Abstract
Objective
To examine associations of average and change in late-life blood pressure (BP) with cerebrovascular and Alzheimer disease (AD) neuropathology in a large group of decedents followed longitudinally in vivo.
Methods
This clinical-pathologic study was derived from prospective, community-based cohort studies of aging with similar design and data collection. Measurements of systolic BP (SBP) and diastolic BP (DBP) were obtained annually (mean follow-up 8 years, SD = 4.8). Postmortem neuropathologic evaluations documented diseases of aging. Using regression analyses, we examined associations of average and decline in late-life SBP, and separately in DBP, with neuropathology.
Results
In 1,288 persons (mean age at death = 88.6 years; 65% women), the mean standardized person-specific SBP across the study was 134 (SD = 13) and DBP was 71 (SD = 8) mm Hg. The odds of brain infarcts were increased for participants with a higher mean SBP. Specifically, a person with a 1 SD SBP above the mean (147 vs 134 mm Hg) would have a 46% increased odds of having one or more infarcts, and an increased odds of gross infarct (46%) and microinfarct (36%). Furthermore, a more rapidly declining SBP slope over time increased the odds of one or more infarcts. Mean DBP, not slope, was related to brain infarcts. AD pathology analyses showed an association of a higher mean SBP with higher number of tangles (p = 0.038) but not plaques or other pathology (all p > 0.06). Changes in BP were not significantly related to AD pathology.
Conclusions
Higher average late-life SBP and DBP, and independently a faster decline in SBP, are associated with increasing number of brain infarcts, including gross and microinfarcts. We found some evidence for a relation of SBP with AD, specifically tangles. Both average and decline in BP are related to brain disease.
While hypertension is a risk factor for stroke, less is known about late-life blood pressure (BP), and in particular change in BP, and the main underling pathology of stroke, brain infarcts. In addition, while there is extensive literature about BP and cerebrovascular disease on neuroimaging,1,2 few human data are available on pathologically proven brain infarcts. Yet, brain infarcts are common and often silent in aging (clinically undetected), and even when neuroimaging is conducted, may be missed, especially when small. These factors underscore the value of clinical-pathologic studies to examine infarcts across a range of sizes, including microinfarcts. To date, data on late-life BP and infarcts are unclear, with some showing a relationship of systolic BP (SBP) but not diastolic BP (DBP) with infarcts found on postmortem neuropathologic evaluation.3 Separately, few studies examine BP and other common brain diseases in aging such as Alzheimer disease (AD), and while suggestions of an association are present, these studies are largely limited to fluid-based surrogate biomarkers.4,5 Despite the promise of emerging neuroimaging and biotechnological advances, research using direct assessment of cerebrovascular and AD pathology remains necessary to examine the association of BP with 2 common pathologies of aging in the brain, some features of which can only be definitely demonstrated by neuropathologic examination.
The overall hypothesis of the study was that late-life BP is associated with common brain pathology of aging, specifically cerebrovascular disease (infarcts) and neurodegeneration (AD). To test the main hypothesis, we used data from nearly 1,300 participants in prospective cohort studies of community-dwelling older women and men who were followed annually during an average of 8 years until death, and came to autopsy, allowing for detailed neuropathologic data to be systematically collected. Because late-life BP is often not static, we investigated the role of both mean and change in late-life BP in relation to pathology. We used ordinal logistic regression analyses adjusted for demographics, to examine associations of both SBP and DBP with infarcts, including by size and location in the brain, and large and small cerebral vessel diseases. We used linear regression analyses to examine associations of both SBP and DBP with overall and individual measures of AD pathology, including plaques and tangles. Secondary analyses considered effects of age and APOE ε4, BP-related variables such as hypertension and antihypertensive medications, as well as vascular risk factors and diseases.
Methods
Design overview
Participants were enrolled in 1 of 3 ongoing, prospective, community-based cohort studies of aging (radc.rush.edu), which have essentially identical recruitment techniques and a large overlap of data collection, allowing for the combination of data from the 3 studies to increase statistical power in the examination of risk factors in aging.6,7
Standard protocol approvals, registrations, and patient consents
Participants signed an informed consent and were asked to sign an anatomical gift act to donate their brain at time of death. The studies were approved by the Rush University Institutional Review Board.
Participants
The Religious Orders Study began enrolling Catholic clergy in 1994 from across the United States.8 Of 1,344 people enrolled at the time of analyses, 732 died and 675 came to autopsy, of which 640 had neuropathologic data collection complete for inclusion in this study. The Rush Memory and Aging Project began in 1997, and of 1,865 lay persons in Chicagoland enrolled, 820 died and 666 came to autopsy, and data from 627 were available to be included.9 The Minority Aging Research Study enrolled 706 black participants in Chicagoland since 2004, of which 118 had died, 19 came to autopsy, and 17 had available data to be included.10 Therefore, 1,288 participants were included in analyses.
Clinical evaluations with BP assessment
Participants undergo annual clinical evaluations in the community setting, and data are recorded directly onto laptop computers. Evaluations include a medical history, physical examination with vital signs, and neuropsychological testing. All medications, including antihypertensives, are visually inspected and documented, and data are coded (Medi-Span, Wolters Kluwer). Annual follow-ups are essentially identical to baseline. The follow-up rates for the 3 cohorts are in the range of 85% to 95%.8–10
BP was measured at baseline and annually by a trained research assistant using an automated sphygmomanometer and in an environment familiar to the research participant (community setting, usually participant's own residence).11 Three values were recorded, with 2 in a seated position (about a minute apart) and another in a standing position (after 1 minute standing), in keeping with a published BP clinical trial protocol.12 Given standing BP was not significantly different from sitting, the average of these 3 values was used to derive a mean SBP and mean DBP, as previously reported.11 For analyses in the current study, we used longitudinal BP data on each individual aligned at death, given that death is a strong biological event with many physiologic changes. We computed the standardized person-specific mean SBP (and separately DBP) over time across the years of the study to represent level, as well as the slope of change in SBP (and separately of DBP) to represent change over time. We model the odds of pathology given a higher mean BP (level) and declining BP (change). As previously reported, data in our cohorts are very complete, lessening concerns about missing data.8–10
Other data related to BP were collected, including a self-reported history of hypertension (dichotomized as present or not, using data from all study years) and use of antihypertensive medications (similarly dichotomized).11 Furthermore, vascular risk factors including diabetes, and vascular diseases including stroke and myocardial infarction allowed computation of a number of factors present.13 The presence of an APOE ε4 allele was determined.
Neuropathologic data
Postmortem neuropathologic evaluations were conducted on autopsied brains (mean postmortem interval = 9.1 [SD = 8.5] hours across the 3 cohorts), blinded to clinical data, as previously described.8–10 Briefly, a uniform gross and histologic evaluation examined for common age-related neuropathologies.
Cerebrovascular disease assessment documented gross (macroscopic) infarcts on gross examination, and classified number, volume, and location of each infarct, which was then dissected and confirmed on microscopy and classified by age (chronic/subacute/acute).14 Microinfarcts were not visible to the naked eye15 and were identified in blocks of at least 9 brain regions that were paraffin-embedded and stained with hematoxylin & eosin. Location and age were also documented. For analyses, only chronic infarcts were considered, and all infarct variables were categorized into 3 levels: no infarct (reference group), one infarct, and 2 or more infarcts. Furthermore, cerebral vessel disease documented the semiquantitative severity of atherosclerosis, assessed on gross examination of vessels in the circle of Willis, and arteriolosclerosis, based on histologic examination using hematoxylin & eosin–stained sections of the anterior basal ganglia.16 To allow for analyses of severity, we categorized level of vessel pathology into the following: not present, mild, moderate, and severe pathology.
Neurodegenerative pathology data were also collected. A continuous, overall AD pathology standardized measure considers counts of neuritic plaques, diffuse plaques, and neuronal neurofibrillary tangles from a 1-mm2 area (of greatest density), from sections of entorhinal, hippocampus, midfrontal, middle temporal, and inferior parietal cortices, using a modified silver stain.8,9 For analyses, given that AD pathology measures were not normally distributed, we transformed data.
Statistical analysis
Descriptive statistics were used in the initial examination of the variables of interest. All subsequent analyses were adjusted for age at death (centered at 89 years), sex, education (centered at 16 years), and years in study. The person-specific mean BP was ascertained from the longitudinal data on BP. Using linear mixed models, we also obtained the person-specific BP slope using time in years from death. The person-specific mean BP was standardized, so that in the interpretation in the models, a value represents a departure of 1 SD from the mean. The person-specific slope in BP remained in the original scale in the models but was inverted to represent a declining BP. We used ordinal logistic regression analyses to determine the odds of separate cerebrovascular outcomes, with outcomes being categorical variables from none to higher levels of pathology. First, we determined the odds of one and of more than one infarct (separately for any, gross, micro, cortical, and subcortical infarcts) as compared to no infarcts, by each of the mean and slope of SBP, and then DBP separately, as continuous variable predictors. We also examined the associations of BP with increasing severity of vessel pathology (atherosclerosis and arteriolosclerosis). We next determined the odds of AD pathology, for the overall and individual AD measures (neuritic plaques, diffuse plaques, neurofibrillary tangles), using linear regression analyses. In these analyses, AD outcomes as continuous measures were transformed by square root to reduce skewness. Because the distribution of AD measures, even when transformed, has some departure from normality and were skewed, to demonstrate consistency of the main findings, we conducted secondary sensitivity analyses in which we used ordinal logistic regressions with AD outcomes categorized into 4 groups, defined by the quartiles. Additional analyses were conducted to examine for effect modification by age and to consider for possible confounding by common vascular factors.
Core models were validated and analyses were programmed in SAS (version 9.4; SAS Institute, Cary, NC).
Data availability
Raw data are available by request from qualified investigators applying through the Rush Alzheimer's Disease Center (RADC) Research Resource Sharing Hub (radc.rush.edu/home.htm).
Results
Participant characteristics
On average, participants were followed annually for 8.0 (SD = 4.8) years and died at an age of 88.6 years (table 1). The mean SBP was 136 mm Hg at baseline and 134 mm Hg when averaged across the years of the study. SBP declined (slope), on average, 0.8 mm Hg (SD = 0.8) per year. The DBP was 72 mm Hg at baseline and 71 mm Hg when averaged across the years. DBP declined, on average, 0.1 mm Hg (SD = 0.4) per year. Comparing subjects who died with autopsy to those who died without autopsy, the age and education ranges were similar and there was no difference in the means of SBP or DBP. Decline in BP was nonlinear, and half of the decline occurred in the last 6 years before death. Over the course of the study, a history of hypertension was present in two-thirds of participants, and most participants (1,122/1,288; 87%) used an antihypertensive medication. Two-thirds of participants had at least one vascular risk factor at baseline, and nearly a third had a vascular disease. Spearman correlations showed that mean SBP and DBP across the years were moderately correlated with one another (rs = 0.42, p < 0.001). Correlation of BP with age was weak (for SBP: rs = 0.07, p = 0.014; for DBP: rs = −0.13, p < 0.001).
Table 1.
Demographic, clinical, and neuropathologic characteristics of participantsa
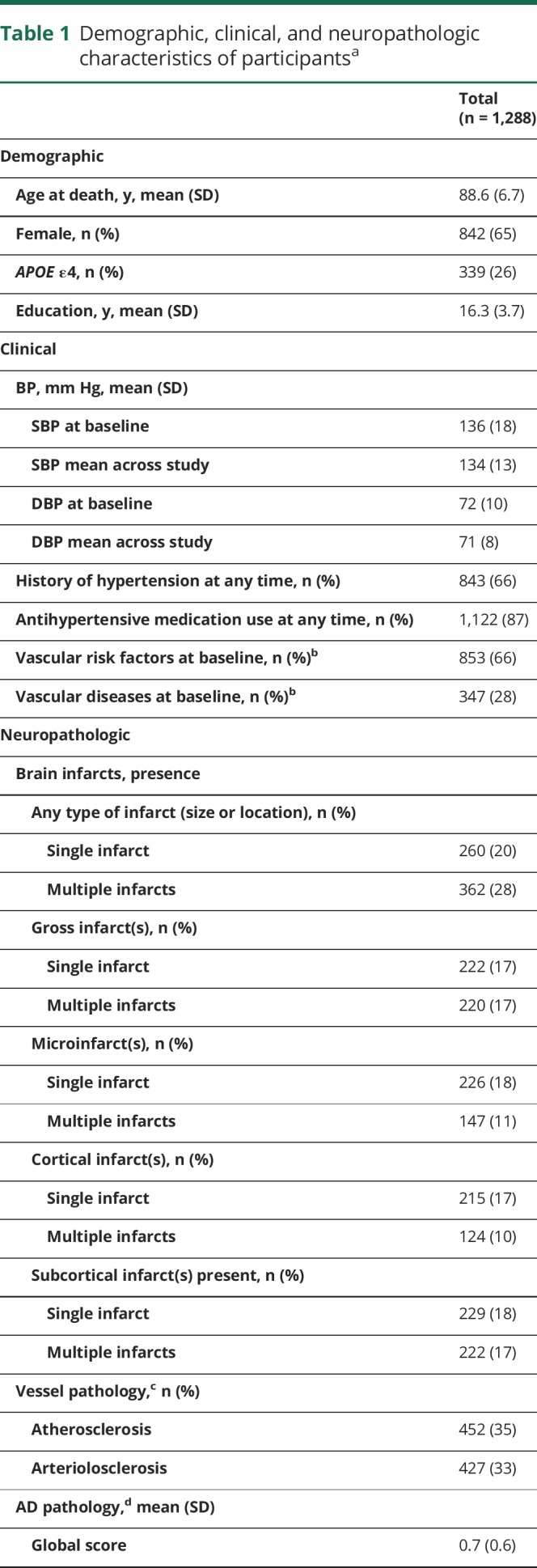
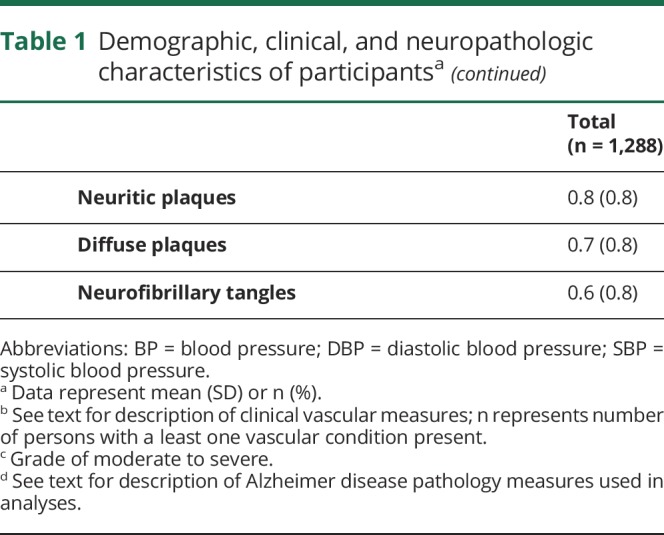
Neuropathologic data showed that half (48%) of subjects had one or more chronic infarcts of any size or location. A third of subjects had gross infarcts and a third had microinfarcts, and 15% (193/1,288) had both gross and microinfarcts. Most subjects had some AD pathology (table 1).
Relation of BP to cerebrovascular disease pathology
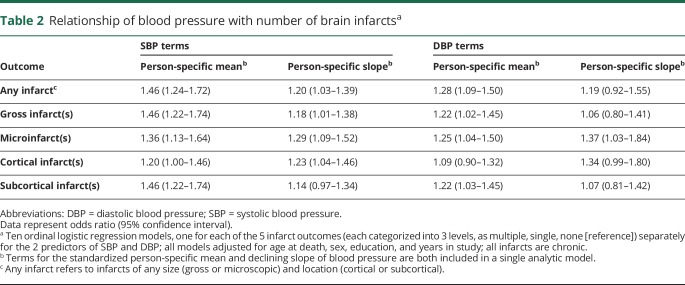
We first examined the relationship of BP with number of brain infarcts (table 2). In an ordinal logistic regression model adjusted for age at death, sex, education, and years in the study, SBP was associated with an increased number of infarcts (one and more than one) of any size or location. Specifically, for an increase by 1 SD in the standardized person-specific mean SBP (e.g., from 134 to 147 mm Hg), there was a 46% increased odds of having one or more than one infarct. For comparison, the effect of an increase by 1 SD in the mean SBP on the odds of increased infarcts was equivalent to that of 9 years of age. We found that a 1-unit-faster decline in the slope in SBP (from a stable BP, slope of zero) increased the odds of having one or more than one infarct by 20% (table 2). In additional sensitivity analyses, first excluding all black participants and then excluding those with a stroke prior to study entry, results were essentially unchanged (data not shown). In 2 additional models, results were consistent for infarct outcomes that were considering number and size of infarcts, with both the mean and slope of SBP being associated with increased odds of having more gross infarcts and, separately, more microinfarcts (table 2). Results in analyses considering infarct location showed that the slope of (but not the mean) SBP was associated with cortical infarcts and the mean (but not the slope) SBP was associated with subcortical infarcts (table 2).
Table 2.
Relationship of blood pressure with number of brain infarctsa
The relationship of DBP to infarcts was weaker and less consistent compared to that of SBP. An increase in 1 SD from the mean DBP value (e.g., from 71 to 79 mm Hg) was associated with a 28% increased odds of any infarct, but the slope in DBP was not associated with infarcts (table 2). In additional sensitivity analyses, first excluding all black participants and then excluding those with a stroke prior to study entry, results were essentially unchanged (data not shown). Analyses by infarct number and size showed that the mean DBP (but not slope) was associated with gross infarcts and that both the mean and slope DBP were associated with microinfarcts. Analyses by infarct location showed that DBP was not associated with cortical infarcts, but the mean (and not the slope) was associated with subcortical infarcts (table 2).
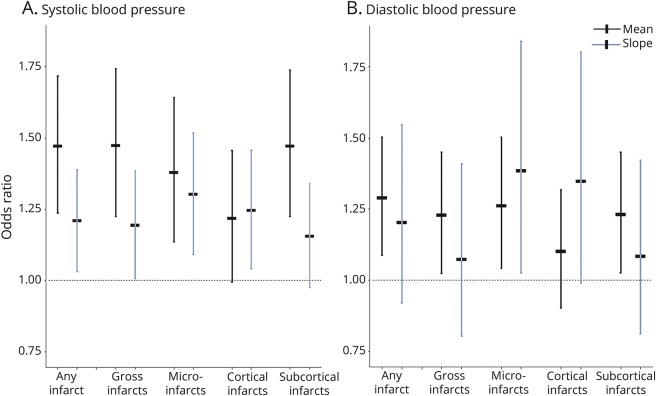
Figure 1 illustrates the relationship of standardized means and declining slopes of SBP and DBP with number of brain infarcts, including any, gross, and microinfarcts, and separately cortical and subcortical infarcts.
Figure 1. Relation of mean and slope of blood pressure with brain infarcts.
Horizontal dashed line represents an odds ratio of 1. (A) Associations between the mean and declining slope of systolic blood pressure with each of the infarct outcomes (categorized as one, and more than one, compared to the reference of none), including any infarcts, gross infarcts and microinfarcts, and cortical and subcortical infarcts. (B) Associations for diastolic blood pressure with the same infarct outcomes.
To further examine cerebrovascular disease, we next conducted additional analyses for large and small cerebral vessel diseases. First, in analyses of atherosclerosis outcomes, we found that the mean SBP was associated with higher severity grades of atherosclerosis (odds ratio [OR] = 1.95; 95% confidence interval [CI]: 1.66–2.29), and there was a borderline relation with the declining slope of SBP with higher severity grades (OR = 1.16; 95% CI: 0.9996–1.339; p = 0.0507). Second, in analyses of arteriolosclerosis, the mean SBP was associated with higher severity grades of arteriolosclerosis (OR = 1.23; 95% CI: 1.05–1.43), but there was no association of the slope of SBP with arteriolosclerosis (p = 0.900). Analyses were repeated using DBP predictors. Both the mean (OR = 1.36; 95% CI: 1.16–1.59) and declining slope of DBP (OR = 1.43; 95% CI: 1.10–1.84) were associated with higher atherosclerosis severity. There was no significant association of the mean or slope of DBP with arteriolosclerosis (both p > 0.078).
Relation of BP to AD pathology
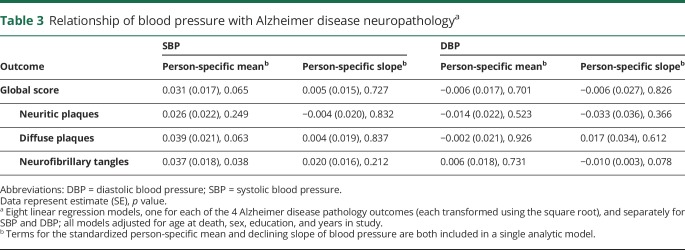
We used 8 linear regression models adjusted for age at death, sex, education, and years in study to examine the relation of mean and slope of SBP and DBP with level of AD pathology (table 3). We found an association of higher mean SBP with higher numbers of tangles (p = 0.038) but no relation of slope of change in SBP with tangles. We did not find relations otherwise between mean or slope of SBP and global or individual measures of AD pathology (including plaques) or between mean and slope of DBP and AD (table 3). In secondary analyses using AD measures categorized into quartiles, results were consistent: higher mean SBP was associated with higher levels of tangle pathology (estimate = 0.168, SD = 0.078; p = 0.031), and no other significant associations of SBP or DBP were present (all p > 0.058).
Table 3.
Relationship of blood pressure with Alzheimer disease neuropathologya
Additional analyses of factors with potential to affect relations
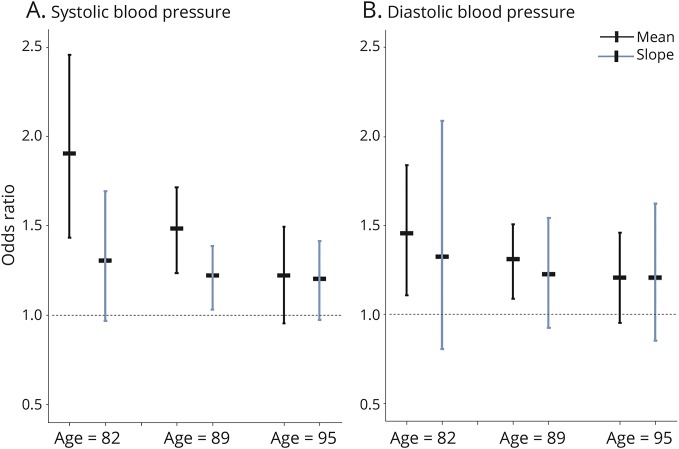
To test for the possibility that age may modify the association of BP with neuropathology, we used ordinal logistic regression models with 2 additional terms for age at death × mean BP and age at death × slope of BP for SBP and DBP separately. In the first analysis with any infarct as the outcome, we found that an increase in the mean SBP was associated with increased odds of having more infarcts, as in the core analyses. In addition, we found an interaction of age × mean SBP, in that the effect of the mean SBP on infarcts was decreased with an increasing age (p = 0.014 for interaction). In the same model, there was no interaction of age with the SBP slope (p = 0.630). In a separate model, there was no interaction of age with the mean or slope DBP (both p > 0.255). The relation of SBP and DBP with any infarcts according to age is shown in figure 2. In separate analyses with AD pathology as the outcome, there were no interactions of age with mean or slope of SBP or DBP, considering overall AD pathology or neurofibrillary tangles specifically (data not shown).
Figure 2. Relation of mean and slope of blood pressure with brain infarcts, according to age.
(A) SBP. (B) DBP. The estimated odds of any infarct, and respective 95% confidence interval, for an increase of 1 SD above the mean SBP (or DBP), or 1-unit-faster decline in the slope of SBP (or DBP), on a participant with a mean age of 89 years (center), a participant 1 SD younger than the mean age or 82 years old (left portion of figure), and a participant 1 SD older than the mean age or 95 years old (right portion of figure). DBP = diastolic blood pressure; SBP = systolic blood pressure.
We conducted additional analyses to control for vascular factors with potential to affect associations. Each of the following terms was added separately to the core model with neuropathology as the outcome: APOE ε4, a history of hypertension, antihypertensive medication use at any time in the study, vascular risk factors, and vascular diseases. The associations of mean and slope SBP and DBP with any infarct were essentially unchanged (data not shown). Furthermore, the associations of mean and slope SBP and DBP with neurofibrillary tangles were also essentially unchanged (data not shown).
Discussion
In this study of nearly 1,300 deceased participants enrolled in a prospective, community-based cohort study, higher person-specific averages across the years in late-life SBP and DBP were each separately associated with the presence and number of brain infarcts at autopsy. While taking into account level of BP, we also found that a faster decline in BP over time, especially in SBP, was associated with increasing number of infarcts. Results were consistent with findings for both the presence and number of gross infarcts, as well as microinfarcts separately. We also found associations of BP, and especially mean SBP, with higher severity of vessel diseases, most notably atherosclerosis. Furthermore, there was some evidence for a relation of SBP with AD pathology, specifically neurofibrillary tangles, but not of SBP or DBP with neuritic (amyloid) plaques. In addition, we found an effect modification by age, such that the effect of mean SBP on infarcts decreased with aging. However, associations of BP with neuropathology remained after controlling for several vascular factors, including APOE ε4, hypertension, and antihypertensive medication use.
BP across a range of values has been shown to be associated with stroke, but most data are available in midlife.17 Furthermore, stroke is a clinical condition for which attribution to underlying pathology is imperfect, and autopsy data allow for the identification of a range of pathologies, vascular and others not currently identifiable in vivo. Some data have shown a relation of midlife BP to autopsy-verified infarcts. In analyses of approximately 750 men who underwent autopsy as part of the Hisayama study, first available DBP (in midlife, but age not specified) was associated with infarcts based on gross examination of the brain only, but the association of SBP with infarcts was not reported in the regression analysis.18 Less is known about late-life BP and pathologically proven infarcts. The recent Adult Changes in Thought study with 250 older participants found an increased relative risk for more than 2 microinfarcts with increasing baseline late-life SBP, but this association was not present when hypertensive medications were controlled for.3 Also, there was no association of baseline SBP with cystic macroinfarcts, or baseline DBP with cerebrovascular pathology, whether micro- or macroinfarcts.3 Our study builds on prior research by including a larger cohort of both women and men, considering SBP and DBP across time (yearly data, during an average of 8 years), using both the person-specific mean and slope from death, and examining additional vascular pathology outcomes including vessel diseases. We found that both higher mean and faster decline in SBP were associated with increasing number of infarcts, and with both gross and microinfarcts separately. In analyses of regional pathology, the slope of SBP was associated with cortical infarcts, and the mean SBP with subcortical infarcts. Relationships of DBP with infarcts were also present but less consistent than those for SBP. The mean but not the slope of DBP was associated with any infarcts, and separately for gross infarcts, and both the mean and slope were associated with microinfarcts. Regional outcomes showed only an association of mean DBP with subcortical pathology. Much work remains to be done to further define the relationship of BP with brain infarcts, including with infarct type such as thrombotic or embolic with or without secondary hemorrhagic conversion, and other studies will need to address these issues.
To better understand BP and cerebrovascular disease, we also directly examined large and small vessel pathology. Prior work has shown that cerebral vessel disease is associated with infarcts.19 We are not aware of any prior systematically conducted, pathologic study of both atherosclerosis and arteriolosclerosis in relation to measured SBP and DBP. We found that mean late-life SBP was associated with atherosclerosis and, separately, arteriolosclerosis. Results with DBP were again less consistent, with the mean and slope of DBP being associated with atherosclerosis but not arteriolosclerosis. In the same cohorts, we have shown that arteriolosclerosis and atherosclerosis are related to brain infarcts.16 Our data suggest that average BP, and especially SBP, may remain an important determinant of cerebrovascular disease in late life.
The association of decline in SBP with infarcts is a novel and important finding. Declining SBP has been previously observed in the very old, and reported to be associated with mortality.20,21 We are aware of only one prior, recent, and much smaller study examining the link between declining BP and brain infarcts, which found a relation of decline in BP with microinfarcts in 297 autopsied persons.22 Other cerebrovascular (e.g., gross infarcts and vessel pathologies) and neurodegenerative pathologies were not examined.22 Our data are consistent and expand on the literature, notably by showing that declining SBP was related to gross infarcts, microinfarcts, and cortical infarcts, but not subcortical infarcts, and there was also a borderline relationship with atherosclerosis. It is intriguing to speculate that declining BP in aging in persons with atherosclerosis could lead to decreased cerebral perfusion and increased risk of specific types of infarcts.
In examining BP and common neuropathology of aging, more recent work has focused on AD pathology, building on observations that high, but also possibly low, BP increases dementia risk, including dementia attributed to AD, and the knowledge that AD is the single most common pathologic cause of dementia in aging, followed by vascular disease as the second most common cause.23–27 Few data on BP and AD pathology are available in humans. In one study of 243 autopsied, Japanese-American men followed longitudinally, midlife SBP was associated with an increased number of neuritic plaques and midlife DBP with increased neurofibrillary tangles.28 Other data suggest a relationship of BP to biomarkers of AD pathology, such as β-amyloid in CSF and atrophy on brain imaging.4,5,29 We are, however, not aware of data on late-life BP and direct measurement of AD pathology in the brain. In our study, we found an association of a higher mean SBP with higher number of tangles, and this association was present using 2 different analytic approaches (with tangles as a continuous variable, and separately as a categorical variable). The mechanism by which higher average late-life SBP may increase tangles in not clear, though vascular factors as a mechanism in AD pathogenesis has long been postulated. Of note, results for the slope of SBP with tangles was not significant, despite some previous data showing an association of decreasing late-life BP with dementia.30 Our finding of an association with tangles is difficult to interpret and will need to be replicated in other cohorts. At this time, there is overall very little evidence that BP increases the odds of amyloid pathology.
Because BP changes with aging and disease,30,31 the potential role of age on the relationship of BP with neuropathology needs evaluation. Few data directly address this question. Published data using stratified models by age showed that only younger-old patients (among an older cohort aged ≥65 years) had an increased risk of microinfarcts with increasing baseline SBP.3 Our data support this observation and expand our knowledge in this area. In addition to controlling for age in all our models, we also specifically examined for effect modification. We found that, in the total group, the effect of the mean SBP over time on infarcts (of any size) was decreased with an increasing age at death (significant interaction of age with mean SBP). By contrast, older age did not affect the analyses using slope of SBP, or mean and slope of DBP, on infarcts or AD pathology. These data may have important public health implications for BP recommendations in late life. As a whole, BP associations with health and disease appear to be important across the lifespan, but associations in late life may become more complex, possibly because of competing risk of vessel disease, cerebral perfusion, and other factors. Further study of BP and brain health in late life is warranted. While our results are not actionable in the clinical care setting, they can meaningfully guide future studies into the biological effects of BP on the brain, clinical trials of BP in aging, and research on the relation of change in BP in aging to important clinical outcomes including cognitive and motor function.
It is important to consider how vascular factors, including hypertension and medications, may affect results. Wang et al.3 reported that SBP was associated with microinfarcts only in those self-reporting as not taking treatment for hypertension. In addition, some data suggest an association of antihypertensive medication use in persons who have hypertension with less AD pathology compared to persons without hypertension.32 Of note, in our study across a range of BP values, we did not find that a diagnosis of hypertension or use of antihypertensives affected the results. Given that most participants were using antihypertensives, it will be important to further study BP trajectory in aging and infarct risk in persons taking and not taking specific treatments. In addition to hypertension and medications, whether other vascular factors have a role in BP and neuropathology also needs consideration. Several previous studies control for vascular factors, including APOE ε4 (e.g., references 5 and 28), and most have not found effects of these on the relation of BP to neuropathology. However, because BP and infarcts are both associated with other vascular factors, we also conducted analyses controlling for potential covariates including APOE ε4, vascular risk factors, and vascular diseases. Our results with infarcts and neurofibrillary tangles were essentially unchanged, suggesting that these vascular factors do not affect the observed associations of BP with neuropathology. Further work is needed to identify at-risk subgroups and associated mechanisms by which mean and slope of BP are associated with neuropathology.
This study has several methodologic strengths. First, the primary predictor was measured BP, considered both SBP and DBP separately, examined the full range of values (rather than only high), and took into account data over an average of 8 years. Second, analyses simultaneously examined the effects of mean BP across the years as well as the slope of change in BP over time. Third, this study of a large sample of nearly 1,300 participants, women and men, who were community-dwelling, followed annually (with high follow-up rates), and came to autopsy, has high internal validity and is more representative of the general population than clinic-based and most other studies on BP and brain infarcts to date. Fourth, systematically collected neuropathologic data were available on both cerebrovascular and AD pathology, blinded to clinical data. However, there are also weaknesses. Most important, we do not have access to midlife BP measurements. Also, BP data from time points in between the annual evaluations are not available, and potential effects of shorter-term changes in BP cannot be determined (e.g., acute episode of hypertension associated with stroke). In addition, most participants were on antihypertensive medications and BP was relatively well controlled, limiting our ability to observe effects of BP with a wider distribution of values. Furthermore, neuropathologic data, while extensive, did not consider other markers such as specific white matter pathology, perhaps best quantified using neuroimaging. Finally, additional data that may shed light on potential indirect mechanisms linking BP to infarcts, including via cardiovascular dysfunction and others, as well as data that would determine when an infarct occurred in life, were not available, and mechanistic analyses were not examined.
Acknowledgment
The authors are sincerely thankful to the thousands of participants in the Religious Orders Study, the Rush Memory and Aging Project, and the Minority Aging Research Study for their considerable altruism over the years. The authors acknowledge the hard work of the study coordinators, data and analytic programmers, and staff and faculty of the Rush Alzheimer's Disease Center.
Glossary
- AD
Alzheimer disease
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- OR
odds ratio
- SBP
systolic blood pressure
Author contributions
Dr. Zoe Arvanitakis: study concept, design, acquisition of data, analysis, interpretation, writing first draft of manuscript, revisions of manuscript, submission of manuscript, responsible for all aspects of the study. Dr. Ana W. Capuano: study concept, analysis, interpretation, revisions of manuscript. Dr. Melissa Lamar: revisions of manuscript. Dr. Raj C. Shah: revisions of manuscript. Dr. Lisa L. Barnes: acquisition of data, revisions of manuscript. Dr. David A. Bennett: acquisition of data, revisions of manuscript. Dr. Julie A. Schneider: study concept, design, acquisition of data, interpretation, revisions of manuscript.
Study funding
This study was supported by the NIH, grants P30 AG010161, RF1 AG015819, R01 AG17917, RF1 AG22018, R01 AG40039, R01 NS084965, and UH2 NS100599.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LY, Larson EB, Sonnen JA, et al. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J Am Geriatr Soc 2009;57:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchet O, Celle S, Roche F, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens 2013;31:1502–1516. [DOI] [PubMed] [Google Scholar]
- 5.Nation DA, Edmonds EC, Bangen KJ, et al. ; Alzheimer's Disease Neuroimaging Initiative Investigators. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol 2015;72:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA. A two-process model for neuropathology of Alzheimer's disease. Neurobiol Aging 2014;35:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruzin JJ, Schneider JA, Capuano AW, et al. Diabetes, hemoglobin A1C, and regional Alzheimer disease and infarct pathology. Alzheimer Dis Assoc Disord 2017;31:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 2012;9:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Relation of blood pressure to risk of incident Alzheimer's disease and change in global cognitive function in older persons. Neuroepidemiology 2006;26:30–36. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Bethesda: National Heart, Lung, and Blood Institute (US); 2004. NIH Publication No. 04-5230. [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care 2006;29:560–565. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 2003;60:1082–1088. [DOI] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2001;42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 2017;27:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannel WB, Dawber TR, Sorlie P, Wolf PA. Components of blood pressure and risk of atherothrombotic brain infarction: the Framingham Study. Stroke 1976;7:327–331. [DOI] [PubMed] [Google Scholar]
- 18.Shinkawa A, Ueda K, Kiyohara Y, et al. Silent cerebral infarction in a community-based autopsy series in Japan. The Hisayama Study. Stroke 1995;26:380–385. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, Vinters HV, Mack WJ, Zarow C, Ellis WG, Chui HC. Cerebral atherosclerosis is associated with cystic infarcts and microinfarcts but not Alzheimer pathologic changes. Stroke 2013;44:2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikinheimo RJ, Haavisto MV, Kaarela RH, Kanto AJ, Koivunen MJ, Rajala SA. Blood pressure in the very old. J Hypertens 1990;8:361–367. [DOI] [PubMed] [Google Scholar]
- 21.Poortvliet RK, de Ruijter W, de Craen AJ, et al. Blood pressure trends and mortality: the Leiden 85-plus Study. J Hypertens 2013;31:63–70. [DOI] [PubMed] [Google Scholar]
- 22.Graff-Radford J, Raman MR, Rabinstein AA, et al. Association between microinfarcts and blood pressure trajectories. JAMA Neurol 2018;75:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 24.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology 2003;61:1667–1672. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Rhew IC, Shofer JB, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc 2007;55:1161–1167. [DOI] [PubMed] [Google Scholar]
- 26.Qiu C, Winblad B, Fratiglioni L. Low diastolic pressure and risk of dementia in very old people: a longitudinal study. Dement Geriatr Cogn Disord 2009;28:213–219. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 28.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57–62. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz A, Pesini P, Espinosa A, et al. Blood amyloid beta levels in healthy, mild cognitive impairment and Alzheimer's disease individuals: replication of diastolic blood pressure correlations and analysis of critical covariates. PLoS One 2013;8:e81334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart R, Xue QL, Masaki K, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension 2009;54:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean Systolic and Diastolic Blood Pressure in Adults Aged 18 and Over in the United States, 2001–2008. National Health Statistics Reports; No. 35. Hyattsville: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 32.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009;72:1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available by request from qualified investigators applying through the Rush Alzheimer's Disease Center (RADC) Research Resource Sharing Hub (radc.rush.edu/home.htm).