Abstract
Two distinct microwave power levels and techniques have been studied in two cases: low-power microwave (LPM) irradiation on in vitro Sequoia plants and high-power microwave (HPM) exposure on recovery rates of cryostored (−196°C) Sequoia shoot apices. Experimental variants for LPM exposure included: (a) in vitro plants grown in regular conditions (at 24 ± 1°C during a 16-h light photoperiod with a light intensity of 39.06 μEm−2 s−1 photosynthetically active radiation), (b) in vitro plants grown in the anechoic chamber with controlled environment without microwave irradiation, and (c) in vitro plants grown in the anechoic chamber with LPM irradiation for various times (5, 15, 30, 40 days). In comparison to control plants, significant differences in shoot multiplication and growth parameters (length of shoots and roots) were observed after 40 days of LPM exposure. An opposite effect was achieved regarding the content of total soluble proteins, which decreased with increasing exposure time to LPM. HPM irradiation was tested as a novel rewarming method following storage in liquid nitrogen. To our knowledge, this is the first report using this type of rewarming method. Although, shoot tips subjected to HPM exposure showed 28% recovery following cryostorage compared to 44% for shoot tips rewarmed in liquid medium at 22 ± 1 °C, we consider that the method represent a basis and can be further improved. The results lead to the overall conclusion that LPM had a stimulating effect on growth and multiplication of in vitro Sequoia plants, while the HPM used for rewarming of cryopreserved apices was not effective to achieve high rates of regrowth after liquid nitrogen exposure.
Keywords: Cryopreservation, Sequoia, Irradiation, Microwaves, Redwood
Introduction
Electromagnetic waves in the range of microwave frequencies (300 MHz to 300 GHz) are widely used in various technical applications. Among them, wireless communications (CDMA - Code Division Multiple Access, WLAN - Wireless Local Area Network, WiFi - Wireless Fidelity, WIMAX - Worldwide Interoperability for Microwave Access, LTE - Long Term Evolution) and domestic heating using commercial microwave ovens are the most common. It has been reported that wireless communications are the main source of electrosmog (the electromagnetic environmental pollution), with possible effects on living systems [1]. Previous reports on biological materials have focused mainly on the effect of high-power electromagnetic waves and only a few studies have reported the effects of low-power electromagnetic radiation on seed germination or growth of plants. Among the most interesting investigation related to practical applications, the following studies can be included: the germ-reducing effect of medicinal plants under microwave irradiation [2], the nondestructive detection of water stress in plants [3], the enhancement of essential oil content [4], stimulation of germination [5, 6], estimation of plant adaptation to saline environment by microwave sensing [7], or the growth enhancement of seeds watered with water exposed to microwave radiation [8]. It was shown that the environmental conditions significantly influenced plant growth and development [9]. In a recent review, it was reported that electromagnetic effects in biology are irreproducible and contradictory [10]. Our previous studies showed that the most common cause of the irreproducibility of the electromagnetic effects is an inappropriate experimental design where the environmental conditions and microwave radiation sources are not accurately controlled [5, 11].
Traditionally, conservation of genetic resources for woody species involves field collections [12]. This form of germplasm preservation requires large spaces, is expensive, and carries the risk of losses due to biotic or abiotic stresses [13]. Therefore, cryopreservation of plant material (shoot apices, somatic or zygotic embryos, axillary buds, etc.) in liquid nitrogen (−196 °C) is now used for the storage of genetic resources as a complementary conservation method. Cryopreservation that completely arrests cell divisions and metabolism has been considered as an ideal means for the long-term storage of woody species [14]. Redwood is a vulnerable conifer species native to the central and northern California coast [15]. Other areas of successful cultivation outside of the native range include Western Europe, New Zealand, and southeastern United States [16]. Although, our environment is surrounded by sources of electromagnetic radiation like GSM communication stations, television and radio transmitters, little is known about the biological effects on plants. In order to limit the level of microwave pollution, ICNIRP international recommendations represent the basis for national rules [17]. It was suggested that most of the microwave irradiation studies on plants led to inconclusive results [18]. Microwaves as nonionizing radiations are part of the electromagnetic spectrum and the physiological impact of nonionizing radiation has long been considered negligible [19]. The effects of low-frequency electromagnetic waves (such as LPM) on biological materials were studied mainly on bacteria, viruses, DNA, and less on in vitro plants. Because in vitro studies are performed outside the normal biological context, a major advantage is that these studies permit a species-specific, more convenient, and detailed analysis than can be done with the whole organism. Studies on the effects of low-power microwaves on plant tissues, and particularly on morphological and physiological parameters, are scarce. Therefore, we decided to study the effects of microwave fields on a woody plant species of high economic value, respectively on in vitro grown Sequoia plants. Our study is focused on two major aspects:
Growth parameters including the length of shoots and roots of in vitro grown plants and the total soluble protein content under various LPM irradiation times (0, 5, 15, 30, 40 days). The LPM field used in the experiment is less than 10 μW/m2 in the 2.41 GHz to 2.48 GHz frequencies range. The reason was to select LPM power densities similar to the average electrosmog power density values surrounding a WLAN router, measured at 1 m distance with specific spectrum analyzer settings;
The effects of HPM irradiation (used for rewarming) on regrowth of shoot apices following cryostorage. As HPM generator, a patented microwave power unit (2.45 GHz ± 50 MHz, 700 W maximum output power) was used, with specific microwave radiation pulse control and a coaxial microwave cavity as a treatment chamber [20]. To our knowledge, this is the first report concerning results about the application of HPM for sample rewarming following cryostorage.
Materials and methods
Microwave irradiation parameters
Low-power microwave (LPM) and high-power microwave (HPM) irradiations are microwave techniques producing distinct effects. LPM produces a non-thermal effect or a negligible thermal effect for a short exposure period. The LPM exposure can be performed in free space or in special enclosures manufactured for experimental purposes [5]. HPM irradiation is a specific technique used for heating or for other physical and chemical microwaves assisted effects in processing of various materials (plasma generation, catalyst, etc.), and the exposure is allowed only in special closed enclosures (treatment chambers) for environmental and operator protection. Based on the above-mentioned reasons, the experimental setup for LPM and HPM will be presented separately.
The LPM experimental setup
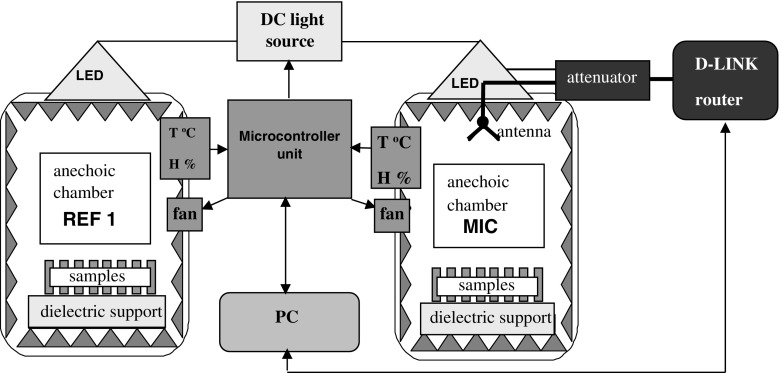
Two identical small (24 cm3 effective volume, L × W × H = 29 × 29 × 29 cm) anechoic chambers [5] (Fig. 1) were connected to a local environment conditioning system based on an embedded system with microcontroller unit in order to maintain the same growing environment: temperature, humidity (sensor type Sensirion SHT75 (±2% RH, ±0.3 °C) and LED illumination (with time cycle control) for two sets of 50 samples; the local environment conditioning is based on fan control to exhaust moisture (type Gembird HD-A3, 0.25 m3/min) so the temperature (T °C) and humidity (H%) have the same values in both anechoic chambers [11, 21]. In the microwave (MIC) anechoic chamber, the in vitro plants were exposed to LPM from a WLAN router while the second anechoic chamber without LPM exposure was used as a reference (REF 1). To exclude the effect of environmental electrosmog pollution, both chambers have a microwave inner-outer barrier of 60 dB in the full range of 10 MHz to 6 GHz. A D-LINK wireless router 802.11 g (2.41–2.48 GHz frequency range, set on channel 6 (2.437 GHz), Pout = max. 100 mW, (19 dBm)) was used as WLAN generator. The router was connected to a miniature microstrip antenna (25 mm highest size) [22] via a coaxial attenuator (3 dB, DC-12.4 GHz, 2 W, SUHNER) to obtain a power density (or an electric field density) in the MIC chamber equal to the average value of the microwaves power density surrounding a WLAN router at 1 m distance. The WLAN router was connected to the PC and set for the repetitive searching mode on selected channel, without LAN connection.
Fig. 1.
The low-power microwave irradiation experimental setup
The microwave power density was measured at the beginning of the irradiation cycle, in the middle of the MIC chamber at approximately 12 cm from the dielectric support, using a portable spectrum analyzer (Aaronia Spectran HF6060, with a measurement inaccuracy of ±2 dB) and an antenna probe of 0 dB gain (D = 25 mm length, 0.8 mm diameter). The spectrum analyzer was set on the following parameters: central frequency band equal with the WLAN channel frequency (f = 2.437GHz, Ch. 6), span 5 MHz, sweep-time 500 ms, radio bandwidth RBW = 1 MHz, video bandwidth VBW = 1 MHz, pulse mode with average (AVG) on 20 sweeps. The power density measured in the middle of the chamber was 7 (+4/−2.6) μW/m2. The reduced dimensions of the emitting microstrip antenna correlated with the router wavelengths (λ ≈ 12.2 cm) gives the Fraunhofer limit (L) at about 10 mm distance from the antenna. We considered the near field boundary to start at least five times the Fraunhofer distance
| 1 |
Thus, both the samples and the measurement location are situated in the far field region, hence the space impedance in this area can be considered equal to the open space impedance (Zo = 377 Ω). The average power density measured at 12 cm above the floor (in the presence of the samples) corresponds to an electric field strength |E| = 51(+30/−19) mV/m. The ICNIRP limit for general public exposure is 61 V/m for the electric field strength [17], the measured value represents 0.08% of this limit. The samples, consisting of shoots (2 cm in length) grown on 20-ml solid culture medium in glass jars, were placed in both anechoic chambers (50 samples per chamber).
The HPM experimental setup
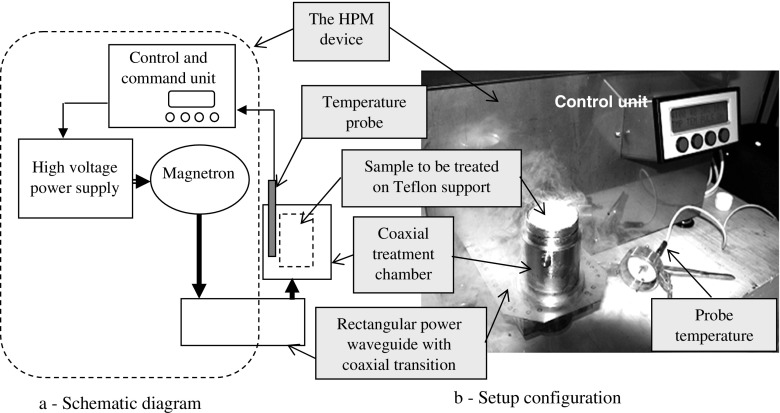
A particular experimental HPM treatment device has been set up for rewarming studies of shoot apices following cryostorage (diagram in Fig. 2a, and the setup in Fig. 2b).
Fig. 2.
The high-power microwave irradiation experimental setup
The HPM treatment device based on a microwave power magnetron generator (700 W max. power supplied by a high-voltage supply (4 kV, 1 A) from a proprietary control unit) delivers on the rectangular waveguide microwave radiation of 2.45 GHz ± 50 MHz and controllable power levels (70 W to 700 W), each step with programmable pulse width modulated time (1 s period/100 mS width step) [20]. A temperature probe (–40 to 120 °C) immersed in the treatment chamber allows the HPM irradiation to turn off if the selected temperature set point is reached and to turn on below the selected set point during the entire treatment period. We used a proprietary patented temperature probe [23] calibrated to an accuracy of ±0.5 °C in the –10 to +80 °C range. Increasing the electromagnetic (EM) immunity was possible thanks to a tubular sheath made of a non-magnetic material, having a copper end, whereon a temperature sensor was fixed with soldering alloy. The probe was inserted into a zone of minimal distribution of microwave field power density as described in the RO125999A2 patent [23].
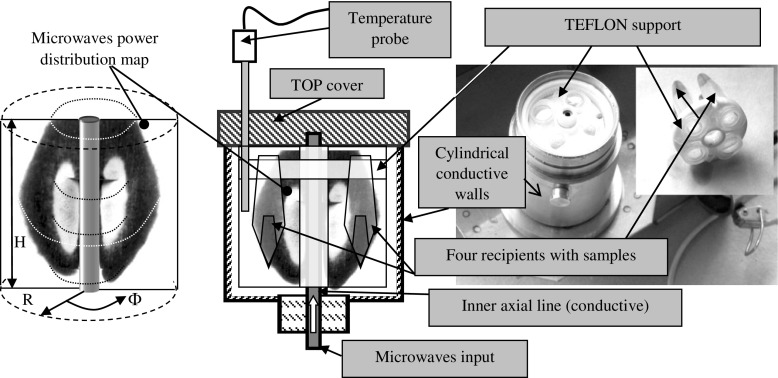
A custom treatment chamber was designed (Fig. 3) for rewarming of biological material following liquid nitrogen storage. The chamber is a coaxial microwave cavity TEM101 coupled to the HPM treatment device with a power coaxial transition to the rectangular waveguide. The dimensions of the cavity allowed a unimodal microwave power distribution characterized by a thermographic transducer sheet [24] (Fig. 3). The samples were placed on a Teflon support (PTFE). The rewarming (after liquid nitrogen storage) method comprises the following steps: (i) the encapsulated redwood shoot apices were stored in a 25-l liquid nitrogen dewar; (ii) the coaxial treatment chamber was frozen to –40 °C using a volume of nitrogen liquid (the procedure was repeated three times until the nitrogen liquid evaporates without bubbles); (iii) the 2-ml cryovial containing samples was placed on the PTFE; (iv) the coaxial treatment chamber was filled to half level with liquid nitrogen and the top was covered; (v) during the HPM heating procedure, the internal temperature of the cavity was measured using the temperature probe while the temperature set point was selected at 40 ± 0.5 °C on an HPM device control unit.
Fig. 3.
The coaxial treatment chamber. The sample location is correlated with the microwave power distribution inside the cavity (TEM101 mode - electric and magnetic field: one maxim upon radius R, constant over angle Φ one maxim over height H); the power distribution map shows the intensity of the radiation in the diametrical section of the cavity; the image suggests a toroidal distribution, where the white color is the highest intensity field and black is the lower intensity field (the area of gray tonalities depends on the thermographic transducer exposure time (120 s) (Surducan et al. 1997)
Plant material
The effects of microwaves have been studied on redwood (Sequoia sempervirens (D. Don) Endl.) in vitro grown plants. The in vitro stock cultures were multiplied by monthly subculturing of nodal stem segments (1.5 cm in length) on [25] culture medium supplemented with 1.0 mg l−1 6-benzylaminopurine (BAP), 0.25 mg l−1 indole-3-butyric acid (IBA), and 30 g l−1 sucrose. The pH was adjusted to 5.7 and the medium was solidified with 8 g l−1 agar before autoclaving at 121 °C for 20 min. The plants were grown at 24 ± 1 °C during a 16-h light photoperiod and a light intensity of 39.06 μEm−2 s−1 photosynthetically active radiation (PAR). For irradiation studies regarding growth parameters, in vitro shoots (2 cm in length) were placed in glass jars on 20 ml of the above-mentioned culture medium. Experimental variants included, (a) in vitro shoots grown in conditions as mentioned above (REF 0), (b) in vitro shoots grown in the anechoic chamber with controlled environment without microwave irradiation (REF 1) and (c) in vitro shoots grown in the anechoic chamber with LPM irradiation (MIC).
Total soluble protein content
Plant material (100 mg) from the three experimental variants (REF 0, REF 1, MIC) was grounded with 1.5 ml phosphate buffer at pH = 6.1 on ice. The extract was centrifuged 15 min at 10000 rpm at 4 °C and the supernatant was stored at 4 °C until use. The Bradford [26] assay (bovine serum albumin as reference) was applied to determine spectrophotometrically (595 nm) the total soluble protein content. The protein concentration was assessed on fresh weight (FW) basis.
Cryopreservation
Cryopreservation of Sequoia shoot apices was performed using the encapsulation-dehydration method. The complete cryopreservation protocol was previously described [27]. In the mentioned study rewarming of samples following cryopreservation was performed in liquid MS medium at 22 ± 1 °C. For rewarming of the samples under HPM exposure, the cryovials with encapsulated apices were transferred to the above-mentioned unit for 3–4 min. Samples rewarmed in liquid MS medium at room temperature, as mentioned in [27], were used as control. Rewarmed beads by both methods were transferred to Petri dishes (5 cm in diameter) on sterile filter paper humidified with liquid medium for regrowth under temperature and light conditions as mentioned in the “Section 2.4”.
Statistical analysis of results
The growth parameters (length of shoots and roots) and the total soluble protein content under various LPM irradiation times (0, 5, 15, 30, 40 days) were measured and analyzed on plant material from the three experimental variants. In studies regarding the HPM irradiation for rewarming following cryopreservation, 50 encapsulated shoot apices were used. Survival of shoot tips was evaluated 40 days after rewarming and was expressed as the percentage of shoots developed from single apices. The results were expressed as the mean ± standard deviation (SD). Data were analyzed by ANOVA using the Tukey test for data comparison.
Results
Growth parameters under LPM irradiation
There were no significant differences in the length of shoots and roots between the three experimental growing categories for 5-, 15-, and 30-day irradiation durations while significant differences between the length of shoots and roots were observed after 40 days of LPM exposure (Table 1). After this period, the shoots from REF0, REF1, and MIC were cut into segments (1.5 cm in length) and transferred to fresh culture medium to examine the long-term effects of microwaves after treatment. After 30 days of culture in optimum growth conditions (24 °C and 16 h light/24-h photoperiod), the irradiated shoots showed an uncommon multiplication rate compared to control shoots (REF 0 and REF 1) (Table 2, Fig. 4a–c). The multiplication started from the base of the irradiated shoots (Fig. 4c) and following 40 days of irradiation, the number of multiplied shoots was significantly higher (23 shoots) compared to shoots grown in the anechoic chamber (six shoots) or in the growth room (seven shoots) (Table 2).
Table 1.
In vitro plant growth and development under LPM exposure
| Growth conditions/Length of shoots and roots (cm ± SD)* | ||||
|---|---|---|---|---|
| Plant material | Irradiation duration (days) | Growth room (REF 0) | Anechoic chamber (REF 1) | LPM exposure (MIC) |
| Shoots | 0 | 2.03 ± 0.05a | 1.96 ± 0.20a | 2.03 ± 0.15a |
| 5 | 2.16 ± 0.11a | 2.16 ± 0.15a | 2.40 ± 0.10a | |
| 15 | 3.53 ± 0.35b | 3.76 ± 0.37a | 4.36 ± 0.11a | |
| 30 | 5.83 ± 0.15b | 6.10 ± 0.20a | 6.83 ± 0.35a | |
| 40 | 10.06 ± 0.30b | 10.33 ± 0.51a | 12.06 ± 0.20b | |
| Roots | 0 | 0.26 ± 0.05a | 0.26 ± 0.05a | 0.30 ± 0.10a |
| 5 | 0.30 ± 0.10a | 0.33 ± 0.11a | 0.46 ± 0.05a | |
| 15 | 1.56 ± 0.11a | 1.43 ± 0.15a | 2.16 ± 0.25a | |
| 30 | 2.46 ± 0.15a | 2.33 ± 0.20a | 2.80 ± 0.10a | |
| 40 | 3.30 ± 0.20a | 3.36 ± 0.15a | 4.10 ± 0.10b | |
*Data represent means ± SD. Values followed by the same letter within a row are not significantly different (p < 0.05).
Table 2.
Effect of LPM exposure on in vitro shoot multiplication
| Irradiation duration/Number of shoots (nr. ± SD)* | ||||
|---|---|---|---|---|
| 5 days | 15 days | 30 days | 40 days | |
| Growth room (REF 0) | 1.66 ± 0.57a | 3.33 ± 1.52b | 7.33 ± 2.30b | 7.33 ± 1.15b |
| Anechoic chamber (REF 1) | 1.33 ± 0.57a | 3.66 ± 0.57b | 5.66 ± 1.15b | 6.00 ± 1.73b |
| LPM exposure (MIC) | 2.66 ± 0.57a | 10.0 ± 2.00a | 15.33 ± 1.52a | 23.33 ± 2.51a |
*Data represent means ± SD. Values followed by the same letter within a column are not significantly different (p < 0.05).
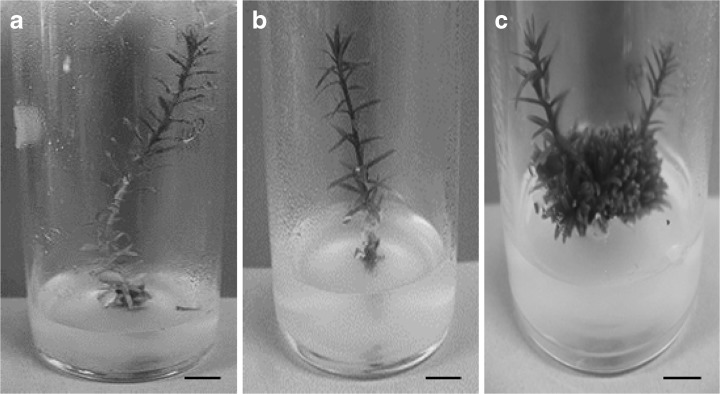
Fig. 4.
In vitro grown Sequoia plants in growth room (REF 0) at 24 ± 1 °C (a), anechoic chamber (REF 1) (b), and under LPM irradiation for 30 days (MIC) (c). Bars = 1 cm
Effects of LPM exposure on total soluble protein content
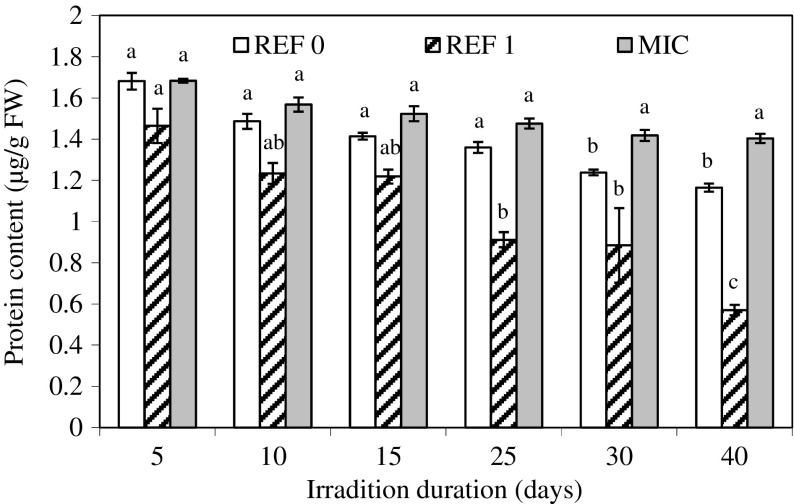
The protein content in plants exposed to different irradiation times and growth conditions showed significant differences between the tested categories (Fig. 5). High values of protein content in irradiated shoots were measured, while a significantly lower protein content was obtained in shoots grown in the anechoic chamber (REF 1) (Fig. 5). The content in total proteins after the 40 days of irradiation ranged between 0.57 μg/g FW (REF 1) and 1.40 μg/g FW (MIC). Significant differences were noticed between shoots grown in the anechoic chamber (REF 1) and in the growth room (REF 0). The protein content in irradiated shoots decreased from 1.68 μg/g FW after 5 treatment days to 1.40 μg/g FW after 40 days of irradiation.
Fig. 5.
Effect of LPM exposure on total soluble protein content in redwood shoots. Growth room (REF 0), Anechoic chamber (REF 1), LPM exposure (MIC)
Rewarming following cryostorage under HPM exposure
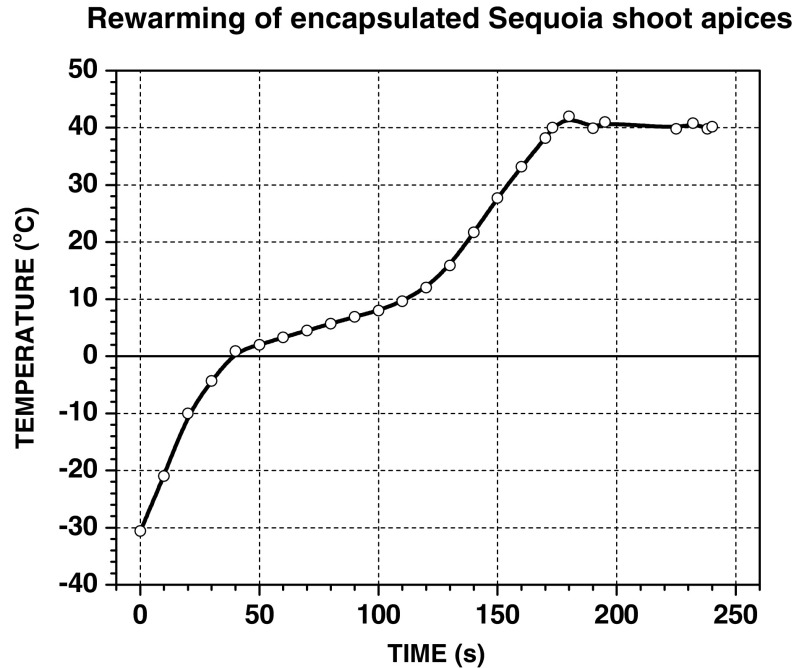
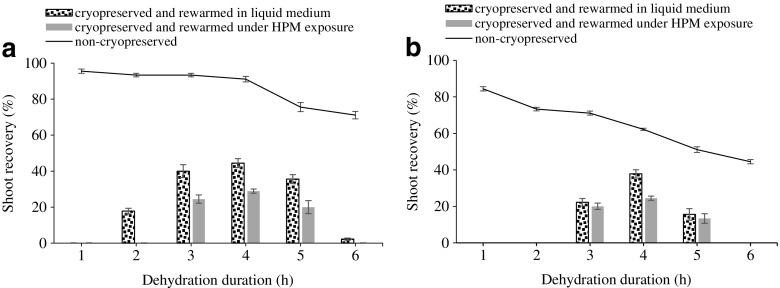
Rewarming of alginate beads following liquid nitrogen storage is usually made either in a water bath at a temperature of 38–40 °C, in liquid medium, or in air at room temperature. We used rewarming of encapsulated Sequoia shoot apices under HPM irradiation as an alternative approach to rewarming in liquid medium. Rewarming conditions using HPM were tested to obtain an optimized rewarming curve so that thawing should take less than 180 s and should not exceed 40 °C (Fig. 6). Recovery of cryopreserved shoot apices started after approximately 25 days following thawing regardless of the rewarming method used, in liquid culture medium or under HPM irradiation (Fig. 7). Significant differences were observed between the rates of recovered shoot tips rewarmed in liquid medium compared to recovered shoot tips rewarmed under microwave exposure (Fig. 8a, b). According to the sucrose concentration used in preculture, the recovery rates varied. So, in case of 0.5 M sucrose, the regrowth was 44% for shoot tips rewarmed in liquid medium and 28% for shoot tips subjected to HPM exposure (Fig. 8a). In case of preculture in 0.75 M sucrose, the recovery rates were lower for both rewarming techniques (Fig. 8b).
Fig. 6.
Thawing curve (after liquid nitrogen storage) using HPM irradiation. The diagram was obtained with the following HPM irradiation parameters: MWP = 700 W, EffMWE = 13.8 Wh, δ = 30%, t = 312 s, Teff = 240 s, TSP = 40 °C, Tin = −30.6 °C, Tfin = 40 °C
Fig. 7.
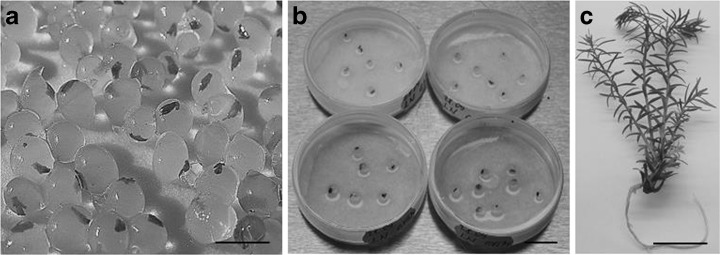
a Redwood shoot apices encapsulated in Na-alginate beads (control); b encapsulated apices after cryopreservation directly after rewarming using HPM exposure; c redwood plant after 3 months following liquid nitrogen storage. Bars = 1 cm
Fig. 8.
Effect of rewarming procedure on shoot recovery following cryostorage related to the sucrose concentration used in preculture, 0.5 M (a) and 0.75 M (b) and dehydration duration. Different letters indicate significantly differences (p < 0.05). Vertical bars represent SD
Discussion
In our study concerning the effects of LPM and HPM irradiation, two major issues need special attention, first the effects of LPM on in vitro grown Sequoia plants seen as a whole biological system, and second the recovery ability under HPM exposure of apical dome meristematic cells, respectively encapsulated shoot tips (2–3 mm in length) following liquid nitrogen storage.
LPM exposure
The effects of LPM exposure duration on the development of shoot and roots showed similar results for both plant organs, shoots and roots (Table 1) and phenotypically the irradiated shoots were similar to the control (REF 0 and REF 1) shoots and no abnormalities were observed. In studies related to growth of plants, it has been proven that the exposed plants developed bigger longitude and weight [28]. The LPM used for irradiation in this experiment induced a high rate of cell division, which resulted in high multiplication rate of irradiated shoots (Table 2). These results are in accordance with previously published data showing an enhanced growth of corn and bean under LPM exposure [21] or with results showing that microwave irradiation was stimulating for seed germination and seedling vigor [6]. On the other hand, our results on LPM irradiation of redwood shoots are in contradiction with previously reported data where environmental radiation on soybean seedlings decreased the growth plants, emphasizing that non-thermal exposure levels for 900-MHz radiation were not beneficial for plant growth [29]. The high multiplication rate of irradiated shoots was similar to other results where increased cell division in irradiated protoplasts was observed [30]. Contrary to the findings of a diminished growth of lens roots exposed to a radiation of 1800 MHz [31], the Sequoia roots showed increased growth under LPM (2410 MHz to 2480 MHz) exposure.
It is well known that stress conditions induce numerous physiological and biochemical changes at various levels of a biological system, such as changes in protein expression [32] or that short exposure to microwave irradiation protects cells from UV radiation lesions [33]. The response of plants depends upon intensity and duration of irradiation, plant species, or the stage of development. In redwood shoots, the high amount of total soluble proteins showed an activated metabolism under LPM irradiation. Only after prolonged irradiation duration (40 days) was the protein content lower. These results are in agreement with our previously reported data on corn and bean [21].
HPM exposure
The physiological mechanisms involved in cellular response to stress factors have generated interest [34]. It is known that in cryopreservation by encapsulation-dehydration, the osmotic dehydration in sucrose solution is usually followed by evaporative desiccation in order to achieve moisture content compatible with cryopreservation [35]. Due to the extreme dehydration of explants, most freezable water is removed from cells, and vitrification of internal solutes takes place during rapid exposure to liquid nitrogen avoiding lethal intracellular ice crystallization [36]. The HPM exposure of encapsulated Sequoia shoot apices had a detrimental effect in terms of survival and regrowth after liquid nitrogen storage. Thereby, significantly lower regeneration rates were obtained after rewarming by HPM irradiation (Fig. 8). A possible explanation of the adverse effects of HPM on redwood plants could be associated either with the power level of HPM irradiation, approx. 500 W in HPM pulsed mode compared with less than 100 mW in LPM mode or with the small size of shoot tips (only a length of 0.2–0.3 cm). It was shown that a certain length of shoot apices is essential to secure further regrowth after cryostorage, otherwise it was reported that too small shoot tips tend to die during the freeze–thaw cycle [37]. Jayasanka and Asaeda [38] in a recent review showed that the effects of microwaves on plants are dependent not only on the plant family and growth stage but also on the exposure duration, frequency, or power density. The complex mechanisms by which plants react to the exposure of electromagnetic fields are not entirely known. A common response to stress factors including irradiation is the increased production of reactive oxygen species, which can damage cell structures by oxidizing proteins, membrane lipids, and nucleic acids [39]. It has been shown that our environment is polluted with waste in the form of dangerous electromagnetic radiation [40] and trees are particularly sensitive and react to environmental changes [41]. The results regarding the effects of microwave treatments on plants are controversial for many reasons, which are related either to the type of plant material (seeds, shoots, plants) or to the duration of exposure, frequency, or the large variety of experimental conditions used.
Conclusions
The results lead to the conclusion that the applied LPM in the range of 2410 MHz to 2480 MHz, (7 μW/m2 power density) significantly enhanced the growth (length of shoot and roots) and multiplication of in vitro Sequoia plants. LPM irradiation led to a decrease in the amount of total soluble proteins with an increasing exposure time.
The HPM (2450 ± 50 MHz, 13.8 Wh) used for rewarming of encapsulated apices was not effective to achieve high rates of survival and regeneration after cryostorage.
To improve this method, further experiments using modified treatment parameters (involving less effective microwave energy) represent a possible approach. This study could be a solid basis for further studies on the effects of microwave irradiation on plant physiological, biochemical, or molecular parameters with extension to animal cells. For example, LPM irradiation could be used on seeds or plants to increase production or to stimulate plant growth and development.
Acknowledgments
Financial support from the National Authority for Scientific Research and Innovation - ANCSI, Core Programme, PN16-30 01 02 (Nucleu-INCDTIM Cluj-Napoca, Romania) and the national program PN16 19 Biodivers (NIRDBS Romania) is gratefully acknowledged.
Author contributions
A.H., E.S., and V.S. planned and conducted the experiments and contributed to the writing of manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.World Health Organization: Electromagnetic fields and public health: mobile phones, Fact sheet N°193 (2011)
- 2.Brantner, A., Lücke, W.: Influence of physical parameters on the germ-reducing effect of microwave irradiation on medicinal plants. Pharmazie 50, 762–766 (1995) [PubMed]
- 3.Shimomachi T, Takemasa T, Takakura T, Kurata K. Nondestructive detection of plant water stress by microwave sensing. Acta Hortic. 2006;710:465–470. doi: 10.17660/ActaHortic.2006.710.57. [DOI] [Google Scholar]
- 4.Soran ML, Stan M, Niinemets Ü, Copolovici L. Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J Plant Physiol. 2014;171:1436–1443. doi: 10.1016/j.jplph.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balint VC, Surducan V, Surducan E, Oroian IG. Plant irradiation device in microwave field with controlled environment. Comput Electron Agric. 2016;121:48–56. doi: 10.1016/j.compag.2015.11.012. [DOI] [Google Scholar]
- 6.Ragha L, Mishra S, Ramachandran V, Bhatia MS. Effects of low-power microwave fields on seed germination and growth rate. J Electromagn Anal Appl. 2011;3:165–171. [Google Scholar]
- 7.Shimomachi, T., Takemasa, T., Kurata, K., Kobashigawa, C., Omoda, E., Takakura, T.: Quantitave estimation method of plant adaptation responses to saline environment using microwave sensing. Acta Hortic. 797, 463–468 (2008)
- 8.Fuangfoong M, Eaipresertsak K, Chim-Oye T, Dugkanya K. Effect of low-power microwave radiation on seed growth rate. Taipei: PIERS Proceedings; 2013. pp. 676–679. [Google Scholar]
- 9.Vian, A., Davies, E., Gendraud, M., Bonnet, P.: Plant responses to high frequency electromagnetic fields. BioMed Res Intl. 2016, pp. 13 (2016) [DOI] [PMC free article] [PubMed]
- 10.Buchachenko, A.: Why magnetic and electromagnetic effects in biology are irreproducible and contradictory. Bioelectromagnetics 37, 1–13 (2016) [DOI] [PubMed]
- 11.Surducan, E., Surducan, V., Halmagyi, A.: Process and installation for stimulating plant growth in microwave field. Romanian patent RO 125068 B1 (2012)
- 12.Lambardi, M.: Cryopreservation of germplasm of Populus (Poplar) species. In: Towill, L.E., Bajaj, Y.P.S. (eds.) Biotechnology in Agriculture and Forestry 50, pp. 269–286. Springer, Berlin (2002)
- 13.Nordin, M.S., Saad, M.S.: Genetic considerations in field genebank conservation. In: Saad, M.S., Rao, V.R. (eds.) Establishment and Management of Field Genebank, pp. 66–72. International Plant Genetic Resources Institute, Serdang (2001)
- 14.Pâques, M., Monod, V., Poissonnier, M., Dereuddre, J.: cryopreservation of Eucalyptus sp. shoot tips by the encapsulation-dehydration procedure. In: Towill, L.E., Bajaj, Y.P.S. (eds.) Biotechnology in Agriculture and Forestry 50, pp. 234–245. Springer, Berlin (2002)
- 15.IUCN: IUCN Red List of Threatened Species. http://www.iucnredlist.org (2007)
- 16.Noss, R.F.: The Redwood Forest: History, Ecology and Conservation of the Coast Redwood. Island Press, Washington DC (2000)
- 17.ICNIRP Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz) Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- 18.Hore, P.J.: Are biochemical reactions affected by weak magnetic field? Proc. Natl. Acad. Sci. U.S.A. 109, 1357–1358 (2012) [DOI] [PMC free article] [PubMed]
- 19.Vian A, Roux D, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G. Microwave irradiation affects gene expression in plants. Plant Signal Behav. 2006;1:67–70. doi: 10.4161/psb.1.2.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surducan, E., Surducan, V.: Process and installation for dynamic substance processing in power microwave field. Romanian patent RO 122063 B1 (2008)
- 21.Surducan, E., Surducan, V., Keul, A., Halmagyi, A.: Microwaves irradiation experiments on biological samples. Studia UBB Biologia. LVIII, 83–98 (2013)
- 22.Surducan, E., Iancu, D., Glossner, J.: Microstrip multi-band composite antenna. International patent (WIPO) WO 2006086194 A2 (2006)
- 23.Surducan, E., Surducan, V.: Method and transducer for measuring the temperature in the microwave power processing treatments. Romanian patent RO 125999 A2 (2009)
- 24.Surducan, E., Surducan, V.: Thermographic transducer for microwave radiation. Romanian patent, RO 116506 B1 (2001)
- 25.Murashige T, Skoog FA. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Halmagyi A, Deliu C. Cryopreservation of redwood (Sequoia sempervirens (D. Don.) Endl.) shoot apices by encapsulation-dehydration. Contrib Bot. 2011;46:117–125. [Google Scholar]
- 28.Martínez, M.T., Ballester, A., Vieitez, A.M.: Cryopreservation of embryogenic cultures of Quercus robur using desiccation and vitrification procedures. Cryobiology 46, 182–189 (2003) [DOI] [PubMed]
- 29.Halgamuge, M.N., Yak, S.K., Eberhardt, J.L.: Reduced growth of soybean seedlings after exposure to weak microwave radiation from GSM 900 mobile phone and base station. Bioelectromagnetics 36, 87–95 (2015) [DOI] [PubMed]
- 30.Haneda, T., Fujimura, Y., Iino, M.: Magnetic field exposure stiffens regenerating plant protoplast cell walls. Bioelectromagnetics 27, 98–104 (2006) [DOI] [PubMed]
- 31.Akbal A, Kiran Y, Sahin A, Turgut-Balik D, Balik HH. Effects of electromagnetic waves emitted by mobile phones on germination, root growth, and root tip cell mitotic division of Lens culinaris Medik. Pol J Environ Stud. 2012;21:23–29. [Google Scholar]
- 32.Chen RD, Tabaeizadeh Z. Alteration of gene expression in tomato plants (Lycopersicon esculentum) by drought and salt stress. Genome. 1992;35:385–391. doi: 10.1139/g92-058. [DOI] [Google Scholar]
- 33.Chen, Y.P.: Microwave treatment of eight seconds protects cells of Isafis indigofica from enhanced UV-8 radiation lesions. Photochem Photobiol. 82, 503–507 (2006) [DOI] [PubMed]
- 34.Fulda, S., Gorman, A.M., Hori, O., Samali, A.: Cellular stress responses: cell survival and cell death. Intl J Cell Biol. (2010). doi:10.1155/2010/245803 [DOI] [PMC free article] [PubMed]
- 35.Dumet D, Grapin A, Bailly C, Dorion N. Revisiting crucial steps of an encapsulation/desiccation based cryopreservation process: importance of thawing method in the case of Pelargonium meristems. Plant Sci. 2002;163:1121–1127. doi: 10.1016/S0168-9452(02)00323-0. [DOI] [Google Scholar]
- 36.Engelmann, F.: In vitro conservation methods. In: Callow, J.A., Ford-Loyd, B.V., Newbury, H.J. (eds.) Biotechnology and Plant Genetic Resources, pp. 119–161. CAB International, Oxford (1997)
- 37.Thinh, N.T., Takagi, H.: Cryopreservation of in vitro grown apical meristems of terrestrial orchids (Cymbidium spp.) by vitrification. In: Engelmann, F., Takagi, H. (eds.) Cryopreservation of Tropical Plant Germplasm: Current Research Progress and Applications, pp. 441–443. JIRCAS, Tsukuba and IPGRI, Rome (2000)
- 38.Jayasanka SMDH, Asaeda T. The significance of microwaves in the environment and its effect on plants. Environ Rev. 2014;22:220–228. doi: 10.1139/er-2013-0061. [DOI] [Google Scholar]
- 39.Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–1238. [Google Scholar]
- 40.Volkrodt, W.: Droht den Mikrowellen ein ähnliches Fiasko wie der Atomenergie? Wetter-Boden-Mensch. 4, 16-23 (1991)
- 41.Balodis V, Brumelis G, Kalviskis K, Nikodemus O, Tjarve D, Znotina V. Does the Skrunda radio location station diminish the radial growth of pine trees? Sci Total Environ. 1996;180:57–64. doi: 10.1016/0048-9697(95)04920-7. [DOI] [Google Scholar]