Abstract
Objective
Biomechanics for rupture risk prediction in abdominal aortic aneurysms (AAA) are gaining popularity. However, their clinical applicability is still doubtful as there is lack of standardization. This study evaluates the added value of biomechanical indices in rupture risk assessment.
Methods
This study included 175 asymptomatic, 11 sAAA and 45 ruptured aneurysms. 3D-geometries were reconstructed using computer tomography angiographies. Subsequently, finite element models were made to calculate peak wall stress (PWS), peak wall rupture index (PWRI) and the rupture risk equivalent diameter (RRED). The indices were determined with a dedicated software to facilitate standardization.
Results
SAAAs showed a trend towards higher PWS, PWRI and RRED compared to asymptomatic AAAs, but PWS (22.0±5.8 vs. 33.4±15.8 N/cm2), PWRI (0.52±0.2 vs. 1.01±0.64), and RRED (65±60 vs. 98±51 mm) were significantly (p = 0.001) higher in ruptured. However, after diameter-matching no significant differences were seen. The ROC-curves for the maximum diameter and all biomechanical indices were similar but it slightly increased when diameter and biomechanical indices were combined.
Conclusions
This study showed no added value for biomechanical indices in AAA rupture risk assessment. Additionally, the difficulty of such an assessment increases. However, as symptomatic aneurysms show a trend towards higher biomechanical indices with similar diameters the indices may provide information about aneurysm growth and development.
Introduction
Abdominal aortic aneurysms (AAAs) occur in approximately five percent of the population and are c potentially life threatening in case of rupture.[1] Unruptured AAAs could be repaired with open or endovascular surgery in an elective setting, but both carry a risk for complications. The decision to intervene is usually based on the clinical condition of the patient, medical imaging, and patient preference. Currently, the maximum aneurysm diameter and expansion rate are the two most important risk factors for AAA rupture. Both have been extensively validated.[2,3] In general, small (maximum diameter below 5.5 cm) and slowly expanding (<0.3cm per year) aneurysms are less likely to rupture. However, aneurysm diameter is a population based risk estimate and thus some small aneurysms rupture while some large aneurysms remain stable.[3–5] Furthermore, small aneurysms are more prevalent than large aneurysms.[6,7] Although the risk is lower in small aneurysms the small fraction of this majority may be a significant part of the ruptured AAAs. Thus there is a clear need for a patient tailored approach and patient-specific decision making.
Diagnostic indices which are able to accurately estimate the rupture risk of a specific aneurysm would greatly improve this patient-specific assessment. For this purpose biomechanical indices were developed.[8–11] These indices are based on the basic principle of material failure; an aneurysm ruptures when wall stress exceeds wall strength. Therefore, they might relate more closely to the pathological process of growth and rupture than the maximum diameter. These indices are extracted using computational models and diagnostic imaging such as ultrasound, computed tomography scanning or magnetic resonance imaging.[12,13] Hereby, several patient-specific biomechanical indices are calculated, such as peak wall stress (PWS), peak wall rupture index (PWRI) and rupture risk equivalent diameter (RRED). PWS is the maximum in plane wall stress. The wall strength is estimated using patient characteristics, subsequently, PWRI can be calculated by dividing calculated wall stress by the estimated wall strength.[10,14,15] The RRED reflects the PWRI translated to equivalent diameters of the average aneurysm patient.[16]
The clinical applicability and additional value of these biomechanical indices compared to the maximum diameter are still unknown, particularly, due to the lack of standardization.[17] However, over recent years dedicated software for the biomechanical analysis of AAA became available. This software might help to implement the biomechanical analysis in daily clinical practice. Previous studies showed that it has low inter- and intra-observer variability.[18]
The aim of this study was to assess the clinical value of a biomechanical analysis method using a patient-specific AAA geometry and finite element analysis. The clinical usability is assessed by comparing the resulting geometric and biomechanical indices between asymptomatic, symptomatic non-ruptured and ruptured AAAs.
Methods
Study design
The study was approved by the Institutional Review Board (METc2016.161). The requirement for informed consent was waived because no diagnostics other than routine clinical imaging were used in this study.
Patients were retrospectively collected between January 2003 and December 2014 from a prospectively held database containing acute and elective endovascular treated patients at the vascular surgery division of a large tertiary referral center. Acute repair was performed in case of rupture or symptomatology.
Patient selection and characteristics
Primary selection consisted of 216 asymptomatic, 11 symptomatic intact and 80 ruptured patients with a non-inflammatory infrarenal AAA which are randomly selected form the database using simple random sampling. An extension to the common iliac arteries was accepted (EUROSTAR classification type D)[19]. Only patients with a suitable preoperative computerized tomography angiography (CTa) were included. The CTa was deemed suitable when the aorta was visible from the renal arteries to the iliac bifurcation, and when the lumen was distinguishable from the intraluminal thrombus. Fig 1 displays the flow diagram for the selection of patients. Older CTa often have thicker slices and less contrast between lumen, thrombus and surroundings. Therefore, only a small part of the scans before 2010 were deemed suitable (5% of the CTa after 2010 were excluded versus 12.5% of the CTa before 2010).
Fig 1. Flowchart patient selection.
The symptomatic non-ruptured group (sAAA) contained patients who presented with AAA associated symptomatology, such as abdominal and/or back pain, but had no signs of rupture on CTa. These patients underwent endovascular repair after ruling out other differential diagnoses. The ruptured group (rAAA) contained patients who presented with acute signs of rupture which were confirmed on preoperative CTa. Notably, the post-rupture scans were used for the ruptured group.
The following risk factors and co-morbidities were registered with definitions according to the guidelines of the American Heart Association[20]: age, sex, diabetes mellitus (yes/no), smoking habits (current smoker yes/no), blood pressure (systolic, diastolic and mean arterial pressure (MAP; systolic pressure + diastolic pressure), body mass index (BMI) and an early family history for AAA (male <55 years of age, female <65 years of age (first-degree relative)), hypercholesterolemia (diagnosis yes/no, (preventative) statins yes/no), and other cardiovascular disease (CVD; stroke, coronary/peripheral artery disease). Clinical data were collected from the last measurement in a non-critical setting within one year before intervention, either during routine check-ups or at hospital admission.
Biomechanical analysis
All biomechanical analyses were performed using commercially available software (A4research™, VASCOPS, Graz, Austria). The software uses several steps. First, AAA geometry is reconstructed by segmenting the vessel lumen, the intraluminal thrombus (ILT), and the vessel wall (Fig 2).[12] Second, a mesh is generated and the finite element analysis (FEA) is executed. Details are described below.
Fig 2. Workflow of the used software.
A. Luminal segmentation in blue B. Luminal (blue) and thrombus (red) segmentation C. Exterior mesh. D. PWRI outcome displayed on the 3D model.
For an accurate segmentation several requirements have to be met. In 4 AAA and 15 RAAA biomechanical analysis was not possible due to limited amount of contrast (n = 4), insurmountable distinction between ILT and hematoma (n = 3) and complex geometries resulting in intersecting faces (n = 12). Consequently, these cases were excluded. A total of 179 asymptomatic AAAs (aAAA), 11 symptomatic AAAs (sAAA) and 60 ruptured AAAs (rAAA) cases were included.
Geometry reconstruction
Segmentation was semi-automatic using deformable snake and balloon models for the 2D and 3D segmentation, respectively. These are objects that deform within the image until they stop at the boundary of a structure (lumen or vessel). The evolution of the object depended on a set of reconstruction parameters and contrast differences. First, a snake model to pre-segment the luminal surface was initialized by manually placing an initialization circle in the lumen of the iliac arteries. Subsequently, the luminal service was perfected using a balloon model and the exterior surface was segmented with a second balloon starting from the luminal surface. Segmented volumes were manually corrected through enriching image data and control polygons. The amount of user interaction depended on the image quality and the complexity of the aneurysm. In general ruptured aneurysms required more manual correction. Finally, the external vessel wall and ILT were automatically segmented by the software.
Mesh generation and finite element analysis
After geometry reconstruction a mesh of the 3D volume containing hexahedral elements was created automatically by the software. Details are presented elsewhere.[12] The FEA region was set from the renal arteries to just distal to the iliac bifurcation. The model was pressurized at the MAP. In case the blood pressure (BP) was not reported a set BP of 140 over 80 mmHg was used (n = 8, 2, 12 for aAAA, sAAA and rAAA cases, respectively). An isotropic constitutive model was used for the ILT and aneurysm wall.[21] The wall strength is estimated using the position of the ILT, sex, family history and the relation between the local diameter and the calculated normal aortic diameter.
Parameter calculation
Several geometric (maximum diameter, volume) and biomechanical parameters (PWS, PWRI, RRED) were extracted. PWS is produced by the blood pressure resulting in an in-plane wall stress and consequently deformation. The PWRI is a calculated index, dividing the calculated wall stress through the estimated wall strength. The latter (RRED) expresses the diameter of an average AAA that has the same PWRI. All parameters were calculated automatically. The maximum diameter was based on measurements perpendicular to the center luminal line. The software produced colored overlays to provide information of the distribution of wall stress and rupture risk (Fig 2). The maximum diameter as determined by an experienced radiologist, using also measurements perpendicular to the center luminal line, was extracted from the patient file to assess the differences with current clinical practice.
Statistical analysis
Statistics were expressed as mean ± standard deviation in case of a normal distribution. Percentages were given for nominal variables. Normality of the data was tested using the one sample Kolmogorov-Smirnov test. Comparison between scans or groups was done using a Student t-test in case of normal distribution. Mann-Whitney U tests were performed to compare skewed variables. Dichotomous variables were compared using the chi square test. Missing values were pair wise excluded. To evaluate the capacity to correctly predict rupture on the basis of the diameter and biomechanical indices a ROC analysis was performed, examining the discrimination between groups under varying thresholds. A combined model of all biomechanical indices (PWRI, PWS and RRED) and the maximum AAA diameter was made using logistic regression. Subsequently, the predicted probability was included into the ROC-analysis. All statistical analyses were performed using SPSS statistics 23 (IBM, New York, NY, United States). Significance was set at p < 0.05. To minimize the false discovery rate we applied Bonferroni correction for multiple analyses on the same dependent variable. The adjusted significance levels was of .017 per test (.05/3).
Diameter matching
A sub analysis comparing only size-matched AAA and RAAA subjects was done. Hereby, providing a more stringent analysis of whether the biomechanical outcomes could differentiate between asymptomatic AAA and RAAAs. The diameters as measured by the software were matched using SPSS Case-Control matching. Match tolerances were set at 5 mm, resulting in 31 matches within the tolerance rate.[22]
Results
All three groups had similar demographic characteristics. No significant differences were observed (Table 1, P > 0.017). The AAA group showed a skewed distribution of the maximum diameter as most values were between 50 and 60 mm due to the current threshold to treat.
Table 1. Demographic variables for aAAA, sAAA and rAAA.
| Variable | AAA | SAAA | RAAA | P-value |
|---|---|---|---|---|
| N | 175 | 11 | 45 | - |
| Age (year) | 72.4 ± 8.7 | 73.4 ± 10.4 | 73.9 ± 8.7 | 0.71a/0.40b/0.95c |
| Male | 156 (89%) | 7 (63%) | 39 (87%) | 0.01a/0.64b/0.07c |
| Blood pressure (mmHg) (n = 209) | ||||
| Systolic | 135 ± 18 | 136 ± 13 | 140 ± 25 | 0.79a/0.29b/0.64c |
| Diastolic | 76 ± 12 | 79 ± 5 | 76 ± 16 | 0.09a/0.23b/0.40c |
| MAP | 96 ± 13 | 98 ± 6 | 96 ± 20 | 0.22a/0.86b/0.54c |
| BMI (n = 178) | 26.9 ± 4.2 | 23.9 ± 3.1 | 26.6 ± 6.9 | 0.20a/0.89b/ 0.91c |
| Diabetes mellitus | 23 (13%) | 1 (9%) | 7 (15%) | 0.70a/0.67b/0.58c |
| Cardiovascular disease | 107 (61%) | 9 (82%) | 24 (51%) | 0.17a/0.14b/0.05c |
| Smoking (n = 211) | 86 (49%) | 3 (27%) | 22 (47%) | 0.16a/0.98b/0.20c |
| Hypercholesterolemia (n = 152) | 0.40a/0.15b/0.92c | |||
| Diagnosis | 31(18%) | 3 (27%) | 10 (21%) | |
| Preventative medication | 66 (38%) | 2 (18%) | 11 (23%) | |
| Positive family history (n = 21) | 10 (5%) | 1 (9%) | 0 (0%) | 0.38a/0.14b/0.08c |
BMI = body mass index. MAP = mean arterial pressure, AAA = asymptomatic abdominal aortic aneurysm, SAAA = symptomatic non-ruptured abdominal aortic aneurysm, RAAA = ruptured abdominal aortic aneurysm.
a: AAA compared to SAAA
b: AAA compared to RAAA
c: SAAA compared to RAAA
Evaluation biomechanical indices and diameter
The sAAA group showed similar geometric values compared to the aAAA group (P > 0.0017). However, a small but insignificant trend towards higher biomechanical indices in the sAAA group was seen (P = 0.08, 0.16, 0.20 for PWS, PWRI and RRED, respectively).
All geometric and biomechanical indices were significantly higher in the rAAA group compared to the aAAA group (P < 0.001), for instance PWS was 22.0 ± 5.8 vs. 33.4 ± 15.8 N/cm2 for the aAAA and rAAA, respectively (Table 2).
Table 2. FEA outcomes of aAAA, sAAA and rAAA.
| AAA | SAAA | RAAA | P-value | |
|---|---|---|---|---|
| n | 175 | 11 | 45 | - |
| Maximum diameter by radiologist (mm) | 60 ± 11 | 56 ± 9 | 77 ± 19 | 0.37a/0.001b/ 0.001c |
| Maximum diameter by software (mm) | 63 ± 13 | 64 ± 14 | 88 ± 24 | 0.88a/0.001b/0.002c |
| Total luminal volume (cm3) | 93 ± 49 | 93 ± 56 | 190 ± 134 | 0.90a/0.001b/ 0.012c |
| Total volume (cm3) | 200 ± 102 | 195 ± 64 | 424 ± 214 | 0.80a/0.001b/0.001c |
| Total ILT volume (cm3) | 83 ± 61 | 65 ± 35 | 186 ± 135 | 0.49a/0.001b/0.001c |
| PWS (N/cm2) | 22.0 ± 5.8 | 24.3 ± 5.4 | 33.4 ± 15.8 | 0.08a/0.001b/ 0.04c |
| PWRI | 0.5 ± 0.2 | 0.6 ± 0.3 | 1.0 ± 0.6 | 0.16a/0.001b/ 0.06c |
| RRED (mm) | 65 ± 60 | 67 ± 24 | 98 ± 51 | 0.20a/0.001b/0.07c |
ILT = intraluminal thrombus, PWS = peak wall stress, PWRI = peak wall rupture index, RRED = rupture risk equivalent diameter, AAA = asymptomatic abdominal aortic aneurysm, SAAA = symptomatic non-ruptured abdominal aortic aneurysm, RAAA = ruptured abdominal aortic aneurysm.
a: AAA compared to SAAA
b: AAA compared to RAAA
c: SAAA compared to RAAA
Significant differences were also seen in geometric indices sAAA and the rAAA group (P<0.017). For instance the total ILT-volume was significantly higher in the rAAA; 65 ± 35 cm3 versus 186 ± 135 cm3 (P = 0.001) for the sAAA and rAAA, respectively. However the biomechanical indices did not show a significant difference after Bonferroni correction. For instance PWS was 24.3 ± 5.4 vs. 33.4 ± 15.8 N/cm2 (P = 0.04) for the sAAA and rAAA, respectively (Table 2).
An overlap was seen between the aAAA and SAAA cases for both the diameter and biomechanical indices. However, a larger overlap was seen between the aAAA and rAAA groups. Thus several ruptured cases could not be distinguished from the asymptomatic cases based on these parameters.
Diameter matching
After diameter matching the demographic characteristics were still similar (P > 0.05) between the aAAA and rAAA group. However, no differences in biomechanical indices were seen between the aAAA and the rAAA group after diameter matching (Table 3; P > 0.05).
Table 3. FEA outcomes of the diameter matched subgroup.
| AAA | RAAA | P-value | |
|---|---|---|---|
| n | 31 | 31 | - |
| Maximum diameter by radiologist (mm) | 71 ± 15 | 72 ± 18 | 0.81 |
| Maximum diameter by software (mm) | 77 ± 16 | 78 ± 17 | 0.67 |
| Total luminal volume (cm3) | 132 ± 79 | 132 ± 76 | 0.76 |
| Total volume (cm3) | 296 ± 150 | 324 ± 148 | 0.34 |
| Total ILT volume (cm3) | 129 ± 91 | 158 ± 117 | 0.34 |
| PWS (N/cm3) | 26.1 ± 8.9 | 26.2 ± 7.5 | 0.99 |
| PWRI | 0.69 ± 0.33 | 0.70 ± 0.27 | 0.61 |
| RRED (mm) | 88 ± 89 | 73 ± 22 | 0.95 |
ILT = intraluminal thrombus, PWS = peak wall stress, PWRI = peak wall rupture index, RRED = rupture risk equivalent diameter, AAA = asymptomatic abdominal aortic aneurysm, SAAA = symptomatic non-ruptured abdominal aortic aneurysm, RAAA = ruptured abdominal aortic aneurysm.
ROC analysis
The ROC-curves are displayed in Fig 3. The maximum diameter shows a slightly higher area under the curve (AUC) compared to the biomechanical indices; 0.843, 0.770, 0.796, and 0.778 for the maximum diameter, PWS, PWRI and RRED, respectively. However, when the maximum diameter was combined with the biomechanical indices the AUC increased a little bit to 0.855.
Fig 3. ROC-curve for the ability to accurately predict rupture.
Maximum diameter (blue), PWS (green), PWRI (brown), RRED (purple) and combination of all parameters (yellow).
Discussion
This study examined three potential biomechanical parameters (PWS, PWRI and RRED) for rupture risk prediction of AAA based on FEA with patient specific geometries segmented from CTa. SAAAs showed a trend towards higher values of these biomechanical indices compared to aAAA while no significant difference in maximum diameter was seen. This trend suggests that biomechanical indices ameliorate rupture risk prediction compared to the maximum diameter alone as symptomatic aneurysms are prone to rupture.[23,24]
The results showed significant higher biomechanical indices in rAAA which is consistent with previous studies.[9,10,11,15,25–30,31] However, the diameter was also significantly higher. After diameter matching no significant differences in geometric or biomechanical parameters between aAAA and rAAA were seen. Three out of four previous studies using diameter matching did show a significant difference between these groups.[9,11,28,30] These studies excluded the large and small diameters to create a diameter matched group (55-75mm). Our study used a true matched subject design as ruptured aneurysms were matched to similar sized aneurysms in the aAAA group, giving a more accurate overview of the total AAA population.
Biomechanical indices are a direct result of several clinical factors such as age, sex, blood pressure, morphology and shape. Therefore, these indices might facilitate the rupture risk estimation of a specific patient. Additionally, the number of patients with small AAAs expands with the introduction of screening programs, increasing the need to identify the small AAAs at risk of rupture.[32] In this group biomechanical indices might be useful.
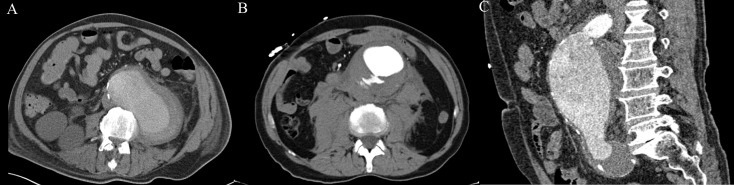
This study has some limitations. In all subjects endovascular infrarenal aneurysm repair was done. Therefore the size of the aAAA was skewed towards the threshold to treat (5.5cm) and thus these AAA already posed a higher risk of rupture. Additionally, the geometries included in this study are geometries suitable for endovascular repair without branches; i.e. an infrarenal AAA with a proper landing zone, sufficient iliac access and limited tortuosity. A quarter of the ruptured geometries analyzed (examples in Fig 4), and two percent of the asymptomatic geometries could not be accurately extracted. Consequently, FEA was not possible. As PWS increases at higher curvatures these cases indicate a possible benefit for the biomechanical analysis over the maximum diameter. However, the segmentation methods should first be optimized to be able to include all cases.
Fig 4. Examples of rAAA cases.
A. AAA and hemorrhagic region could not be distinguished, this case was excluded. B. Contrast extravasation into the ILT, with manual correction a sufficient estimation of pre-rupture state could be acquired. C. Tortuous proximal aneurysm inlet with a decrease in contrast in the aneurysmal lumen and an iliac artery aneurysm, with much manual correction an segmentation could be made.
Previous studies used both pre-rupture and post-rupture geometries[9,15,25,28,33–35]. In these studies it was assumed that biomechanics of post-ruptured could still represent the pre-rupture situation. However, it can be argued that the rupture causes a significant geometrical change in the aneurysm. As a consequence the extracted post-rupture geometry might not result in a realistic estimation of the pre-rupture biomechanical indices. Nonetheless, no study could be found confirming or disapproving this statement. The comparison between aAAA and sAAA is therefore more promising for assessing the clinical usability of biomechanical analysis. However, larger cohorts are needed to confirm the results of this study.
The outcome was not blinded from the observer during the analysis. This is could have resulted in a bias, especially in the aAAA versus sAAA comparison. However, apart from the segmentation the complete analysis process was automated. Therefore bias could only occur when manual correction of the segmentation was needed. During this step both groups were treated as equally as possible. Apart of the segmentation the analysis process was automated and thus bias can only occur when manual correction of the segmentation is needed. As the software showed similar maximum diameters between the experienced radiologist and software for both aAAA and sAAA, the bias is likely to be minimal.
During this study the biomechanical showed similar AUC values for predicting rupture compared to the maximum diameter. The previous study of Fillinger et al. showed similar AUC-values, but the PWS AUC-value was significantly higher (0.88 versus 0.74) compared to the maximum diameter.[25]. Contrary to Fillinger et al. this study included the ILT and a patient specific blood pressure, both influence the wall stress.[36–44] Therefore, it is anticipated that the used model better represents the local geometry and physiology. However, this model did not include calcifications, which could also impact wall stress, especially at the border of the calcified plaque.[36,45,46] Notwithstanding, determining the exact position of the calcified plaque is difficult due to partial volume effect and luminal contrast enhancement. Thus including calcifications into the model will require an additional non-contrast CT or a validated method to quantify and locate the calcified volume.
Furthermore, other assumptions were made, such as a homogenous ILT composition and a uniform wall thickness. These factors might differ between aAAA and rAAA and subsequently might result in a similar PWS and PWRI in the diameter matched cases.
To conclude, this study showed no added value for biomechanical indices in AAA risk assessment. Clinical applicability is reduced by the complexity of the analysis However, as symptomatic aneurysms show a trend towards higher biomechanical indices with similar diameters the indices may provide additional information about aneurysm growth and development, but larger (prospective) studies are needed to truly evaluate the clinical usability.
Data Availability
All relevant data is available within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers R, et al. Aneurysm global epidemiology study public health measures can further reduce abdominal aortic aneurysm mortality. Circulation. 2014;129: 747–753. 10.1161/CIRCULATIONAHA.113.005457 [DOI] [PubMed] [Google Scholar]
- 2.Scott RAP, Tisi PV, Ashton HA, Allen DR. Abdominal aortic aneurysm rupture rates: A 7-year follow-up of the entire abdominal aortic aneurysm population detected by screening. J Vasc Surg. 1998;28: 124–128. 10.1016/S0741-5214(98)70207-1 [DOI] [PubMed] [Google Scholar]
- 3.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41 Suppl 1: S1–S58. 10.1016/j.ejvs.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 4.Nicholls SC, Gardner JB, Meissner MH, Johansen KH. Rupture in small abdominal aortic aneurysms. J Vasc Surg. 1998;28: 884–888. 10.1016/S0741-5214(98)70065-5 [DOI] [PubMed] [Google Scholar]
- 5.Powell JT, Brown LC, Forbes JF, Fowkes FGR, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007;94: 702–708. 10.1002/bjs.5778 [DOI] [PubMed] [Google Scholar]
- 6.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population-a meta-analysis. PLoS One. 2013;8: 1–11. 10.1371/journal.pone.0081260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karthikesalingam A, Vidal-Diez A, Holt PJ, Loftus IM, Schermerhorn ML, Soden PA, et al. Thresholds for Abdominal Aortic Aneurysm Repair in England and the United States. N Engl J Med. 2016;375: 2051–2059. 10.1056/NEJMoa1600931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorp D. Biomechanics of abdominal aortic aneurysm. J Biomech. 2007;40: 1887–902. 10.1016/j.jbiomech.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillinger MF, Raghavan MLL, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg. 2002;36: 589–597. 10.1067/mva.2002.125478 [DOI] [PubMed] [Google Scholar]
- 10.Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J. Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc Surg. England; 2010;40: 176–85. 10.1016/j.ejvs.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 11.Doyle BJ, Callanan A, Walsh MT, Grace P a., McGloughlin TM. A Finite Element Analysis Rupture Index (FEARI) as an additional tool for abdominal aortic aneurysm rupture prediction. Vasc Dis Prev. 2009;6: 114–121. 10.2174/1567270000906010114 [DOI] [Google Scholar]
- 12.Auer M, Gasser C. Reconstruction and finite element mesh generation of abdominal aortic aneurysms from computerized tomography angiography data with minimal user interactions. IEEE Trans Med Imaging. United States; 2010;29: 1022–1028. 10.1109/TMI.2009.2039579 [DOI] [PubMed] [Google Scholar]
- 13.Buijs RVC, Willems TP, Tio R, Boersma HH, Tielliu IFJ, Slart RHJ, et al. Current state of experimental imaging modalities for risk assessment of abdominal aortic aneurysm. J Vasc Surg. Society for Vascular Surgery; 2013;57: 851–9. 10.1016/j.jvs.2012.10.097 [DOI] [PubMed] [Google Scholar]
- 14.Tierney ÁP, Callanan A, McGloughlin TM. Use of regional mechanical properties of abdominal aortic aneurysms to advance finite element modeling of rupture risk. J Endovasc Ther. 2012;19: 100–114. 10.1583/11-3456.1 [DOI] [PubMed] [Google Scholar]
- 15.Vande Geest JP, Di Martino ES, Bohra A, Makaroun MS, Vorp DA. A biomechanics-based rupture potential index for abdominal aortic aneurysm risk assessment: demonstrative application. Ann N Y Acad Sci. United States; 2006;1085: 11–21. 10.1196/annals.1383.046 [DOI] [PubMed] [Google Scholar]
- 16.Gasser TC, Nchimi A, Swedenborg J, Roy J, Sakalihasan N, Bockler D, et al. A novel strategy to translate the biomechanical rupture risk of abdominal aortic aneurysms to their equivalent diameter risk: Method and retrospective validation. Eur J Vasc Endovasc Surg. England; 2014;47: 288–295. 10.1016/j.ejvs.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 17.Leemans EL, Willems TP, van der Laan MJ, Slump CH, Zeebregts CJ. Biomechanical Indices for Rupture Risk Estimation in Abdominal Aortic Aneurysms. J Endovasc Ther. 2017;24: 254–261. 10.1177/1526602816680088 [DOI] [PubMed] [Google Scholar]
- 18.Teutelink A, Cancrinus E, van de Heuvel D, Moll F, de Vries J-P. Preliminary intraobserver and interobserver variability in wall stress and rupture risk assessment of abdominal aortic aneurysms using a semiautomatic finite element model. J Vasc Surg. 2012;55: 326–30. 10.1016/j.jvs.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 19.Harris PL, Buth J, Mialhe C, Myhre HO, Norgren L. The Need for Clinical Trials of Endovascular Abdominal Aortic Aneurysm Stent-Graft Repair: The EUROSTAR Project [Internet]. Journal of Endovascular Surgery. 1997. pp. 72–77. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129 10.1161/01.cir.0000437741.48606.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghavan ML, Vorp DA, Federle MP, Makaroun MS, Webster MW. Wall stress distribution on three-dimensionally reconstructed models of human abdominal aortic aneurysm. J Vasc Surg. 2000;31: 760–769. Available: http://www.scopus.com/inward/record.url?eid=2-s2.0-0033999346&partnerID=tZOtx3y1 10.1067/mva.2000.103971 [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: Comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. W.B. Saunders; 1996;12: 230–237. 10.1016/S1078-5884(96)80112-2 [DOI] [PubMed] [Google Scholar]
- 23.Cambria RA, Gloviczki P, Stanson AW, Cherry KJ, Hallett JW, Bower TC, et al. Symptomatic, nonruptured abdominal aortic aneurysms: Are emergent operations necessary? Ann Vasc Surg. 1994;8: 121–126. 10.1007/BF02018859 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan C a., Rohrer MJ, Cutler BS. Clinical management of the symptomatic but unruptured abdominal aortic aneurysm. J Vasc Surg. 1990;11: 799–803. 10.1067/mva.1990.19420 [DOI] [PubMed] [Google Scholar]
- 25.Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37: 724–732. 10.1067/mva.2003.213 [DOI] [PubMed] [Google Scholar]
- 26.Venkatasubramaniam AK, Fagan MJ, Mehta T, Mylankal KJ, Ray B, Kuhan G, et al. A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2004;28: 168–176. 10.1016/j.ejvs.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 27.Truijers M, Pol JA, Schultzekool LJ, van Sterkenburg SM, Fillinger MF, Blankensteijn JD. Wall stress analysis in small asymptomatic, symptomatic and ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. England; 2007;33: 401–407. 10.1016/j.ejvs.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 28.Raghavan MLL, Vorp DA. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech. UNITED STATES: Elsevier Science Ltd; 2000;33: 475–482. 10.1016/S0021-9290(99)00201-8 [DOI] [PubMed] [Google Scholar]
- 29.Heng MS, Fagan MJ, Collier JW, Desai G, McCollum PT, Chetter IC. Peak wall stress measurement in elective and acute abdominal aortic aneurysms. J Vasc Surg. 2008;47: 17–22; discussion 22. 10.1016/j.jvs.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Maier A, Gee MW, Reeps C, Pongratz J, Eckstein H-H, Wall WA. A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann Biomed Eng. United States; 2010;38: 3124–3134. 10.1007/s10439-010-0067-6 [DOI] [PubMed] [Google Scholar]
- 31.Erhart P, Hyhlik-Dürr A, Geisbüsch P, Kotelis D, Müller-Eschner M, Gasser TC. C, et al. Finite element analysis in asymptomatic, symptomatic, and ruptured abdominal aortic aneurysms: in search of new rupture risk predictors. Eur J Vasc Endovasc Surg. England, England: W.B. Saunders Ltd; 2015;49: 239–245. 10.1016/j.ejvs.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 32.Wanhainen A, Hultgren R, Linné A, Holst J, Gottsäter A, Langenskiöld M, et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation. 2016;134: 1141–1148. 10.1161/CIRCULATIONAHA.116.022305 [DOI] [PubMed] [Google Scholar]
- 33.Vande Geest JP, Schmidt DE, Sacks MS, Vorp DA. The effects of anisotropy on the stress analyses of patient-specific abdominal aortic aneurysms. Ann Biomed Eng. 2008;36: 921–32. 10.1007/s10439-008-9490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonesson B, Sandgren T, Länne T. Abdominal aortic aneurysm wall mechanics and their relation to risk of rupture. Eur J Vasc Endovasc Surg. 1999;18: 487–493. 10.1053/ejvs.1999.0872 [DOI] [PubMed] [Google Scholar]
- 35.Wilson KA, Lee AJ, Hoskins PR, Fowkes GR, Ruckley CV, Bradbury AWA, et al. The relationship between aortic wall distensibility and rupture of infrarenal abdominal aortic aneurysm. J Vasc Surg. United States; 2003;37: 112–117. 10.1067/mva.2003.40 [DOI] [PubMed] [Google Scholar]
- 36.Li Z-Y, U-King-Im J, Tang TY, Soh E, See TC, Gillard JH. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg. 2008;47: 928–935. 10.1016/j.jvs.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 37.Wang DHJ, Makaroun MS, Webster MW, Vorp D. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg. United States; 2002;36: 598–604. Available: http://www.scopus.com/inward/record.url?eid=2-s2.0-0242693879&partnerID=tZOtx3y1 [DOI] [PubMed] [Google Scholar]
- 38.Reeps C, Gee M, Maier A, Gurdan M, Eckstein H-H, Wall WA. The impact of model assumptions on results of computational mechanics in abdominal aortic aneurysm. J Vasc Surg. United States; 2010;51: 679–688. 10.1016/j.jvs.2009.10.048 [DOI] [PubMed] [Google Scholar]
- 39.Maier A, Gee MW, Reeps C, Eckstein HH, Wall WA. Impact of model complexity on patient specific wall stress analyses of abdominal aortic aneurysms. IFMBE Proc. 2009;25: 510–513. 10.1007/978-3-642-03882-2-135 [DOI] [PubMed] [Google Scholar]
- 40.Speelman L, Schurink GWH, Bosboom EMH, Buth J, Breeuwer M, van de Vosse FN, et al. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J Vasc Surg. United States; 2010;51: 19–26. 10.1016/j.jvs.2009.08.075 [DOI] [PubMed] [Google Scholar]
- 41.Polzer S, Gasser TC, Swedenborg J, Bursa J. The impact of intraluminal thrombus failure on the mechanical stress in the wall of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. England; 2011;41: 467–473. 10.1016/j.ejvs.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 42.Bluestein D, Dumont K, De Beule M, Ricotta J, Impellizzeri P, Verhegghe B, et al. Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm–FSI modelling. Comput Methods Biomech Biomed Engin. 2009;12: 73–81. 10.1080/10255840903077170 [DOI] [PubMed] [Google Scholar]
- 43.Rissland P, Alemu Y, Einav S, Ricotta J, Bluestein D. Abdominal aortic aneurysm risk of rupture: patient-specific FSI simulations using anisotropic model. J Biomech Eng. 2009;131: 31001 10.1115/1.3005200 [DOI] [PubMed] [Google Scholar]
- 44.Scotti CM, Finol EA. Compliant biomechanics of abdominal aortic aneurysms: a fluid–structure interaction study. Comput Struct. 2007;85: 1097–1113. 10.1016/j.compstruc.2006.08.041 [DOI] [Google Scholar]
- 45.De Putter S, Van De Vosse FN, Breeuwer M, Gerritsen FA. Local influence of calcifications on the wall mechanics of abdominal aortic aneurysm. In: Manduca A, Amini AA, editors. Progress in Biomedical Optics and Imaging—Proceedings of SPIE. San Diego, CA; 2006. pp. E1–E11. 10.1117/12.650480 [DOI] [Google Scholar]
- 46.Speelman L, Bohra A, Bosboom EMH, Schurink GWH, van de Vosse FN, Makaorun MS, et al. Effects of wall calcifications in patient-specific wall stress analyses of abdominal aortic aneurysms. J Biomech Eng. United States; 2007;129: 105–109. 10.1115/1.2401189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data is available within the paper and its Supporting Information files.