hearts but not N. coriiceps hearts in response to CTmax exposure (Mueller et al., 2012). The current data suggest that an increase in levels of the antioxidants superoxide dismutase (SOD) and peroxiredoxin 3 in response to CTmax may decrease oxidative damage in N. coriiceps hearts during exposure to CTmax. Although total SOD activity does not increase in N. coriiceps hearts in response to CTmax (Mueller et al., 2012), an increase in levels of the Mn mitochondrial isoform, localized to the mitochondrial matrix and measured here, may be more effective in preventing ROS-mediated damage because mitochondria are a major source of ROS production (Boveris, 1977). Elevated levels of α-aminoadipic semialdehyde dehydrogenase, which metabolizes derivatives of lipid peroxidation (Brocker et al., 2010), may also help ameliorate oxidative damage.
The increase in enzymes and proteins related to protein synthesis (elongation factor Tu, aspartate aminotransferase), mitochondrial import (Tim13 subunit) and degradation (Lon protease) suggest there is an increase in the rate of protein turnover in mitochondria in response to exposure to CTmax. Acute warming increases ATP demand, which may stimulate mitochondrial biogenesis (O'Brien, 2011). Hence, increased levels of CDGSH iron sulfur domain-containing protein (mitoNEET), which imports iron into mitochondria, may contribute to the biogenesis of ETC protein complexes (Tamir et al., 2015).
Taken together, changes in cardiac mitochondrial protein expression indicate that N. coriiceps hearts have the capacity to enhance aerobic metabolic capacity and defences against oxidative stress during warming, whereas C. aceratus hearts do not, probably contributing to differences in cardiac performance during warming.
Conclusions and perspectives
The lower aerobic metabolic capacity, enhanced mitochondrial proton leakage and lack of plasticity in mitochondrial protein expression at elevated temperature may hinder cardiac performance of the icefish C. aceratus and contribute to their lower thermal tolerance compared with that of N. coriiceps. Consistent with this, the maximum power output of N. coriiceps hearts is significantly higher than that of C. aceratus hearts at both 1 and 4°C (S. E. Egginton, M. Axelsson, E. L. Crockett, K. M. O'Brien and A. P. Farrell, unpublished). Future experiments will be aimed at determining the thermal plasticity of cardiac performance, which will be crucial to the survival of the fish fauna as the Southern Ocean warms.
Supplementary Material
Acknowledgements
We are grateful for the excellent support from the Master's and crew of the ARSV Laurence M. Gould, and the staff at the US Antarctic Research Station, Palmer Station. We thank Autumn Fish for assistance with measuring lactate.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.M.O., E.L.C.; Methodology: K.M.O., S.E., E.L.C., K.S., R.W.; Validation: K.M.O., K.S., R.W.; Formal analysis: K.M.O., A.S.R., S.E., K.S., R.W., M.H., S.M.; Investigation: K.M.O., A.S.R., S.E., K.S., R.W., S.M.; Resources: K.M.O., S.E., K.S., R.W.; Data curation: K.M.O., A.S.R., S.E., K.S., R.W., M.H., S.M.; Writing - original draft: K.M.O., A.S.R., K.S., S.M.; Writing - review & editing: K.M.O., A.S.R., S.E., A.P.F., E.L.C., K.S., R.W.; Visualization: K.M.O., S.M.; Supervision: K.M.O., E.L.C.; Project administration: K.M.O., E.L.C.; Funding acquisition: K.M.O., E.L.C.
Funding
Funding for this research was provided by grants from the National Science Foundation (ANT 1341663 to K.M.O. and ANT 1341602 to E.L.C.). A.S.R. was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant P20GM103395. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH. The proteomics studies were supported in part by the Nevada INBRE grant from the National Institute of General Medical Sciences (GM103440) to the Mick Hitchcock Nevada Proteomics Center. A.P.F. holds a Canada Research Chair and is funded by the Natural Sciences and Engineering Research Council of Canada. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.177816.supplemental
References
- Acierno R., Agnisola C., Tota B. and Sidell B. D. (1997). Myoglobin enhances cardiac performance in antarctic icefish species that express the protein. Am. J. Physiol. 273, R100-R106. 10.1152/ajpregu.1997.273.1.R100 [DOI] [PubMed] [Google Scholar]
- Almroth B. C., Asker N., Wassmur B., Rosengren M., Jutfelt F., Gräns A., Sundell K., Axelsson M. and Sturve J. (2015). Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J. Exp. Mar. Biol. Ecol. 468, 130-137. 10.1016/j.jembe.2015.02.018 [DOI] [Google Scholar]
- Atkinson D. E. and Walton G. M. (1967). Adenosine triphosphate conservation in metabolic regulation rat liver citrate cleavage enzyme. J. Biol. Chem. 242, 3239-3241. [PubMed] [Google Scholar]
- Atkinson A., Siegel V., Pakhomov E. and Rothery P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100-103. 10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Banh S., Wiens L., Sotiri E. and Treberg J. R. (2016). Mitochondrial reactive oxygen species production by fish muscle mitochondria: Potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 191, 99-107. 10.1016/j.cbpb.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Barrientos A., Fontanesi F. and Diaz F. (2009). Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. Chapter 19, Unit19 3 10.1002/0471142905.hg1903s63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers J. M. and Sidell B. D. (2011). Thermal tolerance of Antarctic notothenioid fishes correlates with level of circulating hemoglobin. Physiol. Biochem. Zool. 84, 353-362. 10.1086/660191 [DOI] [PubMed] [Google Scholar]
- Beitinger T. L., Bennett W. A. and McCauley R. W. (2000). Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 58, 237-275. 10.1023/A:1007676325825 [DOI] [Google Scholar]
- Benard G., Faustin B., Passerieux E., Galinier A., Rocher C., Bellance N., Delage J.-P., Casteilla L., Letellier T. and Rossignol R. (2006). Physiological diversity of mitochondrial oxidative phosphorylation. Am. J. Physiol. Cell Physiol. 291, C1172-C1182. 10.1152/ajpcell.00195.2006 [DOI] [PubMed] [Google Scholar]
- Benjamini Y. and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289-300. [Google Scholar]
- Blier P. U. and Lemieux H. E. E. (2001). The impact of the thermal sensitivity of cytochrome c oxidase on the respiration rate of Arctic charr red muscle mitochondria. J. Comp. Physiol. B 171, 247-253. 10.1007/s003600000169 [DOI] [PubMed] [Google Scholar]
- Boveris A. (1977). Mitochondrial production of superoxide radical and hydrogen peroxide. Adv. Exp. Med. Biol. 78, 67-82. 10.1007/978-1-4615-9035-4_5 [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brand M. D., Turner N., Ocloo A., Else P. L. and Hulbert A. J. (2003). Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem. J. 376, 741-748. 10.1042/bj20030984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker C., Lassen N., Estey T., Pappa A., Cantore M., Orlova V. V., Chavakis T., Kavanagh K. L., Oppermann U. and Vasiliou V. (2010). Aldehyde dehydrogenase 7A1 (ALDH7A1) is a novel enzyme involved in cellular defense against hyperosmotic stress. J. Biol. Chem. 285, 18452-18463. 10.1074/jbc.M109.077925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. A., Fraser K. P. P., Peck L. S., Bishop C. M. and Egginton S. (2007). Life in the fast lane: the free-ranging activity, heart rate and metabolism of an Antarctic fish tracked in temperate waters. J. Exp. Mar. Biol. Ecol. 349, 142-151. 10.1016/j.jembe.2007.05.009 [DOI] [Google Scholar]
- Campbell H. A., Fraser K. P. P., Bishop C. M., Peck L. S. and Egginton S. (2008). Hibernation in an antarctic fish: on ice for winter. PLoS ONE 3, e1743 10.1371/journal.pone.0001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-H., Yang F.-F., Liao S.-A., Miao Y.-T., Ye C.-X., Wang A.-L., Tan J.-W. and Chen X.-Y. (2015). High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 53, 172-179. 10.1016/j.jtherbio.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Cheng L., Trenberth K. E., Fasullo J., Boyer T., Abraham J. and Zhu J. (2017). Improved estimates of ocean heat content from 1960 to 2015. Sci. Adv. 3, e1601545 10.1126/sciadv.1601545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin L.-M., Lande R. and Mace G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 10.1371/journal.pbio.1000357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A., Murphy E. J., Meredith M. P., King J. C., Peck L. S., Barnes D. K. A. and Smith R. C. (2007). Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos. Trans. R Soc. Lond. B Biol. Sci. 362, 149-166. 10.1098/rstb.2006.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor D. P., Kuhn D. E., O'Brien K. M. and Crockett E. L. (2016). Hyperoxia does not extend critical thermal maxima (CTmax) in white- or red-Blooded Antarctic notothenioid fishes. Physiol. Biochem. Zool. 89, 1-9. 10.1086/684812 [DOI] [PubMed] [Google Scholar]
- Driedzic W. R. (1983). The fish heart as a model system for the study of myoglobin. Comp. Biochem. Physiol. A Comp. Physiol. 76, 487-493. 10.1016/0300-9629(83)90451-6 [DOI] [PubMed] [Google Scholar]
- Ducklow H. W., Baker K., Martinson D. G., Quetin L. B., Ross R. M., Smith R. C., Stammerjohn S. E., Vernet M. and Fraser W. (2007). Marine pelagic ecosystems: the west Antarctic Peninsula. Philos. Trans. R Soc. Lond. B Biol. Sci. 362, 67-94. 10.1098/rstb.2006.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S. and Campbell H. A. (2016). Cardiorespiratory responses in an Antarctic fish suggest limited capacity for thermal acclimation. J. Exp. Biol. 219, 1283-1286. 10.1242/jeb.130963 [DOI] [PubMed] [Google Scholar]
- Ekström A., Brijs J., Clark T. D., Gräns A., Jutfelt F. and Sandblom E. (2016). Cardiac oxygen limitation during an acute thermal challenge in the European perch: effects of chronic environmental warming and experimental hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R440-R449. 10.1152/ajpregu.00530.2015 [DOI] [PubMed] [Google Scholar]
- Ekström A., Sandblom E., Blier P. U., Dupont Cyr B.-A., Brijs J. and Pichaud N. (2017). Thermal sensitivity and phenotypic plasticity of cardiac mitochondrial metabolism in European perch, Perca fluviatilis. J. Exp. Biol. 220, 386-396. 10.1242/jeb.150698 [DOI] [PubMed] [Google Scholar]
- Farrell A. P. (2009). Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 212, 3771-3780. 10.1242/jeb.023671 [DOI] [PubMed] [Google Scholar]
- Franklin C. E., Davison W. and Seebacher F. (2007). Antarctic fish can compensate for rising temperatures: thermal acclimation of cardiac performance in Pagothenia borchgrevinki. J. Exp. Biol. 210, 3068-3074. 10.1242/jeb.003137 [DOI] [PubMed] [Google Scholar]
- Galli G. L. J., Lau G. Y. and Richards J. G. (2013). Beating oxygen: chronic anoxia exposure reduces mitochondrial F1FO-ATPase activity in turtle (Trachemys scripta) heart. J. Exp. Biol. 216, 3283-3293. 10.1242/jeb.087155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E., Lassnig B., Kuznetsov A., Rieger G. and Margreiter R. (1998). Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 201, 1129-1139. [DOI] [PubMed] [Google Scholar]
- Hansen C. A. and Sidell B. D. (1983). Atlantic hagfish cardiac muscle: metabolic basis of tolerance to anoxia. Am. J. Physiol. 244, R356-R362. 10.1152/ajpregu.1983.244.3.R356 [DOI] [PubMed] [Google Scholar]
- Hilton Z., Clements K. D. and Hickey A. J. R. (2010). Temperature sensitivity of cardiac mitochondria in intertidal and subtidal triplefin fishes. J. Comp. Physiol. B 180, 979-990. 10.1007/s00360-010-0477-7 [DOI] [PubMed] [Google Scholar]
- Iftikar F. I. and Hickey A. J. R. (2013). Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 8, e64120 10.1371/journal.pone.0064120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikar F. I., MacDonald J. R., Baker D. W., Renshaw G. M. C. and Hickey A. J. R. (2014). Could thermal sensitivity of mitochondria determine species distribution in a changing climate? J. Exp. Biol. 217, 2348-2357. 10.1242/jeb.098798 [DOI] [PubMed] [Google Scholar]
- Iftikar F. I., Morash A. J., Cook D. G., Herbert N. A. and Hickey A. J. R. (2015). Temperature acclimation of mitochondria function from the hearts of a temperate wrasse (Notolabrus celidotus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 184, 46-55. 10.1016/j.cbpa.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Janssen A. J. M., Trijbels F. J. M., Sengers R. C. A., Smeitink J. A. M., van den Heuvel L. P., Wintjes L. T. M., Stoltenborg-Hogenkamp B. J. M. and Rodenburg R. J. T. (2007). Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 53, 729-734. 10.1373/clinchem.2006.078873 [DOI] [PubMed] [Google Scholar]
- Jayasundara N., Healy T. M. and Somero G. N. (2013). Effects of temperature acclimation on cardiorespiratory performance of the Antarctic notothenioid Trematomus bernacchii. Polar Biol. 36, 1047-1057. 10.1007/s00300-013-1327-3 [DOI] [Google Scholar]
- Jayasundara N., Tomanek L., Dowd W. W. and Somero G. N. (2015). Proteomic analysis of cardiac response to thermal acclimation in the eurythermal goby fish Gillichthys mirabilis. J. Exp. Biol. 218, 1359-1372. 10.1242/jeb.118760 [DOI] [PubMed] [Google Scholar]
- Jeffries K. M., Hinch S. G., Sierocinski T., Pavlidis P. and Miller K. M. (2014). Transcriptomic responses to high water temperature in two species of Pacific salmon. Evol. Appl. 7, 286-300. 10.1111/eva.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce W., Egginton S., Farrell A. P., Crockett E. L., O'Brien K. M. and Axelsson M. (2018). Exploring nature's natural knockouts: in vivo cardiorespiratory performance of Antarctic fishes during warming. J. Exp. Biol. 221, jeb183160. 10.1242/jeb.183160 [DOI] [PubMed] [Google Scholar]
- King J. C. (1994). Recent climate variability in the vicinity of the Antarctic Peninsula. Int. J. Climatol. 14, 357-369. 10.1002/joc.3370140402 [DOI] [Google Scholar]
- Leary S. C., Lyons C. N., Rosenberger A. G., Ballantyne J. S., Stillman J. and Moyes C. D. (2003). Fiber-type differences in muscle mitochondrial profiles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R817-R826. 10.1152/ajpregu.00058.2003 [DOI] [PubMed] [Google Scholar]
- Leo E., Kunz K. L., Schmidt M., Storch D., Pörtner H.-O. and Mark F. C. (2017). Mitochondrial acclimation potential to ocean acidification and warming of Polar cod (Boreogadus saida) and Atlantic cod (Gadus morhua). Front. Zool. 14, 21 10.1186/s12983-017-0205-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier T., Malgat M. and Mazat J.-P. (1993). Control of oxidative phosphorylation in rat muscle mitochondria: implications for mitochondrial myopathies. Biochim. Biophys. Acta 1141, 58-64. 10.1016/0005-2728(93)90189-M [DOI] [PubMed] [Google Scholar]
- Lundby A. and Olsen J. V. (2011). GeLCMS for in-depth protein characterization and advanced analysis of proteomes. Methods Mol. Biol. 753, 143-155. 10.1007/978-1-61779-148-2_10 [DOI] [PubMed] [Google Scholar]
- Lutterschmidt W. I. and Hutchinson V. H. (1997). The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561-1574. 10.1139/z97-783 [DOI] [Google Scholar]
- Manfredi G., Yang L. C., Gajewski C. D. and Mattiazzi M. (2002). Measurements of ATP in mammalian cells. Methods 26, 317-326. 10.1016/S1046-2023(02)00037-3 [DOI] [PubMed] [Google Scholar]
- McAlister G. C., Nusinow D. P., Jedrychowski M. P., Wühr M., Huttlin E. L., Erickson B. K., Rad R., Haas W. and Gygi S. P. (2014). MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150-7158. 10.1021/ac502040v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. P. and King J. C. (2005). Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 32, L19604 10.1029/2005GL024042 [DOI] [Google Scholar]
- Moylan T. J. and Sidell B. D. (2000). Concentrations of myoglobin and myoglobin mRNA in heart ventricles from Antarctic fishes. J. Exp. Biol. 203, 1277-1286. [DOI] [PubMed] [Google Scholar]
- Mueller I. A., Grim J. M., Beers J. M., Crockett E. L. and O'Brien K. M. (2011). Inter-relationship between mitochondrial function and susceptibility to oxidative stress in red- and white-blooded Antarctic notothenioid fishes. J. Exp. Biol. 214, 3732-3741. 10.1242/jeb.062042 [DOI] [PubMed] [Google Scholar]
- Mueller I. A., Devor D. P., Grim J. M., Beers J. M., Crockett E. L. and O'Brien K. M. (2012). Exposure to critical thermal maxima causes oxidative stress in hearts of white- but not red-blooded Antarctic notothenioid fishes. J. Exp. Biol. 215, 3655-3664. [DOI] [PubMed] [Google Scholar]
- O'Brien K. M. (2011). Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 214, 275-285. 10.1242/jeb.046854 [DOI] [PubMed] [Google Scholar]
- O'Brien K. M. and Sidell B. D. (2000). The interplay among cardiac ultrastructure, metabolism and the expression of oxygen-binding proteins in Antarctic fishes. J. Exp. Biol. 203, 1287-1297. [DOI] [PubMed] [Google Scholar]
- O'Brien K. M., Mueller I. A., Orczewska J. I., Dullen K. R. and Ortego M. (2014). Hearts of some Antarctic fishes lack mitochondrial creatine kinase. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 178, 30-36. 10.1016/j.cbpa.2014.08.003 [DOI] [PubMed] [Google Scholar]
- O'Brien K. M., Crockett E. L., Philip J., Oldham C. A., Hoffman M., Kuhn D. E., Barry R. and McLaughlin J. (2018). The loss of hemoglobin and myoglobin does not minimize oxidative stress in Antarctic icefishes. J. Exp. Biol. 221, jeb162503. 10.1242/jeb.162503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S., De Rasmo D., Scacco S., Signorile A., Technikova-Dobrova Z., Palmisano G., Sardanelli A. M., Papa F., Panelli D., Scaringi R. et al. (2008). Mammalian complex I: a regulable and vulnerable pacemaker in mitochondrial respiratory function. Biochim. Biophys. Acta 1777, 719-728. 10.1016/j.bbabio.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Payne B. A. I., Chinnery P. F. (2015). Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim. Biophys. Acta 1847, 1347-1353. 10.1016/j.bbabio.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud N., Ekström A., Hellgren K. and Sandblom E. (2017). Dynamic changes in cardiac mitochondrial metabolism during warm acclimation in rainbow trout. J. Exp. Biol. 220, 1674-1683. 10.1242/jeb.152421 [DOI] [PubMed] [Google Scholar]
- Porter R. K., Hulbert A. J. and Brand M. D. (1996). Allometry of mitochondrial proton leak: influence of membrane surface area and fatty acid composition. Am. J. Physiol. 271, R1550-R1560. 10.1152/ajpregu.1996.271.6.R1550 [DOI] [PubMed] [Google Scholar]
- Rardin M. J., Newman J. C., Held J. M., Cusak M. P., Sorensen D. J., Li B., Schilling B., Mooney S. D., Kahn C. R., Verdin E. et al. (2013). Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc. Natl. Acad. Sci. USA 110, 6601-6606. 10.1073/pnas.1302961110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick K. J., Gamperl A. K., Nash G. W. and Syme D. A. (2014). Temperature and sex dependent effects on cardiac mitochondrial metabolism in Atlantic cod (Gadus morhua L.). J. Therm. Biol. 44, 110-118. 10.1016/j.jtherbio.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Sandersfeld T., Davison W., Lamare M. D., Knust R. and Richter C. (2015). Elevated temperature causes metabolic trade-offs at the whole-organism level in the Antarctic fish Trematomus bernacchii. J. Exp. Biol. 218, 2373-2381. 10.1242/jeb.122804 [DOI] [PubMed] [Google Scholar]
- Seebacher F., Davison W., Lowe C. J. and Franklin C. E. (2005). A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fishes. Biol. Lett. 1, 151-154. 10.1098/rsbl.2004.0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F., White C. R. and Franklin C. E. (2015). Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61-66. 10.1038/nclimate2457 [DOI] [Google Scholar]
- Sidell B. D., Driedzic W. R., Stowe D. B. and Johnston I. A. (1987). Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol. Zool. 60, 221-232. 10.1086/physzool.60.2.30158646 [DOI] [Google Scholar]
- Sidell B. D., Vayda M. E., Small D. J., Moylan T. J., Londraville R. L., Yuan M.-L., Rodnick K. J., Eppley Z. A. and Costello L. (1997). Variable expression of myoglobin among the hemoglobinless Antarctic icefishes. Proc. Natl. Acad. Sci. USA 94, 3420-3424. 10.1073/pnas.94.7.3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero G. N. and Childress J. J. (1980). A violation of the metabolism-size scaling paradigm: activities of glycolytic enzymes in muscle increase in larger-size fish. Physiol. Zool. 53, 322-337. 10.1086/physzool.53.3.30155794 [DOI] [Google Scholar]
- Somero G. N. and DeVries A. L. (1967). Temperature tolerance of some Antarctic fishes. Science 156, 257-258. 10.1126/science.156.3772.257 [DOI] [PubMed] [Google Scholar]
- Srere P. A., Brazil H. and Gonen L. (1963). The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta. Chem. Scand. 17, S219-S234. 10.3891/acta.chem.scand.17s-0129 [DOI] [Google Scholar]
- Stillman J. H. and Somero G. N. (2000). A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 73, 200-208. 10.1086/316738 [DOI] [PubMed] [Google Scholar]
- Tamir S., Paddock M. L., Darash-Yahana-Baram M., Holt S. H., Sohn Y. S., Agranat L., Michaeli D., Stofleth J. T., Lipper C. H., Morcos F. et al. (2015). Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease. Biochim. Biophys. Acta 1853, 1294-1315. 10.1016/j.bbamcr.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Trivelpiece W. Z., Hinke J. T., Miller A. K., Reiss C. S., Trivelpiece S. G. and Watters G. M. (2011). Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc. Natl. Acad. Sci. USA 108, 7625-7628. 10.1073/pnas.1016560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J., Barrand N. E., Bracegirdle T. J., Convey P., Hodgson D. A., Jarvis M., Jenkins A., Marshall G. J., Meredith M. P., Roscoe H. et al. (2014). Antarctic climate change and the environment: an update. Polar Rec. 50, 237-259. 10.1017/S0032247413000296 [DOI] [Google Scholar]
- Urschel M. R. and O'Brien K. M. (2009). Mitochondrial function in Antarctic notothenioid fishes that differ in the expression of oxygen-binding proteins. Polar Biol. 32, 1323-1330. 10.1007/s00300-009-0629-y [DOI] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K. and Eppenberger H. M. (1992). Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 281, 21-40. 10.1042/bj2810021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lefevre S., Iversen N. K., Findorf I., Buchanan R. and McKenzie D. J. (2014). Anaemia only causes a small reduction in the upper critical temperature of sea bass: is oxygen delivery the limiting factor for tolerance of acute warming in fishes? J. Exp. Biol. 217, 4275-4278. 10.1242/jeb.104166 [DOI] [PubMed] [Google Scholar]
- Weinstein R. B. and Somero G. N. (1998). Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J. Comp. Physiol. B 168, 190-196. 10.1007/s003600050136 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



