Abstract
Objective
To examine the risk of postpartum hemorrhage (PPH) and neonatal bleeding complications associated with late-pregnancy exposure to anticonvulsant drugs (ACDs) that induce cytochrome P450 enzymes (ACDi) and alter the metabolism of vitamin K compared to other ACDs.
Methods
We used a population-based cohort study stemming from a nationwide sample of publicly insured pregnant women with a liveborn infant from the 2000 to 2010 Medicaid Analytic eXtract. ACDi (carbamazepine, phenobarbital, phenytoin, oxcarbazepine, topiramate) were compared to other ACDs dispensed during the last month of pregnancy. Relative risks (RRs) and 95% confidence intervals (CIs) of PPH and neonatal bleeding complications were estimated using generalized linear models with fine stratification on the propensity score to control for indication and other potential confounders.
Results
Among 11,572 women with an ACD prescription overlapping delivery, 2.6% (135/5,109) in the ACDi group and 3.6% (231/6,463) in the other ACDs group had a diagnosis of PPH: unadjusted RR 0.74 (95% CI 0.60–0.91), adjusted RR 0.77 (95% CI 0.58–1.00). The prevalence of neonatal bleeding complications was 3.1% (157/5,109) in the ACDi group and 3.5% (229/6,463) in the other ACDs group: unadjusted RR 0.87 (95% CI 0.71–1.06), adjusted RR 0.83 (95% CI 0.64–1.08).
Conclusions
Evidence from this large observational study suggests that use of ACDi near delivery does not increase the risk of bleeding complications compared to other ACDs in clinical settings where neonatal intramuscular or oral vitamin K administration is considered standard of care. These findings provide reassurance for clinicians and pregnant women successfully treated with ACDi.
Both maternal and neonatal bleeding are important sources of morbidity and mortality.1–4 Given the major increase in women who consume prescription medications during pregnancy,5 a better understanding of bleeding risks in women and infants exposed to medications that may affect coagulation is needed. One class of medications that can inhibit vitamin K activity, and thus coagulation pathways, is anticonvulsants. There are approximately half a million women of reproductive age with epilepsy in the United States and 24,000 offspring born to these women each year.6 The majority of women with epilepsy are advised to continue medication for epilepsy in pregnancy, and changes or discontinuation of treatment during pregnancy may be dangerous for both the mother and fetus.7 Anticonvulsant drugs (ACDs) are taken by 0.4% of pregnant women for epilepsy.8 Moreover, several ACDs are used to treat mood disorders and prevent migraines.9–11 Some case reports, case series, and small cohorts from birth registries have raised concerns of an elevated risk of obstetric bleeding complications in women on ACDs, but the results are inconsistent.12–15 A recent, large population-based study showed a substantially increased risk of all types of postpartum hemorrhage (PPH) in patients with epilepsy.16 However, this study lacked information on medication use and could not provide information on the comparative safety of ACDs.
Alteration in vitamin K metabolism induced by ACDs has been postulated as a possible mechanism by which ACD use could lead to excess bleeding.17 Phenobarbital, primidone, phenytoin, oxcarbazepine, and carbamazepine have significant enzyme-inducing properties.18 In general, they induce or are substrates for cytochrome P450 (CYP450) isozymes, including CYP1A2, CYP2A6, CYP2B, CYP2C, and CYP3A, as well as the uridine diphosphate glucuronosyltransferase isozymes. Many of the newer ACDs (i.e., gabapentin, levetiracetam, tiagabine, and zonisamide) either have no induction effects or induce only selected enzymes.18 Whether ACDs with enzyme-inducing properties (ACDi) confer an increased risk of obstetric bleeding complications compared to other ACDs remains unknown.
Neonates are at particular risk of vitamin K deficiency bleeding (VKDB), due to insufficient placental transfer.19 An early vitamin K deficiency–related bleed that occurs within 24 hours of birth is almost exclusively seen in infants of mothers taking drugs that inhibit vitamin K.20 The clinical presentation is often severe and can include cephalohematoma, as well as intracranial and intraabdominal hemorrhages. Vitamin K is commonly given prophylactically after every birth for the prevention of VKDB.21 Placental transfer of maternal drugs that inhibit vitamin K activity has been described as a risk factor for early onset of VKDB.22
The aim of this study was to determine whether the use of ACDi in late pregnancy compared to other ACDs is associated with an increased risk of PPH and neonatal bleeding complications when neonatal vitamin K administration is the standard of care.
Methods
Cohort
We used the Medicaid Analytic eXtract database for 46 US states and the District of Columbia for the years 2000 through 2010, as previously described by Palmsten et al.23 The cohort included all pregnancies in women aged 12 to 55 years linked to live-born infants among Medicaid beneficiaries. The date of last menstrual period (LMP) was estimated based on a validated algorithm.24 Additional inclusion criteria were continuous eligibility for Medicaid with no private insurance or restricted benefits from 4 months after the estimated LMP through 3 months after delivery. The linked infants met the same Medicaid eligibility criteria as their mothers for at least 3 months after birth, unless they died. The cohort was then restricted to women with a filled prescription for any of the following most frequently used anticonvulsants from 4 months after the LMP month until delivery: carbamazepine, phenobarbital, phenytoin, oxcarbazepine, topiramate, valproic acid, divalproex, valproate sodium, lamotrigine, levetiracetam, gabapentin, pregabalin, and clonazepam. Pregnant women with one or more filled prescriptions for a blood thinner or anticoagulant (i.e., clopidogrel, aspirin, heparin and derivatives, warfarin, apixaban, dabigatran, rivaroxaban, edoxaban, fondaparinux, bivalirudin, antithrombin, alteplase) from 4 months after the LMP through 3 months after delivery were considered to be at higher risk of bleeding, and thus were excluded.
Standard protocol approvals, registrations, and patient consents
The use of this deidentified database for research was approved by the institutional review board of the Brigham and Women's Hospital. The institutional review board granted a waiver of informed consent.
Exposure
The relevant window for exposures was defined as the last month before delivery. Exposure was therefore defined as a filled prescription for ACDi, with drug supply that overlapped with the delivery date: carbamazepine, phenobarbital, phenytoin, oxcarbazepine, topiramate. The reference group was defined as women who had a prescription for other anticonvulsants (valproate, lamotrigine, levetiracetam, pregabalin, gabapentin, clonazepam) with supply that overlapped the delivery date. Women exposed to both ACDi and other anticonvulsants were not included in the study.
Outcomes
The primary maternal study outcome was the occurrence of PPH from delivery to 1 month post delivery (because in some instances, PPH can occur in a delayed manner). PPH was defined on the basis of ICD-9 diagnostic code 666.xx or any subcode thereof in the inpatient or outpatient claims. Diagnostic codes have been shown to have a positive predictive value higher than 80% for PPH in administrative data.25,26 The primary neonatal complication examined was a composite of neonatal bleeding complications, based on an ICD-9 code of 772.1x (intraventricular hemorrhage of fetus or newborn) in maternal or infant claims from delivery to 1 month post delivery, 772.2–772.9 (subarachnoid or umbilical or gastrointestinal or adrenal cutaneous or other specified or unspecified hemorrhage of fetus or newborn) in maternal or infant claims during the delivery hospitalization, or any of the following ICD-9 codes in infant claims only: 430.xx (subarachnoid hemorrhage), 431.xx (intracerebral hemorrhage), 432.xx (other and unspecified intracranial hemorrhage), 459.0 (other disorders of circulatory system, hemorrhage unspecified), 478.xx (gastrointestinal hemorrhage), 287.8 (other specified hemorrhagic conditions), and 287.9 (unspecified hemorrhagic conditions) from delivery to 1 month post delivery.
Covariates
We considered the following covariates as potential confounders (or proxies for confounders) of the association between ACDi and PPH or neonatal bleeding complications, which were assessed in the maternal claims from 4 months after the LMP until delivery: maternal demographics, potential indication for anticonvulsant medications, other medical comorbidities and obstetric conditions, medications that are risk factors for bleeding or proxies for conditions that might increase the risk of bleeding (see table 1 for a complete list of covariates).
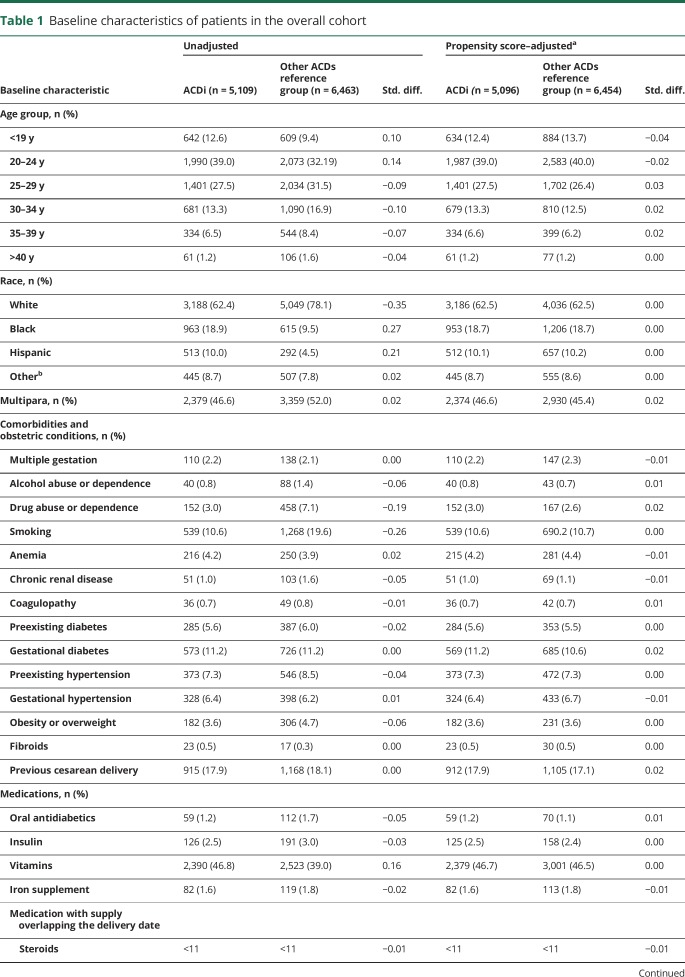
Table 1.
Baseline characteristics of patients in the overall cohort
Statistical analysis
We compared the proportions or means and SDs for potential confounders among women exposed to ACDi at delivery to other ACDs. In addition, we identified relevant obstetric characteristics, including polyhydramnios, placenta previa, preeclampsia, placental abruption, number of hospitalizations >3 days during the last month before delivery, chorioamnionitis, induction of labor, operative delivery, and cesarean delivery, which may increase the risk of PPH, but we did not adjust for these characteristics as they may be on the causal pathway from ACDi use to PPH.27 Absolute risks for any PPH and neonatal bleeding complications and unadjusted risk ratios (RRs) with their 95% confidence intervals (CIs) were calculated. Exposure propensity scores (PS) were estimated as the predicted probability of receiving ACDi vs other ACDs, conditional upon the above specified potential confounders using logistic regression models. The population in the nonoverlapping areas of the PS distributions was trimmed, and 50 PS strata were created based on the distribution of the women receiving ACDi.28 Adjusted RR and 95% CI were estimated in generalized linear models (PROC GENMOD with binomial distribution, log link function, weight statement). All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Sensitivity analysis
To test the robustness of our findings, we conducted several sensitivity analyses. First, we redefined exposure status using prescriptions for the medications of interest with days' supply ending from 30 days before delivery to any time after delivery since it is possible that women with a prescription supply ending shortly before delivery were also exposed at or near delivery. In addition, to further control for the potential effect of the treatment indication, we conducted a sensitivity analysis restricted to patients with a diagnosis of epilepsy (in the main analysis, we adjust for this condition). As a negative control analysis, we redefined exposure status as filling a prescription for the medication of interest with the supply ending more than 30 days before delivery, since there is unlikely to be any carryover effect for medications discontinued at least 30 days before delivery.
Data availability
Because of the data use agreements associated with the use of the Medicaid data, we are not permitted to release the raw data used in our analyses.
Results
Cohort characteristics
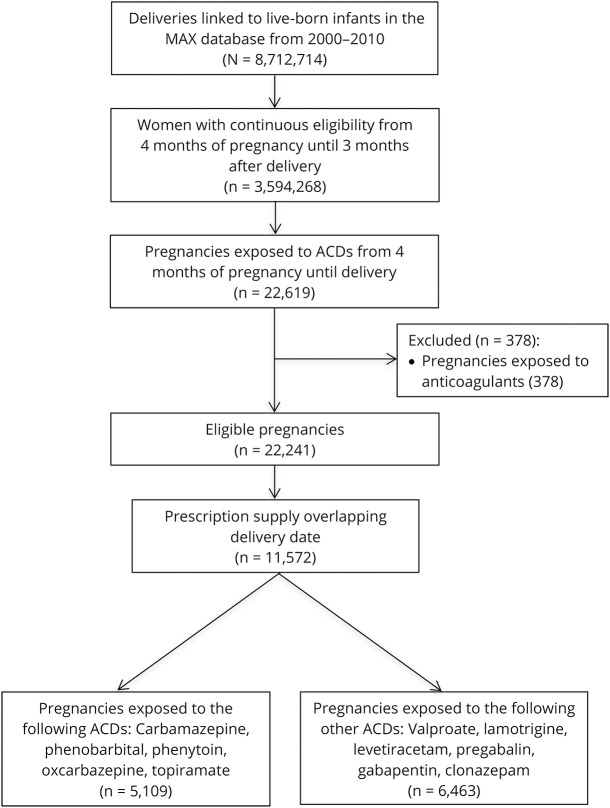
Among 3,594,268 pregnancies available in the Medicaid Analytic eXtract mom-baby linked cohort, 11,572 (0.3%) filled an ACD prescription with days' supply that overlapped with the delivery date for any of the studied anticonvulsants (figure). Among these, 5,109 were exposed to ACDi (1,669 carbamazepine, 666 phenobarbital, 1,686 phenytoin, 513 oxcarbazepine, 765 topiramate) and 6,463 were exposed to other ACDs (900 valproic acid, 1,850 lamotrigine, 809 levetiracetam, 97 pregabalin, 843 gabapentin, 2,263 clonazepam). In the ACDi group, 4,922 were on monotherapy, 184 had 2 different medications prescribed, and 6 had 3 or more, and in the other ACDs group, 6,171 were on monotherapy, 285 had 2 different medications prescribed, and 7 had 3 or more.
Figure. Study flowchart.
ACD = anticonvulsant drug; MAX = Medicaid Analytic eXtract.
There were several differences in the baseline characteristics of the women exposed to ACDi compared with women exposed to noninducing ACD. Women exposed to ACDi were less likely to be white, less likely to have smoking or illicit drug use reported, more likely to carry a diagnosis of epilepsy, and less likely to carry a diagnosis of bipolar disorder, anxiety, depression, or pain. All characteristics were well balanced (as assessed by absolute standardized differences <0.1) after PS stratification weights were applied (table 1).
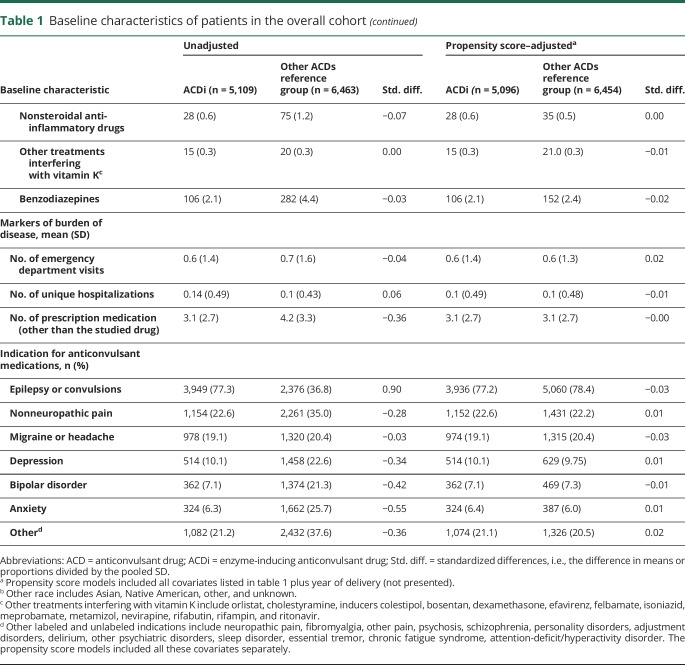
No differences were observed between groups in the unweighted distribution of several pregnancy-associated risk factors for PPH (i.e., polyhydramnios, placenta previa, placental abruption, or chorioamnionitis) and delivery-associated risk factors for PPH (i.e., induction of labor, cesarean delivery, or delivery of a macrosomic infant) (table 2). Other risk factors for neonatal bleeding were also balanced (i.e., preterm, birth trauma).
Table 2.
Obstetric, delivery, and neonatal characteristics of patients in the overall cohort
Association of ACDi and PPH
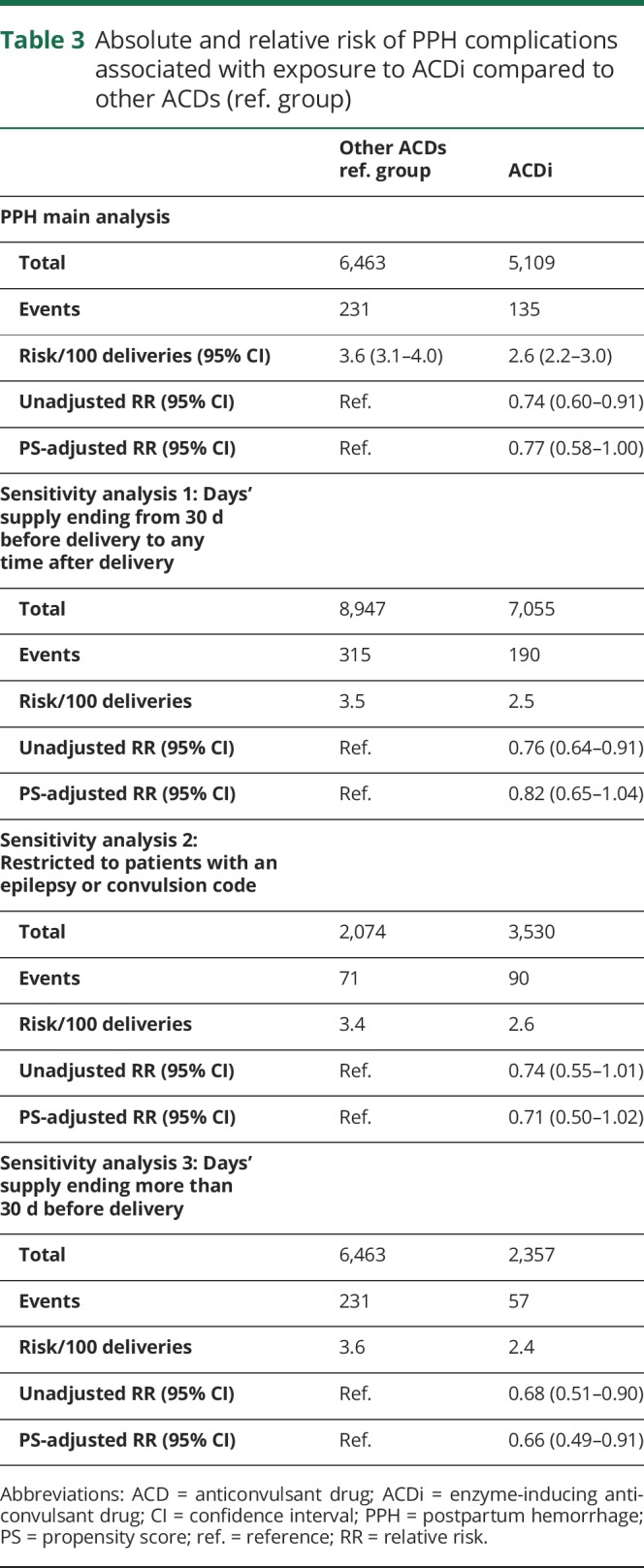
The risk of PPH was 2.6% (95% CI 2.2%–3.0%) in the ACDi group and 3.6% (95% CI 3.1%–4.0%) in the reference group (table 3). The unadjusted RR for PPH associated with exposure to ACDi vs noninducing ACDs was 0.74 (95% CI 0.60–0.91). After adjustment for confounders using PS stratification, the adjusted RR was 0.77 (95% CI 0.58–1.00) (table 3). No substantial differences were noticed among drugs.
Table 3.
Absolute and relative risk of PPH complications associated with exposure to ACDi compared to other ACDs (ref. group)
Association of ACDi and neonatal bleeding complications
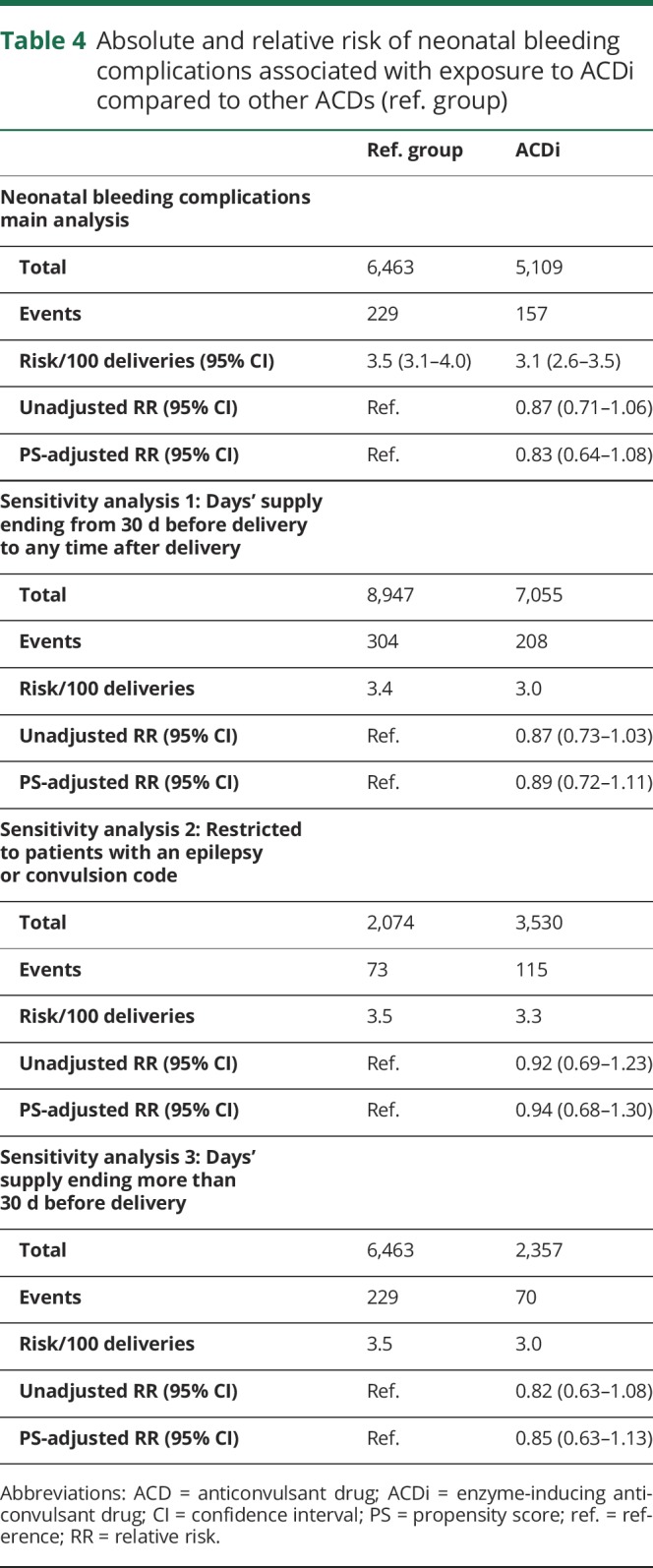
The risk of neonatal bleeding complications was 3.1% (95% CI 2.6%–3.5%) in the ACDi group and 3.5% (95% CI 3.1%–4.0%) in the reference group. The most prevalent were intraventricular hemorrhage (0.69% [35/5,109] in the ACDi group and 0.68% [44/6,463] in the reference group) and cutaneous hemorrhage (0.67% [34/5,109] in the ACDi group and 0.90% [58/6,463] in the reference group). The unadjusted RR was 0.87 (95% CI 0.71–1.06). After adjustment for confounders using PS stratification, the adjusted RR was 0.83 (95% CI 0.64–1.08) (table 4).
Table 4.
Absolute and relative risk of neonatal bleeding complications associated with exposure to ACDi compared to other ACDs (ref. group)
Sensitivity analyses
When we performed our analysis using a less restrictive definition for exposure time window (i.e., with days' supply ending from 30 days before delivery to any time after delivery), the results were similar to those of the main analysis for PPH (RR 0.82, 95% CI 0.65–1.04) and neonatal bleeding complications (RR 0.89, 95% CI 0.72–1.11) after adjustment. Results were also similar after restriction to women with epilepsy: PPH (RR 0.71, 95% CI 0.50–1.02) or neonatal bleeding complications (RR 0.94, 95% CI 0.68–1.30). When we moved the exposure time window to any supply ending before 1 month before delivery as a negative control (assuming no carryover effect), there were no meaningful differences with the results of the main analysis for PPH (RR 0.66, 95% CI 0.48–0.91) or for neonatal bleeding complications (RR 0.85, 95% CI 0.63–1.13).
Discussion
We examined the association between the use of ACDi in late pregnancy and the risks of bleeding complications including PPH and neonatal bleeding complications among 11,572 Medicaid-insured women exposed to ACDi in late pregnancy compared to a reference group of other ACDs during the same period. We did not observe a significantly increased risk for either outcome after controlling for potential confounding conditions and coexposures.
Prior studies assessing the effect of epilepsy on several pregnancy outcomes observed 30% to 50% increases in PPH after exposure to any ACD (i.e., any ACD with or without inducing properties).13,15 In the first study,15 1,350 epileptic women exposed to any ACDs (including ACDi) any time during pregnancy were compared to all singleton births of the Swedish Medical Birth Register. The ACDi carbamazepine was the most prevalent drug (n = 683). The odds ratio for PPH after vaginal delivery was 1.29 (95% CI 1.02–1.63). In the second study,13 2,805 pregnancies in women with current or history of epilepsy were compared to all pregnancies of the Norwegian National Population Register. More than 30% of the women were taking an ACD with or without inducing properties, and among them, the odds ratio for PPH was 1.5 (95% CI 1.3–1.9). Use of vitamin K was not reported in either study but was recommended in Sweden and Norway at the time of the studies. These prior studies assessing the effect of epilepsy had major differences by design in comparison to ours. They used a reference group of nonepileptic women and epileptic women were not systematically treated with an ACD with or without inducing properties. For those exposed to medication, women with an ACD any time during pregnancy were considered as a proxy for ACD exposure later in pregnancy, the pharmacologically most relevant period to assess an effect on PPH. In these 2 studies, as well as others, authors reported increased prevalence of several of the major risk factors for PPH among women with epilepsy, such as use of labor induction, instrumental delivery, preeclampsia, chorioamnionitis, and antepartum hemorrhage.13,15,16 In both studies, these factors—which may be causal intermediates between ACD or epilepsy and PPH—were not well balanced between groups and could thus explain the association observed. These factors appeared to be balanced across comparison groups in our study, which included an active comparator reference group. In contrast (and consistent with our results), a Norwegian group failed to show an association between the estimated blood loss and the use of ACDi.12 In this hospital-based study, 109 patients with epilepsy were compared to 109 controls matched on age, time of delivery, and mode of delivery. Among the patients with epilepsia, 66 were taking ACDi and 20 received vitamin K. The mean amount of bleeding volume was not different among groups (438 mL for patients with epilepsy, 431 mL for those taking ACDi, vs 426 mL for the controls).
One explanation for the lack of association between ACDi and PPH/neonatal bleeding could be that an important fraction of women using ACDi in our study were supplemented with vitamin K at the end of pregnancy. In our study, information on prenatal vitamin K supplementation was not reliable and could not reliably be assessed. Several previous guidelines recommended high-dose vitamin K intake during the last month of pregnancy to prevent hemorrhagic disease of the newborn exposed to ACDi in addition to the routine administration of vitamin K to the neonate at birth to prevent VKDB. These recommendations were not supported by any specific study,29 and as a result, their clinical application was very heterogeneous as shown by some US hospital-based studies.30,31 Later, the lack of evidence to recommend routine maternal use of oral vitamin K to prevent hemorrhagic disease of the newborn and PPH led to the revision of these guidelines.6 A high level of heterogeneity in clinical practice might still be present after these various changes, making any estimation of the fraction of women supplemented with vitamin K at the end of pregnancy difficult.
Similar to other studies comparing ACDi exposed to unexposed neonates, our study failed to show an association between ACDi use in late pregnancy and neonatal bleeding complications.17,31 Here again, the lack of association may be the result of the routine practice of giving intramuscular vitamin K to the neonate at the time of birth. This information was not available in our data, and thus the potential protective effect of neonatal vitamin K administration could not be assessed. This study has several strengths, including its large sample size. Our cohort included 5,109 women exposed to ACDi in late pregnancy, compared with 942 using anticonvulsants overall in the largest study available to date.13 The richness of the data allowed for careful control of potential confounders including more than 50 variables in PS analyses. We specifically addressed confounding by indication through adjustment for all potential clinical indications for AEDs. We also performed a sensitivity analysis restricting to women with a recorded diagnosis of epilepsy, and the results were not affected. However, when we defined exposure as filling prescriptions for ACDi with the supply ending more than 30 days before delivery (i.e., a negative control assuming no carryover), the similar protective association observed could suggest residual confounding by unmeasured characteristics.
To focus on exposure during the etiologically relevant window close to delivery and to minimize the risk of exposure misclassification (i.e., false-positives), we chose an exposure definition requiring women to have filled an ACDi prescription during the last month of pregnancy with the drug supply overlapping the delivery date. A highly specific outcome definition was used, and estimated PPH risks among the unexposed women are in line with the literature; and known risk factors (e.g., placenta previa, chorioamnionitis) were replicated in our data, thus indirectly validating the outcome. Furthermore, diagnostic codes have been shown to have a positive predictive value higher than 80% for PPH in administrative data.25,26 An unbiased estimate of the relative risk is expected as long as the specificity is high and the sensitivity is nondifferential between the exposed and reference groups. Potential confounding by information not captured by the data source (e.g., lifestyle factors) is not a major concern given our null findings. Finally, findings from this study might not be generalizable to other populations if the biological relations studied are affected by characteristics of the studied population (young, racially diverse, vulnerable Medicaid-insured population with a high burden of mental illness) that differ from the general population. However, these limitations do not limit the importance of this study as Medicaid covers nearly 50% of all births in the United States. This investigation left unanswered questions about the potential protective effect of late-pregnancy vitamin K on the outcome, which is a limitation. Numbers were insufficient for a dose-response evaluation and an assessment of the association between numbers of different ACDi prescribed and the outcome.
Given incomplete knowledge regarding potential risks linked to pharmaceutical agents during pregnancy, balancing risks and benefits of treatment during pregnancy is not an easy task for clinicians and patients. Providing any new evidence to facilitate this task is a step forward. Our findings suggest that use of ACDi in late pregnancy does not meaningfully increase the risks of bleeding complications including PPH and neonatal bleeding complications in clinical settings where neonatal intramuscular vitamin K administration is considered standard of care. These findings provide some reassurance with regard to hemorrhagic complications for clinicians and pregnant women successfully treated with ACDi.
Glossary
- ACD
anticonvulsant drug
- ACDi
enzyme-inducing anticonvulsant drug
- CI
confidence interval
- CYP450
cytochrome P450
- ICD-9
International Classification of Diseases, Ninth Revision
- LMP
last menstrual period
- PPH
postpartum hemorrhage
- PS
propensity score
- RR
relative risk
- VKDB
vitamin K deficiency bleeding
Author contributions
Study concept and design: Panchaud, Cohen, Bateman, Huybrechts, Hernandez-Diaz. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Panchaud, Hernandez-Diaz. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Panchaud, Mogun, Hernandez-Diaz. Obtained funding: Panchaud, Huybrechts, Hernandez-Diaz. Administrative, technical, or material support: Panchaud, Mogun, Hernandez-Diaz. Study supervision: Bateman, Huybrechts, Hernandez-Diaz, Gray.
Study funding
This study was supported by an R01 grant (R01 MH100216) from the National Institute of Mental Health and a Swiss National Science Foundation grant P3SMP3-158808/1.
Disclosure
A. Panchaud was supported by Swiss National Science Foundation grant P3SMP3-158808/1. J. Cohen has received salary support from a research grant from GlaxoSmithKline for unrelated work. E. Patorno was supported by a career development grant K08AG055670 from the National Institute on Aging and she is investigator of investigator-initiated grants to the Brigham and Women's Hospital from GSK and Boehringer Ingelheim outside the submitted work. K. Huybrechts was supported by career development grant K01MH099141 from the National Institute of Mental Health and reports research funding from Eli Lilly and Pfizer outside the submitted work. R. Desai reports grants from Merck outside the submitted work. K. Gray was supported by training grant F32HD086948 from the National Institute of Child Health and Human Development. H. Mogun reports no disclosures relevant to the manuscript. S. Hernandez-Diaz has consulted for AstraZeneca and UCB for unrelated topics and has worked with the North American AED pregnancy registry, which is funded by multiple companies. She reports research funding from Eli Lilly, Pfizer, and GSK outside the submitted work. B. Bateman was supported by career development grant K08HD075831 from the National Institute of Child Health and Human Development and reports research funding from Eli Lilly, Pfizer, Pacira, GSK, and Baxalta outside the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Hogan MC, Foreman KJ, Naghavi M, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010;375:1609–1623. [DOI] [PubMed] [Google Scholar]
- 2.Oyelese Y, Ananth CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol 2010;53:147–156. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Maternal Mortality in 2000: Estimates Developed by WHO, UNICEF and UNFPA [online]. Available at: apps.who.int/iris/bitstream/handle/10665/68382/a81531.pdf. Accessed October 6, 2016. [Google Scholar]
- 4.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–1074. [DOI] [PubMed] [Google Scholar]
- 5.Palmsten K, Hernandez-Diaz S, Chambers CD, et al. The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid program. Obstet Gynecol 2015;126:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50:1247–1255. [DOI] [PubMed] [Google Scholar]
- 7.Gedzelman E, Meador KJ. Antiepileptic drugs in women with epilepsy during pregnancy. Ther Adv Drug Saf 2012;3:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011;205:e51–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol 2016;20:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chronicle E, Mulleners W. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev 2004:CD003226. [DOI] [PubMed] [Google Scholar]
- 11.Corrado AC, Walsh JP. Mechanisms underlying the benefits of anticonvulsants over lithium in the treatment of bipolar disorder. Neuroreport 2016;27:131–135. [DOI] [PubMed] [Google Scholar]
- 12.Sveberg L, Vik K, Henriksen T, Taubøll E. Women with epilepsy and post partum bleeding: is there a role for vitamin K supplementation? Seizure 2015;28:85–87. [DOI] [PubMed] [Google Scholar]
- 13.Borthen I, Eide MG, Daltveit AK, Gilhus NE. Delivery outcome of women with epilepsy: a population-based cohort study. BJOG 2010;117:1537–1543. [DOI] [PubMed] [Google Scholar]
- 14.Borthen I, Eide MG, Daltveit AK, Gilhus NE. Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG 2011;118:956–965. [DOI] [PubMed] [Google Scholar]
- 15.Pilo C, Wide K, Winbladh B. Pregnancy, delivery, and neonatal complications after treatment with antiepileptic drugs. Acta Obstet Gynecol Scand 2006;85:643–646. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald SC, Bateman BT, McElrath TF, Hernández-Díaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol 2015;72:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaaja E, Kaaja R, Matila R, Hiilesmaa V. Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology 2002;58:549–553. [DOI] [PubMed] [Google Scholar]
- 18.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006;61:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Winckel M, De Bruyne R, Van De Velde S, Van Biervliet S. Vitamin K, an update for the paediatrician. Eur J Pediatr 2009;168:127–134. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G, Franchini M. Vitamin K in neonates: facts and myths. Blood Transfus 2011;9:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database Syst Rev 2000:CD002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autret-Leca E, Jonville-Béra AP. Vitamin K in neonates: how to administer, when and to whom. Paediatr Drugs 2001;3:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One 2013;8:e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf 2013;22:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romano PS, Yasmeen S, Schembri ME, Keyzer JM, Gilbert WM. Coding of perineal lacerations and other complications of obstetric care in hospital discharge data. Obstet Gynecol 2005;106:717–725. [DOI] [PubMed] [Google Scholar]
- 26.Lain SJ, Roberts CL, Hadfield RM, Bell JC, Morris JM. How accurate is the reporting of obstetric haemorrhage in hospital discharge data? A validation study. Aust N Z J Obstet Gynaecol 2008;48:481–484. [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 28.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017;28:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Practice parameter: management issues for women with epilepsy (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Epilepsia 1998;39:1226–1231. [DOI] [PubMed] [Google Scholar]
- 30.Seale CG, Morrell MJ, Nelson L, Druzin ML. Analysis of prenatal and gestational care given to women with epilepsy. Neurology 1998;51:1039–1045. [DOI] [PubMed] [Google Scholar]
- 31.Choulika S, Grabowski E, Holmes LB. Is antenatal vitamin K prophylaxis needed for pregnant women taking anticonvulsants? Am J Obstet Gynecol 2004;190:882–883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of the data use agreements associated with the use of the Medicaid data, we are not permitted to release the raw data used in our analyses.