Abstract
Objective
To characterize the lipid profile in vaginal discharge of women with vulvovaginal candidiasis, cytolytic vaginosis, or no vaginal infection or dysbiosis.
Design
Cross-sectional study.
Setting
Genital Infections Ambulatory, Department of Tocogynecology, University of Campinas, Campinas, São Paulo–Brazil.
Sample
Twenty-four women were included in this study: eight with vulvovaginal candidiasis, eight with cytolytic vaginosis and eight with no vaginal infections or dysbiosis (control group).
Methods
The lipid profile in vaginal discharge of the different study groups was determined by liquid chromatography-mass spectrometry and further analyzed with MetaboAnalyst 3.0 platform.
Main outcome measures
Vaginal lipids concentration and its correlation with vulvovaginal candidiasis and cytolytic vaginosis.
Results
PCA, PLS-DA and hierarchical clustering analyses indicated 38 potential lipid biomarkers for the different groups, correlating with oxidative stress, inflammation, apoptosis and integrity of the vaginal epithelial tissue. Among these, greater concentrations were found for Glycochenodeoxycholic acid-7-sulfate, O-adipoylcarnitine, 1-eicosyl-2-heptadecanoyl-glycero-3-phosphoserine, undecanoic acid, formyl dodecanoate and lipoic acid in the vulvovaginal candidiasis group; N–(tetradecanoyl)-sphinganine, DL-PPMP, 1-oleoyl-cyclic phosphatidic, palmitic acid and 5-aminopentanoic acid in the cytolytic vaginosis group; and 1-nonadecanoyl-glycero-3-phosphate, eicosadienoic acid, 1-stearoyl-cyclic-phosphatidic acid, 1-(9Z,12Z-heptadecadienoyl)-glycero-3-phosphate, formyl 9Z-tetradecenoate and 7Z,10Z-hexadecadienoic acid in the control group.
Conclusions
Lipids related to oxidative stress and apoptosis were found in higher concentrations in women with vulvovaginal candidiasis and cytolytic vaginosis, while lipids related to epithelial tissue integrity were more pronounced in the control group. Furthermore, in women with cytolytic vaginosis, we observed higher concentrations of lipids related to bacterial overgrowth.
Introduction
The instability surrounding the vaginal ecosystem is a well-known fact in the gynecological practice, and such variations may derive from a variety of physiological or external factors. The vaginal microbiota is constituted by microorganisms that promote local environmental balance and its maintenance is established by complex interactions between the regular microbiota, the microbial metabolic products, the regular hormonal status and the host’s immune response [1].
A high number of women are affected by vulvovaginal diseases such as vulvovaginal candidiasis (VVC) and cytolytic vaginosis (CV). Both conditions present very similar symptomatology and, for this reason, they are commonly mistaken in the clinical practice, leading to misdiagnosis and inappropriate choice of treatment [2]. Nevertheless, subtle distinctions can be made between them. VVC produces a white and thick vaginal discharge, with vulvovaginal pruritus and eventually pain and vulvovaginal fissure. Its intense inflammatory process results from epithelium aggression caused by a fungus, usually Candida albicans [3]. On the other hand, CV contains no sort of infection; instead, it is a dysbiosis, consisting of an intense proliferation of lactobacillus that leads to cell lysis, histamine discharge and, ultimately, vaginal epithelial scaling. Rather than pruritus, CV patients often report vulvovaginal burning that is accentuated in the premenstrual period, thus mimicking vulvovaginal candidiasis. Moreover, CV is not accompanied by a cellular inflammatory process as observed in VVC [2,4,5].
The development of metabolome analysis through mass spectrometry allowed researchers to reach a correct interpretation of the quantitative and qualitative metabolic profile of one organism or biological system, considering the metabolites composition and dynamics with respect to genetic, physiological and environmental factors [6,7]. Lipidomics has emerged as a segment closely related to metabolomics and is dedicated to the global study of lipids, including their biochemical characteristics and networks formed within biological systems [8–11]. Nowadays, lipidomics studies are inclined to consider lipids as part of a broad integrated system of pathophysiological processes, instead of individual molecular structures with isolated functions [8].
The study of lipids composing the vaginal ecosystem is a new and promising perspective for better understanding gynecological conditions and their pathophysiological mechanisms. In this context, the objective of our study was to characterize the lipid profile in vaginal discharge samples of women with VVC, CV or no infection or dysbiosis, in order to enable improvement of diagnosis and treatment success.
Methods
Study population and clinical evaluation
A cross-sectional pilot study carried out at the Genital Infections Ambulatory of the Department of Tocogynecology of the University of Campinas included 24 sexually active, in contraceptive use, non-pregnant women aged 18 to 42 years. Experimental groups were divided and named as follows (n = 8): 1) VVC group: women with vulvovaginal candidiasis; 2) CV group: women with cytolytic vaginosis; 3) NL group: women without any type of vaginal infection or dysbiosis. The study was approved by the Ethics and Research Committee at the University of Campinas, CAAE n° 60648016.8.0000.5404, and written informed consent was obtained from all participants.
The participants were submitted to a specular examination for collection of vaginal samples by Dacron sterile swabs in order to perform lipid analyses and bacterioscopy by Gram stain. The characterization of the VVC group was based on the presence of yeast, pseudohyphae or blastospores in the vaginal discharge by microscopic examination by Gram stain, further confirmed by growth in Sabouraud culture media (Becton-Dickinson, Sparks, MD, USA). The criteria used for the diagnosis of CV included white and flocculated vaginal discharge accompanied by itching and/or burning at clinical evaluation, and Gram stain examination revealing presence of vaginal epithelial cell lysis, high number of Lactobacillus morphotypes, absence of leukocytes or microbial pathogens, and negative Candida sp culture.
Equivocal cases of endocervicitis, with presence of blood, bacterial vaginosis, trichomoniasis and/or mixed infections were ruled out. Bacterial vaginosis was diagnosed by the Amsel criteria [12] and Nugent score ≥ 7[13]. Infection by Trichomonas vaginalis was identified by the visualization of inflammatory cells in the vaginal smear by bacterioscopy (Gram stain) and predominance of coccoid and coccobacillary bacteria, as well as the visualization of the protozoan in fresh microscopy.
Lipid analysis of the vaginal samples
Two samples of the vaginal content of each patient were collected using sterile swabs and immediately stored in dry 10-mL tubes at -80°C, until processing.
For extraction of lipids, the samples were randomized and each sample was resuspended in 1 mL of 1:2 CHCl3: MeOH solution (Sigma, Basel, Switzerland), followed by the addition of 0.33 mL of CHCl3 and 0.33 mL of deionized water. The extraction was made in 15-mL glass tubes. The solution was then stirred for 5 minutes, followed by centrifugation at 13,000 rpm for 5 minutes. The supernatant was discarded, and the bottom layer of the sample containing the lipid fraction was transferred to 1.5-mL glass tubes. All samples were dried using SpeedVac for 30 minutes at 30°C and kept frozen at -80°C until the date of analysis.
Data acquisition
Lipid chromatographic separation was performed by ultra-high performance liquid chromatography (UHPLC) Agilent 1290 Infinity system (Agilent, Santa Clara, California, USA) and chromatographic elution was performed on Kinetex C18 column (4.6 mm x 50 mm x 2.6 μm) (Phenomenex, Torrance, CA, USA). For the positive ion mode, the aqueous mobile phase A solvent was 0.1% formic acid and phase B solvent was methanol; for the negative ion mode, phase A solvent was 5 mM Ammonium Acetate and phase B solvent was methanol. Before injection, the samples were randomized and inserted in the properly order. The mobile phase flow rate was 0.3 mL min-1 and the injection volume was 2 μL. The mobile phase gradient started at 5% of phase B changing linearly to 95% of phase B within 15 minutes and then returning to the initial composition, at which the gradient was kept constant for 5 minutes until the next run. This gradient profile was used for both positive and negative ion modes.
Mass spectrometry
To obtain the mass spectra of samples in positive and negative ion modes, a hybrid mass spectrometer with QTOF 6550 mass analyzer (Agilent, Santa Clara, California, USA) was used. The instrumental parameters of the electrospray ionization source used in this study for both positive and negative ion modes were: VCap of 3,000 V; 100 V shredder voltage, 65 V skimmer voltage, 750 V OCT 1 RF Vpp, 290°C Gas Temperature, 350°C Sheath Gas Temperature, 12 L.min-1 Sheath Gas Flow. The mass spectra were acquired in centroid mode and the mass range used for acquisition was 50–1700 Da.
Data processing
The raw data obtained was converted to the mzData format using the MassHunter Qualitative software (Agilent, Santa Clara, California, USA), eliminating isotopic interference. After conversion, the files were imported into the XCMS online software[14] for peak detection, alignment, retention time correction and other relevant pre-processing steps.
The data obtained from the online XCMS software was converted into an Excel table. Data normalization, scaling, hierarchical clustering into heatmaps and multivariate statistical analysis were performed in the MetaboAnalyst 3.0 platform [15]. Metabolites with a value of p < 0.05 (ANOVA) were considered representative for further investigation. Exploratory multivariate data analysis was performed using the Principal Component Analysis (PCA) unsupervised method and the Partial Least Squares Discriminant Analysis (PLS-DA), allowing selection of molecules with a VIP index higher than 1.0 as potential biomarkers. Putative lipid identification of the selected biomarkers was performed by measurement of their exact mass, retention time and elution profile, and further matching of such compounds in METLIN [14], Human Metabolome Database (HMDB) [16] and Lipid Maps databases [17].
Results
PCA and PLS-DA analyses
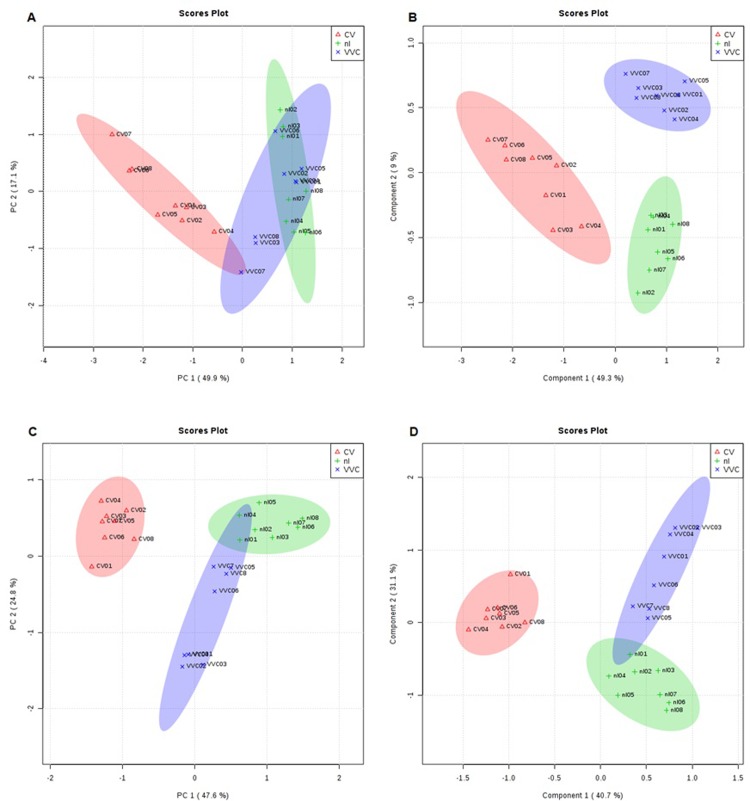
Comparison between VVC, CV and nl groups with regard to the main lipid components was performed by PCA and PLS-DA analyses (Fig 1). Fig 1A and 1C refer to the PCA in positive and negative ion modes, respectively. Both graphs show that the CV ellipses are separated from the other groups, indicating that women with CV manifest a very distinct lipid composition. Larger overlaid area of VVC and nl ellipses in the positive ion mode characterizes a certain similarity between these groups. In the PLS-DA Parameter Score charts (Fig 1B and 1D), separation of the CV group from the VVC and nl groups was more pronounced when compared to the PCA, and this difference was probably due to the unsupervised nature of the latter method.
Fig 1. Score plots analysis of the lipid fraction of vaginal samples from CV (red), nl (green) and VVC (blue) groups.
A: PCA analysis in the positive ion mode. C: PCA analysis in the negative ion mode. B: PLS-DA analysis in the positive ion mode. D: PLS-DA analysis in the negative ion mode.
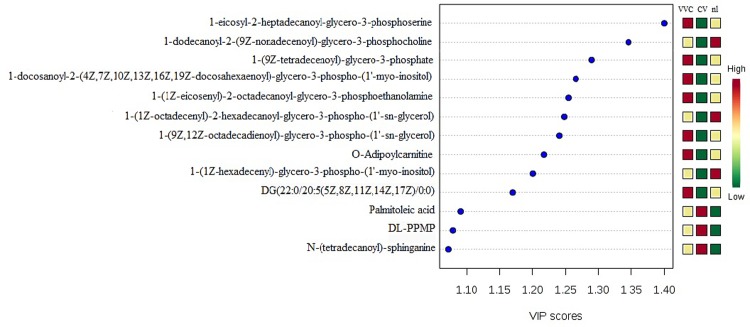
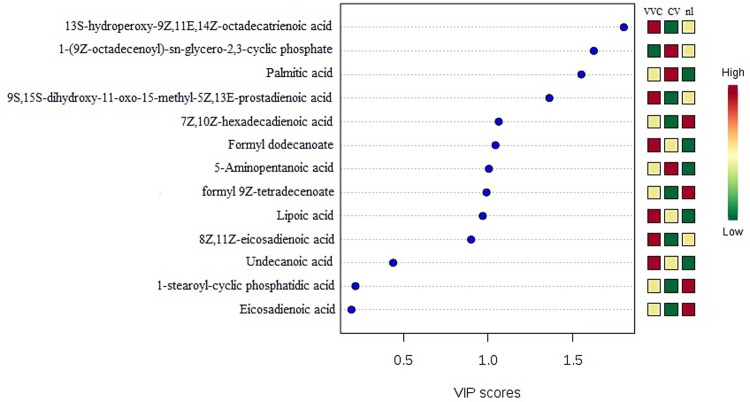
The PLS-DA model also provides the VIP score, which is a measure of a variable's importance in the analysis. Figs 2 and 3 show the lipids with the highest VIP scores and their respective concentrations in the vaginal samples. As these molecules were the most contributory in the model, they were selected as potential biomarkers for the different experimental groups.
Fig 2. PLS-DA analysis in the positive ion mode revealing the 15 lipid molecules with the highest VIP scores and their respective concentrations in the vaginal samples from CV, nl and VVC groups.
Fig 3. PLS-DA analysis in the negative ion mode revealing the 15 lipid molecules with the highest VIP scores and their respective concentrations in the vaginal samples from CV, nl and VVC groups.
Hierarchical clustering of lipids concentration by sample
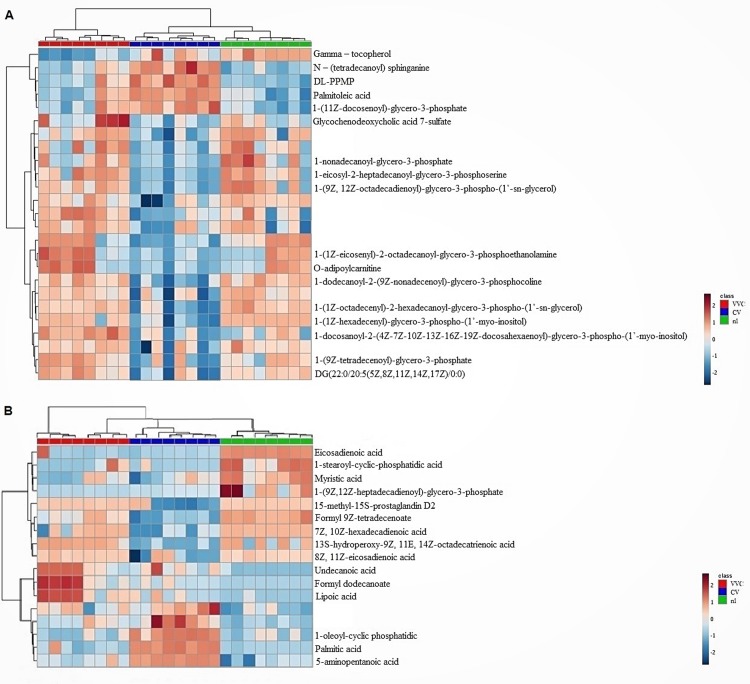
Fig 4 shows the hierarchical clustering of lipids in the form of heatmaps and dendrograms, demonstrating the proportion of significantly altered lipid components, identified as potential lipid biomarkers of the studied groups. Results from this analysis confirmed some of the lipid proportions already evidenced by the former methods, as described below.
Fig 4. Hierarchical clustering of lipids with regard to their relative concentration in each vaginal sample from VVC (red dendrogram columns), CV (blue dendrogram columns) and nl (green dendrogram columns) groups (n = 8).
High concentrations of lipids are shown in shades of red and low concentrations are shown in shades of blue. A: analysis performed in the positive ion mode. B: analysis performed in the negative ion mode.
In VVC women, there was a higher concentration of Glycochenodeoxycholic acid 7-sulfate, 1-(1Z-eicosenyl)-2-octadecanoyl-glycero-3-phosphoethanolamine, O-adipoylcarnitine, 1-eicosyl-2-heptadecanoyl-glycero-3-phosphoserine in comparison with the control and CV groups. On the other hand, CV women showed higher concentrations of N–(tetradecanoyl) sphinganine, DL-PPMP and 1-(11Z-docosenoyl)-glycero-3-phosphate in relation to the other groups. The main potential lipid biomarkers of the control group (nl) were identified as 1-nonadecanoyl-glycero-3-phosphate and 1-(9Z, 12Z-octadecadienoyl)-glycero-3-phospho-(1’-sn-glycerol). Several lipids presented similar concentrations especially between the VVC and nl groups, thus corroborating the overlapping ellipses found in the PCA and PLS-DA analyses.
Moreover, the negative ionization mode showed the highest contrasts of lipid concentrations for VVC and CV groups. In this sense, the most expressive lipid biomarkers were undecanoic acid, formyl dodecanoate and lipoic acid for the VVC group, and 1-oleoyl-cyclic phosphatidic, palmitic acid and 5-aminopentanoic acid for the CV group. In parallel, eicosadienoic acid, 1-stearoyl-cyclic-phosphatidic acid, 1-(9Z,12Z-heptadecadienoyl)-glycero-3-phosphate, formyl 9Z-tetradecenoate and 7Z,10Z-hexadecadienoic acid were the main potential biomarkers for the control group.
Considering all methods of analysis, in both negative and positive ion modes, we found 38 potential biomarkers that allow distinction between the VVC, CV and control groups.
Discussion
Our lipidomics study showed significant differences between women with VVC and CV regarding the lipid composition of vaginal discharge samples. Herein, we showed that lipids play an important role in the maintenance of the vaginal microenvironment homeostasis and that lipids profile can be markedly altered during pathophysiological processes. To our knowledge, this phenomenon had not yet been described in the current literature.
Moreover, several VVC potential lipid biomarkers were possibly related to inflammation. Among them, we found high concentrations of O-Adipoylcarnitine, belonging to the family of Acyl carnitines, which are intermediate oxidative metabolites synthesized by the mitochondria and peroxisomes, with the objective of transporting long-chain fatty acids for the process of β-oxidation [18,19]. Long-chain fatty acids can persistently modify biological processes, such as cellular stress [20], ionic variations [21] and inflammation [22]. Another lipid biomarker found in the VVC group, the 15-methyl-15S-prostaglandin D2 may act as a mediator of inflammation resulting from the vaginal mucosa response to the virulence factor of Candida sp.
Studies have shown that the oxidation of fatty acids typically occurs in tissues submitted to oxidative stress, including sites of inflammation [23,24]. Indeed, in the present investigation, a number of the selected biomarkers were found to be potentially related to oxidative stress. 13S-hydroperoxide-9Z,11E,14Z-octadecatrienoic acid, pointed in our study as the lipid biomarker with the highest VIP score in women with VVC (negative ion mode data), is typically produced by lipid peroxidation and can accumulate in mitochondrial membranes, enabling mitochondrial degradation and cellular damage [25–27]. Consistently, the glycochenodeoxycholic-acid-7-sulfate is the smallest of fatty acids metabolites whose excretion is increased when there are errors in mitochondrial metabolism, being suitable to diagnose mitochondrial beta-oxidation disorders [28, 29]. In our study, this compound was found in considerable proportions in the vaginal discharge of women with VVC and could be associated with mitochondrial deregulations in response to the oxidative stress caused by Candida sp.
A possible biological mechanism against oxidative stress and inflammatory process is the production of antioxidant metabolites or even by oral treatment, such as the lipoic acid [30, 31]. In its reduced form, dihydrolipoic acid, such compound has been described as a metabolite of fundamental importance to counterbalance reactive oxygen species [32–34]. In our study, lipoic acid was found as a potential biomarker of VVC, also possibly related to the vaginal mucosa response against Candida sp. oxidative stress.
Likewise, undecanoic acid was found at higher concentrations in the VVC group and seems to present antifungal and oxidative stress defense properties. This compound is an endogenous fatty acid commonly found in body fluids, and is involved in cell signaling, integrity and stability of biological membranes [35]. Studies have shown that the administration of undecanoic acid together with palmitic acid has resulted in antifungal activity [36] and inhibition of the morphogenesis of Candida albicans, preventing the development of blastospores into hyphae [37]. Thus, the presence of this lipid in women with VVC indicates a tissue response to Candida sp. infection.
Interestingly, palmitic acid was found at high concentrations only in the CV group. This compound is the most prominent fatty acid used by the human body [38]. Studies with rats have shown that palmitic acid increases endoplasmic reticulum stress, apoptosis in endothelial cells [39], increases endothelial nitric oxide synthase phosphorylation [40] and increases the formation of ROS and NADPH oxidase in skeletal muscles [41]. Therefore, CV lipid profile was also influenced by oxidative stress, probably caused by the exacerbated increase of lactobacilli producing lactic acid and other organic acids.
N-(tetradecanoyl) sphinganine and DL-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (DL-PPMP) were also indicated as potential biomarkers for CV in our study. Both are precursors of apoptosis [42–44], possibly due to the homeostatic disturbance related to the low pH (3.5 to 4.5) that is characteristic of this vaginosis [5]. Additionally, the increase in the levels of palmitoleic acid and 1-oleoyl-cyclic phosphatidic acid in the CV group was consistent with the role of these acids on injured epithelial tissues, already described this relation in hepatic cells [45]. Our results showed the presence of these potential lipids biomarkers in CV group which can be inferred the possible relation of injured caused on the vaginal epithelium visualized on the Gram stain.
In the present report, lipid biomarkers such as the phosphatidylserines fatty acids phosphatidylinositol and phosphatidylglycerophosphate, involved in cell signaling such as apoptosis, signal transduction of the plasmatic membrane and cardiolipin precursors [35, 46], showed higher concentrations in the VVC group.
The 5-aminopentanoic acid can be produced endogenously or by the bacterial catabolism of lysine, and it is known to act on the growth of anaerobic bacteria [47, 48]. In contrast, the presence of this biomarker in high concentrations in the CV group, where there is predominance of lactobacilli, raises new questions about the function of this compound in situations where there is an overpopulation of aerobic bacteria.
Among the main lipids identified in the control group (nl), the eicosadienoic acid presented relevant concentrations in comparison to the other study groups. This compound is an omega-6 fatty acid whose synthesis is linked to several receptors that can be found in various tissues of the human body [35, 49, 50]. The eicosadienoic acid is an antagonist of the leukotriene B4 receptor [51], and both leukotrienes and prostanoids act in the body in autocrine and paracrine regulation, influencing many physiological and pathophysiological mechanisms within the cell [52]. This organic acid can be related to anti-inflammatory mechanisms exerting a protective control of inflammatory mediators [53]. In addition, the presence of glycerophospholipids as potential biomarkers in the control group supports the function normality of the vaginal epithelial tissue, as these lipids are commonly found in the human organism [35, 54].
Furthermore, tocopherols are lipids associated with the cell membranes, acting against the most reactive forms of free radicals, besides preventing lipid peroxidation and bacterial translocation, hence reducing tissue injuries [55–58]. Our study demonstrated a higher concentration of gamma-tocopherol in the control group, which is a derivative of vitamin E. Notably, this lipid is important for the vaginal epithelial tissue homeostasis as it acts as a protector against disorders in the local microenvironment, such as the oxidative stress caused by infections and dysbioses. Another antioxidant lipid biomarker found in the control group was myristic acid, which has been described as improving the bioavailability of polar antioxidant molecules in the organism [59], besides exerting antimicrobial activities [60].
Conclusion
From the lipidomic point of view, we are facing three distinguishable vaginal profiles. In women with VVC, we found a higher concentration of lipids related to inflammation and oxidative stress, while in CV women, we observed a higher concentration of lipids related to cellular apoptosis, oxidative stress and bacterial overgrowth. In control women, vaginal lipidome was characterized by the presence of lipids involved in the maintenance of epithelial integrity epithelium and anti-inflammatory and antioxidant functions.
For clinical practice, this work provides some answers and new insights about the lipid metabolism involved in the pathophysiological processes of VVC and CV. Studies with larger population and targeting specifics lipids biomarkers can provide subsidies for better definition of conduct and treatment for women affected by these so frequent gynecological disorders.
Details of ethics approval
The study was approved by the Ethics and Research Committee at the University of Campinas on 16 November 2012 (ref. no. 155.315), and written informed consent was obtained from all participants.
Supporting information
(XLSX)
Acknowledgments
The supervision of the laboratory studies by Gustavo Henrique Bueno Duarte at Thomson Mass Spectrometry Laboratory–Campinas State University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Research Support Foundation of Sao Paulo (FAPESP) (2016/18850-9 to Mr. José Marcos Sanches). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Giraldo P, Amaral R, Gonçalves A, Vicentini R, Martins C, Giraldo H, et al. Influence of frequency of vaginal intercourses and the use of doushing on vaginal microbiota. Rev Bras Ginecol Obstet 2005;27(5):257–62. [Google Scholar]
- 2.Hu Z, Zhou W, Mu L, Kuang L, Su M, Jiang Y. Identification of cytolytic vaginosis versus vulvovaginal candidiasis. J Low Genit Tract Dis 2015;19(2):152–5. 10.1097/LGT.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 3.Sobel J. Vulvovaginal candidosis. Lancet 2007;369(9577):1961–71. 10.1016/S0140-6736(07)60917-9 [DOI] [PubMed] [Google Scholar]
- 4.Cerikcioglu N, Beksac M. Cytolytic vaginosis: misdiagnosed as candidal vaginitis. Infect Dis Obstet Gynecol 2004;12(1):13–6. 10.1080/10647440410001672139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibley L. Cytolytic vaginosis. Am J Obstet Gynecol 1991;165(4 Pt 2):1245–9. [DOI] [PubMed] [Google Scholar]
- 6.Almstetter M, Oefner P, Dettmer K. Comprehensive two-dimensional gas chromatography in metabolomics. Anal Bioanal Chem 2012;402(6):1993–2013. 10.1007/s00216-011-5630-y [DOI] [PubMed] [Google Scholar]
- 7.Putri S, Yamamoto S, Tsugawa H, Fukusaki E. Current metabolomics: technological advances. J Biosci Bioeng 2013;116(1):9–16. 10.1016/j.jbiosc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Han X, Gross R. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res 2003;44(6):1071–9. 10.1194/jlr.R300004-JLR200 [DOI] [PubMed] [Google Scholar]
- 9.Lagarde M, Geloen A, Record M, Vance D, Spener F. Lipidomics is emerging. Biochim Biophys Acta 2003;1634(3):61 [DOI] [PubMed] [Google Scholar]
- 10.Quehenberger O, Dennis E. The human plasma lipidome. N Engl J Med 2011;365(19):1812–23. 10.1056/NEJMra1104901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol 2010;11:593–8. 10.1038/nrm2934 [DOI] [PubMed] [Google Scholar]
- 12.Amsel R, Totten P, Spiegel C, Chen K, Eschenbach D, Holmes K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74(1):14–22. [DOI] [PubMed] [Google Scholar]
- 13.Nugent R, Krohn M, Hillier S. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tautenhahn R, Patti G, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem 2012;84(11):5035–9. 10.1021/ac300698c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia J, Sinelnikov I, Han B, Wishart D. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 2015;43(W1):W251–7. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.hmdb.ca/metabolites. Accessed on 07/14/2017.
- 17.http://www.lipidmaps.org/. Accessed on 07/14/2017.
- 18.Reuter S, Evans A. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet 2012;51(9):553–72. 10.2165/11633940-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 19.Rinaldo P, Matern D, Bennett M. Fatty acid oxidation disorders. Annu Rev Physiol 2002;64:477–502. 10.1146/annurev.physiol.64.082201.154705 [DOI] [PubMed] [Google Scholar]
- 20.McCoin C, Knotts T, Ono-Moore K, Oort P, Adams S. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: calcium-dependent and -independent effects. Am J Physiol Endocrinol Metab 2015;308(11):E990–e1000. 10.1152/ajpendo.00602.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T, Kiyosue T, Arita M. Inhibitory effects of palmitoylcarnitine and lysophosphatidylcholine on the sodium current of cardiac ventricular cells. Pflugers Arch 1992;420(1):94–100. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowsky J, Knotts T, Ono-Moore K, McCoin C, Huang S, Schneider D, et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 2014;306(12):E1378–87. 10.1152/ajpendo.00656.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida Y, Umeno A, Akazawa Y, Shichiri M, Murotomi K, Horie M. Chemistry of lipid peroxidation products and their use as biomarkers in early detection of diseases. J Oleo Sci 2015;64(4):347–56. 10.5650/jos.ess14281 [DOI] [PubMed] [Google Scholar]
- 24.Ramsden C, Ringel A, Feldstein A, Taha A, MacIntosh B, Hibbeln J, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids 2012;87(4–5):135–41. 10.1016/j.plefa.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem G, et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep 2013;3:1349 10.1038/srep01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D, Funk C. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem 1996;271(39):24055–62. [PubMed] [Google Scholar]
- 27.Kuhn H, Brash A. Occurrence of lipoxygenase products in membranes of rabbit reticulocytes. Evidence for a role of the reticulocyte lipoxygenase in the maturation of red cells. J Biol Chem 1990;265(3):1454–8. [PubMed] [Google Scholar]
- 28.Liang S, Su W, Wang Y, Peng W, Nie Y, Li P. Effect of quercetin 7-rhamnoside on glycochenodeoxycholic acid-induced L-02 human normal liver cell apoptosis. Int J Mol Med 2013;32(2):323–30. 10.3892/ijmm.2013.1414 [DOI] [PubMed] [Google Scholar]
- 29.Zhangxue H, Min G, Jinning Z, Yuan S, li W, Huapei S, et al. Glycochenodeoxycholate induces rat alveolar epithelial type II cell death and inhibits surfactant secretion in vitro. Free Radic Biol Med 2012;53(1):122–8. 10.1016/j.freeradbiomed.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 30.Costantino M, Guaraldi C, Costantino D. Resolution of subchorionic hematoma and symptoms of threatened miscarriage using vaginal alpha lipoic acid or progesterone: clinical evidences. Eur Rev Med Pharmacol Sci 2016; 20 (8): 1656–1663. [PubMed] [Google Scholar]
- 31.Parente E, Colannino G, Picconi O, Monastra G. Safety of oral alpha-lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci 2017; 21 (18): 4219–4227 [PubMed] [Google Scholar]
- 32.Tirosh O, Sen C, Roy S, Kobayashi M, Packer L. Neuroprotective effects of alpha-lipoic acid and its positively charged amide analogue. Free Radic Biol Med 1999;26(11–12):1418–26. [DOI] [PubMed] [Google Scholar]
- 33.Behling C, Andrade A, Putti J, Mahl C, Hackenhaar F, Silva A, et al. Treatment of oxidative stress in brain of ovariectomized rats with omega-3 and lipoic acid. Mol. Nutr. Food Res. 2015, 59, 2547–2555. 10.1002/mnfr.201500338 [DOI] [PubMed] [Google Scholar]
- 34.Polyak E, Ostrovsky J, Peng M, Dingley S, Tsukikawa M, Kwon Y, et al. N-acetylcysteine and vitamin E rescue animal longevity and cellular oxidative stress in pre-clinical models of mitochondrial complex I disease. Molecular Genetics and Metabolism. 2018, 02.013, 1096–7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NELSON D, COX M. Princípios de Bioquímica de Lehninger Porto Alegre: Artmed; 2011. [Google Scholar]
- 36.Avrahami D, Shai Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to D,L-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 2003;42(50):14946–56. 10.1021/bi035142v [DOI] [PubMed] [Google Scholar]
- 37.McLain N, Ascanio R, Baker C, Strohaver R, Dolan J. Undecylenic acid inhibits morphogenesis of Candida albicans. Antimicrob Agents Chemother 2000;44(10):2873–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staiger H, Staiger K, Stefan N, Wahl H, Machicao F, Kellerer M, et al. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes 2004;53(12):3209–16. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Gonzalez O, Shen X, Barnhart S, Kramer F, Kanter J, et al. Endothelial acyl-CoA synthetase 1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment. Arterioscler Thromb Vasc Biol 2013;33(2):232–40. 10.1161/ATVBAHA.112.252239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Cheng J, Chen L, Li C, Chen G, Gui L, et al. Endoplasmic reticulum stress involved in high-fat diet and palmitic acid-induced vascular damages and fenofibrate intervention. Biochem Biophys Res Commun 2015;458(1):1–7. 10.1016/j.bbrc.2014.12.123 [DOI] [PubMed] [Google Scholar]
- 41.Lambertucci R, Hirabara S, Silveira L, Levada-Pires A, Curi R, Pithon-Curi T. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 2008;216(3):796–804. 10.1002/jcp.21463 [DOI] [PubMed] [Google Scholar]
- 42.Watters R, Fox T, Tan S, Shanmugavelandy S, Choby J, Broeg K, et al. Targeting glucosylceramide synthase synergizes with C6-ceramide nanoliposomes to induce apoptosis in natural killer cell leukemia. Leuk Lymphoma 2013;54(6):1288–96. 10.3109/10428194.2012.752485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen W, Henry AG, Paumier K, Li L, Mou K, Dunlop J, et al. Inhibition of glucosylceramide synthase stimulates autophagy flux in neurons. J Neurochem 2014;129(5):884–94. 10.1111/jnc.12672 [DOI] [PubMed] [Google Scholar]
- 44.Stefanic S, Spycher C, Morf L, Fabrias G, Casas J, Schraner E, et al. Glucosylceramide synthesis inhibition affects cell cycle progression, membrane trafficking, and stage differentiation in Giardia lamblia. J Lipid Res 2010;51(9):2527–45. 10.1194/jlr.M003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Q, Yang J, Zhu R, Jiang X, Li W, He S, et al. Dihydroceramide-desaturase-1-mediated caspase 9 activation through ceramide plays a pivotal role in palmitic acid-induced HepG2 cell apoptosis. Apoptosis 2016;21(9):1033–44. 10.1007/s10495-016-1267-9 [DOI] [PubMed] [Google Scholar]
- 46.Alberts B.; Johnson A. & Walter P. Molecular Biology of the Cell. 5th Ed, Artmed, 2010. [Google Scholar]
- 47.Fothergill J, Guest J. Catabolism of L-lysine by Pseudomonas aeruginosa. J Gen Microbiol 1977;99(1):139–55. [DOI] [PubMed] [Google Scholar]
- 48.Syrjanen S, Piironen P, Markkanen H. Free amino-acid content of wax-stimulated human whole saliva as related to periodontal disease. Arch Oral Biol 1987;32(9):607–10. [DOI] [PubMed] [Google Scholar]
- 49.Kain V, Ingle K, Kachman M, Baum H, Shanmugam G, Rajasekaran N, et al. Excess Omega-6 Fatty Acids Influx in Aging Drives Metabolic Dysregulation, Electrocardiographic Alterations and Low-grade Chronic Inflammation. Am J Physiol Heart Circ Physiol. 2017:ajpheart.00297.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi J, Park N, Hwang S, Sohn J, Kwak I, Cho K, et al. The antibacterial activity of various saturated and unsaturated fatty acids against several oral pathogens. Environ Biol 2013;34:673–676. [PubMed] [Google Scholar]
- 51.Yagaloff K, Franco L, Simko B, Burghardt B. Essential fatty acids are antagonists of the leukotriene B4 receptor. Prostaglandins Leukot Essent Fatty Acids 1995;52(5):293–7. [DOI] [PubMed] [Google Scholar]
- 52.Smith W. Prostanoid biosynthesis and mechanisms of action. Am J Physiol 1992;263(2 Pt 2):F181–91. [DOI] [PubMed] [Google Scholar]
- 53.Serhan C, Chiang N, Van Dyke T. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8(5):349–61. 10.1038/nri2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park K, Vasko M. Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol Sci 2005;26(11):571–7. 10.1016/j.tips.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 55.Schanaider A, Castro L, Madi K. Effects of alpha- tocopherol on bacterial translocation and lipid peroxidation in rats with intestinal obstruction. Acta Cirúrgica Brasileira 2003;18(4): 283–8. [Google Scholar]
- 56.Dairi S, Carbonneau M, Galeano-Diaz T, Remini H, Dahmoune F, Aoun O, et al. Antioxidant effects of extra virgin olive oil enriched by myrtle phenolic extracts on iron-mediated lipid peroxidation under intestinal conditions model. Food Chem 2017;237:297–304. 10.1016/j.foodchem.2017.04.106 [DOI] [PubMed] [Google Scholar]
- 57.Denniss S, Haffner T, Kroetsch J, Davidson S, Rush J, Hughson R. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc Health Risk Manag 2008;4(1):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palan P, Shaban D, Martino T, Mikhail M. Lipid-soluble antioxidants and pregnancy: maternal serum levels of coenzyme Q10, alpha-tocopherol and gamma-tocopherol in preeclampsia and normal pregnancy. Gynecol Obstet Invest 2004;58(1):8–13. 10.1159/000077011 [DOI] [PubMed] [Google Scholar]
- 59.Prasadani W, Senanayake C, Jayathilaka N, Ekanayake S, Seneviratne K. Effect of three edible oils on the intestinal absorption of caffeic acid: An in vivo and in vitro study. PLoS One 2017;12(6):e0179292 10.1371/journal.pone.0179292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vudhgiri S, Koude D, Veeragoni D, Misra S, Prasad R, Jala R. Synthesis and biological evaluation of 5-fatty-acylamido-1, 3, 4-thiadiazole-2-thioglycosides. Bioorg Med Chem Lett 2017;27(15):3370–3. 10.1016/j.bmcl.2017.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.