Abstract
Objective
To determine whether congenital arhinia/Bosma arhinia microphthalmia syndrome (BAMS) and facioscapulohumeral muscular dystrophy type 2 (FSHD2), 2 seemingly unrelated disorders both caused by heterozygous pathogenic missense variants in the SMCHD1 gene, might represent different ends of a broad single phenotypic spectrum associated with SMCHD1 dysfunction.
Methods
We examined and/or interviewed 14 patients with FSHD2 and 4 unaffected family members with N-terminal SMCHD1 pathogenic missense variants to identify BAMS subphenotypes.
Results
None of the patients with FSHD2 or family members demonstrated any congenital defects or dysmorphic features commonly found in patients with BAMS. One patient became anosmic after nasal surgery and one patient was hyposmic; one man was infertile (unknown cause) but reported normal pubertal development.
Conclusion
These data suggest that arhinia/BAMS and FSHD2 do not represent one phenotypic spectrum and that SMCHD1 pathogenic variants by themselves are insufficient to cause either of the 2 disorders. More likely, both arhinia/BAMS and FSHD2 are caused by complex oligogenic or multifactorial mechanisms that only partially overlap at the level of SMCHD1.
Identical pathogenic variants in the “structural maintenance of chromosomes flexible hinge domain containing 1” (SMCHD1) gene are associated with 2 seemingly unrelated disorders: facioscapulohumeral muscular dystrophy type 2 (FSHD2),1 a rare form of adult-onset muscular dystrophy, and arhinia, a severe congenital malformation often accompanied by reproductive and ocular defects, a triad called Bosma arhinia microphthalmia syndrome (BAMS).2–4
FSHD2 has a complex etiology that involves SMCHD1 (18p11.32) and the D4Z4 macrosatellite repeat array (4q35).1 Loss of SMCHD1 repressive activity leads to partial relaxation of the D4Z4 chromatin structure and derepression of the normally suppressed DUX4 retrogene in the D4Z4 unit. Only specific 4q35 haplotypes provide a polyadenylation signal (DUX4PAS) that stabilizes the DUX4 messenger RNA, permitting translation of the myotoxic DUX4 protein.1,5 Contraction of the D4Z4 repeat array to 1–10 units can also relax the D4Z4 locus and derepress DUX4 expression; this is the mechanism underlying the more common form of FSHD called FSHD type 1 (FSHD1).5
In contrast to FSHD2, where missense and loss-of-function variants are distributed along the entire SMCHD1 locus, in patients with BAMS, the variants are all missense and clustered within or immediately downstream of the adenosine triphosphatase (ATPase) domain.2,3 While D4Z4 hypomethylation akin to FSHD2 in patients with BAMS suggests a loss-of-function mechanism,2 a gain-of-function mode of action has also been proposed.3
To date, only one patient with both arhinia and FSHD2 and one multiplex family with both conditions have been reported.2 There has yet to be a systematic investigation of BAMS-associated features in patients with FSHD2. Therefore, we performed phenotypic and genotypic studies in patients with FSHD2 and their family members with pathogenic missense variants in the N-terminal region of SMCHD1 to identify potential areas of overlap.
Methods
Patients
We identified 23 patients with FSHD with heterozygous pathogenic missense variants near the ATPase domain of SMCHD1 in the FSHD genetic database in the Department of Human Genetics of the Leiden University Medical Center. Family members of one patient were recruited through a cohort study (FSHD-FOCUS study) by the Department of Neurology of the Radboud University Medical Center, Nijmegen. Another 10 sporadic cases were recruited for participation by referring clinicians from the United States, France, United Kingdom, and the Netherlands.
Genetic testing
DNA was extracted from blood samples and analyzed for D4Z4 repeat size and chromosome 4q and 10q haplotypes, as described previously,6 and for SMCHD1 pathogenic variants by Sanger sequencing.1 CpG methylation at the D4Z4 repeat was determined by Southern blot and the methylation-sensitive restriction enzyme FseI. Detailed protocols are freely available from the Fields Center website (urmc.rochester.edu/fields-center). The Delta1 score, a measure of the degree of D4Z4 hypomethylation, was calculated as described previously.7 The Delta1 threshold for FSHD-associated SMCHD1 pathogenic variants lies below −21%.
Clinical assessment
All participants were interviewed regarding nasal and olfactory abnormalities, pubertal development, fertility, eye anatomy and vision, history of maxillofacial surgery, and presence of cleft lip/palate. Photographs were available for 10 participants, which were independently assessed by 3 clinicians.
In addition, 10 members of one family with FSHD were examined in person for (subtle) signs of arhinia or associated comorbidities by a clinical geneticist (M.K.) who was blinded to mutation status. Olfactory function was assessed using the Sniffin' Sticks Screening Test (Burghart Medizintechnik, GmbH, Wedel, Germany), which assigns a sex- and age-adjusted olfactory score. One family member was examined using Skype.
Standard protocol approvals, registrations, and patient consents
This study was conducted according to the principles of the Declaration of Helsinki (version October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). Participants were consented under a protocol approved by the local ethics committee of the Radboud University Medical Center, Nijmegen.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Genetic results
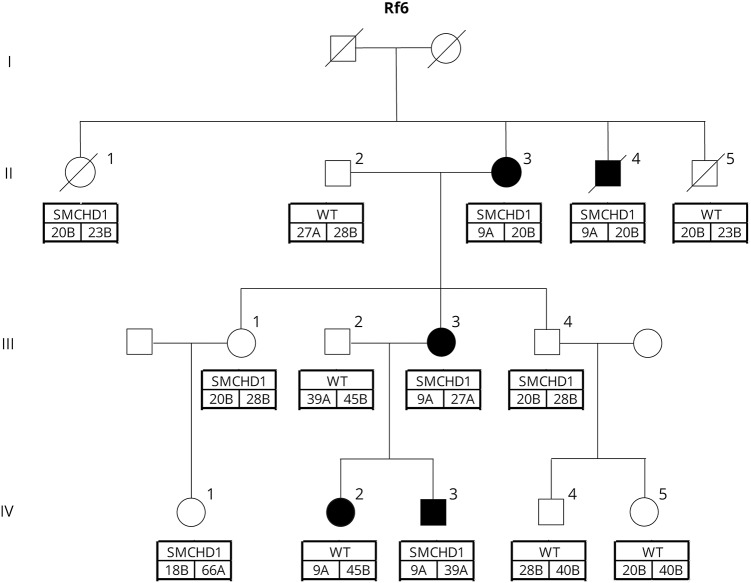
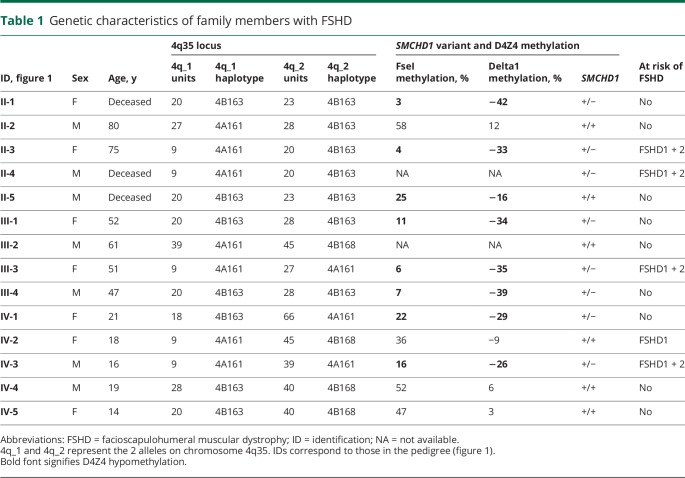
In the large Euro-Caucasian family with FSHD, 8 family members carried a pathogenic missense variant in SMCHD1 (c.320T>C; p.Leu107Pro) (figures 1 and 2, table 1). Of note, this pathogenic variant was previously reported in an unrelated, African American female with BAMS.2 All pathogenic variant carriers showed profound hypomethylation at the D4Z4 locus on chromosome 4q with Delta1 scores below −26%.7 The 5 affected individuals had the FSHD-permissive, 4qA haplotype that contains the somatic DUX4 PAS.6 In addition, 4 of them had a D4Z4 repeat array of 9 units, compatible with an additional molecular diagnosis of FSHD1,5 but also found in 1% to 2% of the Caucasian control population.8–10 The 3 unaffected individuals were homozygous for the 4qB haplotype, which is not FSHD-permissive because of the absence of a somatic DUX4 PAS. One family member who tested negative for the SMCHD1 pathogenic variant did carry a 9-unit repeat on a 4qA haplotype. Her Delta1 score was −9%.
Figure 1. Pedigree for FSHD2 multiplex family with pathogenic variant (p.L107P) in SMCHD1.
Shaded symbols represent family members meeting clinical criteria for FSHD2. Genetic information is listed below each family member: top box is mutation status (SMCHD1 variant present or WT [wild type]); lower boxes indicate the 4q35 haplotype (A or B) and D4Z4 repeat length (units) for each allele. FSHD2 = facioscapulohumeral muscular dystrophy type 2.
Figure 2. Sequence track of the SMCHD1 pathogenic variant in the family with FSHD2 and in a control sample.
The position of the variant in exon 3 is indicated above the sequence traces and is highlighted in yellow. The genomic position is based on reference genome hg19 and the transcript and protein position on accession number NM015295 and NP056110, respectively. FSHD2 = facioscapulohumeral muscular dystrophy type 2.
Table 1.
Genetic characteristics of family members with FSHD
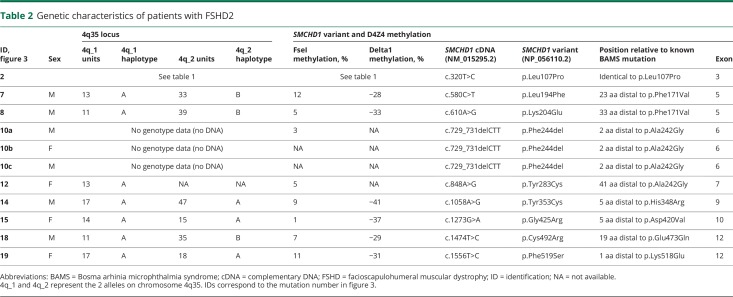
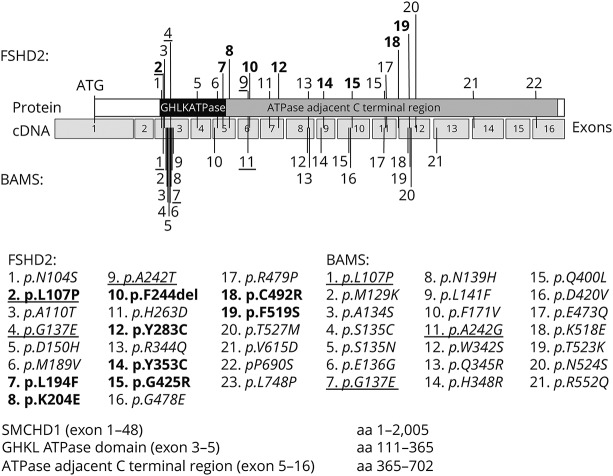
We identified 23 other sporadic FSHD2 patients in the FSHD genetic database with a pathogenic missense variant in close proximity to or identical to those previously identified in patients with arhinia or BAMS,2,3 (Shaw, unpublished observation). All patients with FSHD2 had a permissive haplotype and D4Z4 hypomethylation (table 2). Seventeen of the 20 pathogenic variants in these patients with FSHD2 involved the same SMCHD1 exon as in patients with arhinia and 3 pathogenic variants were identical to those found in patients with arhinia (figure 3). We also identified one family with a heterozygous 3–base pair deletion in exon 6 (c.729_731delCTT; p.Phe244del) resulting in the deletion of a single amino acid just 2 positions downstream of an amino acid affected in patients with BAMS.
Table 2.
Genetic characteristics of patients with FSHD2
Figure 3. Schematic of pathogenic missense variants in the N-terminal region of SMCHD1 associated with FSHD2 and/or arhinia/BAMS.
Pathogenic variants in the FSHD2 cohort in the current study are in bold (table 2), and the pathogenic variants that have been implicated in both FSHD2 and BAMS are underlined. ATPase = adenosine triphosphatase; BAMS = Bosma arhinia microphthalmia syndrome; cDNA = complementary DNA; FSHD2 = facioscapulohumeral muscular dystrophy type 2.
Clinical characteristics
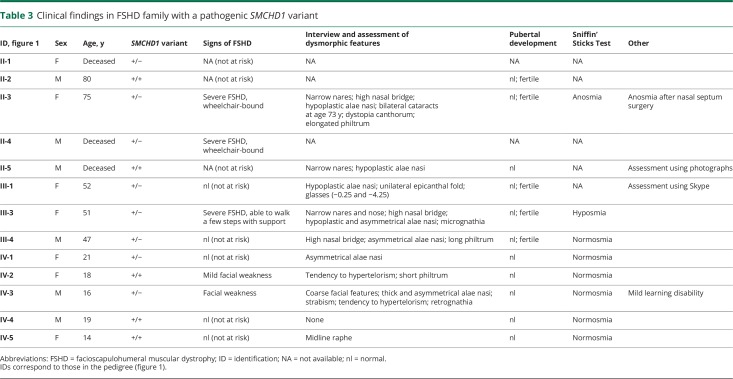
In the large FSHD family, 6 individuals with an N-terminal SMCHD1 pathogenic missense variant were examined (individuals II:1 and II:4 were deceased at the time of the study). None of them had microphthalmia, congenital cataracts, coloboma, nasolacrimal duct atresia, midface hypoplasia, or cleft lip/palate (table 3). Several family members had narrow nares and/or hypoplastic alae nasi (rounded prominence of nostril) but these features did not segregate with the SMCHD1 pathogenic variant, suggesting they were unrelated, familial traits. One family member with FSHD1 and 2 (II-3) developed anosmia shortly after surgery for a deviated nasal septum. A second affected patient with both FSHD1 and 2 (III-3) was hyposmic (Sniffin' Sticks Screening Test result below the 10th percentile). All family members who were questioned reported normal pubertal timing and denied infertility.
Table 3.
Clinical findings in FSHD family with a pathogenic SMCHD1 variant
Four family members had symptoms of FSHD: the 2 older individuals (50 years and older) displayed severe muscle weakness and were wheelchair-dependent, whereas the 2 younger individuals had facial weakness, an early sign of FSHD. Three of them had both FSHD1 and 2, and one of the younger individuals had only FSHD1.
The 10 sporadic FSHD2 patients who were phenotyped did not have physical features consistent with arhinia/BAMS (table 4). One male reported normal pubertal development but had infertility of unknown etiology. He denied other signs of hypogonadism such as cryptorchidism or micropenis and had never required testosterone replacement. Photographs of this patient revealed no signs of a craniofacial defect.
Table 4.
Clinical findings in sporadic FSHD2 patients as determined by interview and photographs
Discussion
We assessed patients with FSHD who had pathogenic missense variants in the N-terminal region of SMCHD1, which were recently shown to cause arhinia/BAMS, to determine whether FSHD2 and BAMS might represent the opposite ends of one broad, phenotypic spectrum or if each condition is caused by SMCHD1 dysfunction in the presence of a genetic background unique to each condition. Only one patient with arhinia has been identified thus far who meets clinical and genetic criteria for FSHD2,2 and until now, patients with FSHD2 had never been specifically assessed for BAMS-like features.
Detailed examination of a large FSHD family with an SMCHD1 pathogenic variant identical to one found in patients with BAMS did not uncover any congenital defects or dysmorphic features commonly found in patients with BAMS. We identified one patient in this family who developed cataracts in her 70s and lost olfaction after nasal surgery. These findings are unlikely to be related to BAMS as cataracts are very common with aging secondary to cumulative photooxidative insults (e.g., ultraviolet B) and she did not have congenital anosmia as occurs in patients with BAMS; rather, she lost olfactory function after nasal surgery, which is a recognized, albeit rare, potential side effect of septoplasty.11,12 We also observed several family members with nasal hypoplasia. The power of our combined genetic and phenotypic approach, however, allowed us to confidently classify this phenotype as a familial rather than SMCHD1-related trait as it did not segregate with the SMCHD1 pathogenic variant.
All other patients with FSHD2 included in this study reported normal olfaction, no craniofacial or ocular abnormalities, and normal pubertal development, and those of reproductive age were fertile with the exception of one male patient with infertility of unknown cause. Thus, we find no evidence for phenotypic overlap in FSHD2 and BAMS patients.
The phenotyping protocol for this study was intentionally simple and noninvasive in design such that all study procedures could be performed by patients from afar. Although we performed detailed, structured interviews to collect phenotypic data on the sporadic cases, it is possible that patients were not fully aware of any subtle BAMS-associated features. Future studies will be required to confirm our findings in a larger number of patients with FSHD using more sophisticated tools, such brain imaging, to assess the integrity of the olfactory bulbs and tracts, dilated eye examinations, and reproductive hormone testing.
Our data support the hypothesis that arhinia/BAMS and FSHD2 represent 2 distinct oligogenic disorders. In both conditions, SMCHD1 dysfunction appears to be necessary but not sufficient to cause disease. In FSHD2, a permissive 4q35 haplotype is one known requirement, but the variability in muscle weakness that is seen among family members with the same SMCHD1 pathogenic variant (and D4Z4 repeat size) suggests that there are other genetic or environmental modifiers yet to be discovered. Incomplete penetrance of SMCHD1 variants in the form of nasal hypoplasia or isolated anosmia has also been observed in multiplex arhinia/BAMS families.2 Modifier genes have not been identified in arhinia, but SMCHD1 binding partners and/or downstream targets are rational candidates. Thus, in the extremely rare chance that a patient has an N-terminal SMCHD1 pathogenic variant and meets the genetic requirements unique to arhinia/BAMS and to FSHD2, he or she can demonstrate both conditions.
Pathogenic variants in the N-terminal region of SMCHD1 have a critical role in the pathogenesis of both FSHD2 and arhinia/BAMS. The complete absence of phenotypic overlap between these 2 disorders, however, suggests that these variants are, by themselves, insufficient to cause either disorder. The current study instead supports an oligogenic or multifactorial disease mechanism for both FSHD2 and arhinia/BAMS.
Acknowledgment
The authors sincerely thank all participants for their contribution to this study.
Glossary
- ATPase
adenosine triphosphatase
- BAMS
Bosma arhinia microphthalmia syndrome
- FSHD
facioscapulohumeral muscular dystrophy
- SMCHD1
structural maintenance of chromosomes flexible hinge domain containing 1
Author contributions
K.M., R.J.L.F.L., B.G.M.v.E., C.G.C.H., N.D.S., and S.M.v.d.M.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and tables/figures. M.K.: acquisition of data, analysis and interpretation of data, drafting of tables/figures, revision of the manuscript. P.J.v.d.V., M.L.v.d.B., U.A.B., J.M.G., A.E.L., H.B., S.A.M., K.J., T.E., A.T., V.S., S.K.G., S.S., R.T., S.J.T., and N.C.V.: acquisition of data, analysis and interpretation of data, revision of the manuscript. B.G.M.v.E., N.D.S., and S.M.v.d.M.: obtained funding for the study.
Study funding
This study was funded by the Prinses Beatrix Spierfonds (W.OR12.22, W.OP14-01, W.OB17-01), the National Institute of Neurological Disorders and Stroke award numbers P01 NS069539 and U54, NS053672, the National Institute of Arthritis Musculoskeletal and Skin Diseases award number R01AR066248, and Stichting Spieren voor Spieren and was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES103315).
Disclosure
K. Mul, R. Lemmers, M. Kriek, P. van der Vliet, and M. van den Boogaard report no disclosures relevant to the manuscript. U. Badrising receives compensation for consultancy for Novartis Pharma A.G. and Argen-X. J. Graham, Jr., A. Lin, H. Brand, S. Moore, K. Johnson, T. Evangelista, A. Töpf, V. Straub, S. Kapetanovic García, S. Sacconi, R. Tawil, S. Tapscott, and N. Voermans report no disclosures relevant to the manuscript. B. van Engelen receives grants from Prinses Beatrix Spierfonds, Association Française contre les Myopathies, Stichting Spieren voor Spieren, FSHD Stichting, and NWO Dutch Organisation for Scientific Research. C. Horlings and N. Shaw report no disclosures relevant to the manuscript. S. van der Maarel receives compensation for consultancy for aTyr Pharma and Fulcrum therapeutics, receives grants from the NIH National Institute of Neurological Disorders and Stroke (P01NS069539), the Prinses Beatrix Spierfonds, the European Union Framework Programme 7 (agreement 2012-305121, NEUROMICS), the FSH Society, and Stichting Spieren voor Spieren. Go to Neurology.org/N for full disclosures.
References
- 1.Lemmers RJ, Tawil R, Petek LM, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet 2012;44:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw ND, Brand H, Kupchinsky ZA, et al. SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated arhinia and Bosma arhinia microphthalmia syndrome. Nat Genet 2017;49:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon CT, Xue S, Yigit G, et al. De novo mutations in SMCHD1 cause Bosma arhinia microphthalmia syndrome and abrogate nasal development. Nat Genet 2017;49:249–255. [DOI] [PubMed] [Google Scholar]
- 4.Bosma JF, Henkin RI, Christiansen RL, Herdt JR. Hypoplasia of the nose and eyes, hyposmia, hypogeusia, and hypogonadotrophic hypogonadism in two males. J Craniofac Genet Dev Biol 1981;1:153–184. [PubMed] [Google Scholar]
- 5.Lemmers RJ, van der Vliet PJ, Klooster R, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 2010;329:1650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemmers RJ, van der Vliet PJ, van der Gaag KJ, et al. Worldwide population analysis of the 4q and 10q subtelomeres identifies only four discrete interchromosomal sequence transfers in human evolution. Am J Hum Genet 2010;86:364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemmers RJ, Goeman JJ, van der Vliet PJ, et al. Inter-individual differences in CpG methylation at D4Z4 correlate with clinical variability in FSHD1 and FSHD2. Hum Mol Genet 2015;24:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacconi S, Lemmers RJ, Balog J, et al. The FSHD2 gene SMCHD1 is a modifier of disease severity in families affected by FSHD1. Am J Hum Genet 2013;93:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemmers RJ, Wohlgemuth M, van der Gaag KJ, et al. Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy. Am J Hum Genet 2007;81:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scionti I, Fabbri G, Fiorillo C, et al. Facioscapulohumeral muscular dystrophy: new insights from compound heterozygotes and implication for prenatal genetic counselling. J Med Genet 2012;49:171–178. [DOI] [PubMed] [Google Scholar]
- 11.Bateman ND, Woolford TJ. Informed consent for septal surgery: the evidence-base. J Laryngol Otol 2003;117:186–189. [DOI] [PubMed] [Google Scholar]
- 12.Briner HR, Simmen D, Jones N. Impaired sense of smell in patients with nasal surgery. Clin Otolaryngol Allied Sci 2003;28:417–419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.