Abstract
This paper describes the production and chemical separation of the 163Ho isotope that will be used in several nuclear physics experiments aiming at measuring the neutrino mass as well as the neutron cross section of the 163Ho isotope. For this purpose, several batches of enriched 162Er have been irradiated at the Institut Laue-Langevin high flux reactor to finally produce 6 mg or 100 MBq of the desired 163Ho isotope. A portion of the Er/Ho mixture is then subjected to a sophisticated chemical separation involving ion exchange chromatography to isolate the Ho product from the Er target material. Before irradiation, a thorough analysis of the impurity content was performed and its implication on the produced nuclide inventory will be discussed.
Introduction
The radioactive isotope 163Ho (t1/2 = 4567 a [1,2]) has gained considerable attention within the physics community due to its very low Q-value for electron capture decay of only 2.8 keV [3,4]. This property and recent developments in high precision calorimetric measurements have facilitated several research projects devoted to measuring the mass of the neutrino. Among them, the HOLMES collaboration aims at implanting 3x105 Bq of isotopically pure 163Ho a grid of 103 transition edge sensor micro-calorimeters to precisely measure the end-point region of the energy spectrum of the 163Ho electron capture decay [5]. With an envisaged statistical mass sensitivity around 1 eV, this measurement will provide an alternative technique to spectrometry to answer the long lasting question in physics about the neutrino mass [6].
The isotope 163Ho is also an interesting nuclide in terms of nuclear physics research in the field of bound state beta decay. It has been experimentally observed that under fully ionized conditions (such as in stellar environments), the previously stable 163Dy isotope becomes radioactive and decays to 163Ho with a half-life of 47 d [7]. This circumstance significantly influences the s-process pathway in the A = 163 mass region, opening an additional branch towards isotopes accessible mainly via the p-process. Within this context, the neutron-capture cross section of the 163Ho isotope is foreseen to be measured at the n_TOF CERN facility at thermal to stellar energies for the first time. While the Maxwellian average neutron capture cross-section (MACS) of 163Ho has been measured in 1995 at stellar energies [8], a new measurement with several mg quantities of 163Ho is envisaged to determine the neutron capture cross section in the eV to keV region typical for stellar environments [9].
In collaboration with the Institut Laue-Langevin (ILL), Grenoble, France, and the Paul Scherrer Institut (PSI), Villigen, Switzerland, the HOLMES project aims at producing roughly 100 MBq of 163Ho by irradiation of enriched 162Er material in a nuclear reactor. The production route 162Er(n,γ)163Er followed by decay to 163Ho is depicted in Fig 1 together with other neutron capture reactions. While neutron irradiation of 162Er has been shown to be the most efficient way of producing the 163Ho isotope [10,11], alternative routes via proton irradiation of natDy or enriched 164Dy foils have also been reported in literature [1,12,13]. The HOLMES collaboration has chosen to pursue the former production method due to higher 163Ho production yields [10] and better availability of irradiation resources, taking into account its unavoidable disadvantage.
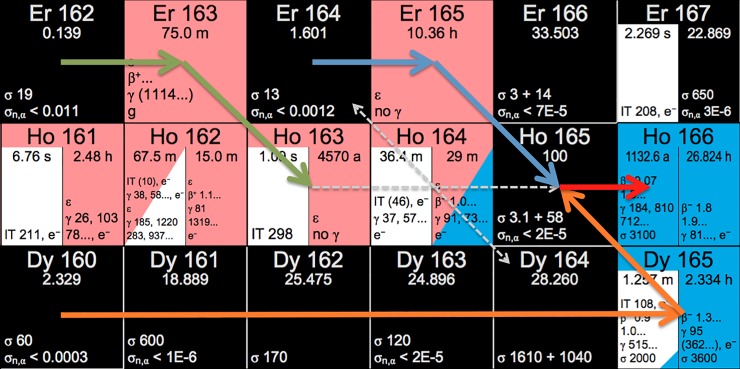
Fig 1. Excerpt from the nuclide chart showing the main production route of 163Ho (green arrows) together with parasitic formation of 166mHo (red arrow) from 165Ho.
The latter is formed by neutron captures from 164Er (blue arrows) or from Dy isotopes (orange arrows) within the irradiated material. Reprinted from nucleonica.com / Karlsruhe Nuclide Chart Online under a CC BY license, with permission from Dr. Joseph Magill, original copyright 10th Edition, 2018.
The main drawback arising from neutron irradiation of enriched Er is the inevitable formation of 166mHo (see Fig 1). This isotope has a half-life (t1/2 = 1132 a [14]) comparable to that of 163Ho and has a complex decay scheme, which drastically deteriorates calorimetric decay measurements of 163Ho contaminated with 166mHo [5]. Since the latter isotope cannot be separated from 163Ho by chemical means, a mass separation of these two isotopes is mandatory before the implantation of Ho into a calorimetric measurement system [5]. The main production pathways of 166mHo may be summarized as follows:
from neutron captures on 165Ho present as impurity in the irradiated Er material;
from the reaction 164Er(n,γ)165Er(EC) towards 165Ho and subsequent reaction 1;
from capture reactions of 164-xDy(xn,γ)165Dy(β-) towards 165Ho and subsequent reaction 1;
from neutron captures on 163Ho to 164g+mHo, that undergo either EC decay feeding reaction 3 or β- decay towards 164Er feeding reaction 2.
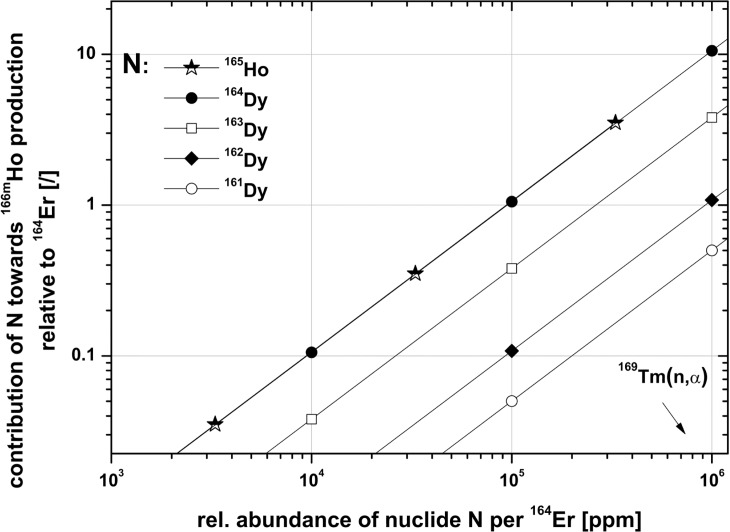
The production rates of 166mHo from 165Ho and the isotopes 161Dy, 162Dy, 163Dy and 164Dy as function of their concentration relative to 164Er for a 50 d irradiation with a thermal neutron flux of 1015 n cm-2 s-1 are given in Fig 2. As can be seen, the dominant impurities transmuting into 166mHo are 165Ho and 164Dy. While the main production pathway surely proceeds over the 164Er(n,γ) reaction, an impurity content of 5% relative to 164Er of 165Ho or 164Dy almost equally contributes to the 166mHo production. Since the thermal neutron capture cross section of 166mHo is in the order of 3 kb [15,16], its production and destruction rate will soon be in equilibrium at a neutron flux of 1015 n cm-2 s-1, resulting in a constant ratio of 165Ho to 166mHo after an irradiation time of 50 d (see also Fig 2 in [17]).
Fig 2. Contribution towards the production of 166mHo from Ho and Dy isotopes as function of their concentration relative to 164Er.
Calculated for a 50 d irradiation in a thermal neutron flux of 1015 n cm-2 s-1. Calculations were performed using the ChainSolver code [20]. Ho and 164Dy come to lie nearly on the same line since under the given flux 164Dy proceeds via reaction 3 almost completely to 166mHo, only a minor fraction leads via double capture reactions to 166Dy(β-)166gHo.
Other production routes of 166mHo, such as 169Tm(n,α)166mHo or 166Er(n,p)166mHo, are of minor importance due to the small reaction cross sections for these reactions [18,19] and the relatively well thermalized neutron flux during irradiation. For example, the fraction of epithermal and fast neutrons in the V4 beam tube of the ILL reactor is below 10%. Yet another production route of 165Ho proceeds via double neutron capture on 163Ho and largely depends on the involved thermal capture cross-sections of 163Ho and 164g+mHo. Up to date, only the former is known as it has been recently measured to be σ163Ho = (156 + 23) b for the formation of 164gHo and 164mHo, respectively [11]. Neutron capture reactions on 163Ho significantly influence the production rate and thus, the total amount of 163Ho after neutron irradiation. In addition, capture reactions on 164g+mHo yield again 165Ho, a pathway that competes with both 164gHo decay modes yielding 164Er and 164Dy (see hashed white lines in Fig 1). Thus, even for a chemically and isotopically pure 162Er material, the production of 163Ho in a high flux reactor is always accompanied by a number of parasitic reactions leading to 166mHo.
In addition to 166mHo, the irradiation will result in substantial amounts of 170Tm and 171Tm from capture reactions of 168Er(n,γ)169Er(β-)169Tm(n,γ)170Tm and 170Er(n,γ)171Er(β-)171Tm, respectively. Both of these isotopes are produced in the GBq range, representing the most significant hazard for handling the irradiated sample material. Moreover, any contamination of the final Ho product with kBq amounts of these isotopes will certainly deteriorate the sensitivity of 163Ho neutrino mass measurements. Additionally, capture reactions on impurity 158Dy will form 159Dy (t1/2 = 144 d). Thus, a sophisticated chemical separation of Ho not only from massive amounts of Er, but also from Tm and Dy has to be accomplished to assure a radiochemically clean product. A decrease in the production of 166mHo is achieved by choosing high enrichment grades of the 162Er material (with low 164Er content) and substantially reducing the amount of impurities (Ho, Dy) present in the initial material [21]. In order to quantify the amount of 163Ho and parasitic 166mHo formed during a reactor irradiation, a careful characterization of the initial Er material including isotope composition and impurity content should thus be performed.
Ultimately, the recovery of irradiated and purified 162Er is an additionally anticipated task. Due to the absence of any Er isotopes having half-lives exceeding 9.4 d (169Er), the recycled 162Er material is essentially free from any radioactivity after a cool-down period of 1 year and might be reused for new irradiations. This is especially desirable considering the price of enriched 162Er (> 100 $/mg). Such recycled material will moreover be depleted in 167Er, the strongest neutron absorber among all stable Er isotopes, which results in self-shielding and neutron flux depression during the first irradiation.
A detailed description of a possible separation procedure has been very recently given in [11]. The authors of this work suggest a pre-purification of the starting 162Er material in order to mitigate impurities lighter than Er. This approach maximizes the isotopic purity of 163Ho, but it is experimentally proven that the final Ho product will always result in a mixture of 163Ho, 165Ho and 166mHo after irradiation. Apart from the described purification, the authors provide a very thorough analysis of the final 163Ho material yielding 1.2x1018 atoms of 163Ho, 6.3x1017 atoms of 165Ho and 7 kBq (3.8x1014 atoms) of 166mHo from irradiating 30 mg of 20.4% enriched 162Er for 54 d in the ILL high flux reactor.
In parallel to results published in [11], this work describes our efforts in the frame of the HOLMES project to define and tune the production and separation process of 163Ho using two test batches of 162Er. We also report on the analysis of a final 470 mg batch of 162Er purchased in 2016 and give results on the separation of 163Ho from irradiated test batches of 2014 and 2015. In contrast to the approach presented in [11], the chemical separation procedure was based on a two-step process involving cation exchange and extraction chromatography, which has already been successfully applied for the separation of neighbouring lanthanides (see [22] for more details). It is experimentally shown that recycling of large quantities of enriched 162Er is feasible and might be continuously performed to satisfy needs of 163Ho for HOLMES. Finally, an analysis of the purified material from both test batches representing in total 1.5 mg (or 5x1018 atoms) of 163Ho will be given. No analysis of the purified 163Ho from the irradiated final 470 mg batch can be given since this material still awaits chemical separation.
Materials and methods
Materials
Three different batches of 162Er material have been processed at PSI between 2014 and 2016 and irradiated at ILL of which only the first two underwent a chemical purification so far. In 2014, 20.6 mg of 162Er2O3 (Isoflex, USA), enriched to 28.2% (164Er– 7.41%, 166Er– 32.24%, 167Er– 14.26%, 168Er– 12.26%, 170Er– 5.63%) and subsequently denoted as batch I, were purified from contaminants as described below and subsequently irradiated at ILL for 55 d. In 2015, 106.5 mg of 162Er2O3 (also Isoflex), enriched to 26.5% (164Er– 7.5%, 166Er– 31.2%, 167Er– 15.3%, 168Er– 13.0%, 170Er– 6.5%), were pre-purified, mixed with the previous irradiated and purified batch I and 119.5 mg of the resulting mixture (batch II) containing 26.8% 162Er2O3 was irradiated at ILL for 53 d. Finally, in 2016, a total mass of 544.2 mg of 25.1% enriched 162Er2O3 (for the isotopic composition refer to Table 1) was purchased from TraceSciences, Canada. This material, subsequently denoted as batch III, was not pre-purified and irradiated as delivered for 44 d. Each oxide from the described batches was provided to ILL in Suprasil 300 high purity quartz ampoules (Heraeus, Germany). Approximately 4 mg of batch III was kept for quantitative analysis of the material using ICP-OES, ICP-MS and neutron activation analysis (NAA).
Table 1. Isotopic composition and determined impurity content of 162Er provided by TraceScience as measured by ICP-MS, ICP-OES and NAA.
The original data provided by the supplier is also given.
| Analysis PSI | TraceScience CoA | |
|---|---|---|
| isotope | isotopic composition1 [%] | |
| Er-162 | 26.1(4) | 25.1(14) |
| Er-164 | 6.0(2) | 6.87 |
| Er-166 | 30.1(7) | 29.75 |
| Er-167 | 15.5(4) | 15.67 |
| Er-168 | 15.6(6) | 15.63 |
| Er-170 | 6.7(2) | 6.98 |
| impurities | concentration relative to Er [at. ppm] | |
| Eu | 15(1)§ 1,3 | / |
| Gd | 29(2)1 | 1000 |
| Tb | 7(1)1 | / |
| Dy | 5160(280)*,2 | 5400 |
| Ho | 235(25)1, 3 | / |
| Tm | 360(30)1 | 900 |
| Yb | 1530(70)*,2 | / |
| Lu | 265(10)§ 1,3 | / |
1 –measured by ICP-MS
2 –measured by ICP-OES
3 –measured by NAA
§ - average value of two independent measurement techniques
*—the isotopic composition of Dy and Yb is non-natural, see Table 2; values denoted by “/” were not stated
Material characterization
For a characterization of the material of batch III, a solution was prepared by dissolving 4 mg of 162Er2O3 in 2 mL of 1 M HNO3 and half of this solution used to prepare dilution series of 1:10, 1:100 and 1:1000 for ICP-OES measurements. For each dilution, 18 MΩ cm MilliQ water was provided by an in-house water purification system. Dy, Ho, Er, Tm and Yb calibration solutions in concentrations ranging from 10 ppb up to 10 ppm were prepared from their respective ICP standard solutions provided by Merck, Germany. A Perkin-Elmer Optima 3000 optical emission spectrometer was used to measure the concentration of these 5 elements in the Er stock solution. The following emission lines of the respective elements were used for analysis: Dy—396.839 nm; Ho– 345.60 nm; Er– 337.217 nm; Tm– 313.126 nm; Yb– 328.937 nm.
Another 400 μL of the stock solution was evaporated in a PE vessel and irradiated for 104 s in the neutron activation facility of the PSI spallation neutron source (SINQ). 3.9 mg of IRMM-527 (Al-0.1%Co) alloy, Sigma Aldrich, USA, was used as flux monitor. Gamma spectra of the 162Er sample were recorded 5h, 24h and 4d after end of irradiation. Then the sample was irradiated with neutrons for another 104 s and immediately underwent chemical separation by ion exchange chromatography as described below. The separation was monitored by γ‑spectrometry using a coaxial p-type HPGe detector, Mirion Technologies, USA. The Genie2000 software, Canberra, was used to evaluate the recorded spectra. Each sample was measured for at least 10 min to provide a statistical uncertainty below 1% for the peak area of interest. The separated fractions of Dy, Er and Yb were then measured for their isotopic composition using a sector-field based mass spectrometer Element 2, Thermo Fischer Scientific, Germany. The ICP mass spectrometer was operated in low resolution mode and wet plasma conditions. All measurement solutions were prepared from high purity nitric acid and water in polymer vessels.
Material separation
For the separation of 163Ho from irradiated 162Er of each test batch I and II, the ampoule containing the oxide was cracked, the material dissolved in 5 mL of 7 M HNO3 and the pH adjusted to 4 with approximately 10 mL NH4OH. Approx. 0.5 mg of 162Er activated at the PSI SINQ facility to yield 171Er (t1/2 = 7.52 h) was added prior to each separation to be able to monitor the elution of Er using γ‑spectrometry. The solution was then loaded onto a column (L = 23 cm, d = 1 cm) containing 19 g of the cation exchange resin Aminex HPX87H from BioRad Laboratories, USA. Batch I containing 20.6 mg of 162Er2O3 was entirely loaded on the column, while the solution of batch II containing 119.5 mg of 162Er2O3 was divided in half to allow two independent separations. The elution of the lanthanides was performed at room temperature using increasing concentrations of α-hydroxy-isobutyric acid (HIBA) in a similar way as described in [22] via a peristaltic pump ISM834C (Ismatec, Switzerland). The gradual elution of the lanthanides was performed in 10 mL steps using a flow rate of 0.66 mL/min and was monitored by γ‑spectrometry. All fractions containing chemically pure Ho were unified, the resulting 30 mL of solution acidified with 6 mL 1 M HNO3 and loaded on a column (L = 16 m, d = 1 cm) containing 4.7 g of LN1 resin (Triskem, France) for final purification from HIBA and residual contaminants using increasing concentrations of HNO3. Fractions containing Er were unified to yield 70 mL of solution, acidified with 15 mL 1 M HNO3 and similarly loaded on LN1 resin for HIBA stripping. After the elution of Er with 4 M HNO3, the acid is evaporated and the residue burned to the oxide in a quartz ampule (Heraeus, Germany) using a flame torch. The total recovery of recycled 162Er is determined gravimetrically using an AE 100 balance, Mettler-Toledo, Switzerland.
The purified Ho fractions were unified, evaporated to dryness and redissolved in 10 mL of 1 M HNO3. This solution was subsequently investigated with ICP-MS and γ‑spectrometry to determine the content of 163Ho, 165Ho and 166mHo. The concentration of 163Ho and 165Ho was measured by dilution series using a Ho standard solution, Merck, Germany, as reference. All uncertainties are reported according to the “guide to the expression of uncertainty in measurement” (GUM) with a coverage factor of k = 1.
Results and discussion
Material composition
The results of the ICP-MS, ICP-OES and neutron activation analysis of batch III is given in Table 1 together with the data from the original Certificate of Analysis provided by the supplier. The results obtained from all three analytical methods agree well within the statistical uncertainties of the measurement. The only significant deviation was found between results obtained by NAA and ICP-MS on Dy and Yb, since both of these impurity elements were found to have a non-natural composition.
The determined isotopic composition of Er tends to agree with the numbers provided by the supplier. It should be noted that the impurity content given by the supplier partially deviates from what was found by the analysis at PSI. This fact is due to isobaric interferences arising from Dy and Yb impurities, which are present in the material in the ‰ range. During the isotope enrichment process, not only the main product is enriched, but also its isobaric impurities such as or . Thus, in isotopically enriched samples of 162Er, the Dy impurity gets enriched in 162Dy. This fact has been confirmed by ICP-MS measurements of the Dy isotopic composition given in Table 2. Apart from much lower Gd content, the analysed sample also contained a significant amount (1530 ppm) of Yb enriched in 176Yb, which has not been reported in the original analysis provided by the supplier. The Yb impurity is believed to originate from previous enrichment runs of 176Yb operated at the same enrichment facility.
Table 2. The isotopic composition of Dy and Yb present as impurities in the analysed 162Er batch III; measured by ICP-MS.
| isotope | abundance | isotope | abundance |
|---|---|---|---|
| Dy-156 | 0.02% | Yb-168 | 0.16% |
| Dy-158 | 0.03% | Yb-170 | 2.17% |
| Dy-160 | 0.35% | Yb-171 | 6.03% |
| Dy-161 | 8.40% | Yb-172 | 8.84% |
| Dy-162 | 64.13% | Yb-173 | 10.42% |
| Dy-163 | 15.84% | Yb-174 | 15.08% |
| Dy-164 | 11.23% | Yb-176 | 57.31% |
From the relative abundance of 164Er and the concentration of the Dy and Ho impurities in the initial batch III material it is possible to deduce their expected contribution towards the 166mHo production according to Fig 2. As calculations show, roughly 80% of parasitic 166mHo will be formed from 164Er, while 8% is expected to be produced from 164Dy. The initial content of 235 ppm Ho as well as 163Dy and 162Dy almost equally contribute with 4% to the total 166mHo inventory. Assuming a 163Ho burn-up as given in [11], the expected 163Ho:166mHo atomic ratio will be in the order of 3x103 according to calculations.
163Ho separation
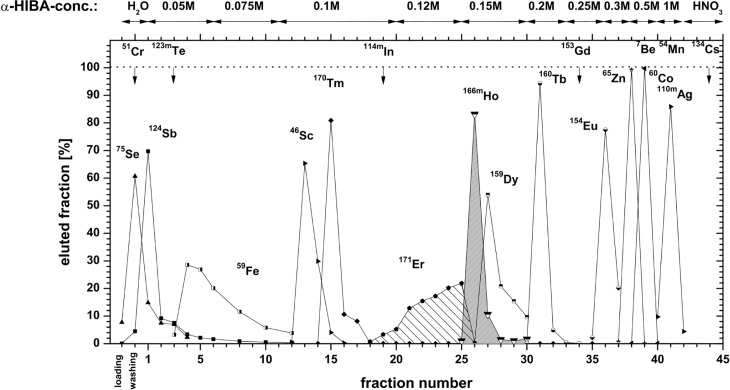
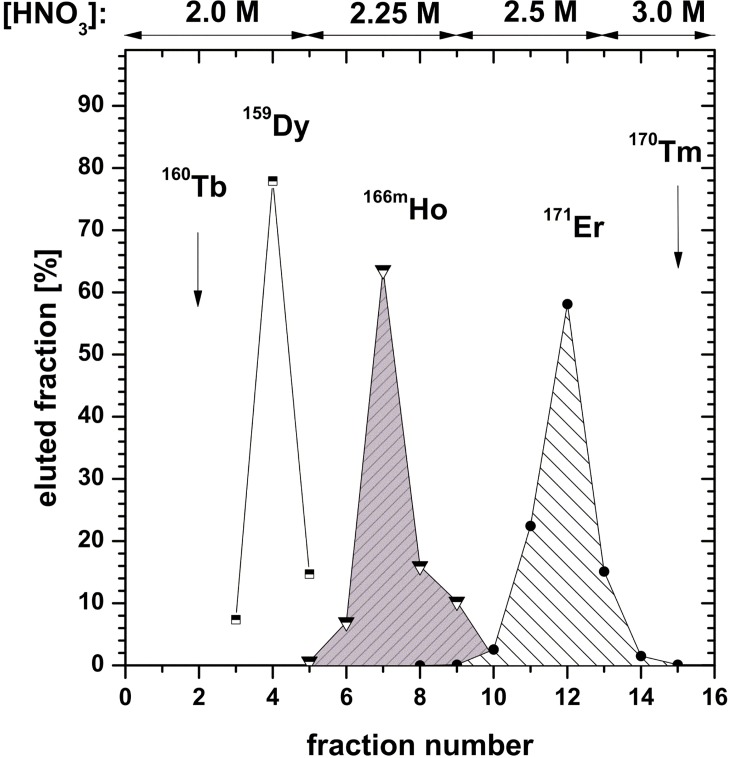
The separation profile of the batch II material irradiated for 53 d in 2015 is given in Fig 3. As it can be seen from Fig 3, a large number of radioisotopes is produced during the irradiation for 53 d at the ILL high flux reactor. Most elements may be efficiently separated on a cation exchange resin from the Ho product, although some overlap with Er and Dy was observed. This is due to the fact of high column loading that leads to inferior separation of the lanthanides [23]. 170/171Tm, 154Eu, 65Zn and 60Co, representing isotopes with the highest contribution to activity and dose rate, are efficiently separated. The absence of their γ-lines in the final Ho product yields decontamination factors exceeding 106. The additional purification of the Ho product on the LN1 resin even further decreased the impurity contribution from Er and Dy. The corresponding separation profile is given in Fig 4.
Fig 3. Separation profile of Ho from irradiated 162Er of batch II using exchange chromatography on Aminex HPX87H resin with increasing concentrations of α-hydroxy-isobutyric acid.
Fig 4. Separation profile of the final Ho purification on the LN1 resin using increasing concentrations of nitric acid.
The final mass of isolated 163Ho, the measured activity of 166mHo and the impurity content originating from the batches I and II are given in Table 3. The cumulative amount of 163Ho produced during these runs is approximately 1.5 mg, which represents roughly 27 MBq or 5x1018 atoms of this isotope. The contribution of signals at m/q = {161–170} relative to m/q = 163 is assigned to the sum of the respective isobars. Conservatively assuming that all signals at m/q = 166 are originating from 166Er, the overall separation factor Ho/Er is calculated to be at least 105 and 3x104 for batches I and II, respectively. The cumulative separation factor for Ho/Dy after both chromatographic separations is difficult to assess, since signals at m/q = {162–164} have isobaric interferences from 162Er, 163Ho and 164Er. The only Dy isotope free from isobaric interferences is 161Dy, of which the abundance, however, was not measured. A more sophisticated analysis of the final product using resonant ionization mass spectrometry in connection with NAA as described in [11] would resolve the issue. In any case it can be stated with certainty that both 163Ho samples processed so far contain less than 1% of (Dy+Er). This indicates that the separation procedure presented in this work yields 163Ho of satisfactory quality similar to results obtained in [11].
Table 3. Overview on 163Ho samples with their respective mass and composition.
| batch number | m(163Ho) [mg]1 |
A(163Ho) [MBq] | m(165Ho) [mg]1 |
163/165Ho at. ratio1 | A(166mHo) [kBq]2 |
163/166mHo at. ratio1,2 |
|---|---|---|---|---|---|---|
| I | 0.25(1)3 | 4.4 | 0.14(1) | 1.83 | 6.3(1) | 2.9x103 |
| II | 1.28(3)4 | 22.7 | 0.84(2) | 1.52 | 37.7(3) | 2.4x103 |
| III | 6* | 100* | 4* | 1.5* | 200* | 2x103* |
1 –measured by ICP-MS
2 –measured by γ‑spectrometry
3 –impurity content: {161,162,164,166,167,168,169,170}/163 < 0.0005
4 –impurity content: 162/163–0.00125; 164/163–0.0025; 166/163–0.0011; 168/163–0.0006
*—estimated numbers calculated using the ChainSolver program
Regarding the overall separation yield a clear dependence on the total mass of 162Er is noteworthy. While for a separation of 163Ho from 20.6 mg Er2O3 (batch I) a total yield of 98.4% was achieved, the yield dropped down to 79.2% in case of the batch II material where 119.5 mg of Er2O3 were processed. This is due to some unwanted overlapping of Er/Ho/Dy elution peaks as seen in Fig 3 as a result of higher column loadings with respect to the previous batch. In order to mitigate this problem in future, it is advisable to partition the column separation step into several runs. The recovery of 162Er was successful in every separation and more than 90% of the material could be recycled and used for new irradiations.
As can be seen from numbers given in Table 3, the production of 163Ho scales linearly with the amount of 162Er irradiated for ≈50 days at the ILL reactor. Due to higher 162Er enrichment of batch I, the total 163/165Ho atomic ratio is consequently higher. Enrichments above 90% of 162Er or much shorter irradiation times would be needed in order to make this ratio exceed 6. It is interesting to note that according to the measured data given in Table 3, the atomic ratio of 165Ho to 166mHo is constant through batch I and II and equals roughly 1.6x103. This is due to a dynamic equilibrium reached after 50 d of irradiation in the production and destruction pathways leading to 165Ho and 166mHo, as has been mentioned earlier.
The 544.2 mg of enriched 162Er2O3 of batch III as well as 110.1 mg of recovered 162Er2O3 from batch II were irradiated at the high flux reactor at ILL in 2017 and will undergo chemical separation soon. The separated material is intended for neutron cross section measurements at the CERN n_TOF facility prior to final delivery to the HOLMES isotope separator. With a total amount of 6 mg 163Ho, this new irradiated of batch III will fully cover the needs of this isotope within the frame of the HOLMES project [5].
Conclusions
We have successfully separated 1.5 mg of 163Ho, corresponding to 27 MBq, from macro amounts of enriched 162Er. This amount of 163Ho is at least one order of magnitude higher than can be reasonably achieved by any other production route involving e.g. proton irradiations of Dy targets [10]. It was also shown that a recycling of 162Er is easily achievable to meet with 163Ho requirements for nuclear physics experiments devoted to measure the mass of the neutrino and to deduce the 163Ho neutron capture cross section at stellar neutron energies. However, this production route comes with the disadvantage of parasitic formation of 166mHo, which cannot be separated from the wanted 163Ho by chemical means. A sophisticated, highly efficient and selective mass separation has to be performed for reliable removal of the isotopic contaminant in order to allow clean calorimetric measurements of the 163Ho electron capture decay. A mass separation involving laser resonant ionization has already been successfully proven to reduce the 166mHo amount by three orders of magnitude [21].
Acknowledgments
Many thanks to S. Tietze and K. Domnanich for the support during ICP-OES measurements. A. Vögele is acknowledged for his help during neutron activation. This work has received funding from the EC FP7 Programme under the CHANDA project (Grant no. 605203). The HOLMES experiment is funded by the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreement no. 340321.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by ERC: European Research Council, https://erc.europa.eu/, grant number 340321, S. Ragazzi; and EC FP7: European Commission, 7th Framework Programme, https://ec.europa.eu/research/fp7, grant number 605203, D. Schumann.
References
- 1.Kawakami O, Masuda A, Fujoka M, Omori T, Yasumi S. Half-Life of 163Ho. Phys Rev C. 1988;38(4):1857–60. [DOI] [PubMed] [Google Scholar]
- 2.Baisden PA, Sisson DH, Niemeyer S, Hudson B, Bennett CL, Naumann RA. Measurement of the Half-Life of 163Ho. Phys Rev C. 1983;28(1):337–41. [Google Scholar]
- 3.Eliseev S, Blaum K, Block M, Chenmarev S, Dorrer H, Duellmann CE, et al. Direct Measurement of the Mass Difference of Ho-163 and Dy-163 Solves the Q-Value Puzzle for the Neutrino Mass Determination. Phys Rev Lett. 2015;115(6). [DOI] [PubMed] [Google Scholar]
- 4.Ranitzsch P. C. O., Hassel C, Wegner M, Hengstler D, Kempf S, Fleischmann A, et al. Characterization of the 163Ho Electron Capture Spectrum: A Step Towards the Electron Neutrino Mass Determination. Phys Rev Lett. American Physical Society; 2017;119(12):122501EP. [DOI] [PubMed] [Google Scholar]
- 5.Alpert B, Balata M, Bennett D, Biasotti M, Boragno C, Brofferio C, et al. HOLMES The electron capture decay of Ho-163 to measure the electron neutrino mass with sub-eV sensitivity. European Physical Journal C. 2015;75(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucciotti A. The Use of Low Temperature Detectors for Direct Measurements of the Mass of the Electron Neutrino. Advances in High Energy Physics. 2016;2016:41. [Google Scholar]
- 7.Jung M, Bosch F, Beckert K, Eickhoff H, Folger H, Franzke B, et al. First observation of bound-state β−decay. Phys Rev Lett. 1992;69(15):2164–7. 10.1103/PhysRevLett.69.2164 [DOI] [PubMed] [Google Scholar]
- 8.Jaag S, Käppeler F. The Stellar (n, gamma) Cross Section of the Unstable Isotope 163Ho and the Origin of 164Er. The Astrophysical Journal. 1996;464:874–83. [Google Scholar]
- 9.Guerrero C, Domingo-Pardo C, Kaeppeler F, Lerendegui-Marco J, Palomo FR, Quesada JM, et al. Prospects for direct neutron capture measurements on s-process branching point isotopes. European Physical Journal A. 2017;53(5). [Google Scholar]
- 10.Engle JW, Birnbaum ER, Trellue HR, John KD, Rabin MW, Nortier FM. Evaluation of 163Ho production options for neutrino mass measurements with microcalorimeter detectors. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. Elsevier B.V; 2013;311(C):131–8. [Google Scholar]
- 11.Dorrer H, Chrysalidis K, Goodacre DT, Düllmann C, Eberhardt K, Enss C, et al. Production, isolation and characterization of radiochemically pure 163Ho samples for the ECHo-project. Radiochim Acta. 2018;106(7):535–48. [Google Scholar]
- 12.Mocko V, Taylor WA, Nortier FM, Engle JW, Barnhart TE, Nickles RJ, et al. Isolation of 163Ho from Dysprosium Target Material by HPLC for Neutrino Mass Measurements. Radiochim Acta. 2015;103(8):577–85. [Google Scholar]
- 13.Croce MP, Rabin MW, Mocko V, Kunde GJ, Birnbaum ER, Bond EM, et al. Development of Holmium-163 Electron-Capture Spectroscopy with Transition-Edge Sensors. Journal of Low Temperature Physics. 2016;184(3–4):958–68. [Google Scholar]
- 14.Nedjadi Y, Bailat C, Caffari Y, Froidevaux P, Wastiel C, Kivel N, et al. A new measurement of the half-life of 166mHo. Appl Rad Isot. Elsevier; 2012;70(9):1990–6. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Katoh T, Furutaka K, Wada H, Harada H, Baba T, et al. Measurement of Thermal Neutron Capture Cross Section and Resonance Integral of 90Sr and 166mHo. Journal of Nuclear Science and Technology. Taylor & Francis; 2014;39(2):258–61. [Google Scholar]
- 16.Katoh T, Nakamura S, Furutaka K, Harada H, Baba T, Fujii T, et al. Measurement of Thermal Neutron Capture Cross Section and Resonance Integral of the 166mHo(n, γ)167Ho Reaction using a Two-Step Irradiation Technique. Journal of Nuclear Science and Technology. 8 ed. Taylor & Francis; 2002;39(7):705–15. [Google Scholar]
- 17.Danilenko VN, Gromova NP, Konstantinov AA, Kurenkov NV, Malinin AB, Mamelin AV, et al. Methods of Producing Radionuclides for Spectrometric Gamma-Ray Sources and Their Standardization—3. Holmium-166m. Appl Rad Isot. 1989;40(9):789–92. [DOI] [PubMed] [Google Scholar]
- 18.Qaim SM. Activation Cross-Sections of (n,alpha) Reactions at 14.7 Mev in the Region of Rare-Earths. Radiochim Acta. 1984;35(1):5–9. [Google Scholar]
- 19.Dzysiuk N, Kadenko A, Kadenko I, Primenko G. Measurement and systematic study of (n, x) cross sections for dysprosium (Dy), erbium (Er), and ytterbium (Yb) isotopes at 14.7 MeV neutron energy. Phys Rev C. 2012;86(3):034609. [Google Scholar]
- 20.Romanov EG, Tarasov VA, Vahetov FZ. ORIP-21 Computer Programs for Isotope Transmutation Simulations. Avignon; 2005. [Google Scholar]
- 21.Gastaldo L, Blaum K, Chrysalidis K, Day Goodacre T, Domula A, Door M, et al. The electron capture in 163Ho experiment–ECHo. The European Physical Journal Special Topics. 2017;226(8):1623–94. [Google Scholar]
- 22.Heinitz S, Maugeri EA, Schumann D, Dressler R, Kivel N, Guerrero C, et al. Production, separation and target preparation of 171Tm and 147Pm for neutron cross section measurements. Radiochim Acta. 2017;105(10):801–11. [Google Scholar]
- 23.Qaim SM, Ollig H, Blessing G. Separation of Lanthanides by Preparative High-Pressure Liquid-Chromatography. Radiochim Acta. 1979;26(1):59–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.