Abstract
OBJECTIVE
We assessed whether poor glycemic control is associated with an increase in myocardial fibrosis among adults with diabetes.
RESEARCH DESIGN AND METHODS
We studied 47 adults with type 2 diabetes and stratified them into three groups according to their hemoglobin A1c (HbA1c) level: <6.5% (group 1; n = 12), 6.5–7.5% (group 2; n = 20), and >7.5% (group 3; n = 15). Left ventricular (LV) mass was assessed using cardiac MRI. The extracellular volume fraction (ECVF), an index of myocardial fibrosis, was measured by using myocardial T1 mapping before and after the administration of a gadolinium-based contrast agent.
RESULTS
Mean HbA1c was 5.84 ± 0.16%, 6.89 ± 0.14%, and 8.57 ± 0.2% in groups 1, 2, and 3, respectively. LV mass was not significantly different between the groups. The myocardial ECVF was significantly greater in groups 2 (mean 27.6% [95% CI 24.8–30.3]) and 3 (27.6% [24.4–30.8]) than in group 1 (21.1% [17.5–24.7]; P = 0.015). After adjusting for age, sex, BMI, blood pressure, and estimated glomerular filtration rate, the myocardial ECVF was significantly greater in groups 2 (27.4% [24.4–30.4]) and 3 (28% [24.5–31.5]) than in group 1 (20.9% [17.1–24.6]; P = 0.0156, ANCOVA).
CONCLUSIONS
An increased myocardial ECVF, suggesting myocardial fibrosis, is independently associated with poor glycemic control among adults with diabetes. Further research should assess whether tight glycemic control can revert fibrosis to healthy myocardium or ameliorate it and its adverse clinical consequences.
Introduction
Individuals with diabetes are at high risk of developing cardiovascular events, including heart failure, myocardial infarction, and death. Prior studies have demonstrated that poor glycemic control can increase the risk of cardiovascular complications and hospitalizations for heart failure (1,2). However, the mechanistic pathways that contribute to this elevated risk are complex and not well understood. Previous studies have shown the presence of myocardial fibrosis in subjects with diabetes (3–5). Although elevations in HbA1c are associated with early left ventricular (LV) dysfunction in subjects with diabetes (5), the relation between glycemic control and the extent of myocardial fibrosis is unknown.
Cardiac MRI allows precise measurements of LV structure and function (6). MRI can also accurately measure diffuse myocardial fibrosis, which causes extracellular matrix expansion (7). The latter can be assessed on the basis of myocardial extracellular volume fraction (ECVF), which is the proportion of tissue volume that corresponds to extracellular, rather than intracellular, space. High ECVF indicates myocardial interstitial fibrosis (7).
In this study we investigated the association between glycemic control and measures of macroscopic LV remodeling (LV mass) and myocardial fibrosis, measured with cardiac MRI, among adults with diabetes. We hypothesized that poor glycemic control is associated with increases in myocardial fibrosis and extracellular volume.
Research Design and Methods
Study Population
We prospectively enrolled a convenience sample of 47 adults with type 2 diabetes at the Corporal Michael J. Crescenz Veterans Affairs (VA) Medical Center (Philadelphia, PA). The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and all subjects provided written informed consent.
Key exclusion criteria were 1) claustrophobia; 2) presence of metallic objects or medical devices implanted in the body; 3) atrial fibrillation; 4) LV ejection fraction (EF) <50%; 5) congestive heart failure; 6) conditions that would make the study measurements less accurate or unreliable (e.g., arrhythmia affecting cardiac gating, inability to adequately hold breath during cardiac MRI); and 7) known infiltrative or hypertrophic cardiomyopathy, or a history of extracardiac amyloidosis or sarcoidosis. The presence of ischemic heart disease (in the absence of a low EF or congestive heart failure) was not an exclusion criterion.
Measurement of LV Mass
Participants underwent cardiac MRI to assess LV structure and function; we used a 1.5-Tesla whole-body MRI scanner (Avanto or Espree; Siemens, Malvern, PA) equipped with a phased-array cardiac coil. LV volume and EF were determined using balanced steady-state, free-precession cine imaging. Typical parameters were repetition time 30.6 ms, echo time 1.3 ms, 30 phases, 8-mm slice thickness, matrix size 192 × 192, and an integrated parallel imaging technique (iPAT) factor of 2. Short-axis stack cine images of the LV were manually traced at end diastole and end systole using CMR42 software (Circle CVI, Calgary, Alberta, Canada). LV mass was computed as the difference between epicardial and endocardial volumes, multiplied by myocardial density (1.06 g/mL). LV mass was normalized for 1) body surface area (BSA) and 2) body height (meters raised to the allometric power of 1.7) (8).
Assessment of ECVF
We assessed ECVF (an index of myocardial fibrosis) with cardiac MRI. We used modified Look-Locker inversion recovery (MOLLI) (9), which can assess T1 times in a midventricular short-axis slice before and after intravenous administration of gadolinium contrast (gadopentetate dimeglumine 0.15 mmol/kg or equivalent). Scan parameters for the MOLLI protocol included field of view ∼340 mm, matrix size 144 × 192, 6-mm slice thickness, repetition time 24.9 ms, echo time 1.18 ms, and flip angle 30 degrees. MOLLI was performed with two inversions and a 5‐3‐3 schema (five inversion times after inversion 1, three T1 recovery heartbeats, and three inversion times after inversion 2). Myocardial T1 measurements were performed before and ∼6–10, 15, 20, 25, and 30–40 min after gadolinium administration. While determining myocardial regions of interest in MOLLI images, we avoided areas that were immediately adjacent to the blood-endocardium interface in order to avoid potential partial volume effects. The myocardium-blood partition coefficient (λ) was computed as the slope of the blood 1/T1 change to the myocardial 1/T1 change, as computed with linear regression using all available measurements, as previously described (10). Hematocrit was measured from venous blood the day of the MRI scan. The myocardial ECVF was computed as λ × (1 − hematocrit). LV extracellular volume was computed as LV wall volume × ECVF. LV cellular volume was computed as LV wall volume × (1 − ECVF). In addition, indexed extracellular volume and cellular volume were computed by dividing the respective volumes by BSA, as previously described (11).
Statistical Analysis
Continuous variables are presented as the mean (95% CI). Categorical variables are shown as total counts with percentages and were compared using the χ2 test or the Fisher exact test. Continuous variables were compared between the groups with the use of ANOVA. Adjusted comparisons of ECVF and other LV measures between subjects (categorized by HbA1c strata) were made with ANCOVA. Analyses were adjusted for age, sex, and BMI. Additional adjustments were performed for systolic and diastolic blood pressures, history of hypertension, coronary artery disease, ACE inhibitor use, spironolactone use, angiotensin receptor blocker use, and estimated glomerular filtration rate (eGFR) because these variables may themselves influence myocardial fibrosis. Post hoc pairwise comparisons were made and Bonferroni correction for α error was applied. Statistical significance was defined as a two-tailed P < 0.05. Analyses were performed using SPSS version 24 for Mac (IBM, Chicago, IL) and the Statistics and Machine Learning Toolbox in MATLAB version 2016b (The Mathworks, Inc., Natick, MA).
Results
General characteristics of study subjects are summarized in Table 1. Our study included 12 subjects with HbA1c <6.5% (48 mmol/mol; group 1), 20 subjects with HbA1c between 6.5% and 7.5% (48–58 mmol/mol; group 2), and 15 subjects with HbA1c >7.5% (>58 mmol/mol; group 3). Mean age was 66, 67, and 64 years in subjects in groups 1, 2 and 3, respectively (P = 0.69); mean BMI was 29.1, 33.2, and 32.7 kg/m2, respectively (P = 0.91). The population comprised predominantly men, and no significant gender differences existed between the groups. No significant differences in systolic blood pressure, antihypertensive medication use, eGFR, HDL-cholesterol, or LDL-cholesterol existed between the groups. Insulin was used significantly more frequently with higher HbA1c levels (17%, 32%, and 80% in groups 1, 2, and 3, respectively; P = 0.002). Diastolic blood pressure was slightly but significantly higher among subjects with HbA1c >7.5% (P = 0.047).
Table 1.
General characteristics of study subjects stratified by HbA1c level
| HbA1c (%) |
P value | |||
|---|---|---|---|---|
| <6.5 (n = 12) | 6.5–7.5 (n = 20) | >7.5 (n = 15) | ||
| Age, years | 66 (61–72) | 67 (62–71) | 64 (59–69) | 0.69 |
| Male sex | 11 (92) | 18 (90) | 13 (87) | 0.91 |
| BMI, kg/m2 | 29.1 (24.2–34) | 33.2 (28.9–37.4) | 32.7 (27.8–37.5) | 0.41 |
| BSA, m2 | 2.08 (1.89–2.26) | 2.28 (2.12–2.44) | 2.26 (2.08–2.45) | 0.21 |
| SBP, mmHg | 142 (131–154) | 145 (136–153) | 149 (139–160) | 0.62 |
| DBP, mmHg | 81 (76–87) | 80 (76–85) | 89 (83–94) | 0.047 |
| History of hypertension | 11 (91.67) | 18 (90.00) | 15 (100.00) | 0.46 |
| Coronary artery disease | 3 (25.00) | 5 (25.00) | 6 (40.00) | 0.58 |
| Medication use | ||||
| β-Blockers | 3 (25.00) | 11 (55.00) | 9 (60.00) | 0.15 |
| Aspirin | 5 (42.00) | 15 (75.00) | 11 (73.00) | 0.12 |
| ACE inhibitors | 8 (67.00) | 16 (80.00) | 8 (53.00) | 0.24 |
| Spironolactone | 0 (0.00) | 1 (5.00) | 0 (0.00) | 0.50 |
| Calcium channel blockers | 4 (33.00) | 8 (40.00) | 7 (47.00) | 0.78 |
| Insulin | 2 (17.00) | 6 (32.00) | 12 (80.00) | 0.002 |
| Metformin | 7 (58.33) | 10 (50.00) | 9 (64.29) | 0.70 |
| Sulfonylureas | 1 (8.33) | 8 (40.00) | 3 (21.43) | 0.13 |
| Thiazolidinediones | 0 (0.00) | 2 (10.00) | 1 (7.14) | 0.54 |
| GLP-1 agonists | 0 (0.00) | 1 (5.00) | 0 (0.00) | 0.51 |
| DPP-4 inhibitors | 1 (8.33) | 2 (10.00) | 1 (7.14) | 0.96 |
| eGFR, mL/min/1.73 m2 | 87.5 (69.9–105.1) | 71.3 (60.4–82.2) | 85.4 (70.2–100.6) | 0.14 |
| HbA1c, % | 5.84 (5.53–6.15) | 6.89 (6.6–7.17) | 8.57 (8.16–8.98) | — |
| HbA1c, mmol/mol | 40 (37–44) | 52 (49–54) | 70 (66–73) | |
| HDL-cholesterol, mg/dL | 49.3 (39.3–59.3) | 42 (35.8–48.2) | 38.1 (31.5–44.6) | 0.13 |
| LDL-cholesterol, mg/dL | 39.6 (0–86.9) | 61.5 (22.8–100.1) | 71.4 (18.8–123.9) | 0.49 |
Data are the mean (95% CI) or n (%) of subjects. No subjects were receiving meglitinides, α-glucosidase inhibitors, or sodium–glucose cotransporter 2 inhibitors. DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; SBP, systolic blood pressure.
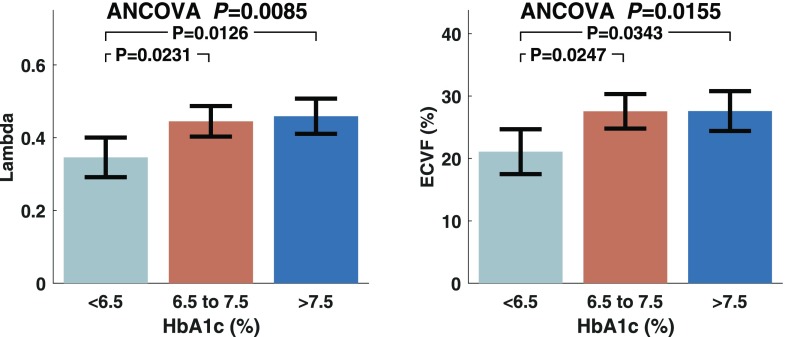
Figure 1 and Table 2 show comparisons of various parameters of LV remodeling and fibrosis between subjects stratified according to HbA1c level; the comparisons are adjusted for age, sex, and BMI. The gadolinium partition coefficient (λ) was significantly higher in group 2 (mean 0.45 [95% CI 0.40–0.49]) and group 3 (0.46 [0.41–0.51]) than in group 1 (0.35 [0.29–0.40]; P = 0.0085, ANCOVA). The myocardial ECVF was significantly greater in groups 2 (mean 27.6% [95% CI 24.8–30.3]) and 3 (27.6% [24.4–30.8]) than in group 1 (21.1% [17.5–24.7]; P = 0.0155). LV mass was not significantly different between the groups. Extracellular volume, however, was significantly larger in group 2 (mean 41.4 mL [95% CI 34.3–48.5]) and group 3 (39.3 mL [31.7–46.9]) than in group 1 (24.7 mL [19.3–30.2]; P < 0.001, ANCOVA), with no significant differences in cellular volume. Similar to LV mass, cellular volume indexed to BSA was not significantly different between the groups (P = 0.52), whereas indexed extracellular volume was significantly lower in group 1 (mean 13 mL/m2 [95% CI 9.6–16.3]) than in group 2 (20.4 mL/m2 [17.7–23.1]; P = 0.006, ANCOVA).
Figure 1.
Gadolinium partition coefficient (Lambda) and extracellular volume coefficient (ECVF) in subjects stratified according to HbA1c level, adjusted for age, sex, and BMI.
Table 2.
Comparison of various parameters of LV remodeling between subjects stratified according to HbA1c level adjusted for age, sex, and BMI
| HbA1c (%) |
P value | |||
|---|---|---|---|---|
| ≤6.5 (Group 1) | 6.5–7.5 (Group 2) | >7.5 (Group 3) | ||
| LV mass, g | 131 (114–149) | 158 (141–174) | 152 (134–170) | 0.08 |
| LV mass index | ||||
| By BSA, g/m2 | 62.5 (54.1–70.9) | 74.3 (67.6–80.9) | 68 (60.6–75.5) | 0.12 |
| By height, g/m1.7 | 52.5 (45.1–59.9) | 61.6 (55.7–67.4) | 58.7 (52.1–65.3) | 0.19 |
| Gadolinium partition coefficient (λ) | 0.35 (0.29–0.4) | 0.45 (0.40–0.49) | 0.46 (0.41–0.51) | 0.009*,# |
| ECVF | 21.1 (17.5–24.7) | 27.6 (24.8–30.3) | 27.6 (24.4–30.8) | 0.016*,# |
| Cellular volume, mL | 102 (86–117) | 113 (101–125) | 107 (94–121) | 0.5472 |
| Extracellular volume, mL | 24.7 (19.3–30.2) | 41.4 (34.3–48.5) | 39.3 (31.7–46.9) | <0.001*,# |
| Indexed cellular volume, mL/BSA (m2) | 47.2 (40.8–53.5) | 51 (46–56) | 47.1 (41.5–52.7) | 0.52 |
| Indexed extracellular volume, mL/BSA (m2) | 13 (9.6–16.3) | 20.4 (17.7–23.1) | 18.3 (15.4–21.3) | 0.006* |
Data are mean (95% CI).
*Pairwise comparison between groups 1 and 2 (significant at P < 0.05).
#Pairwise comparison between groups 1 and 3 (significant at P < 0.05).
After further adjustment for systolic and diastolic blood pressures and eGFR (Table 3), the gadolinium partition coefficient (λ) was significantly higher in group 2 (mean 0.44 [95% CI 0.39–0.48]) and group 3 (0.47 [0.42–0.52]) than in group 1 (0.34 [0.29–0.40]; P = 0.005, ANCOVA). The myocardial ECVF also was significantly greater in groups 2 (mean 27.4% [95% CI 24.4–30.4]) and 3 (28% [24.5–31.5]) than in group 1 (20.9% [17.1–24.6]; P = 0.0156, ANCOVA), as was extracellular volume: mean 42.8 mL (95% CI 34.9–50.7) in group 2 and 40.7 mL (32–49.5) in group 3 versus 26.1 mL (20–32.1) in group 1 (P = 0.0021, ANCOVA); we found no significant differences in cellular volume. Finally, indexed extracellular volume was significantly larger in group 2 than in group 1 (Table 3), without significant differences in indexed cellular volume.
Table 3.
Comparison of various parameters of LV remodeling between subjects stratified according to HbA1c level adjusted for age, sex, BMI, systolic and diastolic blood pressures, and eGFR
| HbA1c (%) | P value | |||
|---|---|---|---|---|
| ≤6.5 (Group 1) | 6.5–7.5 (Group 2) | >7.5 (Group 3) | ||
| LV mass, g | 134 (116–152) | 156 (139–173) | 151 (132–170) | 0.1815 |
| LV mass index | ||||
| By BSA, g/m2 | 64 (55.5–72.5) | 74 (67.1–80.9) | 67.2 (59.3–75.1) | 0.1987 |
| By height, g/m1.7 | 53.6 (46–61.2) | 61 (54.8–67.2) | 58.5 (51.4–65.6) | 0.3466 |
| Gadolinium partition coefficient (λ) | 0.34 (0.29–0.40) | 0.44 (0.39–0.48) | 0.47 (0.42–0.52) | 0.0050*,# |
| ECVF | 20.9 (17.1–24.6) | 27.4 (24.4–30.4) | 28 (24.5–31.5) | 0.0156*,# |
| Cellular volume, mL | 109 (93–125) | 117 (103–130) | 110 (95–125) | 0.7417 |
| Extracellular volume, mL | 26.1 (20–32.1) | 42.8 (34.9–50.7) | 40.7 (32–49.5) | 0.0021*,# |
| Indexed cellular volume, mL/BSA (m2) | 50.3 (43.7–56.9) | 53 (47.6–58.4) | 48.1 (41.9–54.3) | 0.5421 |
| Indexed extracellular volume, mL/BSA (m2) | 13.7 (10.1–17.3) | 21 (18.1–24) | 19.1 (15.8–22.5) | 0.0131* |
Data are mean (95% CI).
*Pairwise comparison between groups 1 and 2 (significant at P < 0.05).
#Pairwise comparison between groups 1 and 3 (significant at P < 0.05).
Only four participants demonstrated at least one area of focal myocardial delayed enhancement (three consistent with focal infarcts and one with a nonischemic, nonspecific midwall pattern). The results were not appreciably different after these participants were excluded. The ECVF was significantly greater in group 2 (mean 28.4% [95% CI 25.4–31.5]) and group 3 (27.5% [24.4–30.6]) than in group 1 (20.9% [17.3–24.4]; P = 0.0087, ANCOVA).
Conclusions
In this study we demonstrated—to our knowledge for the first time—that poor glycemic control is independently associated with increased ECVF (suggesting myocardial fibrosis) in adults with type 2 diabetes. We demonstrated, using noninvasive quantification of LV mass (with cine MRI) and ECVF (with myocardial T1 mapping, a well-validated technique for characterizing tissue) (11,12), that poor glycemic control is associated with increased ECVF and extracellular volume (indicative of myocardial fibrosis), but not with cellular volume. These findings increase our understanding of the implications of poor glycemic control for the myocardium.
LV mass has been reported to be higher in subjects with diabetes than in those without diabetes (13), although large studies found concentric remodeling of the LV, rather than LV hypertrophy (i.e., increased LV mass), to be independently associated with insulin resistance (14,15). It is interesting to note that among subjects with diabetes LV mass was not associated with HbA1c, whereas ECVF (i.e., the fraction of myocardial tissue corresponding to the extracellular compartment) and the total extracellular volume (i.e., the extracellular volume in the LV wall, expressed in milliliters) were significantly associated with poor glycemic control. In contrast, cellular volume was not associated with glycemic control. This indicates that expansion of the extracellular compartment is a key component of the adverse myocardial remodeling associated with poor glycemic control in diabetes. Furthermore, our findings indicate that assessments of the extracellular and intracellular compartments (via T1 mapping) provide additional information to that provided by simple measurements of macroscopic hypertrophy (e.g., LV mass).
Myocardial fibrosis occurs in patients with diabetes more often than in control subjects without diabetes, and does so independently of coronary atherosclerosis or other cardiac risk factors (16,17). Myocardial fibrosis also has been well documented in animal models of type 1 and type 2 diabetes (18–22), and it was confirmed through the use of myocardial biopsies from patients with diabetes (23). Diabetes with suboptimal glycemic control (HbA1c ≥6.5%) has been shown to be associated with diastolic dysfunction, aortic stiffness, and the development of heart failure (13,24,25). Hyperglycemia also has been associated with circumferential myocardial dysfunction in young adults with type 1 diabetes (5) and with subclinical LV diastolic dysfunction, an early manifestation of heart disease in diabetes (26–29). However, the association between glycemic control and myocardial fibrosis in adults with diabetes has not, to our knowledge, been previously investigated. We showed that subjects with diabetes who exhibit poor glycemic control demonstrate increased myocardial fibrosis, independent of various confounders, compared with subjects with diabetes who exhibit tight glycemic control.
Insulin resistance with hyperinsulinemia (either endogenous or from exogenous administration) and hyperglycemia/glucotoxicity may be involved in the observed associations between HbA1c level and ECVF (30,31). These collective metabolic disturbances can promote cardiac remodeling, fibrosis, and myocardial dysfunction. Impaired insulin signaling via the mammalian target of rapamycin–S6 kinase 1 pathway can impair nitric production and promote profibrotic responses (30–32).
Hyperglycemia and glucotoxicity can also exert profibrotic effects. Hyperglycemia induces nonenzymatic glycosylation of lipids, lipoproteins, and amino acids, leading to increased amounts of advanced glycation end products (AGEs). AGE deposition contributes to increased connective tissue crosslinking and fibrosis, with increased resistance to enzymatic proteolysis in connective tissues (30,31). AGEs may also bind to the cell surface receptor for AGE (RAGE), thereby inducing increased matrix protein expression via the mitogen-activated protein kinase and Janus kinase pathways in vascular and cardiac tissues. AGEs may also promote the formation of reactive oxygen species, which can promote fibrosis. In a mouse model of type 1 diabetes, administration of a RAGE antagonist prevented AGEs and RAGE signaling–mediated increases in myocardial fibrosis (33). Finally, activation of both the systemic renin-angiotensin-aldosterone system and that in cardiac tissue, which occurs in states of insulin resistance and hyperglycemia, also contributes to profibrotic responses (30–32). The relative role of hyperglycemia and hyperinsulinemia (endogenous or exogenous) in myocardial fibrosis in human diabetes remains to be assessed in future studies with larger sample sizes and/or experimental designs. In particular, studies should investigate whether exogenous insulin use (which is intimately associated with poor glycemic control in current clinical practice) has an impact on myocardial fibrosis.
Myocardial fibrosis has important consequences for ventricular function. LV dysfunction, including impaired ventricular relaxation and stiffness, is linked to collagen accumulation in the myocardium (34,35). Myocardial fibrosis can also lead to coronary microvascular dysfunction (36). Increased myocardial stiffness, diastolic dysfunction, and microvascular dysfunction are thought to play important roles in heart failure with preserved EF, an epidemic condition for which no effective pharmacologic therapies are currently available and for which diabetes is an important risk factor (37). Whether myocardial fibrosis predicts risk of future heart failure with preserved EF among subjects with diabetes remains to be assessed in future studies. It is clear that further studies are needed to assess the relation between glycemic control, extracellular volume, myocardial function, and outcomes.
The duration of diabetes and HbA1c level are important predictors of heart failure development in subjects with diabetes (38). Our findings bring up the possibility that myocardial fibrosis (a known pathologic process contributing to heart failure) may at least partially mediate the association between HbA1c and heart failure risk. Parry et al. (39) found that, among subjects with type 2 diabetes, HbA1c <6% or >7% was associated with an increased number of hospitalizations for heart failure, creating a U-shaped relationship between HbA1c level and the risk of heart failure–related hospital admissions. The relation between tight diabetes control and heart failure might be explained by 1) frequent episodes of hypoglycemia, which were previously found to constitute an independent cardiovascular risk factor (40), or 2) the effects of oral antidiabetes medications (i.e., high-dose sulfonylureas [41] and thiazolidinediones [42]), which might be linked to the development of heart failure independent of glycemic control per se. Heart failure risk likely develops differently between subjects with tight glycemic control and those with poor glycemic control. Whether the institution of tight glycemic control can revert fibrosis to healthy myocardium or ameliorate it and its adverse clinical consequences in patients with diabetes remains to be assessed in future intervention studies. In a similar way, extracellular volume measurements may help stratify risk in patients with diabetes and identify those who may benefit from novel antifibrotic strategies, which should be the focus of future research.
The observation that increased cellular volume was not related to HbA1c level may have several explanations. First, our sample size may have been too limited to detect subtle increases in intracellular volume. Second, the pathologic processes associated with insulin resistance and hypoglycemia may lead to myocardial cell loss. For instance, myocardial lipid accumulation and lipotoxicity, which occur in states of insulin resistance, may promote cardiomyocyte apoptosis via increased production of reactive oxygen species and endoplasmic reticulum stress (30,31).
Our study should be interpreted in the context of its strengths and limitations. Strengths include the use of cardiac MRI to measure LV mass and ECVF and to derive cellular and extracellular volumes. Our groups matched relatively well with regard to characteristics that may confound myocardial fibrosis measurements; furthermore, we performed comprehensive adjustments in our comparisons, which adds confidence to our findings. Our study also has limitations: It used a convenience sample of participants at a VA Medical Center. Most participants were middle-aged or elderly men, and our results might not be generalizable to women and younger cohorts. HbA1c only reflects recent glucose control, whereas myocardial changes probably result from longer periods of altered metabolic states that are not well characterized by this measurement. ECVF is affected not only by fibrosis but also by edema and amyloid deposition. In this clinical context, however, it is reasonable to assume that the variability in ECVF represents myocardial fibrosis, rather than edema or amyloid deposits. Our cross-sectional study cannot address causality, and therefore the associations between poor glycemic control and myocardial fibrosis need to be interpreted cautiously and in the context of other available evidence. Finally, residual confounding from unmeasured factors cannot be ruled out.
In conclusion, diffuse myocardial fibrosis quantified by cardiac MRI is independently associated with poor glycemic control in subjects with diabetes. In contrast, poor glycemic control is not associated with increases in LV mass or expansion of myocardial cellular volume. Further studies are needed to investigate the causal role of glycemic control in the development of fibrosis and the molecular mechanisms behind this association. It is important that experimental trials be conducted to assess whether tight glycemic control represents a therapeutic strategy to ameliorate myocardial fibrosis, and its adverse clinical consequences, in human diabetes.
Article Information
Funding. This study was supported by the National Heart, Lung, and Blood Institute (grant no. R00HL108157 to W.R.W.), the National Institutes of Health (grant nos. R56HL124073-01A1 and R01HL121510-01A1 to J.A.C.), and a VISN-4 research grant from the U.S. Department of Veterans Affairs (to J.A.C.). A.A.-B is supported by a Ruth L. Kirschstein Institutional National Research Service Award Training Grant (5T32HL007745).
Duality of Interest. J.A.C. has received consulting honoraria from Bristol-Myers Squibb, OPKO Health, Fukuda Denshi Co., Laboratoris Sanifit S.L., Microsoft Corp., Vital Laboratories, Pfizer, and Merck & Co.; research grants from the National Institutes of Health, the American College of Radiology Network, Fukuda Denshi Co., Bristol-Myers Squibb, Microsoft Corp., and CVRx Inc.; and device loans from AtCor Medical. J.A.C. is named as inventor on a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction. No other conflicts of interest relevant to this article have been reported.
Author Contributions. A.A.-B. and J.A.C. wrote the manuscript. G.H.O., R.M., K.J., S.R.A., and J.A.C. obtained data. Z.H., A.A.S., B.A., S.G., W.R.W., and J.A.C. processed imaging data. Z.H., A.A.S., S.G., and W.R.W. performed critical review of the manuscript. S.R.A. and J.A.C. obtained and administered funding. J.A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 67th Annual Scientific Session & Expo of the American College of Cardiology, Orlando, FL, 12–14 March 2018.
Footnotes
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 2.Cavender MA, Steg PG, Smith SC Jr., et al.; REACH Registry Investigators . Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation 2015;132:923–931 [DOI] [PubMed] [Google Scholar]
- 3.Wong TC, Piehler KM, Kang IA, et al. . Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014;35:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasanji Z, Sigal RJ, Eves ND, et al. . Increased left ventricular extracellular volume and enhanced twist function in type 1 diabetic individuals. J Appl Physiol (1985) 2017;123:394–401 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AC, Ambale-Venkatesh B, Turkbey E, et al.; DCCT/EDIC Research Group . Association of cardiovascular risk factors and myocardial fibrosis with early cardiac dysfunction in type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2017;40:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah RV, Abbasi SA, Kwong RY. Role of cardiac MRI in diabetes. Curr Cardiol Rep 2014;16:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirinos JA, Segers P, De Buyzere ML, et al. . Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension 2010;56:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified Look-Locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081–1086 [DOI] [PubMed] [Google Scholar]
- 10.Chirinos JA, Akers SR, Trieu L, et al. . Heart failure, left ventricular remodeling, and circulating nitric oxide metabolites. J Am Heart Assoc 2016;5:e004133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CWL, Everett RJ, Kwiecinski J, et al. . Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White SK, Sado DM, Fontana M, et al. . T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging 2013;6:955–962 [DOI] [PubMed] [Google Scholar]
- 13.Kozakova M, Morizzo C, Fraser AG, Palombo C. Impact of glycemic control on aortic stiffness, left ventricular mass and diastolic longitudinal function in type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RV, Abbasi SA, Heydari B, et al. . Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1698–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velagaleti RS, Gona P, Chuang ML, et al. . Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging 2010;3:257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 2016;90:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan TJ, Lyons MM, Ahmed SS, et al. . Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 1977;60:884–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh K, McMullen JR, Julius TL, et al. . Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 2010;59:1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ares-Carrasco S, Picatoste B, Benito-Martín A, et al. . Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol 2009;297:H2109–H2119 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 2010;59:2033–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen X, Bornfeldt KE. Mouse models for studies of cardiovascular complications of type 1 diabetes. Ann N Y Acad Sci 2007;1103:202–217 [DOI] [PubMed] [Google Scholar]
- 22.Zaman AK, Fujii S, Goto D, et al. . Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol 2004;37:525–535 [DOI] [PubMed] [Google Scholar]
- 23.Nunoda S, Genda A, Sugihara N, Nakayama A, Mizuno S, Takeda R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels 1985;1:43–47 [DOI] [PubMed] [Google Scholar]
- 24.Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–146 [DOI] [PubMed] [Google Scholar]
- 25.Konduracka E, Cieslik G, Galicka-Latala D, et al. . Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: a 7-year prospective cohort study. Acta Diabetol 2013;50:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia 2010;53:1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging 2016;9:e003643. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Barriga JJ, Rangel A, Castañeda R, et al. . Left ventricular diastolic dysfunction secondary to hyperglycemia in patients with type II diabetes. Arch Med Res 2001;32:44–47 [DOI] [PubMed] [Google Scholar]
- 29.Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res 2011;2:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018;61:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 2012;302:E201–E208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H, Li SY, Xu P, et al. . Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med 2009;13(8B):1751–1764 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Regan TJ, Wu CF, Yeh CK, Oldewurtel HA, Haider B. Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res 1981;49:1268–1277 [DOI] [PubMed] [Google Scholar]
- 35.Sakakibara M, Hirashiki A, Cheng XW, et al. . Association of diabetes mellitus with myocardial collagen accumulation and relaxation impairment in patients with dilated cardiomyopathy. Diabetes Res Clin Pract 2011;92:348–355 [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi M, Techigawara M, Ishihata T, et al. . A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels 1997;12:267–274 [DOI] [PubMed] [Google Scholar]
- 37.Zakeri R, Cowie MR. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart 2018;104:377–384 [DOI] [PubMed] [Google Scholar]
- 38.Shang Y, Zhang X, Chen L, et al. . Assessment of left ventricular structural remodelling in patients with diabetic cardiomyopathy by cardiovascular magnetic resonance. J Diabetes Res 2016;2016:4786925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parry HM, Deshmukh H, Levin D, et al. . Both high and low HbA1c predict incident heart failure in type 2 diabetes mellitus. Circ Heart Fail 2015;8:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011;34(Suppl. 2):S132–S137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAlister FA, Eurich DT, Majumdar SR, Johnson JA. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur J Heart Fail 2008;10:703–708 [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care 2007;30:2148–2153 [DOI] [PubMed] [Google Scholar]