Abstract
OBJECTIVE
To compare the effectiveness of two interventions to reduce diabetes distress (DD) and improve glycemic control among adults with type 1 diabetes (T1D).
RESEARCH DESIGN AND METHODS
Individuals with T1D (n = 301) with elevated DD and HbA1c were recruited from multiple settings and randomly assigned to OnTrack, an emotion-focused intervention, or to KnowIt, an educational/behavioral intervention. Each group attended a full-day workshop plus four online meetings over 3 months. Assessments occurred at baseline and 3 and 9 months. Primary and secondary outcomes were change in DD and change in HbA1c, respectively.
RESULTS
With 12% attrition, both groups demonstrated dramatic reductions in DD (effect size d = 1.06; 78.4% demonstrated a reduction of at least one minimal clinically important difference). There were, however, no significant differences in DD reduction between OnTrack and KnowIt. Moderator analyses indicated that OnTrack provided greater DD reduction to those with initially poorer cognitive or emotion regulation skills, higher baseline DD, or greater initial diabetes knowledge than those in KnowIt. Significant but modest reductions in HbA1c occurred with no between-group differences. Change in DD was modestly associated with change in HbA1c (r = 0.14, P = 0.01), with no significant between-group differences.
CONCLUSIONS
DD can be successfully reduced among distressed individuals with T1D with elevated HbA1c using both education/behavioral and emotion-focused approaches. Reductions in DD are only modestly associated with reductions in HbA1c. These findings point to the importance of tailoring interventions to address affective, knowledge, and cognitive skills when intervening to reduce DD and improve glycemic control.
Introduction
Diabetes distress (DD) refers to the often hidden emotional burdens, stresses, and worries that result from managing a demanding chronic disease like type 1 diabetes (T1D) (1). DD is highly prevalent, with ∼42% of adults with T1D manifesting elevated DD (2), is distinct from clinical depression (3), tends to be chronic rather than episodic (2), and has been significantly associated with poor glycemic control and problematic self-care behavior in both cross-sectional and longitudinal studies (4–8). There is growing evidence of associative and causative linkages among DD, self-management, and glycemic control, making DD a significant clinical problem.
Surprisingly, there have been few systematic studies that have directly evaluated interventions to reduce DD in clinical populations. A recent meta-analysis of 41 randomized controlled trials by Sturt et al. (9) showed significant reductions in DD, with the strongest effects coming from interventions that targeted the emotional side of diabetes directly, rather than focusing exclusively on behavior change or education. Reductions in HbA1c in these studies were only marginal. Sturt et al. (9) highlighted three major problems with these studies, to which we add one more: most did not target DD directly (most targeted education and behavior change); few studies compared different approaches to reduce DD; baseline levels of DD and HbA1c were rarely controlled, yielding many participants who were neither distressed nor in poor control at baseline; and only 4 of the 41 studies focused on adults with T1D. Thus, although DD appears to be responsive to intervention, few studies have directly compared DD-targeted interventions in highly distressed, poorly controlled patients with T1D.
Most DD intervention studies use one of two general strategies. The diabetes management approach focuses on education, medication management, and behavior change to improve glycemic control, according to the rationale that improved glycemic control will also decrease underlying DD as disease status improves, whereas the emotional approach suggests that the key to alleviating DD is to address the underlying feelings, beliefs, and expectations that promote DD (10).
A crucial omission in the literature is the identification of a conceptual framework that can be used as a platform to develop effective emotion-based interventions. One promising approach is “emotion regulation,” a term used to describe the mechanisms people use to manage the emotions that emerge in response to diabetes-related threats and fears (11). For example, an adaptive response to a hypoglycemic episode might be to mobilize problem-solving resources to determine the cause, whereas a maladaptive response might be to become ruminative or self-blaming. The chronic use of maladaptive emotion regulation mechanisms often leads to significant negative outcomes: the emergence of chronic negative emotions, a narrowing of cognitions, and a reduction in effective problem-solving skills (12–14). Thus, an escalating cycle occurs over time that upregulates negative affect and downregulates positive affect. The negative effects of poor emotion regulation mechanisms in diabetes, such as more hypoglycemic episodes, elevated HbA1c, and less frequent blood glucose monitoring, are well documented (15,16), and there are data to suggest that poor emotion management is associated with high DD (17). Thus, emotion regulation may provide a useful framework for designing effective interventions to reduce DD in T1Ds.
Reducing Distress and Enhancing Effective Management for T1D Adults (T1-REDEEM) was a 9-month, randomized control trial for adults with T1D with elevated DD and HbA1c designed to compare the effectiveness of an intensive education/behavior change intervention, called KnowIt, with an intervention that focused on improving emotion regulation skills, called OnTrack. We herein address the following research questions: 1) which of the two interventions was most effective in reducing DD and HbA1c; 2) how did the effectiveness of each intervention vary as a function of initial level of DD, emotion regulation, cognitive skills, and diabetes knowledge on changes in both DD and HbA1c (moderator analyses); and 3) were reductions in DD as a result of an intervention associated with improvements in glycemic control?
Research Design and Methods
Sample and Recruitment
Using patient registries, support groups, and contacts through social media with online diabetes organizations, we recruited a diverse sample of adults with T1D in California (San Francisco Bay Area, Los Angeles, Sacramento, and San Diego); Tucson, Arizona; Portland, Oregon; and Toronto, Ontario, Canada. Inclusion criteria were as follows: patient ≥19 years of age; diagnosis of T1D for at least 12 months; ability to read, write, and speak English; mean item score of ≥2 on the Type 1 Diabetes Distress Scale (T1-DDS), indicating elevated DD (18); a recently recorded HbA1c ≥7.5%; no severe complications (end-stage renal disease); absence of psychosis or dementia; and availability of a computer with Internet access.
For participants recruited from nonclinic, community settings, we used a protocol approved by the University of California, San Francisco (UCSF) Review Board, which included only opt-in procedures. For participants recruited through clinical settings, human subject approval was received from the appropriate review board and recruitment followed a combination of opt-in and opt-out procedures. Using opt-out procedures, the research team mailed letters to patients informing them of the study and telling them that a project representative would contact them by phone within 2 weeks unless they opted out of the call by returning an enclosed postcard or calling a toll-free telephone number. Using opt-in procedures, individuals receiving letters were encouraged to call our toll-free number or send an e-mail expressing interest. During initial contact with those identified by both recruitment procedures, the project was explained, informed consent was obtained, and initial screening commenced, including administration of the T1-DDS and permission to obtain their latest clinic-recorded HbA1c. If a timely HbA1c was not available, a prepaid lab slip was mailed to the participant for HbA1c collection at a local facility. All potentially eligible participants were then sent an e-mail with a unique personal code to access a Health Insurance Portability and Accountability Act of 1996 (HIPAA)–compliant online survey to complete assessment of remaining inclusion criteria and baseline assessment. Upon completion of the survey and a recorded HbA1c, participants received a $25 gift card for their time and eligible participants were randomly assigned to either KnowIt or OnTrack using a computer-generated, random number protocol. According to a pragmatic research strategy and to address ethical concerns about maintaining highly distressed participants in the study without intervention, no noninterventional control group was included (19). Data were collected in 2014–2017 and analyzed in 2017.
Both KnowIt and OnTrack required the same participant time commitment: attendance at a 1-day group workshop with a trained group leader (a Certified Diabetes Educator for KnowIt and a psychologist with diabetes experience for OnTrack) and participation in four 1-h online video meetings with group members and leader over the following 3 months. Follow-up assessment via online survey and HbA1c testing occurred at 3 and 9 months after the workshop. Follow-up qualitative interviews with 10 OnTrack and 10 KnowIt participants were conducted at the project’s conclusion and will be the subject of a later report.
To assure consistency in program presentation, fidelity tracking checklists were developed separately for OnTrack and KnowIt based on key preidentified intervention content areas. Observers recorded an overall mean of 95% fidelity across all groups, with no between-group differences.
Interventions
KnowIt included a diabetes update of key factors regarding the causes and management of T1D, based on a UCSF Diabetes Education Program. For example, it addressed the etiology of T1D, tips on carb counting, and use of a toolbox of strategies to address specific management problems. Each of the 1-h online meetings subsequent to the workshop reviewed action plans and addressed a specific topic: continuous glucose monitoring, new developments in islet and pancreas transplantation, hypoglycemia, and travel.
OnTrack used a variety of scenarios and exercises, based on emotion regulation, that focused on ways to deal with the emotional side of diabetes and how to develop personalized emotion management techniques to get “unstuck” about behavioral change. Specific techniques were drawn from programs of empowerment-based communication (20), AASAP (a technique to enhance motivation) (21), and motivational interviewing (22) and included several key distress-related, emotion regulation elements (for example, overtly labeling feelings, keeping feelings in perspective to reduce overreactions, and separating feelings from appraisals of self-worth). Participants also completed a personalized action plan that included attention to the positive and negative feelings that one might experience with each aspect of behavior change. Action plans were reviewed at each of the four subsequent online meetings, along with discussions about dealing with T1D 24 h a day, coping with frustrating blood glucose numbers, and dealing with family and friends.
Measures
Age and education in years, sex, ethnicity (white or nonwhite), years with T1D, and number of complications from a list of 14 (5) were recorded. HbA1c was obtained from clinic records for tests within 3 months of survey completion and at 3 and 9 months. If unavailable, a lab slip for HbA1c collection at a community site was provided.
DD was assessed by the T1-DDS, a 28-item scale (α = 0.84) (23) with seven subscales: powerlessness (five items), management distress (four items), hypoglycemia distress (four items), negative social perceptions (four items), eating distress (three items), physician distress (four items), and family/friend distress (four items). Response options ranged from 1 (“not a problem”) to 6 (“a very serious problem”).
Two scales from the Five Facet Mindfulness Scale (24) assessed emotion regulation. The Nonjudging of Inner Experience Scale (NonJudge) is an eight-item subscale (α = 0.95). Items were reverse scored on a 5-point scale from “never or rarely true” to “very often or always true.” The Nonreactivity to Inner Experience Scale (NonReact) is a seven-item subscale (α = 0.89). These two subscales reflect acceptance of emotion without self-criticism and nonimpulsive, planned reactions to emotions, respectively. Item responses were averaged for each subscale score.
Because emotion regulation and cognitions can interact with each other, two cognitive scales were included. The Personal Control subscale from the Revised Illness Perception Questionnaire (25) (α = 0.80) is a six-item scale with five response options from “strongly disagree” to “strongly agree” (α = 0.90). Item scores were reverse scored and summed to create subscale scores. The second cognitive scale was the nine-item Effective Problem-Solving subscale (α = 0.86) of the Health Problem-Solving Scale. Responses are on a 5-point Likert-type scale from 1 (“not at all true of me”) to 5 (“extremely true of me”). Scores were calculated by summing across items.
A 27-item diabetes knowledge assessment, derived from the Revised Brief Diabetes Knowledge Test (DKT2) (26), was included. Content was expanded to assess the information presented in KnowIt.
Data Analysis
Data were analyzed using SPSS version 19 software (27). χ2 or Student t tests, as appropriate, compared the two treatment conditions on participant characteristics and baseline values of outcome variables. These analyses were repeated to test for differences between dropouts and completers. Missing values were imputed using SPSS multiple-imputation procedures. Multiple imputation was minimal: 11.6% of survey responses and 13.2% of HbA1c values at 3 months and 14.1% of survey responses and 18.2% of HbA1c values at 9 months.
Sample size and power estimates are based on two-sided α = 0.05 and Student t tests on change from baseline to 3 and 9 months. Conservatively estimating a 20% attrition rate, a sample of 145 per group allows for detection of small to moderate DD effect sizes (d = 0.35–0.40 SD unit differences) and mean changes in HbA1c of 0.48% or larger (5,9). Sample size estimates for moderator effects are based upon a group-by-moderator strata regression interaction test with sample size powered >0.80 (5,9).
Repeated-measures analyses of variance were conducted to test for time and treatment effects in DD total and subscales and in HbA1c between time periods. There were no differences between HbA1c levels recorded from clinic registries and from community laboratories, so the data were combined. Each model specified time and treatment as within- and between-subject main effects, respectively, and a two-way time-by-treatment interactive effect to determine whether time effects depended on treatment assignment.
Separate regression models evaluated the impact of six potential baseline moderators (baseline DD, NonJudge, NonReact, personal control, problem solving, and diabetes knowledge) on 9-month total DD and HbA1c using continuous variables. For ease of explication, the results are presented by median splits. In each model, the 9-month value of the outcome was specified as the dependent variable, and predictors were the baseline value of the outcome, the baseline value of the moderator, treatment condition, and the multiplicative interaction between the baseline moderator and treatment group.
Results
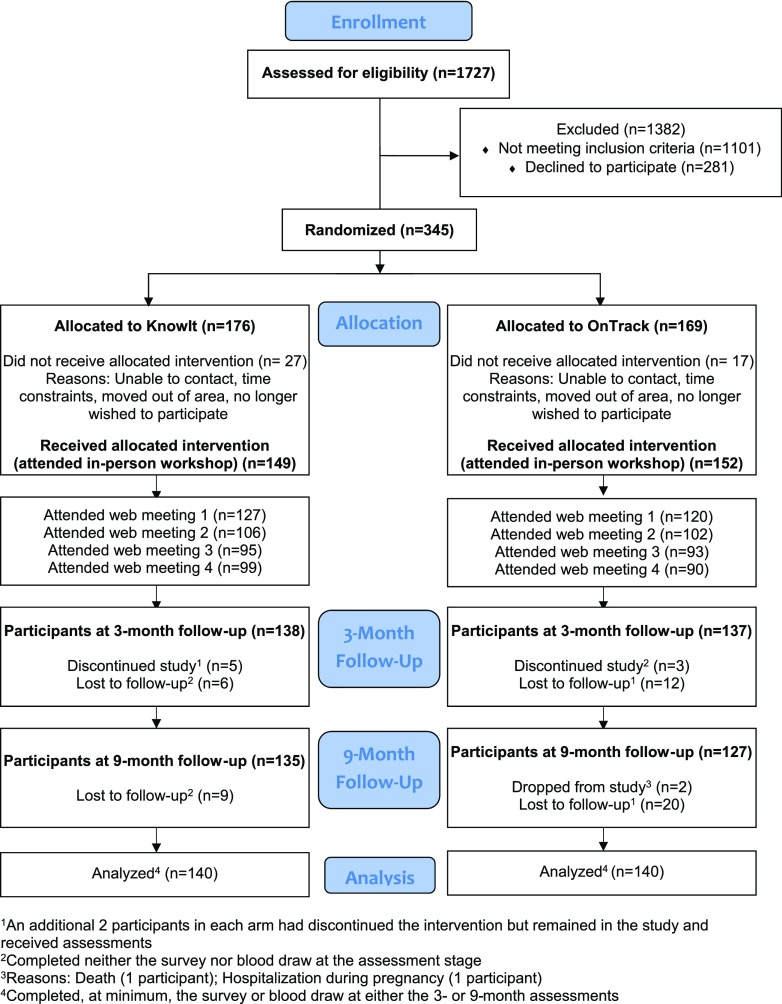
Of 4,188 individuals who were initially identified from clinic registries, we located and contacted 1,727. Of these, 1,101 were ineligible and 281 declined or did not complete screening, and 345 agreed to be randomized (Fig. 1). Of these, 301 (86%) began the intervention by attending the workshop (KnowIt = 149; OnTrack = 152). Among those randomized, those who did not attend a workshop (n = 44) were on average significantly younger (P = 0.002), less educated (P = 0.03), more often a minority (P = 0.03), and had a higher HbA1c (P = 0.04) than those who attended a workshop. The percentages who attended each of the four online meetings were, respectively, 82%, 69%, 62%, and 63%, with no significant between-group differences.
Figure 1.
CONSORT diagram.
Attrition at 3 months was 8%, with an additional 4% loss by 9 months (12% total). Attrition did not differ by study arm across any time period; those who dropped out had significantly higher baseline DD and HbA1c, had more complications, and were younger than those who remained in the study.
Mean (SD) age was 45.1 years (15.0), 69.1% were female, and mean (SD) baseline HbA1c was 8.80% (1.12) (73 mmol [15.5]) (Table 1). Two between-group baseline differences were found: KnowIt participants were slightly older (mean [SD] 47.3 years [14.5]) than OnTrack participants (42.8 [15.1]; P < 0.009), and OnTrack participants scored slightly higher on diabetes knowledge (55% [16]) than KnowIt participants (51% [15]; P = 0.04).
Table 1.
Baseline characteristics by treatment group (n = 301)
| Variable | KnowIt (n = 149) | OnTrack (n = 152) | P value |
|---|---|---|---|
| Age, years | 47.3 (14.5) | 42.8 (15.1) | 0.009 |
| Education, years | 15.7 (3.6) | 15.2 (3.6) | 0.32 |
| Number of children | 1.1 (1.3) | 0.93 (1.0) | 0.20 |
| Age at diagnosis, years | 21.2 (14.4) | 19.5 (13.7) | 0.28 |
| Years with diabetes | 26.12 (13.97) | 23.17 (13.26) | 0.06 |
| Number of complications | 2.84 (2.57) | 2.65 (2.47) | 0.51 |
| % Female | 70.5 | 67.8 | 0.61 |
| % White | 82.6 | 77.6 | 0.29 |
| % With partner | 61.7 | 67.5 | 0.29 |
| % With insulin pump | 63.8 | 67.8 | 0.46 |
| % With continuous glucose monitoring | 37.6 | 38.88 | 0.83 |
| DD, total | 2.87 (0.63) | 2.90 (0.60) | 0.73 |
| HbA1c, % | 8.77 (1.13) | 8.83 (1.11) | 0.65 |
| NonJudge | 3.58 (1.00) | 3.47 (1.05) | 0.39 |
| NonReact | 3.17 (0.78) | 3.16 (0.75) | 0.88 |
| Hypoglycemia problem solving | 26.95 (5.62) | 27.36 (6.27) | 0.56 |
| Diabetes knowledge, % correct | 51 (15) | 55 (16) | 0.04 |
| IPQ-R personal control | 24.62 (3.35) | 25.00 (3.50) | 0.33 |
Data are mean (SD) unless otherwise specified.
Student t test or χ2 test, as appropriate. IPQ-R, Revised Illness Perception Questionnaire.
Changes in DD
For the total sample, statistically significant reductions in total DD and all seven subscales occurred between baseline and 3 months (Table 2). These reductions were sustained between 3 and 9 months, with additional significant reductions for total DD (P < 0.02) and hypoglycemia distress (P < 0.003). Baseline to 9-month DD change effect sizes (28) averaged d = 0.69 (range 0.23–1.06) (d = 0.70 in KnowIt, d = 0.69 in OnTrack), indicating relatively large effects. Also calculated was the number of participants who displayed a reduction of at least one minimal clinically important difference (MCID) in DD versus those who did not (2). For the total sample, a reduction of at least one MCID occurred for 222 (73.8%) participants at 3 months and for 236 (78.4%) at 9 months. Only 21 (7.0%) showed an increase of at least one MCID at 9 months.
Table 2.
Predictors of DD total score and subscales and HbA1c (n = 301)
| Descriptive statistics |
Effect size (d) | Model results (F value) |
|||||
|---|---|---|---|---|---|---|---|
| All subjects (n = 301) | KnowIt (n = 149) | OnTrack (n = 152) | Time | Treatment group | Time × treatment | ||
| Total DD | |||||||
| Baseline | 2.88 (0.61) | 2.87 (0.63) | 2.90 (0.60) | ||||
| 3 months | 2.24 (0.63) | 2.25 (0.65) | 2.23 (0.62) | ||||
| 9 months | 2.17 (0.59) | 2.18 (0.65) | 2.15 (0.52) | ||||
| 0–3 months | 281.26*** | 0.00 | 0.36 | ||||
| 3–9 months | 5.44* | 0.13 | 0.00 | ||||
| 0–9 months | 1.06 | 338.98*** | 0.00 | 1.85 | |||
| Powerlessness | |||||||
| Baseline | 4.09 (0.98) | 4.04 (0.98) | 4.14 (0.97) | ||||
| 3 months | 3.02 (1.05) | 3.04 (1.10) | 3.00 (1.01) | ||||
| 9 months | 2.91 (1.03) | 2.98 (1.11) | 2.97 (0.94) | ||||
| 0–3 months | 257.47*** | 0.10 | 1.13 | ||||
| 3–9 months | 2.93 | 0.40 | 0.14 | ||||
| 0–9 months | 0.98 | 286.93*** | 0.00 | 1.85 | |||
| Diabetes management | |||||||
| Baseline | 3.38 (1.13) | 3.29 (1.10) | 3.46 (1.15) | ||||
| 3 months | 2.41 (1.03) | 2.35 (0.99) | 2.47 (1.07) | ||||
| 9 months | 2.93 (0.95) | 2.37 (0.94) | 2.42 (0.96) | ||||
| 0–3 months | 237.41*** | 1.73 | 0.19 | ||||
| 3–9 months | 0.10 | 0.67 | 0.32 | ||||
| 0–9 months | 0.92 | 256.74*** | 1.13 | 0.93 | |||
| Hypoglycemia distress | |||||||
| Baseline | 2.66 (1.17) | 2.64 (1.18) | 2.68 (1.17) | ||||
| 3 months | 2.18 (1.03) | 2.18 (1.07) | 2.18 (0.98) | ||||
| 9 months | 2.02 (0.86) | 2.00 (0.84) | 2.05 (0.89) | ||||
| 0–3 months | 65.07*** | 0.2 | 0.03 | ||||
| 3–9 months | 9.25** | 0.08 | 0.20 | ||||
| 0–9 months | 0.61 | 111.69*** | 0.14 | 0.05 | |||
| Negative social perceptions | |||||||
| Baseline | 2.26 (1.14) | 2.30 (1.04) | 2.22 (1.23) | ||||
| 3 months | 1.83 (0.98) | 1.90 (1.04) | 1.75 (0.92) | ||||
| 9 months | 1.73 (0.93) | 1.76 (0.96) | 1.70 (0.89) | ||||
| 0–3 months | 63.58*** | 1.14 | 0.46 | ||||
| 3–9 months | 3.29 | 1.20 | 0.86 | ||||
| 0–9 months | 0.55 | 88.70*** | 0.44 | 0.03 | |||
| Eating distress | |||||||
| Baseline | 3.45 (1.23) | 3.42 (1.25) | 3.48 (1.22) | ||||
| 3 months | 2.63 (1.12) | 2.67 (1.15) | 2.59 (1.11) | ||||
| 9 months | 2.63 (1.11) | 2.60 (1.11) | 2.65 (1.12) | ||||
| 0–3 months | 131.67*** | 0.01 | 0.95 | ||||
| 3–9 months | 0.00 | 0.01 | 1.08 | ||||
| 0–9 months | 0.65 | 127.02*** | 0.25 | 0.00 | |||
| Physician distress | |||||||
| Baseline | 1.96 (1.10) | 2.00 (1.11) | 1.92 (1.08) | ||||
| 3 months | 1.77 (1.05) | 1.74 (1.04) | 1.81 (1.07) | ||||
| 9 months | 1.71 (0.96) | 1.81 (1.04) | 1.62 (0.86) | ||||
| 0–3 months | 10.73** | 0.1 | 1.80 | ||||
| 3–9 months | 1.13 | 0.38 | 5.27* | ||||
| 0–9 months | 0.23 | 15.85*** | 1.92 | 0.70 | |||
| Friend and family distress | |||||||
| Baseline | 2.23 (1.14) | 2.24 (1.19) | 2.22 (1.10) | ||||
| 3 months | 1.78 (0.90) | 1.81 (0.91) | 1.74 (0.89) | ||||
| 9 months | 1.69 (0.88) | 1.66 (0.92) | 1.72 (0.85) | ||||
| 0–3 months | 72.16*** | 0.18 | 0.13 | ||||
| 3–9 months | 3.29 | 0.00 | 1.59 | ||||
| 0–9 months | 0.53 | 85.11*** | 0.02 | 0.45 | |||
| HbA1c | |||||||
| Baseline | 8.80 (1.12) | 8.77 (1.13) | 8.83 (1.11) | ||||
| 3 months | 8.67 (1.19) | 8.60 (1.18) | 8.74 (1.21) | ||||
| 9 months | 8.62 (1.22) | 8.59 (1.25) | 8.65 (1.19) | ||||
| 0–3 months | 8.95** | 0.60 | 0.83 | ||||
| 3–9 months | 1.30 | 0.53 | 0.84 | ||||
| 0–9 months | 14.90*** | 0.20 | 0.00 | ||||
Data are mean (SD) unless otherwise specified.
DD scores range from 1 to 6, with ≥2.0 indicating significant distress.
*P < 0.05.
**P < 0.01.
***P < 0.001.
There were, however, no significant between-group, time-by-treatment interactions for change in any DD score between any time period, with the exception of physician distress from 3 to 9 months (Table 2). Thus, substantive change in DD over time was not differential by study arm. Also, there were no significant associations between the number of online meetings attended and change in DD.
All six baseline moderator variables yielded significant results for baseline to 9-month change in T1-DDS total. Average effect size was d = 0.27 (range 0.01–0.51). Simple effects showed that those with higher baseline DD (P < 0.05), poorer problem solving (P = 0.01), poorer nonjudgmental attitudes toward affect (P < 0.01), less nonreactivity to affect (P = 0.07), or higher diabetes knowledge (P = 0.02) who were in OnTrack displayed greater reductions in DD over time than those with similar baseline scores in KnowIt. However, those with more nonjudgmental attitudes toward affect (P = 0.05) or more nonreactivity (P = 0.06) at baseline showed significantly greater reductions in DD in KnowIt compared with OnTrack. Although an overall significant moderator effect occurred for personal control, no specific within-treatment comparisons reached significance. Thus, OnTrack provided greater DD reduction benefits to those with initially poorer cognitive and emotion regulation skills, higher baseline DD, or greater initial diabetes knowledge than those in KnowIt.
Changes in HbA1c
Significant, though modest, reductions in HbA1c occurred between baseline and 3 months (P = 0.003), which were sustained at 9 months without significant change (Table 2). There were no between-group differences or significant effects of any of the tested moderators on change in HbA1c over time. Reductions in T1-DDS total from baseline to 9 months were significantly but modestly associated with reductions in HbA1c (r = 0.14, P = 0.01), with no between-group differences.
Conclusions
We find that in this sample of distressed adults with T1D, DD is notably malleable and can display marked reductions when subject to systematic intervention. Both an emotion-focused and an educational-behavioral approach led to dramatic reductions in DD at 3 months that were sustained at 9 months with further modest improvements. Thus, we see no decrement in gains made over the 6 months after intervention. Furthermore, the relatively large DD change effect sizes (DD total d = 1.06) and the MCID analyses indicate that the gains made were substantive and were not restricted to a subset of participants whose high change scores might have spuriously inflated group means. Hence, both the magnitude and reach of the interventions are notable. These substantive DD reductions are most likely not due to the effects of regression to the mean. A previous noninterventional study with adults with T1D over a similar 9-month period showed that DD was surprisingly stable. In fact, of those who scored within the elevated DD range at baseline, 71% remained in the elevated range at 9 months (2).
The absence of between-group differences in distress reduction was unexpected. Direct observations of both OnTrack and KnowIt workshops by the investigators and an overview of 20 poststudy participant interviews may help explain these findings. Participants from both study arms told us how meaningful it was to interact with other adults with T1D. The sense of community that was experienced was significant, as reflected by the pace and intensity of discussion throughout the program. A sense of reassurance and support that was palpable emerged immediately, which may account in part for the relatively quick, rather than gradual, impact of intervention effects on DD. Even many months after the conclusion of the program, participants recalled that although their worries and fears did not disappear, they were placed in perspective. Although the descriptors used by members of the two groups were different (OnTrackers used emotion-based descriptors and KnowIters used terms reflecting shared management tips and experiences), both groups reported viewing their diabetes experiences as “normal” under the circumstances and that there was little reason to “beat themselves up” about their disease-related problems or to allow themselves to become immobilized by them. We suggest that the power of the group experience, in which personal sharing and interaction are encouraged by expert, sensitive group leadership, can have dramatic DD-reduction effects for distressed adults with T1D.
The moderator analyses indicate that different patients benefit somewhat differently from each of the two interventions, providing further support that the observed DD changes did not result from regression to the mean. Participants who began the intervention with poor emotion regulation, poor cognitive skills, very high DD, or high diabetes knowledge benefited most from OnTrack, an emotion-focused intervention. Those participants with already high baseline skills in these areas did significantly better in KnowIt, where the emphasis was on knowledge and not on emotion regulation and cognitive skills. These results are consistent with Sturt et al. (9) who found that patients with high DD do significantly better in programs that address emotion management and cognitive deficits directly (17).
We also find a statistically significant, although modest, reduction in HbA1c after intervention, with no between-group differences. A previous study with adults with type 2 diabetes with modestly elevated DD but no HbA1c eligibility requirement found no significant reduction in HbA1c after DD intervention (5). We suspect that the difference in findings between studies may be accounted for by the lower baseline levels of both DD and HbA1c in the type 2 diabetes study, a finding also noted by Sturt et al. (9). Because both DD and HbA1c are elevated at baseline in T1-REDEEM, both may be more responsive to intervention.
Finding only a modest reduction in HbA1c after a dramatic decrease in DD raises questions about the potential causative linkages between these two variables. In previous studies, these two variables have shown only a bidirectional and not a prospective relationship over time (29). Two potential areas of exploration might be considered to address this question. First, it could be the case that reductions in HbA1c as a result of a DD intervention are only partially “caused” by the DD reduction per se, given the documented effect of reduced stress on glycemic control (30). More likely is the effect of improved management that may occur once DD falls to more tolerable levels. High DD can limit available energy and a willingness to engage in disease management. A greater emphasis on disease management after or in combination with an emotion-focused DD intervention might further enhance HbA1c reductions, as participants have more energy to engage in the kinds of disease management that leads to better glycemic control. In this sense, DD might serve as a brake on the effectiveness of education and management interventions. Second, it could be the case that DD reductions might occur after improvements in glycemic control, as the reasons for worry and fear are reduced (31). This approach argues for a focus on improved management and glycemic control over time, all the while clocking the effects of improvements in subsequent change in DD. Each of these options is testable by varying the sequence of interventions and including a greater focus on direct disease management in conjunction with emotion-focused DD-reduction strategies.
These findings have significant clinical implications by suggesting a variety of approaches that might be undertaken in clinical care: for example, utilization of a stepped-care approach, with all individuals receiving KnowIt initially and those not responding subsequently receiving OnTrack; tailoring interventions based on earlier emotion regulation, cognitive skill, and diabetes knowledge screening; or a program of the same length that combines OnTrack and KnowIt in a meaningful way. Furthermore, the techniques derived from emotion regulation, which form the basis of OnTrack, may provide a template for developing both screening and intervention protocols, especially for application at critical times in a patient’s diabetes career (at diagnosis, change in medications, addition of insulin, and emergence of a complication) (1).
This study has several strengths. It included a diverse, significantly distressed sample with elevated HbA1c from several settings. It followed a randomized controlled design, and attrition was low with no between-group differences and no differential effect on outcomes. Several study limitations, however, are noteworthy. First, all participants were required to have computer and Internet access, which might have limited generalizability. However, <2% of prospective participants were excluded because of the absence of a computer with Internet connection. Second, the sample was collected using different methods of recruitment. Subsamples within each city were too small to assess unique recruitment effects directly. Third, we were unable to collect any follow-up data for those who were randomized but did not attend the workshop. Such data would be helpful to assess generalizability. Fourth, the interventions were packaged to include a full-day workshop plus four online video meetings. The intervention design did not permit further analysis of the active ingredients of the interventions, such as workshop alone versus workshop plus video meetings, and it did not permit us to separate the effects of the content of the interventions (affect vs. knowledge) from the effect of intervention modality (group interaction vs. one-on-one or other contact effects).
T1-REDEEM demonstrates that DD can be successfully addressed among highly distressed adults with T1D with elevated glycemic levels using both educational/behavioral and emotion-focused approaches. It also highlights the potential importance of emotion regulation, diabetes knowledge, and cognitive skills and directs attention to tailoring DD interventions to address specific participant needs. This investigation provides useful strategies and tools to enhance DD intervention programs in clinical care.
Article Information
Acknowledgments. The authors thank Liana Abscal, Tracie Dalton, Cathy Moller, JoAnne Robb, Mindy Schwartz, and Geralyn da Silva, who served as intervention group leaders, and Marlene Bedrich (University of California, San Francisco), who helped develop the KnowIt curriculum. The authors also thank Dr. Ingrid Block and Dr. Kent Ishihara (University of California, Davis) who assisted with participant recruitment.
Funding. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-094863).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.F. conceived and designed the study and wrote the manuscript. D.H., W.H.P., and U.M. conceived and designed the study. S.G. designed the study and executed the interventions. V.B. executed the interventions. L.S. conducted the statistical analyses. A.A., M.B., I.B., C.C., S.K., A.L.P., M.S., K.W., and P.W. assisted with patient recruitment and made critical revisions to the manuscript for important intellectual content. L.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. no. NCT02175732, clinicaltrials.gov.
References
- 1.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher L, Hessler D, Polonsky W, Strycker L, Masharani U, Peters A. Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications 2016;30:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care 2011;34:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahanty LM, Grant RW, Wittenberg E, et al. . Association of diabetes-related emotional distress with diabetes treatment in primary care patients with type 2 diabetes. Diabet Med 2007;24:48–54 [DOI] [PubMed] [Google Scholar]
- 5.Fisher L, Hessler D, Glasgow RE, et al. . REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd CE, Smith JKW. Stress and diabetes: a review of the literature. Diabetes Spectr 2005;18:121–127 [Google Scholar]
- 7.Hessler D, Fisher L, Strycker LA, Arean PA, Bowyer V. Causal and bidirectional linkages over time between depression and diabetes regimen distress in adults with type 2 diabetes. Diabetes Res Clin Pract 2015;108:360–366 [DOI] [PubMed] [Google Scholar]
- 8.Hessler DM, Fisher L, Polonsky WH, et al. . Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med 2017;34:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturt J, Dennick K, Hessler D, Hunter BM, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. Int Diabetes Nurs 2015;12:40–55 [Google Scholar]
- 10.Frederickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cogn Emot 2005;19:313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quoidbach J, Mikolajczak M, Gross JJ. Positive interventions: an emotion regulation perspective. Psychol Bull 2015;141:655–693 [DOI] [PubMed] [Google Scholar]
- 12.Blair C, Diamond A. Biological processes in prevention and intervention: the promotion of self-regulation as a means of preventing school failure. Dev Psychopathol 2008;20:899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventhal H, Brissette I, Leventhasl EA. The common-sense model of self-regulation of health and illness. In The Self-Regulation of Health and Illness Behavior. Cameron LD, Levelthal H, Eds. New York, NY, Routledge Press, 2003, p. 42–65 [Google Scholar]
- 14.Nigg JT. Annual Research Review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry 2017;58:361–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guendelman S, Medeiros S, Rampes H. Mindfulness and emotion regulation: insights from neurobiological, psychological, and clinical studies. Front Psychol 2017;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansing AH, Berg CA. Adolescent self-regulation as a foundation for chronic illness self-management. J Pediatr Psychol 2014;39:1091–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher L, Hessler D, Polonsky W, et al. . Emotion regulation contributes to the development of diabetes distress among adults with type 1 diabetes. Patient Educ Couns 2018;101:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwarenstein M, Treweek S, Gagnier JJ, et al.; CONSORT Group; Pragmatic Trials in Healthcare (Practihc) Group . Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes 2004;22:123–127 [Google Scholar]
- 21.Fisher L, Hessler D, Naranjo D, Polonsky W. AASAP: a program to increase recruitment and retention in clinical trials. Patient Educ Couns 2012;86:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed New York, NY, Guilford Press, 2013 [Google Scholar]
- 23.Fisher L, Polonsky WH, Hessler DM, et al. . Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications 2015;29:572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13:27–45 [DOI] [PubMed] [Google Scholar]
- 25.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised Illness Perception Questionnaire (IPQ-R). Psychol Health 2002;17:1–16 [Google Scholar]
- 26.Fitzgerald JT, Funnell MM, Anderson RM, Nwankwo R, Stansfield RB, Piatt GA. Validation of the revised brief Diabetes Knowledge Test (DKT2). Diabetes Educ 2016;42:178–187 [DOI] [PubMed] [Google Scholar]
- 27.IBM Corporation IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, IBM Corp., 2010 [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ, Lawrence Erlbaum, 1988 [Google Scholar]
- 29.Hessler D, Fisher L, Glasgow RE, et al. . Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care 2014;37:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilliard ME, Yi-Frazier JP, Hessler D, Butler AM, Anderson BJ, Jaser S. Stress and A1c among people with diabetes across the lifespan. Curr Diab Rep 2016;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polonsky WH, Hessler D, Ruedy KJ, Beck RW; DIAMOND Study Group . The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]