Abstract
Background
Reducing unnecessary prescribing remains a key priority for tackling the global rise of antibiotic-resistant infections.
Aim
The authors sought to update a 2011 qualitative synthesis of GPs’ experiences of antibiotic prescribing for acute respiratory tract infections (ARTIs), including their views of interventions aimed at more prudent prescribing. They expanded the original scope to encompass all primary care professionals (PCPs) who can prescribe or dispense antibiotics for ARTIs (for example, nurses and pharmacists).
Design and setting
Systematic review and meta-ethnography of qualitative studies.
Method
A systematic search was conducted on MEDLINE, EMBASE, PsycINFO, CINAHL, ASSIA, and Web of Science. No date or language restrictions were used. Identified studies were grouped according to their thematic focus (usual care versus intervention), and two separate syntheses were performed.
Results
In all, 53 articles reporting the experiences of >1200 PCPs were included. Analysis of usual-care studies showed that PCPs tend to assume multiple roles in the context of ARTI consultations (the expert self, the benevolent self, the practical self), depending on the range of intrapersonal, interpersonal, and contextual situations in which they find themselves. Analysis of intervention studies identified four possible ways in which PCPs may experience quality improvement interventions (compromise, ‘supportive aids’, source of distress, and unnecessary).
Conclusion
Contrary to the original review, these results suggest that the use of the same intervention is experienced in a totally different way by different PCPs, and that the same elements that are perceived as benefits by some could be viewed as drawbacks by others. Acceptability of interventions is likely to increase if these are context sensitive and take into account PCPs’ varying roles and changing priorities.
Keywords: antibacterial agents, inappropriate prescribing, interventions, primary health care, qualitative research, respiratory tract infections
INTRODUCTION
Antimicrobial resistance has been characterised as ‘one of the world’s most pressing public health problems’.1 Over the last decade, a number of national and international organisations have called for action, but often with a limited impact due to lack of coordination or failure to recognise the global dimensions of the problem.2 On 21 January 2016, the joint Declaration on Combating Antimicrobial Resistance,3 signed by >80 leading pharmaceutical, diagnostics, and biotechnology companies, fuelled a new momentum in the global response to these challenges. A few months later, the publication of two high-profile reports,4,5 the first commissioned by the UK government and the second authored by the World Bank Group, attracted both scientific and media attention, as they provided estimates of the immense global burden and financial impact of drug-resistant infections if urgent action is not taken.
Excessive prescribing of antibiotics remains an important driver of antimicrobial resistance. The bulk of antibiotic prescribing occurs in primary care, with acute respiratory tract infections (ARTIs) representing the most common indication.6 Although ARTIs are often self-limiting and seldom require antibiotics for treatment,7 primary care clinicians have been found to overprescribe for a variety of clinical and, predominantly, non-clinical factors (for example, prior negative experience of non-antibiotic management, or perceived patient pressure).8–10 Qualitative research, focusing on the meanings that people attach to their experiences, is uniquely situated to explain this ‘non-pharmacological’ basis of prescribing, and primary qualitative studies have offered, to date, valuable insights into the reasons why clinicians may choose not to follow evidence-based guideline recommendations. Synthesising the findings from diverse and often small-scale qualitative studies has the potential not only to situate them in a larger interpretative context, but also to make them more ‘ready-to-use’ for healthcare practice and policymaking.11
Similar to statistical meta-analyses, however, qualitative syntheses may also become out of date, as beliefs, experiences, healthcare contexts, and social phenomena are bound to change.12 Thus, the goal of this study was to update a 2011 qualitative synthesis of GPs’ experiences of antibiotic prescribing for ARTIs, including their views of interventions aimed at more prudent prescribing.13 Tonkin-Crine et al were the first to review and synthesise published qualitative literature on processes involved in management decisions for ARTIs. However, most studies included in their review came from the UK and Scandinavia, limiting the transferability of findings to countries with different healthcare systems and antibiotic consumption rates.13 In an effort to address the global aspects of the problem (that is, several disciplines are involved in the delivery of primary care and their involvement/role/tasks vary widely across countries),14 the authors expanded the original focus to encompass all primary care professionals (PCPs) who can prescribe or dispense antibiotics for ARTIs (for example, nurses and pharmacists).
How this fits in
A 2011 systematic review and qualitative synthesis of GPs’ views and experiences of antibiotic prescribing for acute respiratory tract infections (ARTIs) concluded that, to maximise acceptability, interventions aimed at more prudent prescribing should incorporate five aspects: allow GPs to reflect on their own prescribing, help decrease uncertainty about appropriate ARTI management, educate GPs about appropriate prescribing, facilitate more patient-centred care, and be beneficial to implement in practice. However, several new studies have been published since then, and the continued relevance of these findings cannot be assumed. The authors performed an update of this work, while expanding the initial focus to encompass all primary care professionals (PCPs) who can prescribe or dispense antibiotics for ARTIs. The study produced an up-to-date conceptual model of antibiotic prescribing in primary care, and a typology of ARTI intervention acceptance, which could serve as valuable tools for current policy and practice.
METHOD
The reporting of this review is in accordance with the ENTREQ statement (further information available from the authors on request).15 The review protocol was registered with PROSPERO (CRD42016042861).
Search strategy and selection criteria
An information specialist revised the original search strategy to fit the purpose of the updated review (updated search strategy available from the authors on request). The authors performed a systematic, all-language search of the following databases from inception to 29 June 2016: MEDLINE, EMBASE, PsycINFO, CINAHL, ASSIA, and Web of Science. They also hand searched reference lists of included papers and used Web of Science to do forward citation tracking. Studies were eligible for inclusion if they were published as original research articles in peer-reviewed journals, used both qualitative data collection and analysis methods, and reported findings about PCPs’ attitudes and experiences of antibiotic prescribing/dispensing for ARTIs, or their views of interventions aimed at reducing inappropriate prescribing/dispensing. Mixed-methods studies were also eligible for inclusion, provided that the qualitative findings were adequately reported and discussed separately from the quantitative findings. Studies that did not provide participant quotations (raw data) to illustrate main themes/findings were excluded. The authors also excluded studies involving mixed participant groups (for example, patients and PCPs) that did not present separately, or in detail, findings from PCPs.
Data screening and quality assessment
Titles and abstracts of identified references were uploaded into EndNote and screened independently by two reviewers: the same author served as Reviewer 1, whereas three different authors served as Reviewer 2, each screening one-third of the articles. Full texts of potentially eligible articles were retrieved and assessed independently by the same reviewers, as previously described. In both stages, disagreements were resolved by consensus. Key study details (for example, country, sample) were extracted into a Microsoft Excel spreadsheet. Recognising the tension between reporting quality and potential contribution of a paper to the synthesis,16,17 the authors eventually opted not to use a formal appraisal checklist (for example, Critical Appraisal Skills Programme [CASP]) to exclude papers on the basis of their reporting quality. Instead, drawing on the categorisation of Dixon-Woods et al,18 the authors classified studies as follows:
key — that is, papers that were likely to make an important contribution to the synthesis due to their high analytic/explanatory power and high relevance;
satisfactory — that is, papers with high analytic/explanatory power but sufficient relevance, or papers with high relevance but sufficient analytic/explanatory power;
unsure — that is, papers that the authors were unsure of their potential contribution to the synthesis due to either limited analytic/explanatory power or borderline relevance;
fatally flawed — that is, papers that either presented their findings in a numerical way or did not provide participant quotations; and
irrelevant — that is, papers that were not relevant to the review question.
Papers judged as irrelevant and fatally flawed were excluded during full-text screening, whereas the authors followed an all-inclusive strategy for the remainder.
Data analysis
In line with the original synthesis, the authors used the technique of meta-ethnography to synthesise available findings. Meta-ethnography is the qualitative equivalent to meta-analysis, but rather than aggregating findings it focuses on the translation of individual studies into one another and the development of new interpretations.19 Given the large amount of identified papers, the authors began by organising them into groups according to their thematic focus (usual care versus intervention) and then, within each group, by date of publication. To allow for the exploration of potential intervention-specific differences, the authors further classified intervention studies based on type of intervention (clinical, educational, system-level, or multifaceted), using McDonagh et al’s framework,20 and use of intervention (naturalistic, controlled trial, or hypothetical). Starting from the usual-care group, the authors repeatedly read all studies, noting first-order (PCPs’ quotations) and second-order (authors’ interpretations of PCPs’ experiences) constructs. To understand how the studies related to each other, they created a grid and juxtaposed all identified second-order constructs. This enabled them to determine which type of synthesis was most appropriate: a reciprocal (where concepts from one study can easily encompass another) or refutational translation (where concepts are contested across papers), or line-of-argument synthesis, which involves first translating the studies into each other and then constructing an overarching argument about the whole set of studies. Considering the different thematic focus of the two groups of papers, the authors conducted two separate line-of-argument syntheses.
RESULTS
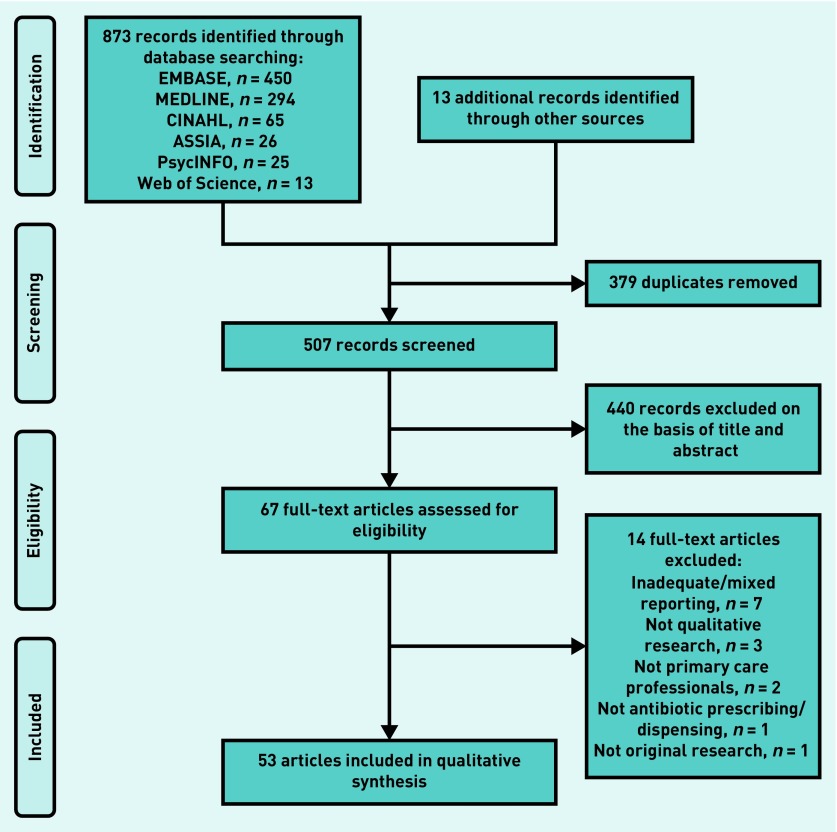
Of 507 unique citations found, 67 were eligible for full-text review and 53 were included in the synthesis (Figure 1). The 53 included papers10,21–72 corresponded to 45 different studies, and reported the experiences of >1200 PCPs (that is, 1113 GPs/family physicians, 74 nurses, 41 paediatricians, 33 pharmacists, one physiotherapist, and one physician assistant) practising in 21 countries. The earliest paper was published in 1998. However, more than half (28) were published after the publication of the original synthesis; 25 papers discussed PCPs’ experiences of antibiotic prescribing/dispensing in usual care, 22 focused on their views of an intervention (or combination of interventions), and six reported mixed information. Those six papers contributed to both syntheses.
Figure 1.
Flow diagram of study selection.
Among the 28 intervention/mixed studies, 13 focused on a clinical intervention (for example, point-of-care testing, delayed prescribing, and clinical scoring tools), seven described a system-level intervention (for example, electronic decision support and antimicrobial stewardship programmes), four were about an educational intervention (for example, communication skills training), and another four discussed a multifaceted intervention (for example, point-of-care testing in combination with communication skills training). Moreover, in the same group of papers, 12 focused on an intervention implemented in naturalistic (real-life) settings, another 12 on an intervention implemented in controlled trial settings, and the remaining four discussed participants’ views about the hypothetical introduction of an intervention (either in naturalistic or in controlled trial settings).
Synthesis of Group 1 (usual care) studies
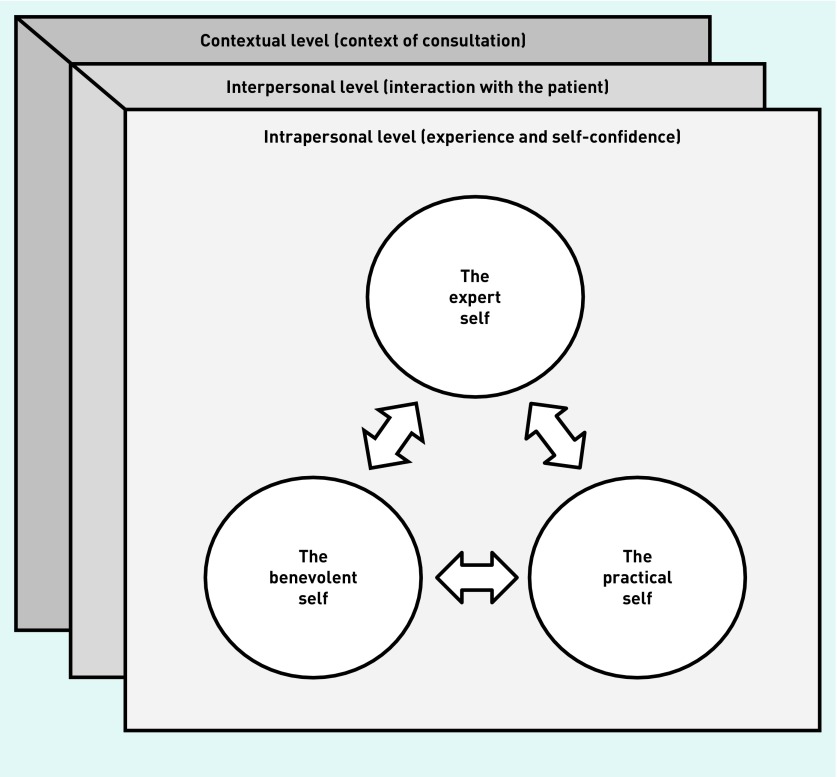
Box 1 presents a list of all second-order constructs that the authors identified from the 31 usual-care studies, along with a narrative translation of each construct (that is, a description that encompasses all the papers from which it was drawn). By re-ordering, re-linking, and re-analysing identified second-order constructs, the authors were able to generate six third-order constructs, which constitute their own interpretations of included studies. Based on these, the authors created a conceptual model showing how PCPs may choose to present themselves differently in the context of ARTI consultations (the expert self, the benevolent self, and the practical self), depending on the range of intrapersonal, interpersonal, and contextual situations in which they find themselves, and according to the function that a certain identity serves at a given moment (Figure 2).
Box 1. Group 1 (usual care) studies: translation of second-order constructs and third-order constructs.
| Third-order constructs | Second-order constructs | Summary definition (translation) of second-order construct | Papers that include the second-order construct |
|---|---|---|---|
| The expert self | Patient history and physical examination | Antibiotic prescribing decisions are, to a large extent, guided by (and justified in reference to) history taking and physical examination. Sometimes, patient complaints or the medical history of the patient might form the ‘diagnostic basis’. In other cases, decisions are guided by clinical signs and presenting symptoms, such as fever and discoloured sputum, which are interpreted in light of relevant risk factors (for example, older age) and comorbidities | 22–25,29,42,46,51,60,64,68,69,72 |
| General impression and ‘gut feeling’ | Many PCPs admit that, apart from the structured examination, the overall assessment of how the patient seems on the day plays a major role in their decision making. Assessments such as ‘very ill’, ‘weakened’, and ‘miserable’ are common, whereas primary care professionals’ ‘gut feeling’ can, in many cases, override a decision based purely on clinical factors | 22,24,42,62,64,72 | |
| The benevolent self | Dissatisfaction in not meeting patient expectations | Many PCPs feel that, once a patient makes the effort to come into the clinic, it is unsatisfying not to be able to offer a solution. Concerns of being perceived as ‘having done nothing’ for the patients, or not being ‘proper doctors’ if they do not prescribe antibiotics, are common | 10,29,30,43,72 |
| Desire to avoid conflict and maintain a good relationship with patients | Building and maintaining a good relationship with their patients is viewed as a priority for healthcare professionals working in primary care and several admit that they would not jeopardise this ‘for the sake of a prescription for penicillin V’ | 10,24,25,33,43,64,71,72 | |
| Beneficence/non-maleficence | PCPs justify their prescribing decisions on the basis of a desire to do their best for the patients. Although some report prioritising potential resistance problems and longer-term issues, the majority feels that their priority should be ‘the patient in front of them’ and their immediate needs. The desire to ‘help’ the patient is not restricted to treating a patient that is ill, but involves a broader consideration of the circumstances in an individual’s life, such as the environment in which they live, and their socioeconomic status or vulnerability on the job market, as well as plans for leisure activities | 10,55,60,61,62,64,66,67,71,72 | |
| The practical self | Patient retention and financial considerations | Many PCPs fear that their patients will not be satisfied if they do not receive a prescription and, as a consequence, they will not return to the clinic again. In this way, prescribing is seen as a means of ensuring self-preservation, especially in the case of professionals who collect on a fee-for-service basis | 10,25,30,39,46,55 61,67,69 |
| Medicolegal concerns | The possibility of ‘missing something’ in a patient is seen as a potential threat to PCPs’ expertise or standing and many express fear of overlooking something, making a mistake, and being sued. Patients’ increasing power in medical encounters and knowledge of the opportunity for legal action are commented as important factors influencing prescribing decisions | 10,24,27,44,71,72 | |
| Confidence and experience | Confidence and experience | PCPs report increased confidence in more accurately differentiating between patients who need treatment and those who can be safely monitored, as they see more patients over time with similar symptoms. On the other hand, they admit that previous bad experience of non-antibiotic management can have substantial impact on current prescribing practices | 22–24,27,29,43,45,46,61,69 |
| Interaction with the patient | Mutual trust and confidence with the patient | The degree of confidence and trust that PCPs have with their patients shapes prescribing decisions. The more insecure they feel about patients’ ability to recognise a worsening illness and re-consult, the more inclined they become to an antibiotic prescription | 22,24,27,40 |
| Patient pressure | Pressure in the form of a clear demand or gesture, or of a patient’s obvious fear (for example, anxiety, repeated consultations for the same episode), is regarded as a main reason for unnecessary antibiotic prescribing. Although explicit requests for antibiotics seem to be less frequent in developed, as compared to developing, countries, most healthcare professionals report ‘giving in’ occasionally to (actual or perceived) patient pressure, either for their own and the patient’s reassurance, or because they feel they cannot do anything else | 10,24,27,30,39,55,62,66–68,70,71 | |
| Context of consultation | Diagnostic uncertainty | The lack of conclusive evidence to support diagnosis and management of ARTIs in primary care creates uncertainty and many prescribers report difficulties in differentiating between viral and bacterial infections on clinical grounds alone. This might often lead to a tendency to ‘play it safe’, namely adopt a defensive practice and prescribe antibiotics, as they fear the possibility of missing a serious diagnosis (especially for children or people with comorbidities) | 10,22,24,25,27,29,30,42,43,45 46,51,55,61,63,66,67,69,72 |
| Continuity of care | Continuity of care promotes diagnostic accuracy and confidence in prescribing decisions through personal knowledge. Through familiarity with what is normal for the patient, PCPs are able to make a more informed evaluation of usual health status. On the other hand, lack of continuous care creates insecurity and often leads to unnecessary ‘just-in-case’ prescribing | 10,22,24,40,42,45 | |
| Work pressure and fatigue | PCPs acknowledge the impact of work pressure and fatigue on their prescribing habits, and several report changing their prescribing practices according to different contexts (for example, prescribing more when on-call or at the emergency centre). It is primarily lack of time that makes them lower their threshold of tolerance. An antibiotic prescription is seen, in such cases, as ‘the easiest way out’, a tool to conclude the consultation as quickly as possible without endangering a good doctor–patient relationship | 10,24,29,30,40,55,60,64,66,69,71,72 | |
| Timing of consultations | PCPs report feeling more pressure to prescribe if patients consult on the eve of a weekend (‘Friday prescriptions’) or holiday. It is important for them to help their patients so that they will not have to seek after-hours care or medical care abroad, in case their condition deteriorates | 10,22,24,43 | |
| System factors | Non-clinical factors imposed by healthcare systems, such as over-the-counter sales of antibiotics or lack of formal, consistently available national guidelines on antibiotic prescribing, are regarded by PCPs as important in prescribing decision making. Considered equally important by many are the incentives from the pharmaceutical industry, which influence prescribing practices both directly (through visits to medical practitioners) and indirectly (through support of continuing medical education) | 25,30,37,43,44,46,55,61,71 |
ARTI = acute respiratory tract infection. PCP = primary care professional.
Figure 2.
Line-of-argument synthesis of Group 1 (usual care) studies: a model of antibiotic prescribing and dispensing for acute respiratory tract infections in primary care.
In particular, the expert self corresponds to the self who holds the expertise and is constructed through formal (that is, history taking and physical examination) and non-formal (that is, overall impression of how the patient looks on the day and ‘gut feeling’) methods of clinical assessment. Antibiotic decisions were, to a large extent, guided by and communicated (or justified) in reference to: problematic or potentially worsening signs and symptoms (for example, discoloured sputum or high or persistent fever), clinical findings (for example, chest sounds on auscultation), or ‘expert’ assessments (for example, the patient looked ‘toxic’ or ‘miserable’). Several PCPs reported recognising ‘the sick patient in among the just unwell as they walked through the door’,24 whereas others described the development of individual ‘guidelines’ or ‘rules-of-thumb’ as a mark of expertise.51,67
The benevolent self corresponds to the self who wants to please and ‘help’. It is constructed through the interaction with the patients, and validated by prevailing social norms and role expectations associated with what constitutes a ‘good’ PCP. In this context, building and maintaining a good relationship with the patient was viewed as a top priority for most PCPs, and some admitted that they were not willing to jeopardise this ‘for the sake of a prescription for penicillin V’.72 Concerns about being perceived as ‘having done nothing’ 30 for the patient or not being ‘proper doctors’ 10 if they did not prescribe antibiotics were also common among GPs, and several expressed dissatisfaction when not being able to offer a tangible solution. Furthermore, PCPs’ desire to ‘do their best for the patient in front of them’ 60 seemed, in many cases, to override expert judgement: pharmacists admitted giving out antibiotics to patients who could not afford to pay fees or private medical consultations, whereas clinicians described how the circumstances in an individual’s life (ranging from the environment in which they lived to plans for leisure activities) often led to unnecessary prescribing.
Lastly, the practical self corresponds to the self who thinks practically (for example, avoiding a lawsuit in case of a mistake), but also to the self who has to cope with specific system demands and practical considerations (for example, patient retention and financial considerations):
‘You shouldn’t be treating all respiratory infections with antibiotics? Certainly. Is it practical? Probably not. I probably wouldn’t have as good of a collection rate. I truly think that part of what you’re doing is consumer-based medicine.’ 61
Legitimised by broader system factors, prescribing in this context was seen as the ‘safest’ or ‘easiest’ choice. GPs in Lithuania, for example, reported occasionally giving in to patient pressure for antibiotics, as they felt ‘unsafe’ and threatened by current legislation on patients’ rights,44 whereas GPs in Iceland described themselves as ‘slaves of the green forms’,67 because their salary consisted of a mixture of wages and a fee-for-service part, collected by means of green forms.
Although the expert self constitutes the ‘default’ identity of PCPs, how they choose to present themselves in the context of ARTI consultations is dependent on a mixture of intrapersonal, interpersonal, and contextual factors. Specifically, PCPs’ prior experience of ARTI management and their level of self-confidence were found to have a considerable impact on their current prescribing practices: in general, experience in ARTI management increased self-confidence and reinforced the expert identity, whereas a previous negative case of non-antibiotic management challenged clinical expertise and often led to overprescribing ‘to be on the safe side’.27 Likewise, both characteristics of the PCP–patient interaction (that is, actual or perceived patient pressure, mutual trust, and confidence with the patient) and contextual factors (that is, degree of diagnostic uncertainty accompanying each patient case, presence or lack of continuous care, work pressure and fatigue, timing of consultation, and system factors) influenced the identity that PCPs chose to articulate.
Although this shifting of identities may allow for flexibility in decision making, it is not always voluntary. In such cases, PCPs may feel pressured to assume a role that they do not wish to assume and experience ambivalence, or even frustration, regarding their management decisions. As a study participant put it, ‘I’m Dr Jekyll and Mr Hyde.’ 40
Synthesis of Group 2 (intervention) studies
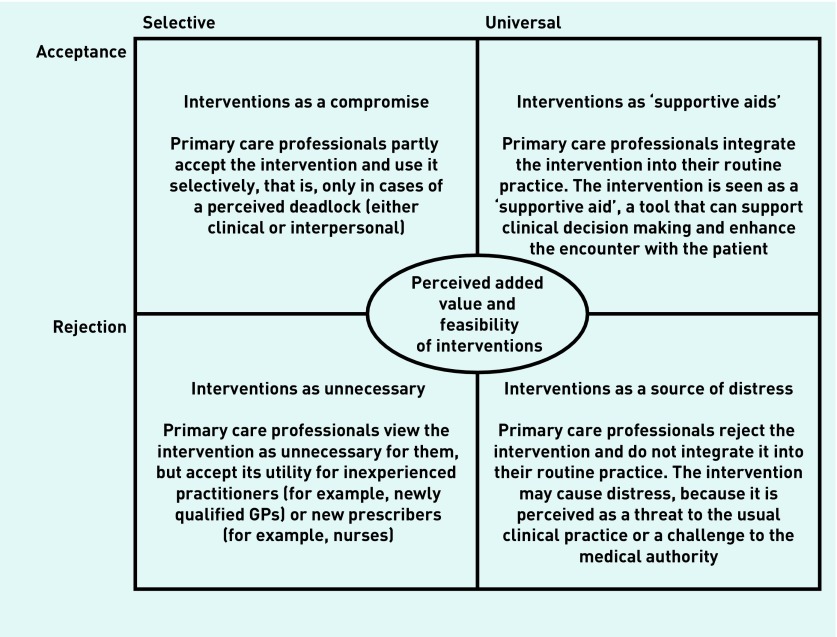
Similarly, the authors’ synthesis of intervention studies identified 13 second-order constructs, which were further abstracted to generate four third-order constructs (Box 2). Such analysis led to the development of a typology of ARTI intervention acceptance in primary care that depicts four possible ways in which PCPs may experience interventions (Figure 3).
Box 2. Group 2 (intervention) studies: translation of second-order constructs and third-order constructs.
| Third-order constructs | Second-order constructs | Summary definition (translation) of the second-order construct | Papers that include the second-order construct |
|---|---|---|---|
| Interventions as a compromise | Managing clinical uncertainty | Clinical interventions can decrease uncertainty related to the diagnosis and treatment of ARTIs, while minimising PCPs’ fear of bad outcomes. Although the usefulness of having additional diagnostic tools is valued, most practitioners prefer to rely on clinical findings when deciding about antibiotic treatment and use interventions only in cases of clinical doubt (‘when unsure’). In such cases, interventions are perceived as providing a safety net for both the practitioner and the patient | 21,26,28,29,47,48,51,52,58,64 |
| Coping with potentially confrontational encounters | Interventions can help practitioners cope with the pressure they experience from patients expecting antibiotics. They are often viewed as a negotiation tool within the practitioner–patient encounter, a compromise needed to avoid or limit conflict, as well as a way for managing patient expectations for antibiotics and demonstrating that their illness is being taken seriously | 24,47,54,58,59,65 | |
| Interventions as ‘supportive aids’ | Enhancing confidence in clinical decision making | Interventions can augment clinical assessment and authority and enhance both practitioners’ and patients’ confidence in clinical decision making. Confirming prescribing decisions from several angles can be reassuring both for the practitioner and for the patient, whereas it can lead to improved satisfaction with care, as well as increased patient compliance with the treatment disposal | 26,29,30–32,35,36,38,41,48–50,52,56–58 |
| Educating and empowering the patient | Interventions are viewed as ‘golden moments’ for patient education. They can provide a stimulus for opening a discussion about the necessity or effectiveness of antibiotics, and empower patients to become more involved in their own healthcare management | 26,38,48,49,52,54,59,65 | |
| Provision of more patient-centred care | Interventions can help PCPs gain greater insight into patients’ perspectives, needs, and expectations, and work together to achieve shared antibiotic prescribing decisions | 35,36,50,57,64,65 | |
| Improved management/treatment | Interventions can provide more effective targeted treatment, prevent unnecessary prescription of antibiotics, and reduce the likelihood of re-consultation | 26,32,35,36,38,41,42,49,50,52,53,64,65 | |
| Perceived ease of use and feasibility of interventions | Perceived ease of use and feasibility of incorporating an intervention into clinical practice increases its actual use | 29,32,38,52,56 | |
| Interventions as a source of distress | Giving mixed messages to patients | Certain interventions, such as delayed prescribing, may convey contradictory messages to patients about the competence of the physician or the efficacy of antibiotics for ARTIs. On the other hand, the increased availability of clinical interventions, such as near-patient tests, might lead patients to consider ARTIs as more serious than they actually are | 26,38,47,64,65 |
| Resulting in a paternalistic approach | Interventions might diminish the trust between the patient and the clinician, disrupt the usual quality of rapport, and lead to the use of a paternalistic (rather than shared decision making) approach | 36,41,64 | |
| Fear of inappropriate management/treatment | The reliability of interventions is often questioned, and many primary care professionals express concerns related to inappropriate management/treatment of ARTIs, such as missing the diagnosis of a serious infection or prescribing unnecessary antibiotics due to a false-positive test result | 38,48,49,51,58,65 | |
| Tension and possible disconnect with clinical assessment and intuition | Interventions are perceived as a threat to the clinical assessment and intuition (especially in cases where there is a conflict between what the prescriber thinks as clinically best and what the intervention indicates as clinically best), and many PCPs express the fear of ‘treating test results rather than patients’ | 34,38,42,48,49,52,53 | |
| Time and cost concerns | Interventions are perceived as too costly or too time consuming to fit into usual practice | 26,32,35,36,38,49,51,53,57,58 | |
| Interventions as unnecessary | Useful for inexperienced practitioners/unnecessary for experienced practitioners | Interventions are viewed as useful for inexperienced practitioners (for example, newly qualified GPs) or new prescribers (for example, nurses), but are considered unnecessary for experienced practitioners | 32,38,56 |
ARTI = acute respiratory tract infection. PCP = primary care professional.
Figure 3.
Line-of-argument synthesis of Group 2 (intervention) studies: a typology of acute respiratory tract infections intervention acceptance in primary care.
In the first cell of the authors’ typology, ‘Interventions as a compromise’, PCPs tend to view interventions solely as a compromise and use them selectively, that is, only in cases of a perceived deadlock (either clinical or interpersonal). This was commonly the case with clinical interventions, such as near-patient tests and delayed prescribing, which were used by many PCPs either in cases of clinical doubt, as a means of managing diagnostic uncertainty and safety netting against the condition worsening, or in cases of potentially confrontational encounters with patients who had strong expectations for antibiotics:
‘I have used [wait-and-see prescriptions] for several years, but to a small extent only. It is for those who want medication, though you argue that they don’t need it, but then they win at the end and I say: “Can you at least wait for a couple of days and see how it develops?” You become somewhat pragmatic with the years.’ 54
In the second cell, ‘Interventions as “supportive aids”’, PCPs choose to integrate interventions into their routine practice, as they perceive them mostly as tools that can support clinical decision making and enhance the encounter with the patient. Although some concerns could still be present, interventions here were typically seen as easy to use and feasible to implement in practice, and their use was thought to:
augment clinical authority and enhance both practitioners’ and patients’ confidence in prescribing decisions;
provide an opportunity for educating and empowering the patient;
help practitioners gain greater insight into patients’ perspectives and provide more patient-centred care; and
contribute to more effective targeted treatment, prevention of unnecessary prescribing, and reduced re-consultation rates.
All of the above perceived benefits of interventions are viewed from the exact opposite angle by PCPs representing the third cell, ‘Interventions as a source of distress’. For them, interventions appeared to constitute a source of distress, as they were considered to convey mixed messages to patients about the competence of the physician or the efficacy of antibiotics for ARTIs, diminish provider–patient trust and result in a paternalistic approach, and lead to possible disconnect with clinical assessment and intuition, as well as to potentially inappropriate management of ARTIs. Moreover, PCPs belonging in this group typically saw interventions as too costly or too time consuming to fit into usual practice:
‘In an ideal world, yes. I have seen 17 patients [so far] today. And each is given 10 minutes of appointment. If you end up admitting one, or end up doing some examinations, it takes longer. So in an ideal world, yes, I could test urines. I could test various things. H. pylori and various other things.’ 58
In the last cell, ‘Interventions as unnecessary’, PCPs choose not to integrate interventions into their own practice, but to accept their utility for other, mostly inexperienced, groups of prescribers. Specifically, this was the case with certain PCPs who reported that, although interventions were unnecessary for them, they did have ‘a place within primary care’,38 as they could prove a useful tool for inexperienced practitioners (for example, newly qualified GPs) or new prescribers (for example, nurses).
However, the proposed typology is neither static nor decontextualised. PCPs continuously evaluate both the added value and the feasibility of a specific intervention, meaning that the proposed types are rather dynamic and may change over time or depending on the characteristics of the encounter. The same practitioner, for instance, who on one occasion may view the implementation of an intervention as particularly distressing (for example, issuing a wait-and-see prescription in the after-hours care setting, where they do not know the patients and the scope for follow-up is limited), could, on another, see it as a ‘supportive aid’ (for example, issuing a wait-and-see prescription in their regular list patient practice, where they know their patients and can start a discussion about the necessity or effectiveness of antibiotics).
DISCUSSION
Summary
This work constitutes one of the very few updates of qualitative syntheses currently available12,73–75 and, to the authors’ knowledge, the first conducted by a different team of reviewers. This updated review incorporated findings from 53 papers (that is, 41 more than the original review), published over the span of two decades, and reporting the experiences of >1200 PCPs practising in 21 countries around the world. By expanding the search beyond GPs, the authors were able to incorporate in their analysis a range of perspectives, while capturing more of a global context, given international differences in the involvement of various disciplines in the delivery of primary care. Most of the factors identified in the original review as responsible for inappropriate prescribing were found to be still pertinent. However, identification of more studies added depth to these concepts and led to a better understanding of the phenomenon. Specifically, the authors were able to show how PCPs manage their professional identity in the context of ARTI consultations (the expert self, the benevolent self, and the practical self), depending on the range of intrapersonal, interpersonal, and contextual situations in which they find themselves. Furthermore, inclusion of recent evidence on PCPs’ experiences of interventions (used not only as part of randomised controlled trials, but also in real-life settings) allowed the authors to draw important conclusions about the possible ways that these may be employed. Contrary to Tonkin-Crine et al,13 the authors found that the use of the same intervention might be experienced in a totally different way by different PCPs, and that the same elements that are perceived as benefits by some could be viewed as drawbacks by others. Most importantly, the authors created a typology of ARTI intervention acceptance, which could serve as a valuable tool for current policy and practice. Their typology presents four different stances towards the use of interventions (compromise, ‘supportive aids’, source of distress, and unnecessary), which are, however, dynamic and mutable, as PCPs seem to continuously evaluate both their feasibility and their added value.
Strengths and limitations
The development and spread of antibiotic-resistant infections has predominantly been a clinical problem in hospital settings, and much of available social science research has focused on investigating norms of practice among hospital-based professionals.76,77 However, resistance to primary care-prescribed antibiotics is also common, so the transmission potential of these infections across healthcare settings could be substantial. This study offers a comprehensive, up-to-date overview of available qualitative literature on PCPs’ management decisions for ARTIs, while highlighting the complexity of the problem in primary care. Nevertheless, certain limitations need to be acknowledged. First, although the authors expanded the original review question to encompass all PCPs who can prescribe or dispense antibiotics, their search located only one study focusing on pharmacists’ dispensing practices. The authors cannot, therefore, be confident that their findings hold relevance for understanding antibiotic dispensing in primary care. Likewise, although they included evidence from a wide range of countries and were able to identify themes that were consistent across different healthcare systems and prescribing contexts, their synthesis of intervention studies relied solely on findings from high-income countries, meaning that they are unaware of whether their typology of ARTI intervention acceptance can be extrapolated to low- or middle-income countries. Finally, the authors recognise that the process of synthesising qualitative research is essentially interpretative. Therefore, it could be argued that differences in conclusions reached might be due to differences in interpretations between the original and the new team. In an effort to check their interpretations, they contacted the original team and asked them to clarify previously emergent concepts, as well as to provide feedback on draft versions of updated models.
Comparison with existing literature
The need to approach antibiotic prescribing as a simultaneously medical, social, and cultural practice has already been stressed by Ackerman and Gonzales,78 who argued that the case of antibiotic overuse powerfully illustrates the importance of context for clinical practice. Indeed, the authors’ synthesis of usual care studies unveils the complex interplay of clinical experience, social norms and expectations, and cultural trends, as well as broader system factors (for example, organisation and financing of primary care). Most importantly, it offers an in-depth understanding of how PCPs perceive their role in such a context, while accounting for the wide variation that exists in the acceptability of interventions (an indicative list of possible scenarios is available from the authors on request). Although the notion of multiple identities as a result of expectations and negotiations goes back several years, the recent shift towards consumerism seems to have sparked a renewed interest in how the emerging model of the patient as ‘reflexive consumer’ has impacted on physicians’ professional identity.79 The authors’ results suggest that, in the highly uncertain context of ARTI consultations, the traditional role of the PCP as the ‘expert’ whose job is to ‘treat infections’ is expanded to include a benevolent identity that wants to satisfy and ‘help’ the patient, as well as a practical identity that has to cope with system demands and real-life considerations.
The practice of benevolence constitutes the implicit basis on which all healthcare professionals operate. How benevolence may interfere with antibiotic decision making and what the practical consequences of this might be, however, is a topic that necessitates further investigation. Broom et al 76 found that benevolence constitutes a core principle of action among medical doctors, justifying suboptimal prescribing practices in the hospital. Yet, it could be argued that in primary care, where the duty of the professional is to serve as the patient’s first contact with the healthcare system, expectations around the performance of benevolence may become even more salient. In this context, for instance, the act of issuing an unnecessary prescription as a way of demonstrating concern and consideration for the patient’s life circumstances (regardless of whether these involve living under insanitary conditions in poor areas of India55 or having to attend an important meeting the following day)62 might not only be legitimate, but also valued and encouraged by social and professional norms around what constitutes a ‘good’ PCP.
Another topic that has been remarkably overlooked in current efforts to optimise prescribing patterns is the role of ‘gut feelings’ in PCPs’ diagnostic reasoning. A growing body of evidence indicates that ‘gut feelings’ are common among PCPs and constitute an integral part of clinical decision making.80–82 The authors’ results resonate with prior findings, while also emphasising the symbolic effect that intuition may have in reinforcing PCPs’ expert identity. Considering that problems presenting in primary care are often early in their natural history, with vague symptoms and a broad range of diagnostic possibilities, being able to ‘recognise the sick patient as they walk through the door’ 24 may be crucial in allowing PCPs to re-establish themselves as the competent technical experts in a shifting context of power relations. This also relates to the authors’ finding that fear of possible disconnect with clinical intuition was a major barrier to the routine use of clinical interventions.
Implications for research and practice
Updating of quantitative systematic reviews and statistical meta-analyses is now mainstream practice. However, the same does not apply to systematic reviews and syntheses of qualitative evidence, for which, to date, the process of updating has remained largely unexplored. The authors’ work provides empirical evidence for the necessity of regularly updating qualitative syntheses, and shows that, in the same way that updated meta-analyses can inform about whether healthcare interventions continue to be safe and effective, updated qualitative syntheses can provide evidence on whether these continue to remain relevant to target audiences’ changing needs, preferences, and experiences. Moreover, identifying and incorporating new evidence into a previously completed qualitative synthesis may lead to new conceptual insights and a more nuanced understanding of the phenomenon under study, which is not something that an updated meta-analysis is able (or aims) to achieve.
The original meta-ethnography concluded that, to maximise acceptability, interventions aimed at more prudent prescribing for ARTIs should incorporate five aspects: allow GPs to reflect on their own prescribing, help decrease uncertainty about appropriate ARTI management, educate GPs about appropriate prescribing, facilitate more patient-centred care, and be beneficial to implement in practice.13 Seven years later, the authors’ updated synthesis suggests that one-size-fits-all approaches are doomed to result in variable uptake, as different professionals experience the same elements in a very different way. The authors argue that acceptability of interventions is likely to increase if these are context sensitive and take into account PCPs’ varying roles and changing priorities. Similar to Ackerman and Gonzales,78 the authors embrace a wider definition of ‘context’ that can account for both specific situational factors (for example, setting and timing of consultation) and broader socioeconomic, cultural, and system influences.
Several context-specific differences that link (either directly or indirectly) to the acceptability of interventions were apparent in this work. First, PCPs practising in countries with fee-for-service payment systems often reported feeling pressured to over-prescribe due to business concerns.41,61,67 Similarly, for PCPs practising in countries where antibiotic use still remains largely unregulated, they extensively discussed how patients’ direct access to antibiotics and self-medication restricted their management options and led to unnecessary prescribing.25,37,43,44,46,55 By comparison, PCPs from Belgium, Iceland, and the UK emphasised how systems to reduce patient expectations, such as public information campaigns, had made their work easier over the last few years.43,51 Of note, in the only study that included a follow-up (that is, the same Icelandic GPs were interviewed in 1995 and again in 2006), Björnsdóttir et al 51 found increased use of point-of-care tests and the perception by GPs that patients were more willing to ‘wait and see’.
In conclusion, the results suggest that to work towards achieving more impactful outcomes, pragmatically tailoring interventions to better fit them to specific PCP groups and local conditions might be a necessary first step. In countries, for example, where over-the-counter sales of antibiotics are allowed, it might be rather difficult to implement clinical interventions. Instead, promoting tailored educational interventions, such as physician communication skills, training, and public campaigns, might be more efficient. Likewise, building flexibility into the design of interventions, so that these can be adjusted according to different circumstances and priorities (for example, time-pressured settings), could eliminate situational barriers and ensure a more consistent use of interventions. The solution of a global problem might not lie in the development of a universal, multifaceted approach, but in addressing deep-rooted, local modus operandi.
Funding
Evi Germeni was supported by an Advanced Postdoc Mobility grant from the Swiss National Science Foundation (P300P1_164574). Ruth Garside, Morwenna Rogers, and Nicky Britten were partially supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula. The content is solely the responsibility of the authors and does not necessarily represent the views of the Swiss National Science Foundation, the UK NIHR, the UK NHS, or the UK Department of Health.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Centers for Disease Control and Prevention Antibiotic prescribing and use in doctor’s offices. 2016 https://www.cdc.gov/getsmart/community/about/fast-facts.html (accessed 24 May 2018) [Google Scholar]
- 2.Carlet J, Jarlier V, Harbarth S, et al. Ready for a world without antibiotics? The Pensieres Antibiotic Resistance Call to Action. Antimicrob Resist Infect Control. 2012;1(1):11. doi: 10.1186/2047-2994-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Federation of Pharmaceutical Manufacturers & Associations. AMR industry alliance. United against antimicrobial resistance 2016. https://www.ifpma.org/partners-2/declaration-by-the-pharmaceutical-biotechnology-and-diagnostics-industries-on-combating-antimicrobial-resistance-amr/ (accessed 24 May 2018)
- 4.O’Neill J. Tackling drug resistant infections globally: Final report and recommendations. 2016. Review On Antimicrobial Resistance. http://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed 24 May 2018)
- 5.World Bank Group Drug resistant infections: a threat to our economic future. 2017. http://documents.worldbank.org/curated/en/323311493396993758/pdf/114679-REVISED-v2-Drug-Resistant-Infections-Final-Report.pdf (accessed 24 May 2018)
- 6.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Chen R, Wu T, et al. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: a systematic review and meta-analysis of primary care studies. Br J Gen Pract. 2013. . [DOI] [PMC free article] [PubMed]
- 8.Strumilo J, Chlabicz S, Pytel-Krolczuk B, et al. Combined assessment of clinical and patient factors on doctors’ decisions to prescribe antibiotics. BMC Fam Pract. 2016;17:63. doi: 10.1186/s12875-016-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akkerman AE, Kuyvenhoven MM, van der Wouden JC, Verheij TJ. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. J Antimicrob Chemother. 2005;56(5):930–936. doi: 10.1093/jac/dki283. [DOI] [PubMed] [Google Scholar]
- 10.Petursson P. GPs’ reasons for ‘non-pharmacological’ prescribing of antibiotics. A phenomenological study. Scand J Prim Health Care. 2005;23(2):120–125. doi: 10.1080/02813430510018491. [DOI] [PubMed] [Google Scholar]
- 11.Sandelowski M, Docherty S, Emden C. Focus on qualitative methods. Qualitative metasynthesis: issues and techniques. Res Nurs Health. 1997;20(4):365–371. doi: 10.1002/(sici)1098-240x(199708)20:4<365::aid-nur9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.France EF, Wells M, Lang H, Williams B. Why, when and how to update a meta-ethnography qualitative synthesis. Syst Rev. 2016;5:44. doi: 10.1186/s13643-016-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother. 2011;66(10):2215–2223. doi: 10.1093/jac/dkr279. [DOI] [PubMed] [Google Scholar]
- 14.Freund T, Everett C, Griffiths P, et al. Skill mix, roles and remuneration in the primary care workforce: who are the healthcare professionals in the primary care teams across the world? Int J Nurs Stud. 2015;52(3):727–743. doi: 10.1016/j.ijnurstu.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181. doi: 10.1186/1471-2288-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2005;366(9488):825–831. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 17.Malpass A, Shaw A, Sharp D, et al. ‘Medication career’ or ‘moral career’? Two sides of managing antidepressants: a meta-ethnography of patients’ experience of antidepressants. Soc Sci Med. 2009;68(1):154–168. doi: 10.1016/j.socscimed.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 18.Dixon-Woods M, Sutton A, Shaw R, et al. Appraising qualitative research for inclusion in systematic reviews: a quantitative and qualitative comparison of three methods. J Health Serv Res Policy. 2007;12(1):42–47. doi: 10.1258/135581907779497486. [DOI] [PubMed] [Google Scholar]
- 19.Britten N, Campbell R, Pope C, et al. Using meta ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy. 2002;7(4):209–215. doi: 10.1258/135581902320432732. [DOI] [PubMed] [Google Scholar]
- 20.McDonagh M, Peterson K, Winthrop K, et al. Improving antibiotic prescribing for uncomplicated acute respiratory tract infections. Rockville, MD: AHRQ; 2016. Comparative effectiveness review No. 163. Agency for Healthcare Research and Quality Comparative Effectiveness Review Publication 15(16)-EHC033-EF. [PubMed] [Google Scholar]
- 21.Andre M, Grondal H, Strandberg EL, et al. Uncertainty in clinical practice — an interview study with Swedish GPs on patients with sore throat. BMC Fam Pract. 2016;17:56. doi: 10.1186/s12875-016-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashdown HF, Raisanen U, Wang K, et al. Prescribing antibiotics to ‘at-risk’ children with influenza-like illness in primary care: qualitative study. BMJ Open. 2016;6(6):e011497. doi: 10.1136/bmjopen-2016-011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabral C, Ingram J, Lucas PJ, et al. Influence of clinical communication on parents’ antibiotic expectations for children with respiratory tract infections. Ann Fam Med. 2016;14(2):141–147. doi: 10.1370/afm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract. 2016. . [DOI] [PMC free article] [PubMed]
- 25.Zhang Z, Zhan X, Zhou H, et al. Antibiotic prescribing of village doctors for children under 15 years with upper respiratory tract infections in rural China: a qualitative study. Medicine (Baltimore) 2016;95(23):e3803. doi: 10.1097/MD.0000000000003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthierens S, Tonkin-Crine S, Cals JW, et al. Clinicians’ views and experiences of interventions to enhance the quality of antibiotic prescribing for acute respiratory tract infections. J Gen Intern Med. 2015;30(4):408–416. doi: 10.1007/s11606-014-3076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabral C, Lucas PJ, Ingram J, et al. ‘It’s safer to ...’ parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc Sci Med. 2015;136–137:156–164. doi: 10.1016/j.socscimed.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Grondal H, Hedin K, Strandberg EL, et al. Near-patient tests and the clinical gaze in decision-making of Swedish GPs not following current guidelines for sore throat — a qualitative interview study. BMC Fam Pract. 2015;16:81. doi: 10.1186/s12875-015-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallas A, van Driel M, van de Mortel T, Magin P. Antibiotic prescribing for the future: exploring the attitudes of trainees in general practice. Br J Gen Pract. 2014. . [DOI] [PMC free article] [PubMed]
- 30.Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194. doi: 10.1186/s12875-014-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedin K, Strandberg EL, Grondal H, et al. Management of patients with sore throats in relation to guidelines: an interview study in Sweden. Scand J Prim Health Care. 2014;32(4):193–199. doi: 10.3109/02813432.2014.972046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott L, Yardley L, Little P, et al. Process evaluation of a point-of-care cluster randomised trial using a computer-delivered intervention to reduce antibiotic prescribing in primary care. BMC Health Serv Res. 2014;14:594. doi: 10.1186/s12913-014-0594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustafa M, Wood F, Butler CC, Elwyn G. Managing expectations of antibiotics for upper respiratory tract infections: a qualitative study. Ann Fam Med. 2014;12(1):29–36. doi: 10.1370/afm.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szymczak JE, Feemster KA, Zaoutis TE, Gerber JS. Pediatrician perceptions of an outpatient antimicrobial stewardship intervention. Infect Control Hosp Epidemiol. 2014;35(Suppl 3):S69–S78. doi: 10.1086/677826. [DOI] [PubMed] [Google Scholar]
- 35.Cals JW, van Leeuwen ME, Chappin FH, et al. ‘How do you feel about antibiotics for this?’ A qualitative study of physician attitudes towards a context-rich communication skills method. Antibiotics (Basel) 2013;2(3):439–449. doi: 10.3390/antibiotics2030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis NA, Phillips R, Wood F, et al. Parents’ and clinicians’ views of an interactive booklet about respiratory tract infections in children: a qualitative process evaluation of the EQUIP randomised controlled trial. BMC Fam Pract. 2013;14:182. doi: 10.1186/1471-2296-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaruseviciene L, Radzeviciene Jurgute R, Bjerrum L, et al. Enabling factors for antibiotic prescribing for upper respiratory tract infections: perspectives of Lithuanian and Russian general practitioners. Ups J Med Sci. 2013;118(2):98–104. doi: 10.3109/03009734.2013.778925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leydon GM, McDermott L, Moore M, et al. A qualitative study of GP, NP, and patient views about the use of rapid streptococcal antigen detection tests (RADTs) in primary care: ‘swamped with sore throats?’. BMJ Open. 2013;3(4):e002460. doi: 10.1136/bmjopen-2012-002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roque F, Soares S, Breitenfeld L, et al. Attitudes of community pharmacists to antibiotic dispensing and microbial resistance: a qualitative study in Portugal. Int J Clin Pharm. 2013;35(3):417–424. doi: 10.1007/s11096-013-9753-4. [DOI] [PubMed] [Google Scholar]
- 40.Strandberg EL, Brorsson A, Hagstam C, et al. ‘I’m Dr Jekyll and Mr Hyde’: are GPs’ antibiotic prescribing patterns contextually dependent? A qualitative focus group study. Scand J Prim Health Care. 2013;31(3):158–165. doi: 10.3109/02813432.2013.824156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anthierens S, Tonkin-Crine S, Douglas E, et al. General practitioners’ views on the acceptability and applicability of a web-based intervention to reduce antibiotic prescribing for acute cough in multiple European countries: a qualitative study prior to a randomised trial. BMC Fam Pract. 2012;13:101. doi: 10.1186/1471-2296-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brookes-Howell L, Hood K, Cooper L, et al. Clinical influences on antibiotic prescribing decisions for lower respiratory tract infection: a nine country qualitative study of variation in care. BMJ Open. 2012;2(3):e000795. doi: 10.1136/bmjopen-2011-000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brookes-Howell L, Hood K, Cooper L, et al. Understanding variation in primary medical care: a nine-country qualitative study of clinicians’ accounts of the non-clinical factors that shape antibiotic prescribing decisions for lower respiratory tract infection. BMJ Open. 2012;2:e000796. doi: 10.1136/bmjopen-2011-000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaruseviciene L, Radzeviciene-Jurgute R, Lazarus JV, et al. A study of antibiotic prescribing: the experience of Lithuanian and Russian GPs. Cent Eur J Med. 2012;7:790–799. [Google Scholar]
- 45.Rowbotham S, Chisholm A, Moschogianis S, et al. Challenges to nurse prescribers of a no-antibiotic prescribing strategy for managing self-limiting respiratory tract infections. J Adv Nurs. 2012;68(12):2622–2632. doi: 10.1111/j.1365-2648.2012.05960.x. [DOI] [PubMed] [Google Scholar]
- 46.Vazquez-Lago JM, Lopez-Vazquez P, Lopez-Duran A, et al. Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360. doi: 10.1093/fampra/cmr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters S, Rowbotham S, Chisholm A, et al. Managing self-limiting respiratory tract infections: a qualitative study of the usefulness of the delayed prescribing strategy. Br J Gen Pract. 2011. . [DOI] [PMC free article] [PubMed]
- 48.Tonkin-Crine S, Yardley L, Coenen S, et al. GPs’ views in five European countries of interventions to promote prudent antibiotic use. Br J Gen Pract. 2011. . [DOI] [PMC free article] [PubMed]
- 49.Wood F, Brookes-Howell L, Hood K, et al. A multi-country qualitative study of clinicians’ and patients’ views on point-of-care tests for lower respiratory tract infection. Fam Pract. 2011;28(6):661–669. doi: 10.1093/fampra/cmr031. [DOI] [PubMed] [Google Scholar]
- 50.Bekkers MJ, Simpson SA, Dunstan F, et al. Enhancing the quality of antibiotic prescribing in primary care: qualitative evaluation of a blended learning intervention. BMC Fam Pract. 2010;11:34. doi: 10.1186/1471-2296-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Björnsdóttir I, Kristinsson KG, Hansen EH. Diagnosing infections: a qualitative view on prescription decisions in general practice over time. Pharm World Sci. 2010;32(6):805–814. doi: 10.1007/s11096-010-9441-6. [DOI] [PubMed] [Google Scholar]
- 52.Cals JW, Chappin FH, Hopstaken RM, et al. C-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPs. Fam Pract. 2010;27(2):212–218. doi: 10.1093/fampra/cmp088. [DOI] [PubMed] [Google Scholar]
- 53.Frich JC, Høye S, Lindbaek M, Straand J. General practitioners and tutors’ experiences with peer group academic detailing: a qualitative study. BMC Fam Pract. 2010;11:12. doi: 10.1186/1471-2296-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Høye S, Frich J, Lindboek M. Delayed prescribing for upper respiratory tract infections: a qualitative study of GPs’ views and experiences. Br J Gen Pract. 2010. . [DOI] [PMC free article] [PubMed]
- 55.Kotwani A, Wattal C, Katewa S, et al. Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Fam Pract. 2010;27(6):684–690. doi: 10.1093/fampra/cmq059. [DOI] [PubMed] [Google Scholar]
- 56.McDermott L, Yardley L, Little P, et al. Developing a computer delivered, theory based intervention for guideline implementation in general practice. BMC Fam Pract. 2010;11:90. doi: 10.1186/1471-2296-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cals JW, Butler CC, Dinant GJ. ‘Experience talks’: physician prioritisation of contrasting interventions to optimise management of acute cough in general practice. Implement Sci. 2009;4:57. doi: 10.1186/1748-5908-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler CC, Simpson S, Wood F. General practitioners’ perceptions of introducing near-patient testing for common infections into routine primary care: a qualitative study. Scand J Prim Health Care. 2008;26(1):17–21. doi: 10.1080/02813430701726285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stock K, Wollny A, Brockmann S, et al. Qualitativer Blick in die Blackbox: edukative Intervention zur senkung unnötiger Antibiotikaverordnungen (CHANGE) (Qualitative glance into the blackbox: educational intervention to reduce unnecessary antibiotic prescriptions) Z Allg Med. 2008;84:444–450. [Google Scholar]
- 60.Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluroquinolone antibiotics. Fam Pract. 2007;24(5):427–434. doi: 10.1093/fampra/cmm040. [DOI] [PubMed] [Google Scholar]
- 61.Hart AM, Pepper GA, Gonzales R. Balancing acts: deciding for or against antibiotics in acute respiratory infections. J Fam Pract. 2006;55(4):320–325. [PubMed] [Google Scholar]
- 62.Altiner A, Knauf A, Moebes J, et al. Acute cough: a qualitative analysis of how GPs manage the consultation when patients explicitly or implicitly expect antibiotic prescriptions. Fam Pract. 2004;21(5):500–506. doi: 10.1093/fampra/cmh505. [DOI] [PubMed] [Google Scholar]
- 63.Varonen H, Sainio S. Patients’ and physicians’ views on the management of acute maxillary sinusitis. Scand J Prim Health Care. 2004;22(1):22–26. doi: 10.1080/02813430310003147. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S, Little P, Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ. 2003;326(7381):138. doi: 10.1136/bmj.326.7381.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arroll B, Goodyear-Smith F, Thomas DR, Kerse N. Delayed antibiotic prescriptions: what are the experiences and attitudes of physicians and patients? J Fam Pract. 2002;51(11):954–959. [PubMed] [Google Scholar]
- 66.Björnsdóttir I, Hansen EH. Intentions, strategies, and uncertainty inherent in antibiotic prescribing. Eur J Gen Pract. 2002;8:18–24. [Google Scholar]
- 67.Björnsdóttir I, Hansen EH. Ethical dilemmas in antibiotic prescribing: analysis of everyday practice. J Clin Pharm Ther. 2002;27(6):431–440. doi: 10.1046/j.1365-2710.2002.00442.x. [DOI] [PubMed] [Google Scholar]
- 68.Rollnick S, Seale C, Rees M, et al. Inside the routine general practice consultation: an observational study of consultations for sore throats. Fam Pract. 2001;18(5):506–510. doi: 10.1093/fampra/18.5.506. [DOI] [PubMed] [Google Scholar]
- 69.Coenen S, Van Royen P, Vermeire E, et al. Antibiotics for coughing in general practice: a qualitative decision analysis. Fam Pract. 2000;17(5):380–385. doi: 10.1093/fampra/17.5.380. [DOI] [PubMed] [Google Scholar]
- 70.Elwyn G, Gwyn R, Edwards A, Grol R. Is ‘shared decision-making’ feasible in consultations for upper respiratory tract infections? Assessing the influence of antibiotic expectations using discourse analysis. Health Expect. 1999;2(2):105–117. doi: 10.1046/j.1369-6513.1999.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barden LS, Dowell SF, Schwartz B, Lackey C. Current attitudes regarding use of antimicrobial agents: results from physician’s and parents’ focus group discussions. Clin Pediatr (Phila) 1998;37(11):665–671. doi: 10.1177/000992289803701104. [DOI] [PubMed] [Google Scholar]
- 72.Butler CC, Rollnick S, Pill R, et al. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998;317(7159):637–642. doi: 10.1136/bmj.317.7159.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daker-White G, Donovan J, Campbell R. Redefined by illness: meta-ethnography of qualitative studies on the experience of rheumatoid arthritis. Disabil Rehabil. 2014;36(13):1061–1071. doi: 10.3109/09638288.2013.829531. [DOI] [PubMed] [Google Scholar]
- 74.McCann S, Campbell M, Entwistle V. Recruitment to clinical trials: a meta-ethnographic synthesis of studies of reasons for participation. J Health Serv Res Policy. 2013;18(4):233–241. doi: 10.1177/1355819613483126. [DOI] [PubMed] [Google Scholar]
- 75.Britten N, Riley R, Morgan M. Resisting psychotropic medicines: a synthesis of qualitative studies of medicine-taking. Adv Psychiatr Treat. 2010;16:207–218. [Google Scholar]
- 76.Broom A, Broom J, Kirby E. Cultures of resistance? A Bourdieusian analysis of doctors’ antibiotic prescribing. Soc Sci Med. 2014;110:81–88. doi: 10.1016/j.socscimed.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 77.Charani E, Castro-Sanchez E, Sevdalis N, et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of ‘prescribing etiquette’. Clin Infect Dis. 2013;57(2):188–196. doi: 10.1093/cid/cit212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackerman S, Gonzales R. The context of antibiotic overuse. Ann Intern Med. 2012;157(3):211–212. doi: 10.7326/0003-4819-157-3-201208070-00013. [DOI] [PubMed] [Google Scholar]
- 79.Yagil D, Medler-Liraz H. Clinical expert or service provider? Physicians’ identity work in the context of counterprofessional patient requests. Qual Health Res. 2015;25(9):1199–1211. doi: 10.1177/1049732314557088. [DOI] [PubMed] [Google Scholar]
- 80.Stolper E, Van de Wiel M, Van Royen P, et al. Gut feelings as a third track in general practitioners’ diagnostic reasoning. J Gen Intern Med. 2011;26(2):197–203. doi: 10.1007/s11606-010-1524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stolper E, van Royen P, Dinant GJ. The ‘sense of alarm’ (‘gut feeling’) in clinical practice. A survey among European general practitioners on recognition and expression. Eur J Gen Pract. 2010;16(2):72–74. doi: 10.3109/13814781003653424. [DOI] [PubMed] [Google Scholar]
- 82.McCutcheon HH, Pincombe J. Intuition: an important tool in the practice of nursing. J Adv Nurs. 2001;35(3):342–348. doi: 10.1046/j.1365-2648.2001.01882.x. [DOI] [PubMed] [Google Scholar]