Abstract
Background
Intravaginal rings (IVRs) can deliver antiretroviral (ARV) agents for HIV pre-exposure prophylaxis (PrEP), theoretically overcoming adherence concerns associated with frequent dosing. However, topical vaginal ARV drug delivery has not simultaneously led to sufficient rectal drug exposure to likely protect from HIV infection as a result of receptive anal intercourse (RAI). Unprotected RAI has a higher risk of infection per sex act and, for women, also can be associated with vaginal exposure during a single sexual encounter, especially in higher-risk subsets of women. The physiologically inflamed, activated, immune-cell dense colorectal mucosa is increasingly appreciated as the sexual compartment with highly significant risk; this risk is increased in the setting of co-infections. Ex vivo studies have shown that colorectal tissue and rectal fluid concentrations correlated with HIV protection. Given these important results, efforts to document colorectal compartment ARV drug concentration from pod-IVR delivery was assessed to determine if vaginal application could provide protective ARV levels in both compartments.
Methodology/Principal findings
A crossover clinical trial (N = 6) evaluated 7 d of continuous TDF pod-IVR use, a wash-out phase, followed by 7 d with a TDF-FTC pod-IVR. A subsequent clinical trial (N = 6) consisted of 7 d of continuous TDF-FTC-MVC pod-IVR use. Rectal fluids were collected on Day 7 at IVR removal in all three ARV-exposures (two Phase 1 trials) and drug concentrations quantified by LC-MS/MS.
Median rectal fluid concentrations of TFV, the hydrolysis product of the prodrug TDF, were between 0.66 ng mg-1 (TDF pod-IVR group) and 1.11 ng mg-1 (TDF-FTC pod-IVR group), but below the analytical lower limit of quantitation in 5/6 samples in the TDF-FTC-MVC pod-IVR group. Unexpectedly, median FTC (TDF-FTC pod-IVR, 20.3 ng mg-1; TDF-FTC-MVC pod-IVR, 0.18 ng mg-1), and MVC rectal fluid concentrations (0.84 ng mg-1) were quantifiable and higher than their respective in vitro EC50 values in most samples. Due to participant burden in these exploratory trials, rectal fluid was used as a surrogate for rectal tissue, where drug concentrations are expected to be higher.
Conclusions/Significance
The concentrations of FTC and MVC in rectal fluids obtained in two exploratory clinical trials of IVRs delivering ARV combinations exceeded levels associated with in vitro efficacy in HIV inhibition. Unexpectedly, MVC appeared to depress the distribution of TFV and FTC into the rectal lumen. Here we show that vaginal delivery of ARV combinations may provide adherence and coitally independent dual-compartment protection from HIV infection during both vaginal and receptive anal intercourse.
Introduction
Topical dosing of antiretroviral (ARV) agents using products formulated for rectal or vaginal application have the potential of protecting against sexually transmitted infections, including HIV. Intravaginal rings (IVRs) can deliver antiretroviral (ARV) agents for HIV pre-exposure prophylaxis (PrEP) [1–3], theoretically overcoming adherence concerns associated with frequent dosing [4]. We have developed an innovative IVR technology, the pod-IVR [1, 5], that enables accelerated development of products capable of delivering multiple agents over a wide range of aqueous solubilities and target delivery rates into the cervicovaginal compartment [2, 3, 6–8].
We have evaluated the clinical pharmacokinetics (PKs) and safety of pod-IVRs delivering the nucleoside reverse transcriptase inhibitor (NRTI) tenofovir disoproxil fumarate (TDF) alone and in combination with emtricitabine (FTC) [9], another NRTI. We subsequently conducted a clinical trial with the first triple combination ARV pod-IVR simultaneously delivering TDF, FTC, and maraviroc (MVC), an inhibitor/antagonist of chemokine receptor CCR5 [9].
Topical vaginal ARV drug delivery has not been well-evaluated to determine if this route of administration could lead to simultaneous rectal drug exposure sufficient to protect from HIV infection as a result of receptive anal intercourse (RAI) [10–14]. Exposure through RAI has a higher risk of infection per sex act and, for women, especially those in higher-risk subsets, there is the possibility of both vaginal and rectal exposure during a single sex act [15–17]. The physiologically inflamed, activated, immune-cell dense colorectal mucosa is increasingly appreciated as the sexual compartment with highly significant risk. Ex vivo HIV challenge of colorectal biopsies from humans receiving oral, vaginal, and rectal ARV drugs have demonstrated suppression of HIV infection, sometimes with clear concentration-response relationships [18–26].
The risk of HIV acquisition associated with both vaginal and rectal sexual intercourse, along with the above results informing potential efficacious ARV drug concentrations needed for rectal protection, have led us to collect rectal fluids in the above clinical trials evaluating pod-IVRs delivering TDF, TDF-FTC, and TDF-FTC-MVC. Colorectal compartment drug concentrations are assessed here to determine if vaginal application could simultaneously provide protective ARV levels in both compartments.
Materials and methods
Ethics statement
All human research was approved by the University of Texas Medical Branch Institutional Review Board (IRB # 14–0479), conducted according to the Declaration of Helsinki, and registered in clinicaltrials.gov (NCT02431273; https://clinicaltrials.gov/ct2/show/NCT02431273?term=NCT02431273&rank=1). All participants provided written informed consent.
Clinical trial design
The early Phase I clinical studies were performed between June 2015 and July 2016 at the Clinical Research Center of the University of Texas Medical Branch in Galveston, Texas. Women were recruited by announcements and word of mouth and underwent a phone pre-screen prior to attending a screening visit where informed consent was obtained and inclusion/exclusion criteria were confirmed. Inclusion criteria included age 18–45, regular menstrual cycles, use of contraception, and agreement to abstain from sexual intercourse during use of the IVR until the follow up exam 1–2 weeks after discontinuation of IVR use. Exclusion criteria included HIV, Heptatitis B, or sexually transmitted disease (gonorrhea, chlamydia, trichomonas) at the time of screening, abnormal liver or kidney function tests, and pregnancy/lactation.
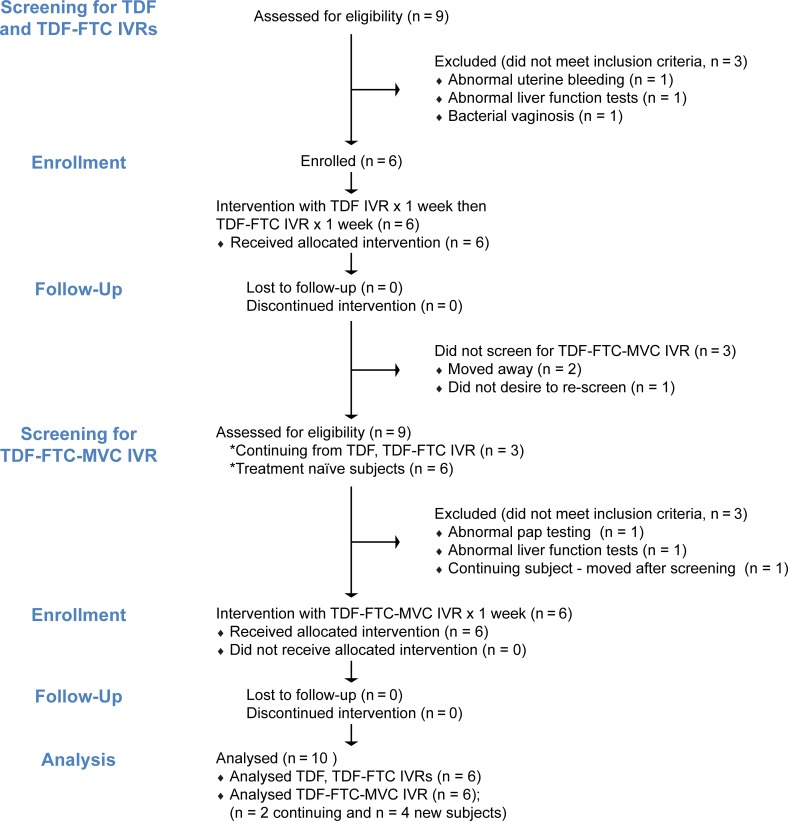
Women initially participated in a clinical trial where they used a pod-IVR releasing TDF and a TDF-FTC combination for 7 days in a cross-over, open label design, with at least 2 weeks of washout between IVRs (n = 6) (Fig 1) [9]. After the study team ensured that the IVRs were safe and provided adequate drug release, the women were invited to re-screen for a continuation of the study where they used a pod-IVR releasing the TDF-FTC-MVC combination for 7 days in an open label design, with new subjects additionally recruited in order to enroll a total of 6 women to use this IVR (Fig 1) [9]. In all three arms, vaginal fluid (Dacron swab), vaginal biopsy, and rectal fluid (Dacron swab) samples were collected on Day 7, immediately after IVR removal. Rectal biopsies were not acquired during these studies due to the increased participant burden. [20]
Fig 1. Participant flow diagram.
Chemicals, reagents, and fabrication of pod-IVRs
Materials and methods related to the IVR fabrication have been provided elsewhere [9] Briefly, TDF and FTC (labeled for human use) were purchased from commercial vendors with a Drug Master File (DMF) registered with the FDA. MVC was isolated from the commercial formulation (Pfizer, Inc., New York, NY), which consists of film-coated tablets for oral administration containing 300 mg of MVC and inactive ingredients, as described previously [2]. All other reagents were obtained from Sigma-Aldrich, unless otherwise noted.
Polydimethylsiloxane (PDMS, silicone) pod-IVRs were fabricated has according to methods described in detail elsewhere [2, 3, 5, 27]. The IVR drug content was as follows: TDF, 180 mg; FTC, 135 mg; MVC, 90 mg. The amount of residual drug remaining in used IVRs was measured by HPLC according to published methods [2, 27].
Bioanalysis of in vivo samples
Concentrations of TDF, TFV, TFV-DP, FTC, and MVC in fluids/tissue were determined via previously described liquid chromatographic-tandem mass spectrometric (LC-MS/MS) assays [11, 28–30]. All assays were developed and validated following the Food and Drug Administration Guidance for Industry, Bioanalytical Method Validation recommendations and met all acceptability criteria [31]. Isotopically labeled internal standards were used for all compounds and the determination of drug concentrations in all specimen sources.
The lower limits of quantification for these methods were as follows: cervicovaginal fluids (CVFs), TDF (0.0625 ng/sample), TFV (0.25 ng/sample), FTC (1.0 ng/sample), MVC (0.05 ng/sample); vaginal tissue homogenate, TFV (0.05 ng/sample), FTC (0.25 ng/sample), MVC (0.05 ng/sample); rectal fluids, TFV (0.25 ng/sample), FTC (1.0 ng/sample), MVC (0.05 ng/sample). Drug concentrations in luminal fluid and tissue samples were ultimately reported as ng mg-1 or fmol mg-1, respectively, following normalization to net biopsy or Dacron swab weight. Post-dose concentrations below the corresponding LLOQs (CLLQ) were imputed as follows in all analyses:
Results included here to provide intra-subject and between-group context for interpreting the comparative utility of rectal fluid results necessarily include CVF and VT concentration data from these trials that was also published separately [9].
Statistical analysis
Data were analyzed using GraphPad Prism (version 7.00, GraphPad Software, Inc., La Jolla, CA). Statistical significance is defined as P < 0.05.
Results
Details on IVR safety, drug concentrations in vaginal fluid and tissue, and user perception have been provided elsewhere [9]. In brief, no significant AEs or behavioral concerns were identified with use of any of the pod-IVRs. All participants completed all study visits and there were no missed visits, drop-outs, or loss to follow-up (Fig 1).
Drug concentration measurements
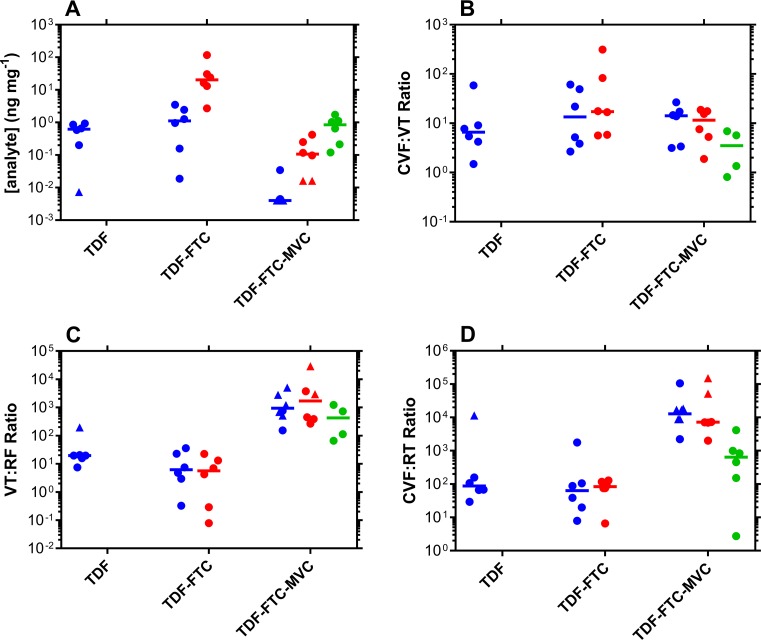
Drug concentrations in key anatomic compartments on Day 7 are summarized in Table 1 and Fig 2A. All Rectal fluid TFV concentrations resulting from either the TDF or the TDF-FTC pod-IVRs were not significantly different (P = 0.20), using a two-tailed paired t test. While important on its own, rectal fluid TFV concentrations in the TDF-FTC-MVC group was quantifiable in only one out of six samples (Table 1). Rectal fluid FTC concentrations resulting from either the TDF-FTC or the TDF-FTC-MVC pod-IVRs were not significantly different (P = 0.10), using an unpaired t test with Welch’s correction (i.e., do not assume equal standard deviations). While TFV concentrations in rectal fluid were similar in the TDF (median, 0.62 ng mg-1; IQR, 0.30–0.81 ng mg-1) and TDF-FTC (median, 1.11 ng mg-1; IQR, 0.36–2.15 ng mg-1) groups, median FTC concentrations in rectal fluid in the TDF-FTC (median, 20.3 ng mg-1; IQR, 14.0–28.9 ng mg-1) group were 190 times higher than in the TDF-FTC-MVC (median, 0.11 ng mg-1; IQR, 0.04–0.22 ng mg-1) group.
Table 1. Summary of drug concentrations in key anatomic compartments on Day 7, immediately after pod-IVR removal (six participants in each group).
CVF, cervicovaginal fluid; VT, vaginal tissue; RF, rectal fluid. Measurements outside of the analytical ranges were not included in the analysis.
| Pod-IVR Typea | |||
|---|---|---|---|
| Analyte, matrix | TDF | TDF-FTC | TDF-FTC-MVC |
| Total TFV,b CVF, ng mg-1 | 51.3 (34.8–61.5) | 40.8 (28.9–49.1) | 67.4 (43.4–72.1) |
| FTC, CVF, ng mg-1 | 1,458 (881–2,073) | 839 (818–1,572) | |
| MVC, CVF, ng mg-1 | 367 (224–489) | ||
| TFV, VT c, ng mg-1 | 8.4 (4.7–11.2) | 5.1 (0.77–10.1) | 5.1 (3.3–9.7) |
| FTC, VT c, ng mg-1 | 74.5 (11.6–193) | 104 (63.7–302) | |
| MVC, VT c, ng mg-1 | 142 (82.5–212)d | ||
| TFV, RF, ng mg-1 | 0.62 (0.30–0.81)e | 1.11 (0.36–2.15) | 0.004f |
| FTC, RF, ng mg-1 | 20.3 (14.0–28.9) | 0.11 (0.04–0.22)g | |
| MVC, RF, ng mg-1 | 0.84 (0.32–1.10) | ||
aMedian (Interquartile range, 25th to 75th percentile).
bMolar sum of paired TDF and TFV concentrations, reported as TFV.
cUsed with permission [9]
d33% of samples above the analytical quantification limit.
e83% of samples were above the analytical LLQ.
fOnly one sample was above the analytical LLQ.
g67% of samples were above the analytical LLQ.
Fig 2. Rectal fluid ARV drug exposure and distribution following IVR delivery.
Every circular datum represents an individual sample from one of the participants (n = 6); horizontal lines represent group medians; blue, TFV; red, FTC; green, MVC. (A) rectal fluid drug concentrations on Day 7. Paired Day 7 concentration ratios across anatomic compartments provide a measure of drug distribution; (B) cervicovaginal fluid to vaginal tissue (CVF:VT), with two VT MVC samples omitted as these were ALQ; (C) vaginal tissue to rectal fluid (VT:RF); (D) cervicovaginal fluid to rectal fluid (CVF:RF). Triangles represent samples where the rectal fluid drug concentration was BLQ.
The collection and analysis of cervicovaginal fluid (CVF), vaginal tissue (VT), and rectal fluid (RF) samples from all six study participants allows paired drug concentration ratios (i.e., CVF:VT, VT:RF, and CVF:RF) to be compared across all three compartments in Table 2 and Fig 2B and 2C. The TFV CVF:VT ratios across all three groups were compared with a nonparametric, Kruskal-Wallis test and determined not to be significantly different (P = 0.85). The FTC CVF:VT ratios in the TDF-FTC and TDF-FTC-MVC pod-IVR groups also were not significantly different (P = 0.26), using an unpaired t test with Welch’s correction.
Table 2. Paired drug concentration ratios by anatomic compartment on Day 7, immediately after pod-IVR removal (six participants in each group).
CVF, cervicovaginal fluid; VT, vaginal tissue; RF, rectal fluid. Data consist of medians (interquartile range) and only measurements within the analytical range of the assay are included.
| Pod-IVR Type | |||
|---|---|---|---|
| Analyte, Ratio | TDF | TDF-FTC | TDF-FTC-MVC |
| TFV,a CVF:VT | 6.6 (4.5–8.8) | 14 (4.2–42) | 14 (6.0–17) |
| FTC, CVF:VT | 17 (8.6–67) | 12 (5.8–17) | |
| MVC, CVF:VT | 3.5 (1.2–6.0) | ||
| TFV, VT:RF | 20 (17–20) | 6.2 (3.4–19) | 946 (559–2,396) |
| FTC, VT:RF | 5.6 (1.3–12) | 1,702 (404–3,565) | |
| MVC, VT:RF | 423 (101–855) | ||
| TFV,a CVF:RF | 87 (67–145) | 63 (25–100) | 12,763 (8,835–16,813) |
| FTC, CVF:RF | 83 (75–111) | 7,181 (6,978–40,726) | |
| MVC, CVF:RF | 644 (228–950) | ||
aMolar sum of paired TDF and TFV concentrations in CVF, reported as TFV.
Tenofovir VT:RF ratios in the TDF (median, 20; IQR, 17–20) group were numerically 3.2 times higher than in the TDF-FTC (median, 6.2; IQR, 3.4–19) group, and 47 times lower than in the TDF-FTC-MVC (median, 946; IQR, 559–2,396) group, where most measurements were BLQ. Emtricitabine VT:RF ratios in the TDF-FTC (median, 5.6; IQR, 1.3–12) group were 304 times lower overall than in the TDF-FTC-MVC (median, 1,702; IQR, 404–3,565) group. However, with the small sample size, the VT:RF ratios were not significantly different for TFV (P = 0.31) in the TDF and TDF-FTC pod-IVR groups and for FTC (P = 0.24) in the TDF-FTC and TDF-FTC-MVC pod-IVR groups, using an unpaired t test with Welch’s correction.
Discussion
Antiretroviral drug concentrations in rectal fluids were higher than expected following pod-IVR (i.e., vaginal) drug delivery in all three clinical trial arms. Tenofovir RF concentrations in the TDF (median, 0.62 ng mg-1; IQR, 0.30–0.81 ng mg-1) and TDF-FTC groups (median, 1.11 ng mg-1; IQR, 0.36–1.25 ng mg-1) were comparable to those reported on Day 7 in another clinical trial (median, 0.44 ng mg-1; IQR, 0.22–1.94 ng mg-1) evaluating a reservoir TDF IVR [14]. Our Day 7 TFV CVF:RF concentration ratios (Table 2, Fig 1D) in the TDF (median, 87; IQR, 67–145) and TDF-FTC groups (median, 63; IQR, 25–100) were lower than the corresponding ratios measured on Day 7 (median, 104; IQR, 59–505) in the clinical study involving the reservoir TDF IVR [14]. The lowest rectal fluid drug exposure was obtained with TFV, especially in the TDF-FTC-MVC group where only one of six samples was above the lower limit of quantitation of the assay.
In MTN-001, the pharmacokinetics of a 1% TFV (note, not TDF) vaginal gel were evaluated in a randomized, cross-over study involving 144 HIV-uninfected women, of which a subset of 12 had rectal sampling [11]. Comparing the end-of-period visit measurements of median CVF (3.1×103 ng mg-1, estimated from CVL samples assuming a 20-fold dilution of the CVF) and RF (6.0 ng mg-1, estimated from rectal sponge measurements reported in ng/sponge and a median mass of rectal fluid collected of 20 mg) concentrations affords a median CFV:RF TFV concentration ratio of 517. In MTN-014, 14 HIV-uninfected women received daily vaginal TFV 1% (reduced glycerin formulation) gel for 2 weeks. The median TFV concentration in CVF swabs (7,138 ng/swab) and RF swabs (4.4 ng/swab) indicate a CVF:RF TFV ratio of ca. 1,622 [13]. Taken together, these two TFV vaginal gel dosing studies report CVF:RF TFV ratios roughly 10–25 times higher than those measured with our pod-IVR. The lower the CFV:RF concentration ratio, the more efficiently the drug is thought to partition from the vaginal to rectal lumen. Clinical data on vaginal to rectal drug distribution for FTC and MVC are not available outside of the current report.
The RF ARV exposure measured here following vaginal drug delivery may hold important implications for HIV PrEP efficacy following receptive anal intercourse (RAI). While EC50, or EC90, drug concentrations are not used here as target concentrations for HIV prevention, they provide a preliminary guide to gauge possible efficacy. The in vitro EC50 of TFV against HIV-1 was 0.18 μM in peripheral blood mononuclear cells (PBMCs) [32] and 0.5 μM in MT-2 cells [33]. Median in vivo TFV RF concentrations in the TDF and TDF-FTC pod-IVR groups were ca. 3.5 μM (1 ng mg-1), in the range currently thought to be needed to provide some level of protection from HIV infection. Median FTC RF concentrations in the TDF-FTC pod-IVR group (20 ng mg-1, 81 μM) and in the TDF-FTC-MVC pod-IVR group (0.11 ng mg-1, 0.43 μM) compare favorably to the range of EC50 (1×10−3–0.64 μM) and EC90 concentrations (0.06–0.35 μM) against HIV reported for a variety of in vitro assays [34–38]. Median in vivo MVC RF concentrations (0.84 ng mg-1, 1.6 μM) measured here were conservatively higher than the in vitro anti-HIV EC50 (0.1×10−3–6×10−3 μM) and EC90 concentrations (0.5×10−3–0.013 μM) reported in the literature [37, 39].
The above preliminary analysis suggests that dual compartment protection from HIV infection may be possible with combination pod-IVRs, with the following important caveats. First, adding MVC to the TDF-FTC combination was associated with a decrease in measurable levels of TFV and FTC in rectal fluids (Fig 2A), despite similar concentrations of TDF/TFV and FTC across groups in CFV and VT (Table 1). While these two drugs work intracellularly in target cells, underscoring the importance of direct tissue measurements, the underlying mechanism for TFV and FTC RF reductions in the presence of vaginally-administered MVC is unknown, but could involve changes in molecular transporter expression [40, 41], or transporter inhibition, in the rectovaginal septum (i.e., layer of tissue between the vagina and the rectum). Second, colorectal tissue, not the lumen, is the likely pharmacologic compartment determining HIV PrEP efficacy.
While ARV drug concentrations in rectal tissues were not measured here, the drug concentration ratios across compartments (Table 2, Fig 1B–1D) suggest that the agents distribute across the rectovaginal septum, rather first than through systemic circulation on the way to the rectal tissue. This hypothesis suggests that anterior (i.e., closer in proximity to the vagina) colorectal tissue drug concentrations will be higher than in rectal fluids, which in turn may exceed posterior colorectal tissue drug concentrations. It is theoretically possible that vaginal to rectal drug distribution could occur through a combination passive and active transport along with local venous and lymphatic drainage. The luminal and mucosal colorectal drug distribution is critical in determining potential efficacy in HIV prevention, as discussed in Weld et al. [42], and needs to be studied further in future pod-IVR trials. Topical dosing has the advantage of producing very high local drug concentrations that may be sufficient to achieve diffusion to a second compartment such that, even with a steep concentration gradient, a protective level might be obtained, especially if activation of the drug is different in the second compartment. For example, colon versus cervicovaginal intracellular phosphorylation differences could completely compensate for diffusion-dependent concentration gradients of the parent drug by producing more phosphorylated TFV or FTC in the second compartment.
In conclusion, rectal fluid ARV drug concentrations resulting from vaginal dosing using three different pod-IVR formulations were detectable and, higher than anticipated. The results suggest that dual compartment protection from sexual HIV infection may be possible while maintaining a low systemic drug exposure. These important findings warrant further investigation in larger clinical trials.
Supporting information
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Research reported in this publication was supported in part by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R44HD075636 and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U19AI113048. Additionally, this study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch and by the Institute for Clinical and Translational Research (ICTR) at Johns Hopkins University, supported in part by Clinical and Translational Science Awards (UL1 TR001439 and UL1 TR001079, respectively) from the National Center for Advancing Translational Sciences, National Institutes of Health and by the Johns Hopkins Center for AIDS Research (P30AI094189). The funders did not play a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by: The Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (https://www.nichd.nih.gov/Pages/index.aspx Award Number R44HD075636); National Institute of Allergy and Infectious Diseases of the National Institutes of Health (https://www.niaid.nih.gov/ Award Number U19AI113048, MMB); Institute for Translational Sciences at the University of Texas Medical Branch, from the National Center for Advancing Translational Sciences, National Institutes of Health (https://ncats.nih.gov/ Award number UL1 TR001439); Institute for Clinical and Translational Research (ICTR) at Johns Hopkins University, from the National Center for Advancing Translational Sciences, National Institutes of Health (https://ncats.nih.gov/ Award Number UL1 TR001079); and Johns Hopkins Center for AIDS Research (http://hopkinscfar.org/ Award Number P30AI094189). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Auritec Pharmaceuticals, Inc. was the sponsor of the study and prime recipient of the NIH grant (Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R44HD075636) that funded part of the study. Auritec played a role in study design, but had a limited role in data collection and analysis, and no role in preparation of the manuscript. Study materials (e.g., IVRs) were provided to UTMB (clinical site) by Oak Crest in collaboration with Auritec.
References
- 1.Moss JA, Baum MM. Microbicide Vaginal Rings In: das Neves J, Sarmento B, editors. Drug Delivery and Development of Anti-HIV Microbicides. Singapore: Pan Stanford Publishing; 2014. p. 221–90. [Google Scholar]
- 2.Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, et al. Pharmacokinetics and Preliminary Safety Study of Pod-Intravaginal Rings Delivering Antiretroviral Combinations for HIV Prophylaxis in a Macaque Model. Antimicrob Agents Chemother. 2014;58(9):5125–35. PubMed Central PMCID: PMCPMC4135875. 10.1128/AAC.02871-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss JA, Butkyavichene I, Churchman SA, Gunawardana M, Fanter R, Miller CS, et al. Combination Pod-intravaginal Ring Delivers Antiretroviral Agents for HIV Prophylaxis: Pharmacokinetic Evaluation in an Ovine Model. Antimicrob Agents Chemother. 2016;60(6):3759–66. 10.1128/AAC.00391-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, et al. Vaginal Ring Adherence in Sub-Saharan Africa: Expulsion, Removal, and Perfect Use. AIDS Behav. 2012;16(7):1787–98. 10.1007/s10461-012-0248-4 [DOI] [PubMed] [Google Scholar]
- 5.Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, et al. An Intravaginal Ring for the Simultaneous Delivery of Multiple Drugs. J Pharm Sci. 2012;101(8):2833–43. PubMed Central PMCID: PMCPMC3857731. 10.1002/jps.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, et al. Tenofovir and Tenofovir Disoproxil Pharmacokinetics from Intravaginal Rings. Aids. 2012;26(6):707–10. PubMed Central PMCID: PMCPMC3855348. 10.1097/QAD.0b013e3283509abb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, et al. Safety and Pharmacokinetics of Intravaginal Rings Delivering Tenofovir in Pig-tailed Macaques. Antimicrob Agents Chemother. 2012;56(11):5952–60. PubMed Central PMCID: PMCPMC3486594. 10.1128/AAC.01198-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, et al. Simultaneous Delivery of Tenofovir and Acyclovir via an Intravaginal Ring. Antimicrob Agents Chemother. 2012;56(2):875–82. PubMed Central PMCID: PMCPMC3264253. 10.1128/AAC.05662-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent KL, Moss JA, Marzinke MA, Hendrix CW, Anton PA, Pyles RB, et al. Phase I Trial of Pod-intravaginal Rings Delivering Antiretroviral Agents for HIV-1 Prevention: Safety and Pharmacokinetics of Single (TDF), Dual (TDF-FTC), and Triple (TDF-FTC-MVC) Drug Formulations. PLoS Med. 2018:under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuttall J, Kashuba A, Wang R, White N, Allen P, Roberts J, et al. The Pharmacokinetics of Tenofovir Following Intravaginal and Intrarectal Administration of Tenofovir Gel to Rhesus Macaques. Antimicrob Agents Chemother. 2012;56(1):103–9. 10.1128/AAC.00597-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: Randomized Pharmacokinetic Cross-over Study Comparing Tenofovir Vaginal Gel and Oral Tablets in Vaginal Tissue and Other Compartments. PLoS One. 2013;8(1):e55013 10.1371/journal.pone.0055013 PubMed PMID: WOS:000315563800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt JD, Cameron D, Dias N, Holding J, Muntendam A, Oostebring F, et al. The Sheep as a Model of Preclinical Safety and Pharmacokinetic Evaluations of Candidate Microbicides. Antimicrob Agents Chemother. 2015;59(7):3761–70. 10.1128/AAC.04954-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair G, Justman JE, Piper J, Marzinke M, Hendrix C, Dai J, et al., editors. Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: a Cross-compartmental Study with Directly Observed Dosing. 8th IAS Conference on Pathogenesis Treatment & Prevention; 2015 July 21; Vancouver, Canada.
- 14.Keller MJ, Mesquita PM, Marzinke MA, Teller R, Espinoza L, Atrio JM, et al. A Phase 1 Randomized Placebo-controlled Safety and Pharmacokinetic Trial of a Tenofovir Disoproxil Fumarate Vaginal Ring. Aids. 2016;30(5):743–51. 10.1097/QAD.0000000000000979 PubMed PMID: WOS:000371309700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javanbakht M, Guerry S, Gorbach PM, Stirland A, Chien M, Anton P, et al. Prevalence and Correlates of Heterosexual Anal Intercourse Among Clients Attending Public Sexually Transmitted Disease Clinics in Los Angeles County. Sex Transm Dis. 2010;37(6):369–76. 10.1097/OLQ.0b013e3181cbf77d PubMed PMID: WOS:000278379700007. [DOI] [PubMed] [Google Scholar]
- 16.Gorbach PM, Pines H, Javanbakht M, Weiss RE, Jeffries R, Cranston RD, et al. Order of Orifices: Sequence of Condom Use and Ejaculation by Orifice During Anal Intercourse Among Women: Implications for HIV Transmission. J Acquir Immune Defic Syndr. 2014;67(4):424–9. PubMed PMID: WOS:000344771900015. 10.1097/QAI.0000000000000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen BN, Brock PM, Butler AR, Pickles M, Brisson M, Baggaley RF, et al. Prevalence and Frequency of Heterosexual Anal Intercourse Among Young People: A Systematic Review and Meta-analysis. AIDS Behav. 2015;19(7):1338–60. 10.1007/s10461-015-0997-y PubMed PMID: WOS:000358248600018. [DOI] [PubMed] [Google Scholar]
- 18.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, et al. RMP-02/MTN-006: A Phase 1 Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Tenofovir 1% Gel Compared with Oral Tenofovir Disoproxil Fumarate. AIDS Res Hum Retrovir. 2012;28(11):1412–21. 10.1089/AID.2012.0262 PubMed PMID: WOS:000310754100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson-Harman N, Mauck C, McGowan I, Anton P. Dose-response Relationship Between Tissue Concentrations of UC781 and Explant Infectibility with HIV Type 1 in the RMP-01 Rectal Safety Study. AIDS Res Hum Retrovir. 2012;28(11):1422–33. 10.1089/AID.2012.0073 PubMed PMID: WOS:000310754100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang KH, Elliot J, Hendrix C, Anton P, Cranston R, McGowan I, et al. Population PK Model of Rectal 1% Tenofovir (TFV) Gel in the Rectum and Plasma. J Pharmacokinet Pharmacodyn. 2013;40:S146–S8. PubMed PMID: WOS:000330126000159. [Google Scholar]
- 21.McGowan I, Cranston RD, Duffill K, Siegel A, Engstrom JC, Nikiforov A, et al. A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study). PLoS One. 2015;10(5):e0125363 10.1371/journal.pone.0125363 PubMed PMID: WOS:000353943400032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunge KE, Dezzutti CS, Rohan LC, Hendrix CW, Marzinke MA, Richardson-Harman N, et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. J Acquir Immune Defic Syndr. 2016;71(5):498–505. 10.1097/QAI.0000000000000897 PubMed PMID: WOS:000372350300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dezzutti CS, Richardson-Harman N, Rohan LC, Marzinke MA, Hoesley CJ, Panther L, et al. Pharmacodynamic Correlations Using Fresh and Cryopreserved Tissue Following Use of Vaginal Rings Containing Dapivirine and/or Maraviroc in a Randomized, Placebo Controlled Trial. Medicine (Baltimore). 2016;95(28). 10.1097/md.0000000000004174 PubMed PMID: WOS:000380767200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, et al. Long-acting Rilpivirine as Potential Pre-exposure Prophylaxis for HIV-1 Prevention (the MWRI-01 study): an Open-label, Phase 1, Compartmental, Pharmacokinetic and Pharmacodynamic Assessment. Lancet HIV. 2016;3(12):E569–E78. 10.1016/S2352-3018(16)30113-8 PubMed PMID: WOS:000389072800011. [DOI] [PubMed] [Google Scholar]
- 25.Richardson-Harman N, Parody R, Anton P, McGowan I, Doncel G, Thurman AR, et al. Analytical Advances in the Ex Vivo Challenge Efficacy Assay. AIDS Res Hum Retroviruses. 2016:Epub ahead of print Dec. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson JA, Marzinke MA, Bakshi RP, Fuchs EJ, Radebaugh CL, Aung W, et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B). AIDS Res Hum Retrovir. 2017;33(4):339–46. 10.1089/AID.2016.0040 PubMed PMID: WOS:000397584200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum MM, Butkyavichene I, Churchman SA, Lopez G, Miller CS, Smith TJ, et al. An Intravaginal Ring for the Sustained Delivery of Tenofovir Disoproxil Fumarate. Int J Pharm. 2015;495(1):579–87. PubMed Central PMCID: PMCPMCID: PMC4609628. 10.1016/j.ijpharm.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons TL, Emory JF, Seserko LA, Aung WS, Marzinke MA. Dual Quantification of Dapivirine and Maraviroc in Cervicovaginal Secretions from Ophthalmic Tear Strips and Polyester-based Swabs via Liquid Chromatographic-tandem Mass Spectrometric (LC-MS/MS) Analysis. J Pharm Biomed Anal. 2014;98:407–16. 10.1016/j.jpba.2014.06.018 PubMed PMID: WOS:000339859800055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retrovir. 2016;32(1):32–43. 10.1089/AID.2015.0182 PubMed PMID: WOS:000367335100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons TL, Marzinke MA. Development and Validation of a Liquid Chromatographic-tandem Mass Spectrometric Method for the Multiplexed Quantification of Etravirine, Maraviroc, Raltegravir, and Rilpivirine in Human Plasma and Tissue. J Pharm Biomed Anal. 2016;131:333–44. 10.1016/j.jpba.2016.08.016 PubMed PMID: WOS:000387628400044. [DOI] [PubMed] [Google Scholar]
- 31.US FDA. Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), 2001 May. Report No.
- 32.Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human Immunodeficiency Virus Activity and Cellular Metabolism of a Potential Prodrug of the Acyclic Nucleoside Phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42(3):612–7. PubMed PMID: WOS:000072311000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arimilli MN, Kim CU, Dougherty J, Mulato A, Oliyai R, Shaw JP, et al. Synthesis, in Vitro Biological Evaluation and Oral Bioavailability of 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) Prodrugs. Antivir Chem Chemother. 1997;8(6):557–64. PubMed PMID: ISI:A1997YJ91700010. [Google Scholar]
- 34.TRUVADA (Emtricitabine and Tenofovir Disoproxil Fumarate) Silver Spring, MD: U.S. Food and Drug Administration; [cited 2014 Oct. 14]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021752s043lbl.pdf.
- 35.Wang LH, Begley J, St Claire RL, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and Pharmacodynamic Characteristics of Emtricitabine Support Its Once Daily Dosing for the Treatment of HIV Infection. AIDS Res Hum Retrovir. 2004;20(11):1173–82. 10.1089/aid.2004.20.1173 PubMed PMID: WOS:000225576400005. [DOI] [PubMed] [Google Scholar]
- 36.Shen L, Peterson S, Sedaghat AR, McMahon MA, Callender M, Zhang HL, et al. Dose-response Curve Slope Sets Class-specific Limits on Inhibitory Potential of Anti-HIV Drugs. Nature Med. 2008;14(7):762–6. 10.1038/nm1777 PubMed PMID: WOS:000257452700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, et al. A Quantitative Basis for Antiretroviral Therapy for HIV-1 Infection. Nat Med. 2012;18(3):446–51. 10.1038/nm.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agosto LM, Zhong P, Munro J, Mothes W. Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission. PLoS Pathog. 2014;10(2):e1003982 10.1371/journal.ppat.1003982 PubMed PMID: WOS:000332085900053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-molecule Inhibitor of Chemokine Receptor CCR5 with Broad-spectrum Anti-human Immunodeficiency Virus Type 1 Activity. Antimicrob Agents Chemother. 2005;49(11):4721–32. 10.1128/AAC.49.11.4721-4732.2005 PubMed PMID: ISI:000233020900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kis O, Robillard K, Chan GNY, Bendayan R. The Complexities of Antiretroviral Drug-drug Interactions: Role of ABC and SLC Transporters. Trends Pharmacol Sci. 2010;31(1):22–35. PubMed PMID: WOS:000274377700005. 10.1016/j.tips.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 41.Pyles RB, Moss JA, Baum MM. Vaginal Mucosal HIV Prep: Fundamental Insights and Practical Considerations 2015. In: Frontiers in Clinical Drug Research: HIV [Internet]. Oak Park, IL: Bentham Science Publishers; [33–165]. [Google Scholar]
- 42.Weld ED, Hiruy H, Guthrie KM, Fava JL, Vargas SE, Buckheit K, et al. A Comparative Pre-Phase I Study of the Impact of Gel Vehicle Volume on Distal Colon Distribution, User Experience, and Acceptability. AIDS Res Hum Retrovir. 2017;33(5):440–7. 10.1089/AID.2016.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.