Abstract
OBJECTIVE
Recent large trials yield conflicting results on the association between incretin-based therapies (IBTs) and diabetic retinopathy (DR). We examined whether IBTs increase DR risk compared with other antihyperglycemics.
RESEARCH DESIGN AND METHODS
We implemented an active comparator, new-user cohort design using a nationwide 20% random sample of fee-for-service U.S. Medicare beneficiaries aged 65 years or older with Parts A, B, and D coverage between 2007 and 2015. We identified the following cohorts without prior treatment for retinopathy: dipeptidyl peptidase 4 inhibitors (DPP4i) versus sulfonylureas (SU), DPP4i versus thiazolidinediones (TZD), glucagon-like peptide-1 receptor agonists (GLP1RA) versus long-acting insulin (LAI), and GLP1RA versus TZD. Primary outcome was advanced diabetic retinopathy requiring treatment (ADRRT), defined as a procedure code for retinopathy treatment. Incident diabetic retinopathy (IDR), identified by a diagnosis code, was a secondary outcome. We estimated propensity scores to balance confounders and adjusted hazard ratios (95% CI) using weighted Cox proportional hazards models.
RESULTS
We identified 213,652 eligible patients. During a median duration of 0.58 to 0.87 years across comparisons, with a rate from 6.0 to 12.8 per 1,000 person-years, IBTs were not associated with increased ADRRT or IDR risk. The adjusted hazard ratios (95% CI) for ADRRT were 0.91 (0.79–1.04) by comparing DPP4i to SU (n = 39,292 and 87,073); 0.91 (0.75–1.11), DPP4i to TZD (n = 51,410 and 22,231); 0.50 (0.39–0.65), GLP1RA to LAI (n = 9,561 and 82,849); and 0.75 (0.53–1.06), GLP1RA to TZD (n = 10,355 and 27,345).
CONCLUSIONS
Our population-based cohort study of older U.S. adults with diabetes suggests that IBTs used for approximately 1 year do not increase the DR risk.
Introduction
Incretin-based therapies (IBTs), including glucagon-like peptide-1 receptor agonists (GLP1RA) and dipeptidyl peptidase 4 inhibitors (DPP4i), control blood glucose by potentiation of incretin receptor signaling (1). Safety concerns regarding IBTs have focused on pancreatitis (2), pancreatic cancer (3), medullary thyroid cancer (1), and heart failure (4,5). Recent cardiovascular trials have led to concerns that IBTs may increase the risk of diabetic retinopathy (DR) compared with placebo. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) reported a significantly higher rate of retinopathy complications with the GLP1RA semaglutide (14.9 vs. 8.6 events/1,000 person-years over 2.1 years of treatment; hazard ratio [HR] 1.76 [95% CI 1.11–2.78]) (6). The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial showed a statistically nonsignificant higher incidence of retinopathy complications with another GLP1RA, liraglutide (6 vs. 5 events/1,000 person-years over a median 3.8 years of treatment; HR 1.15 [0.87–1.52]) (7). The Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) study showed patients on DPP4i sitagliptin had a higher retinopathy frequency (2.8% vs. 2.2%, median follow-up of 3.0 years) (8), whereas the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial showed no increased retinopathy frequency in patients on GLP1RA exenatide (2.9% vs. 3.2%, median follow-up of 3.2 years) (9). The low retinopathy frequency in TECOS and EXSCEL trials could be explained by the absence of systematic examinations; none of the published trials systematically assessed retinopathy complications. Therefore, we conducted this large active comparator, new-user (ACNU) cohort study to examine whether the use of IBTs, as compared with therapeutic alternatives, is associated with an increased risk of DR.
Research Design and Methods
Data Source
Medicare provides medical coverage for the U.S. population aged 65 years and older. The University of North Carolina (UNC) has access to longitudinal claims data for a 20% random sample of all fee-for-service Medicare beneficiaries with Parts A (inpatient), B (outpatient physician services), and D (dispensed prescription drugs) coverage for at least 1 month from January 2007 to September 2015. The study protocol was registered in the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) electronic register of studies (www.encepp.eu/encepp/viewResource.htm?id=17777) and approved by the UNC institutional review board.
Study Population
The eligible population consisted of Medicare enrollees aged 65 years or older with at least 12 months of continuous enrollment in Medicare Parts A, B and D and without Health Maintenance Organization coverage before initiation and during follow-up. We identified all new users of a therapy of interest based on the first dispensing of a prescription in a given drug class after a 12-month washout for that drug class. Patients entered the cohort on the date of dispensing of the first prescription. As we obtained prescription data (Part D) starting from 1 January 2007, thus the earliest index date was 1 January 2008. As IBTs are typically second-line therapies (10) and the DR risk increases with increased diabetes duration (11), it is necessary to compare IBTs with drugs used at a similar stage of diabetes (12). Using an active comparator study design helps to mitigate bias by only selecting individuals with an indication for initiating a second-line antidiabetes treatment, identifying individuals at a similar point in their disease management and ensuring only adverse events after drug initiation are evaluated (12). We identified new users of the following comparisons, DPP4i versus sulfonylureas (SU), DPP4i versus thiazolidinediones (TZD), GLP1RA versus long-acting insulin (LAI), and GLP1RA versus TZD, and we excluded new users who took the other drug in the 12 months prior to initiation. Thus, the number of participants and events for a specific drug class varied across comparisons. Given these antihyperglycemia agents are primarily indicated for diabetes treatment (10), we did not restrict cohorts to those with a diagnosis code for diabetes. As early detection can reduce DR progression and prevent vision loss, patients with diabetes in the U.S. are recommended to have an eye exam at least every 2 years (10,11). As only patients with diabetes who had an eye exam could receive eye disease diagnoses or codes for specific treatments, we required cohort participants to have at least one eye examination in the 12 months prior to the index date. We identified eye examinations using Current Procedural Terminology (CPT) codes (Supplementary Table 1) and excluded patients with blindness and low vision (ICD-9-CM 369.XX), retinopathy treatment (described below), or vitrectomy before the index date. As current guidelines recommend treatment only for severe DR (12), we did not exclude patients with a DR diagnosis (ICD-9-CM 362.0X) without treatment. Last, as patients with congestive heart failure are less likely to initiate TZD (4), we excluded patients with a diagnosis of congestive heart failure in the 12 months prior to the first prescription when comparing IBTs to TZD.
DR Outcome
Our primary outcome was advanced diabetic retinopathy requiring treatment (ADRRT) as a proxy for incident retinopathy severe enough to require treatment (in patients without retinopathy at cohort entry) and worsening of preexisting retinopathy requiring incident treatment (in patients with DR but no retinopathy treatment at cohort entry). We used CPT and Healthcare Common Procedure Coding System (HCPCS) code to identify these treatments (Supplementary Table 1) (11). The primary definition was based on receipt of one of the following procedures with a DR or diabetes diagnosis code on the same claim: 1) photocoagulation (CPT 67228, 67210), 2) vitrectomy (CPT 67036, 67038–67042), and 3) intravitreal injection (CPT 67028) of anti–vascular endothelial growth factor agents (ranibizumab, aflibercept, or bevacizumab) or corticosteroid (triamcinolone, dexamethasone, or fluocinolone). Because medication-specific codes were not available immediately after U.S. Food and Drug Administration approval and bevacizumab lacks a code specific to ocular use (13), we also included claims for intravitreal injection of unclassified/miscellaneous drug codes. To minimize miscoding or misclassification bias, we required all HCPCS drug codes to be jointly used with an intravitreal injection CPT code. As some of these therapies are approved to treat age-related macular degeneration (AMD) (13), we excluded treatments with the following AMD diagnosis codes, ICD-9-CM 362.50, 362.52, 362.42, or 362.43. To capture untreated retinopathy (11), we defined our secondary outcome as incident diabetic retinopathy (IDR), identified by diagnosis ICD-9-CM 362.0X. When assessing IDR, patients with DR at baseline were excluded.
Follow-up
Follow-up started at the first prescription and ended with the earliest of the following events: 1) 30 days after treatment discontinuation (the date of last prescription plus a 90-day grace period), 30 days after switching to or adding the comparator drug class, or 30 days after the end of Medicare Part D enrollment, whichever came first; 2) end of enrollment for Medicare Parts A or B; 3) death; 4) end of study (30 September 2015); or 5) a claim for retinopathy treatment (for secondary outcomes analyses, a claim with a retinopathy diagnosis). We used the first DR treatment date to define the outcome date (first DR diagnosis for secondary outcome). Our primary analysis was as treated (Supplementary Fig. 1), using follow-up based on actual exposure to the initial antihyperglycemia treatment. Patients were defined as exposed to the initial treatment until treatment changed, including index drug class discontinuation or switching to or addition of a drug from the alternative class in the comparison. Treatment discontinuation was defined as no refills within a period equal to the prescribed duration of the last filled prescription plus a 90-day grace period (14). In the SUSTAIN-6 trial, increased retinopathy events with semaglutide occurred within the first 2 months (7). The mechanisms for incretin-associated DR complications are not clear, but one theory is that it results from a rapid glycemic control (15). Thus, we assumed treatment-related retinopathy would occur soon after treatment initiation. To account for the time required between noticing ocular disturbances and receiving an eye exam (the “latency” period), we considered retinopathy events within 30 days after treatment changes to be related to the initial treatment.
Statistical Analyses
We examined a variety of baseline characteristics for each cohort, defined based on claims during the 12 months prior to the index prescription (Table 1). These covariates included demographics, diabetes severity, comorbidities, comedications, health care utilization, and socioeconomic status (16). To control for factors that may influence the decision to prescribe a given treatment, we estimated propensity scores (PS) for each patient in each comparison using these variables. We did not use PS for the comparison on three groups simultaneously (DPP4i, SU, and TZD group and GLP1RA, TZD, and LAI group), as IBT initiators in each comparison are not the same population. To directly compare the estimates across two comparator groups for each of the IBTs, we standardized the covariate distribution of comparator initiators to the covariate distribution of IBT initiators using standardized mortality/morbidity ratio (SMR) weights [PS/(1 − PS)] (17). SMR weighting creates comparator cohorts with the same covariate distribution as in the IBT cohorts, as evinced by standardized differences of covariates close to zero after weighting.
Table 1.
Patient characteristics of IBT and comparator initiators*
| DPP4i vs. SU |
DPP4i vs. TZD |
GLP1RA vs. LAI |
GLP1RA vs. TZD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPP4i (N = 39,292)† | SU (N = 87,073) | Weighted SU‡ (N = 39,482) | DPP4i (N = 51,410)† | TZD (N = 22,231) | Weighted TZD‡ (N = 51,450) | GLP1R (N = 9,561)† | LAI (N = 82,849) | Weighted LAI‡ (N = 9,595) | GLP1RA (N = 10,355)† | TZD (N = 27,345) | Weighted TZD‡ (N = 10,768) | |
| Age, mean ± SD |
76.5 ± 7.01 |
76.7 ± 7.19 |
76.4 ± 6.99 |
76.0 ± 6.73 |
75.4 ± 6.51 |
76.0 ± 6.75 |
73.0 ± 5.17 |
76.9 ± 7.49 |
73.0 ± 5.18 |
72.7 ± 5.01 |
75.5 ± 6.51 |
72.5 ± 5.01 |
| Male |
15,461 (39.3) |
35,631 (40.9) |
15,504 (39.3) |
20,307 (39.5) |
9,262 (41.7) |
20,642 (40.1) |
3,970 (41.5) |
35,069 (42.3) |
3,986 (41.5) |
4,018 (38.8) |
11,424 (41.8) |
4,242 (39.4) |
| Race |
||||||||||||
| White |
30,064 (76.5) |
69,824 (80.2) |
30,268 (76.7) |
39,951 (77.7) |
16,708 (75.2) |
40,663 (79.0) |
8,279 (86.6) |
64,568 (77.9) |
8,304 (86.5) |
8,809 (85.1) |
20,234 (74.0) |
9,294 (86.3) |
| Black |
4,081 (10.4) |
9,300 (10.7) |
4,066 (10.3) |
5,112 (9.9) |
2,301 (10.4) |
4,698 (9.1) |
599 (6.3) |
10,434 (12.6) |
606 (6.3) |
795 (7.7) |
2,819 (10.3) |
747 (6.9) |
| Others |
5,147 (13.1) |
7,949 (9.1) |
5,148 (13.0) |
6,347 (12.3) |
3,222 (14.5) |
6,089 (11.8) |
683 (7.1) |
7,847 (9.5) |
685 (7.1) |
751 (7.3) |
4,292 (15.7) |
727 (6.7) |
| Calendar year of drug initiation |
||||||||||||
| 2008 |
2,280 (5.8) |
9,077 (10.4) |
2,287 (5.8) |
2,557 (5.0) |
4,004 (18.0) |
2,561 (5.0) |
527 (5.5) |
7,583 (9.2) |
524 (5.5) |
288 (2.8) |
4,280 (15.7) |
285 (2.6) |
| 2009 |
3,065 (7.8) |
12,520 (14.4) |
3,057 (7.7) |
3,840 (7.5) |
5,200 (23.4) |
3,836 (7.5) |
590 (6.2) |
10,474 (12.6) |
587 (6.1) |
433 (4.2) |
5,860 (21.4) |
429 (4.0) |
| 2010 |
3,993 (10.2) |
11,933 (13.7) |
4,008 (10.2) |
4,925 (9.6) |
4,306 (19.4) |
4,921 (9.6) |
775 (8.1) |
10,400 (12.6) |
773 (8.1) |
557 (5.4) |
5,094 (18.6) |
551 (5.1) |
| 2011 |
6,039 (15.4) |
11,487 (13.2) |
6,072 (15.4) |
6,926 (13.5) |
2,454 (11.0) |
6,959 (13.5) |
1,108 (11.6) |
10,762 (13.0) |
1,100 (11.5) |
923 (8.9) |
3,113 (11.4) |
914 (8.5) |
| 2012 |
6,316 (16.1) |
10,774 (12.4) |
6,370 (16.1) |
7,888 (15.3) |
1,272 (5.7) |
7,843 (15.2) |
1,361 (14.2) |
11,361 (13.7) |
1,364 (14.2) |
1,410 (13.6) |
1,691 (6.2) |
1,383 (12.8) |
| 2013 |
5,605 (14.3) |
10,989 (12.6) |
5,623 (14.2) |
8,162 (15.9) |
1,556 (7.0) |
8,115 (15.8) |
1,674 (17.5) |
11,471 (13.8) |
1,701 (17.7) |
1,984 (19.2) |
2,164 (7.9) |
2,105 (19.6) |
| 2014 |
6,417 (16.3) |
11,221 (12.9) |
6,464 (16.4) |
9,243 (18.0) |
1,776 (8.0) |
9,307 (18.1) |
1,713 (17.9) |
11,373 (13.7) |
1,719 (17.9) |
2,296 (22.2) |
2,686 (9.8) |
2,451 (22.8) |
| 2015 |
5,577 (14.2) |
9,072 (10.4) |
5,601 (14.2) |
7,869 (15.3) |
1,663 (7.5) |
7,909 (15.4) |
1,813 (19.0) |
9,425 (11.4) |
1,827 (19.0) |
2,464 (23.8) |
2,457 (9.0) |
2,650 (24.6) |
| Eye comorbidities |
||||||||||||
| Retinopathy |
4,555 (11.6) |
8,156 (9.4) |
4,594 (11.6) |
6,094 (11.9) |
2,711 (12.2) |
6,120 (11.9) |
1,025 (10.7) |
11,734 (14.2) |
1,032 (10.8) |
1,382 (13.3) |
3,447 (12.6) |
1,402 (13.0) |
| AMD |
5,173 (13.2) |
11,650 (13.4) |
5,177 (13.1) |
6,243 (12.1) |
2,479 (11.2) |
6,135 (11.9) |
885 (9.3) |
10,975 (13.2) |
886 (9.2) |
951 (9.2) |
3,074 (11.2) |
935 (8.7) |
| Retinal detachment and defects |
562 (1.4) |
1,271 (1.5) |
558 (1.4) |
708 (1.4) |
327 (1.5) |
710 (1.4) |
153 (1.6) |
1,168 (1.4) |
151 (1.6) |
176 (1.7) |
399 (1.5) |
178 (1.7) |
| Other retinal disorders |
6,671 (17.0) |
13,434 (15.4) |
6,656 (16.9) |
8,229 (16.0) |
3,275 (14.7) |
8,497 (16.5) |
1,561 (16.3) |
13,871 (16.7) |
1,568 (16.3) |
1,702 (16.4) |
4,098 (15.0) |
1,796 (16.7) |
| Cataracts |
20,852 (53.1) |
47,001 (54.0) |
20,884 (52.9) |
27,721 (53.9) |
12,281 (55.2) |
27,812 (54.1) |
5,439 (56.9) |
42,068 (50.8) |
5,457 (56.9) |
5,817 (56.2) |
14,983 (54.8) |
6,095 (56.6) |
| Glaucoma |
11,470 (29.2) |
23,446 (26.9) |
11,454 (29.0) |
14,646 (28.5) |
6,031 (27.1) |
14,467 (28.1) |
2,374 (24.8) |
22,038 (26.6) |
2,402 (25.0) |
2,554 (24.7) |
7,619 (27.9) |
2,662 (24.7) |
| Other eye diseases§ |
20,202 (51.4) |
42,616 (48.9) |
20,201 (51.2) |
25,172 (49.0) |
10,144 (45.6) |
25,078 (48.7) |
4,514 (47.2) |
39,448 (47.6) |
4,536 (47.3) |
4,863 (47.0) |
12,722 (46.5) |
5,067 (47.1) |
| Diabetes comorbidities |
||||||||||||
| Nephropathy |
4,152 (10.6) |
6,981 (8.0) |
4,231 (10.7) |
4,920 (9.6) |
1,784 (8.0) |
4,997 (9.7) |
1,031 (10.8) |
13,331 (16.1) |
1,030 (10.7) |
1,332 (12.9) |
2,382 (8.7) |
1,367 (12.7) |
| Neuropathy |
9,359 (23.8) |
16,053 (18.4) |
9,497 (24.1) |
11,646 (22.7) |
4,334 (19.5) |
11,775 (22.9) |
2,497 (26.1) |
23,903 (28.9) |
2,515 (26.2) |
3,106 (30.0) |
5,622 (20.6) |
3,332 (30.9) |
| Cardiovascular comorbidities |
||||||||||||
| Hypertension |
36,239 (92.2) |
79,172 (90.9) |
36,430 (92.3) |
47,316 (92.0) |
19,959 (89.8) |
47,251 (91.8) |
8,797 (92.0) |
77,018 (93.0) |
8,833 (92.1) |
9,589 (92.6) |
24,663 (90.2) |
10,018 (93.0) |
| Dyslipidemia |
34,813 (88.6) |
72,664 (83.5) |
35,048 (88.8) |
45,699 (88.9) |
19,107 (85.9) |
45,930 (89.3) |
8,742 (91.4) |
69,448 (83.8) |
8,785 (91.6) |
9,436 (91.1) |
23,736 (86.8) |
9,852 (91.5) |
| Coronary artery disease |
17,011 (43.3) |
35,638 (40.9) |
17,106 (43.3) |
17,843 (34.7) |
6,881 (31.0) |
17,777 (34.6) |
3,560 (37.2) |
41,977 (50.7) |
3,569 (37.2) |
3,596 (34.7) |
8,592 (31.4) |
3,681 (34.2) |
| Cerebrovascular disease |
9,814 (25.0) |
20,423 (23.5) |
9,854 (25.0) |
10,517 (20.5) |
4,066 (18.3) |
10,256 (19.9) |
1,629 (17.0) |
25,924 (31.3) |
1,622 (16.9) |
1,728 (16.7) |
4,991 (18.3) |
1,811 (16.8) |
| Peripheral vascular disease |
9,258 (23.6) |
17,991 (20.7) |
9,259 (23.5) |
9,722 (18.9) |
3,644 (16.4) |
9,535 (18.5) |
1,632 (17.1) |
23,533 (28.4) |
1,633 (17.0) |
1,689 (16.3) |
4,630 (16.9) |
1,732 (16.1) |
| Congestive heart failure‖ |
9,067 (23.1) |
20,131 (23.1) |
9,127 (23.1) |
NA |
NA |
NA |
1,501 (15.7) |
28,941 (34.9) |
1,494 (15.6) |
NA |
NA |
NA |
| Other comorbidities |
||||||||||||
| Chronic obstructive pulmonary disease |
8,264 (21.0) |
19,171 (22.0) |
8,271 (20.9) |
7,186 (14.0) |
2,903 (13.1) |
7,053 (13.7) |
1,543 (16.1) |
23,423 (28.3) |
1,550 (16.2) |
1,408 (13.6) |
3,539 (12.9) |
1,464 (13.6) |
| Depression |
7,223 (18.4) |
14,971 (17.2) |
7,274 (18.4) |
7,511 (14.6) |
2,839 (12.8) |
7,429 (14.4) |
1,570 (16.4) |
19,073 (23.0) |
1,568 (16.3) |
1,838 (17.7) |
3,443 (12.6) |
1,944 (18.1) |
| Cancer |
7,918 (20.2) |
17,489 (20.1) |
7,908 (20.0) |
9,608 (18.7) |
3,660 (16.5) |
9,723 (18.9) |
1,636 (17.1) |
18,537 (22.4) |
1,632 (17.0) |
1,767 (17.1) |
4,544 (16.6) |
1,814 (16.8) |
| Chronic kidney disease¶ |
11,913 (30.3) |
24,822 (28.5) |
11,994 (30.4) |
12,879 (25.1) |
4,640 (20.9) |
12,949 (25.2) |
2,327 (24.3) |
36,390 (43.9) |
2,340 (24.4) |
2,593 (25.0) |
6,032 (22.1) |
2,709 (25.2) |
| Comedications |
||||||||||||
| Metformin |
25,399 (64.6) |
49,479 (56.8) |
25,782 (65.3) |
37,162 (72.3) |
14,924 (67.1) |
37,247 (72.4) |
6,969 (72.9) |
44,333 (53.5) |
7,016 (73.1) |
7,038 (68.0) |
18,736 (68.5) |
7,303 (67.8) |
| SU‖ |
NA |
87,073 (100.0) |
39,482 (100.0) |
26,736 (52.0) |
12,047 (54.2) |
27,086 (52.6) |
5,695 (59.6) |
49,977 (60.3) |
5,831 (60.8) |
5,036 (48.6) |
15,461 (56.5) |
5,195 (48.2) |
| TZD‖ |
7,384 (18.8) |
10,736 (12.3) |
7,586 (19.2) |
NA |
22,231 (100.0) |
51,450 (100.0) |
2,180 (22.8) |
14,537 (17.5) |
2,237 (23.3) |
NA |
27,345 (100.0) |
10,768 (100.0) |
| DPP4i‖ |
39,292 (100.0) |
NA |
NA |
51,410 (100.0) |
NA |
NA |
3,594 (37.6) |
20,581 (24.8) |
3,716 (38.7) |
4,942 (30.1) |
9,093 (18.7) |
31.6 |
| GLP1RA‖ |
943 (2.4) |
1,480 (1.7) |
991 (2.5) |
1,219 (2.4) |
623 (2.8) |
1,249 (2.4) |
9,561 (100.0) |
NA |
NA |
16,444 (100.0) |
NA |
NA |
| LAI‖ |
8,363 (21.3) |
12,108 (13.9) |
8,612 (21.8) |
8,187 (15.9) |
3,355 (15.1) |
8,282 (16.1) |
NA |
82,849 (100.0) |
9,595 (100.0) |
4,229 (40.8) |
4,006 (14.6) |
4,657 (43.2) |
| α-Glucosidase inhibitors |
225 (0.6) |
295 (0.3) |
234 (0.6) |
423 (0.8) |
170 (0.8) |
426 (0.8) |
148 (1.5) |
979 (1.2) |
154 (1.6) |
132 (1.3) |
269 (1.0) |
188 (1.7) |
| Meglitinides |
1,819 (4.6) |
2,207 (2.5) |
1,906 (4.8) |
1,548 (3.0) |
577 (2.6) |
1,681 (3.3) |
374 (3.9) |
3,846 (4.6) |
393 (4.1) |
326 (3.1) |
869 (3.2) |
333 (3.1) |
| ACE inhibitors |
17,133 (43.6) |
40,642 (46.7) |
17,242 (43.7) |
24,076 (46.8) |
11,020 (49.6) |
23,940 (46.5) |
4,401 (46.0) |
40,539 (48.9) |
4,419 (46.1) |
4,849 (46.8) |
13,504 (49.4) |
4,996 (46.4) |
| ARBs |
13,857 (35.3) |
23,190 (26.6) |
13,996 (35.4) |
17,281 (33.6) |
6,293 (28.3) |
17,227 (33.5) |
3,532 (36.9) |
22,644 (27.3) |
3,576 (37.3) |
3,838 (37.1) |
8,196 (30.0) |
4,070 (37.8) |
| β-Blockers |
21,930 (55.8) |
48,452 (55.6) |
21,992 (55.7) |
26,899 (52.3) |
10,351 (46.6) |
26,867 (52.2) |
5,002 (52.3) |
49,548 (59.8) |
5,032 (52.4) |
5,325 (51.4) |
12,904 (47.2) |
5,596 (52.0) |
| Calcium-channel blockers |
15,060 (38.3) |
32,393 (37.2) |
15,101 (38.2) |
19,400 (37.7) |
7,758 (34.9) |
19,149 (37.2) |
3,289 (34.4) |
33,556 (40.5) |
3,318 (34.6) |
3,634 (35.1) |
9,706 (35.5) |
3,724 (34.6) |
| Statins |
28,536 (72.6) |
56,987 (65.4) |
28,741 (72.8) |
37,329 (72.6) |
15,120 (68.0) |
37,390 (72.7) |
7,230 (75.6) |
56,132 (67.8) |
7,254 (75.6) |
7,882 (76.1) |
19,024 (69.6) |
8,134 (75.5) |
| Loop diuretics |
10,891 (27.7) |
25,207 (28.9) |
10,939 (27.7) |
8,865 (17.2) |
3,290 (14.8) |
9,099 (17.7) |
2,403 (25.1) |
33,119 (40.0) |
2,415 (25.2) |
2,164 (20.9) |
3,979 (14.6) |
2,350 (21.8) |
| Other diuretics |
15,861 (40.4) |
34,387 (39.5) |
15,971 (40.5) |
20,844 (40.5) |
8,764 (39.4) |
20,802 (40.4) |
4,172 (43.6) |
30,603 (36.9) |
4,201 (43.8) |
4,414 (42.6) |
10,821 (39.6) |
4,643 (43.1) |
| Fenofibrate |
3,312 (8.4) |
5,641 (6.5) |
3,388 (8.6) |
4,498 (8.7) |
1,675 (7.5) |
4,585 (8.9) |
1,035 (10.8) |
5,797 (7.0) |
1,062 (11.1) |
1,138 (11.0) |
2,170 (7.9) |
1,195 (11.1) |
| Any drugs may induce retinopathy or macular edema# |
7,983 (20.3) |
17,846 (20.5) |
8,030 (20.3) |
8,762 (17.0) |
3,600 (16.2) |
9,090 (17.7) |
1,741 (18.2) |
18,794 (22.7) |
1,758 (18.3) |
1,685 (16.3) |
4,448 (16.3) |
1,866 (17.3) |
| Health care utilizations |
||||||||||||
|
N of hyperglycemia diagnosis |
||||||||||||
| 0 |
20,302 (51.7) |
51,432 (59.1) |
20,248 (51.3) |
25,128 (48.9) |
11,653 (52.4) |
25,234 (49.0) |
4,270 (44.7) |
31,322 (37.8) |
4,258 (44.4) |
3,947 (38.1) |
13,788 (50.4) |
3,958 (36.8) |
| 1 |
5,249 (13.4) |
11,714 (13.5) |
5,271 (13.4) |
7,029 (13.7) |
2,902 (13.1) |
7,035 (13.7) |
1,312 (13.7) |
12,410 (15.0) |
1,311 (13.7) |
1,283 (12.4) |
3,548 (13.0) |
1,330 (12.4) |
| 2 |
3,106 (7.9) |
6,149 (7.1) |
3,133 (7.9) |
4,231 (8.2) |
1,711 (7.7) |
4,157 (8.1) |
833 (8.7) |
7,993 (9.6) |
843 (8.8) |
898 (8.7) |
2,163 (7.9) |
889 (8.3) |
| ≥3 |
10,635 (27.1) |
17,778 (20.4) |
10,830 (27.4) |
15,022 (29.2) |
5,965 (26.8) |
15,025 (29.2) |
3,146 (32.9) |
31,124 (37.6) |
3,182 (33.2) |
4,227 (40.8) |
7,846 (28.7) |
4,591 (42.6) |
|
N of hospitalizations due to diabetes |
||||||||||||
| 0 |
38,627 (98.3) |
85,759 (98.5) |
38,810 (98.3) |
50,730 (98.7) |
21,991 (98.9) |
50,850 (98.8) |
9,521 (99.6) |
78,850 (95.2) |
9,554 (99.6) |
10,270 (99.2) |
27,037 (98.9) |
10,692 (99.3) |
| 1 |
592 (1.5) |
1,177 (1.4) |
595 (1.5) |
613 (1.2) |
221 (1.0) |
530 (1.0) |
39 (0.4) |
3,660 (4.4) |
40 (0.4) |
76 (0.7) |
282 (1.0) |
71 (0.7) |
| ≥2 |
73 (0.2) |
137 (0.2) |
77 (0.2) |
67 (0.1) |
19 (0.1) |
71 (0.1) |
NTSR |
339 (0.4) |
NTSR |
NTSR |
26 (0.1) |
NTSR |
|
N of ED visits due to diabetes |
||||||||||||
| 0 |
37,929 (96.5) |
84,164 (96.7) |
38,104 (96.5) |
49,715 (96.7) |
21,506 (96.7) |
49,804 (96.8) |
9,393 (98.2) |
75,432 (91.0) |
9,426 (98.2) |
10,071 (97.3) |
26,434 (96.7) |
10,467 (97.2) |
| 1 |
1,072 (2.7) |
2,417 (2.8) |
1,084 (2.7) |
1,388 (2.7) |
610 (2.7) |
1,351 (2.6) |
151 (1.6) |
6,111 (7.4) |
152 (1.6) |
245 (2.4) |
756 (2.8) |
253 (2.4) |
| ≥2 |
291 (0.7) |
492 (0.6) |
293 (0.7) |
307 (0.6) |
115 (0.5) |
295 (0.6) |
17 (0.2) |
1,306 (1.6) |
17 (0.2) |
39 (0.4) |
155 (0.6) |
48 (0.4) |
| N of physician encounters |
||||||||||||
| 0 |
863 (2.2) |
2,813 (3.2) |
853 (2.2) |
935 (1.8) |
553 (2.5) |
853 (1.7) |
76 (0.8) |
4,173 (5.0) |
75 (0.8) |
95 (0.9) |
612 (2.2) |
103 (1.0) |
| 1–3 |
3,105 (7.9) |
9,546 (11.0) |
3,083 (7.8) |
4,088 (8.0) |
2,381 (10.7) |
4,030 (7.8) |
567 (5.9) |
8,828 (10.7) |
554 (5.8) |
564 (5.4) |
2,750 (10.1) |
583 (5.4) |
| 4–6 |
6,103 (15.5) |
15,914 (18.3) |
6,124 (15.5) |
9,183 (17.9) |
4,614 (20.8) |
9,197 (17.9) |
1,499 (15.7) |
12,005 (14.5) |
1,479 (15.4) |
1,585 (15.3) |
5,502 (20.1) |
1,559 (14.5) |
| ≥7 |
29,221 (74.4) |
58,800 (67.5) |
29,421 (74.5) |
37,204 (72.4) |
14,683 (66.0) |
37,370 (72.6) |
7,419 (77.6) |
57,843 (69.8) |
7,486 (78.0) |
8,111 (78.3) |
18,481 (67.6) |
8,523 (79.2) |
| N of ED visit (any reason) |
||||||||||||
| 0 |
23,382 (59.5) |
49,093 (56.4) |
23,475 (59.5) |
34,385 (66.9) |
15,311 (68.9) |
34,616 (67.3) |
6,808 (71.2) |
35,073 (42.3) |
6,859 (71.5) |
7,348 (71.0) |
18,947 (69.3) |
7,581 (70.4) |
| 1 |
7,396 (18.8) |
17,173 (19.7) |
7,454 (18.9) |
9,376 (18.2) |
3,908 (17.6) |
9,308 (18.1) |
1,561 (16.3) |
17,823 (21.5) |
1,554 (16.2) |
1,768 (17.1) |
4,728 (17.3) |
1,918 (17.8) |
| ≥2 |
8,514 (21.7) |
20,807 (23.9) |
8,553 (21.7) |
7,649 (14.9) |
3,012 (13.5) |
7,526 (14.6) |
1,192 (12.5) |
29,953 (36.2) |
1,181 (12.3) |
1,239 (12.0) |
3,670 (13.4) |
1,269 (11.8) |
| Flu vaccine |
24,181 (61.5) |
51,775 (59.5) |
24,306 (61.6) |
31,935 (62.1) |
12,989 (58.4) |
32,402 (63.0) |
6,193 (64.8) |
47,641 (57.5) |
6,217 (64.8) |
6,683 (64.5) |
16,084 (58.8) |
7,014 (65.1) |
| Low-income subsidy |
16,130 (41.1) |
31,169 (35.8) |
16,117 (40.8) |
18,562 (36.1) |
8,598 (38.7) |
17,360 (33.7) |
2,572 (26.9) |
36,133 (43.6) |
2,578 (26.9) |
2,803 (27.1) |
10,864 (39.7) |
2,792 (25.9) |
| Laboratory results** |
||||||||||||
| HbA1c available†† |
3,025 (7.7) |
6,126 (7.0) |
4,553 (8.9) |
1,643 (7.4) |
1,129 (11.8) |
6,654 (8.0) |
1,386 (13.4) |
2,055 (7.5) |
||||
| <7% (53 mmol/mol) |
1,228 (40.6) |
2,530 (41.3) |
1,381 (30.3) |
542 (33.0) |
342 (30.3) |
1,711 (25.7) |
329 (23.7) |
637 (31.0) |
||||
| 7–9% (53–75 mmol/mol) |
1,220 (40.3) |
2,474 (40.4) |
2,281 (50.1) |
779 (47.4) |
566 (50.1) |
3,033 (45.6) |
692 (49.9) |
1,012 (49.2) |
||||
| >9% (75 mmol/mol) |
577 (19.1) |
1,122 (18.3) |
891 (19.6) |
322 (19.6) |
221 (19.6) |
1,910 (28.7) |
365 (26.3) |
406 (19.8) |
||||
| SBP available†† |
1,621 (4.1) |
3,895 (4.5) |
2,364 (4.6) |
1,196 (5.4) |
575 (6.0) |
3,988 (4.8) |
553 (5.3) |
1,428 (5.2) |
||||
| <130 mmHg |
662 (40.8) |
1,467 (37.7) |
890 (37.6) |
395 (33.0) |
221 (38.4) |
1,541 (38.6) |
203 (36.7) |
473 (33.1) |
||||
| 130–139 mmHg |
479 (29.5) |
1,172 (30.1) |
722 (30.5) |
369 (30.9) |
196 (34.1) |
1,156 (29.0) |
180 (32.5) |
444 (31.1) |
||||
| ≥140 mmHg |
480 (29.6) |
1,256 (32.2) |
752 (31.8) |
432 (36.1) |
158 (27.5) |
1,291 (32.4) |
170 (30.7) |
511 (35.8) |
||||
| DBP available†† |
1,596 (4.1) |
3,849 (4.4) |
2,339 (4.5) |
1,172 (5.3) |
559 (5.8) |
3,921 (4.7) |
546 (5.3) |
1,397 (5.1) |
||||
| <80 mmHg |
1,018 (63.8) |
2,417 (62.8) |
1,410 (60.3) |
718 (61.3) |
349 (62.4) |
2,544 (64.9) |
343 (62.8) |
853 (61.1) |
||||
| 80–89 mmHg |
473 (29.6) |
1,212 (31.5) |
777 (33.2) |
376 (32.1) |
187 (33.5) |
1,141 (29.1) |
181 (33.2) |
453 (32.4) |
||||
| ≥90 mmHg |
105 (6.6) |
220 (5.7) |
152 (6.5) |
78 (6.7) |
23 (4.1) |
236 (6.0) |
22 (4.0) |
91 (6.5) |
||||
| LDL cholesterol available†† |
2,405 (6.1) |
5,176 (5.9) |
3,647 (7.1) |
1,443 (6.5) |
915 (9.6) |
5,454 (6.6) |
1,075 (10.4) |
1,787 (6.55) |
||||
| <100 mg/dL |
1,818 (75.6) |
3,883 (75.0) |
2,762 (75.7) |
1,085 (75.2) |
717 (78.4) |
4,254 (78.0) |
848 (78.9) |
1,347 (75.4) |
||||
| 100–129 mg/dL |
385 (16.0) |
791 (15.3) |
571 (15.7) |
231 (16.0) |
132 (14.4) |
743 (13.6) |
154 (14.3) |
283 (15.8) |
||||
| ≥130 mg/dL | 202 (8.4) | 502 (9.7) | 314 (8.6) | 127 (8.8) | 66 (7.2) | 457 (8.4) | 73 (6.8) | 157 (8.8) | ||||
Data are n (%) unless otherwise stated. DBP, diastolic blood pressure; ED, emergency department; NA, not applicable; NTSR, numbers too small (<11) to report based on Centers for Medicare & Medicaid Services rules and data use agreement; SBP, systolic blood pressure.
*The comparisons were defined by use of IBT and PS-weighted comparator. Covariates were measured in the 12 months before cohort entry including the index date (100% of new users have the treatment at baseline). Initiation defined as having no prescriptions of either drug class during the 12 months prior to initiation.
†The size of the population for a specific drug differed across cohorts because of the requirement not to have been treated prior to index date with the comparator drug class (Supplementary Fig. 1).
‡Weighted by standardizing to their distribution in IBT initiators by using weights of 1 for IBT initiators and the odds of the estimated PS for comparator initiators.
§Other eye diseases included disorders of globe (ICD-9-CM 360), chorioretinal inflammation (ICD-9-CM 363), disorder of the iris or ciliary body (ICD-9-CM 364), visual disturbance (ICD-9-CM 368), keratitis (ICD-9-CM 370), corneal disorders (ICD-9-CM 371), disorders of the conjunctiva (ICD-9-CM 372), inflammation of eyelid (ICD-9-CM 373), other disorders of eyelid (ICD-9-CM 374), disorder of lacrimal system (ICD-9-CM 375), disorder of orbit (ICD-9-CM 376), optic nerve disorder (ICD-9-CM 377), strabismus (ICD-9-CM 378), and other disorders of eye (ICD-9-CM 379).
‖Patients with congestive heart failure at baseline were excluded for GLP1RA vs. TZD and DPP4i vs. TZD comparisons, and patients were required not to have been treated prior to index date with the comparator drug class.
¶Diabetic nephropathy codes (250.40–250.43) were not included to identify chronic kidney disease (ICD-9-CM codes 016.0, 095.4, 189.0, 189.9, 223.0, 236.91, 271.4, 274.1, 283.11, 403, 404, 440.1, 442.1, 572.4, 581–588, 591, 753.12–753.19, 753.2, and 794.4).
#Drugs that may induce DR or macular edema included tamoxifen, quinine, chloroquine, hydroxychloroquine, mefloquine, digoxin, ethambutol, peginterferon alfa-2a, peginterferon alfa-2b, interferon alfa-2b, interferon alfa-n3, interferon alfacon-1, interferon beta-1a, interferon alfa-1b, isocarboxazid, sildenafil, isotretinoin, vigabatrin, fingolimod, doxetaxel, niacin, and latanoprost (ophthalmic).
**Based on the measure closest to index date. Covariates distribution level were not weighted because they are well balanced in the crude comparison (except in GLP1RA vs. LAI comparison) and data are available for only a small portion of the population.
††The percentages for patients with clinical measures levels were based on the sample size of cohort, whereas for each level of clinical measures, the denominator for the percentage is the total number patients with HbA1c available. The clinical measures were not included in PS model due to missing data. We imputed for these missing clinical measures and the analyzed data in sensitivity analysis, and the distributions of imputed clinical measures are shown in Supplementary Tables 3 and 4.
We calculated crude incidence by dividing the number of patients with the outcome by the total amount of observation time for each exposure group, with CIs based on the Poisson distribution. We constructed SMR-weighted (adjusted) Kaplan-Meier curves to compare the cumulative incidence of ADRRT (18). We fit Cox proportional hazards models in the PS-weighted populations to estimate HRs and 95% CIs for DR associated with the use of IBTs versus comparators.
Secondary Analyses
Approximately 10% of fee-for-service Medicare beneficiaries initiating the drugs of interest had CPT II codes for HbA1c, blood pressure, and cholesterol categories (Table 1). These patients were likely seen by providers who participated in programs such as Healthcare Effectiveness Data and Information Set performance measurement in the accountable care organizations or the Physician Quality Reporting System, which require physicians to regularly report these clinical measures to Centers for Medicare & Medicaid Services (19,20). We performed 20 multiple imputations for these clinical measures to control for confounding using fully conditional specification with logistic regression (21,22), then estimated adjusted HRs balancing measured and imputed covariates in each of the 20 imputed data sets using PS weighting, and then pooled the results across the imputations. We then stratified by HbA1c tertiles (<7% [53 mmol/mol], 7–9% [53–75 mmol/mol], and >9% [75 mmol/mol]). To assess whether the risk varied with duration of use, we estimated separate HRs for the first 6 months, 6–12 months, and after 12 months. Additionally, we evaluated whether the risk varied across patients with and without preexisting untreated DR, hypertension, and use of ACE inhibitors or angiotensin receptor blockers (ARBs) at cohort entry and the risk for each individual IBT.
Sensitivity Analyses
To assess the robustness of estimated DR risk, we performed several sensitivity analyses. First, we changed the latency period from 30 days to 0, 60, 90, and 180 days. Second, we performed an analysis based on initial treatment (Supplementary Fig. 2), ignoring treatment changes during follow-up. This approach mimics the intention-to-treat analysis in a randomized trial. The follow-up ends with the earliest of the following events: 3 years after initiation, death, end of enrollment for Medicare Parts A or B, end of study (30 September 2015), or a retinopathy treatment claim. Third, we modified our primary outcome to require a DR diagnosis in the primary, secondary, or tertiary position within the procedure claim. Fourth, as some patients with diabetes undergo eye exams less frequently than recommended (22) and only patients who had an eye exam could receive treatment for retinopathy, we excluded patients without an eye exam after cohort entry, recognizing this introduces selection bias. Fifth, as intensive use of insulin has an “early worsening” effect on retinopathy (23), we censored patients receiving LAI during follow-up when compared to SU or TZD. Sixth, we censored patients when they received medications that may induce or worsen (24,25) or slow retinopathy progression (fenofibrate [11]) (Supplementary Table 2). The third through sixth sensitivity analyses were based on our primary analysis (as treated, 30-day latency period). Seventh, we conducted analysis using multivariable-adjusted Cox regression. Last, we assessed our secondary outcome, IDR, using as-treated analysis with different latency periods (0, 30, 60, 90, and 180 days).
Results
Study Population
We identified 213,652 eligible patients from 487,057 initiators of at least one drug of interest, including 126,365 initiators for the DPP4i versus SU comparison, 73,641 initiators for DPP4i versus TZD comparison, 92,410 initiators for GLP1RA versus LAI comparison, and 37,700 initiators for GLP1RA versus TZD comparison (Supplementary Fig. 3). The median duration from last eye exam to index date was about 120 days with interquartile range from 50 to 221 days: DPP4i vs. SU 117 (49–214) vs. 122 (51–221), DPP4i vs. TZD 119 (50–217) vs. 116 (48–218), GLP1RA vs. LAI 120 (50–220) vs. 122 (51–221), and GLP1RA vs. TZD 118 (49–216) vs. 116 (49–216). We present the crude and weighted baseline covariate distributions in Table 1. The mean age ranged between 72.5 and 76.9 years, 38.8% to 42.3% were men, and the frequency of preexisting retinopathy ranged from 10.7% (GLP1RA initiators) to 14.2% (LAI initiators). Overall, the prevalence of comorbidities was similar in the comparison cohorts except LAI initiator cohort, who was more likely to have diabetes complications, cardiovascular diseases, chronic obstructive pulmonary disease, and chronic kidney disease compared with the GLP1RA cohort. In the third column of each comparison in Table 1, we present PS-weighted covariate distributions for the comparator drug initiators. The virtually identical distribution of the covariates in IBT initiators and the PS-weighted comparator initiators shows that we were able to balance all measured covariates and, thus, remove confounding by these covariates. The standardized differences before and after weighting are presented in Supplementary Tables 3 and 4. In the small subset of patients with information on categories of HbA1c, blood pressure, and LDL cholesterol, the distribution of these clinical measures was similar between IBTs and comparators, except that the LAI initiator cohort was more likely to have a higher HbA1c level (>9% [75 mmol/mol]) compared with the GLP1RA initiator cohort. The distribution of clinical covariates was well balanced by PS weighting after multiple imputation.
IBTs and ADRRT
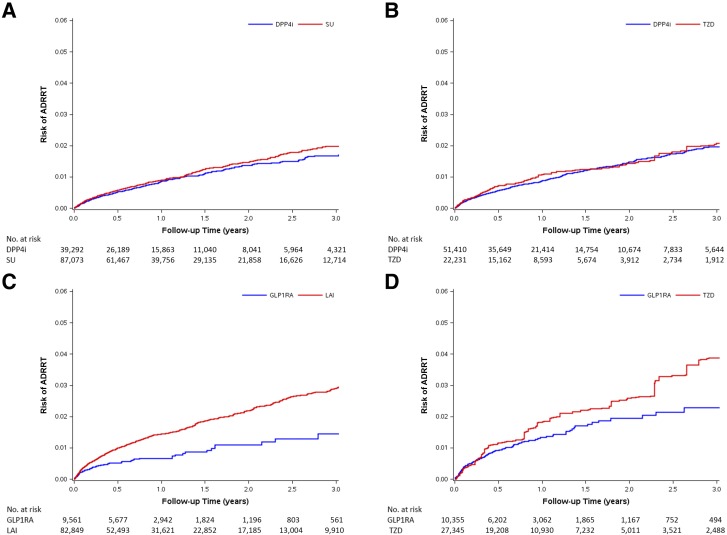
In Table 2, we present results of the primary as-treated analysis for ADRRT risk. Among the eight cohorts, the median treatment duration ranged from 0.58 to 0.87 years, the crude incidence of ADRRT ranged from 6.0 to 12.8 events per 1,000 person-years, and the average incidence was 8.7 per 1,000 person-years. After adjusting for confounding, DPP4i was not associated with a risk of ADRRT when compared to SU (HR 0.91 [95% CI 0.79–1.04]) or TZD (0.91 [0.75–1.11]), and GLP1RA was not associated with increased risk when compared to TZD (0.75 [0.53–1.06]) but showed a decreased risk compared to LAI (0.50 [0.39–0.65]). We present weighted Kaplan-Meier curves for IBTs and comparators in Fig. 1. The curves are similar except for the GLP1RA versus LAI comparisons, in which LAI was associated with a higher risk of ADRRT.
Table 2.
Crude and adjusted HRs for ADRRT associated with use of IBTs compared with therapeutic alternatives*
| Comparison | Cohort | Patients, n | Median duration (years) of treatment (IQR) | Person-years | ADRRT events, n | ADRRT incidence per 1,000 person-years (95% CI) | Crude HR (95% CI) | PS weighting,† HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| DPP4i vs. SU |
DPP4i |
39,292 |
0.75 (0.41–1.67) |
50,222 |
349 |
6.9 (6.3–7.7) |
1.10 (0.97–1.25) |
0.91 (0.79–1.04) |
| SU |
87,073 |
0.87 (0.42–2.01) |
129,099 |
772 |
6.0 (5.6–6.4) |
1.00 (reference) |
1.00 (reference) |
|
| DPP4i vs. TZD |
DPP4i |
51,410 |
0.80 (0.41–1.70) |
67,327 |
520 |
7.7 (7.1–8.4) |
0.85 (0.73–0.98) |
0.91 (0.75–1.11) |
| TZD |
22,231 |
0.74 (0.41–1.52) |
26,984 |
253 |
9.4 (8.3–10.6) |
1.00 (reference) |
1.00 (reference) |
|
| GLP1RA vs. LAI |
GLP1RA |
9,561 |
0.59 (0.41–1.21) |
9,462 |
66 |
7.0 (5.5–8.9) |
0.49 (0.39–0.63) |
0.50 (0.39–0.65) |
| LAI |
82,849 |
0.67 (0.41–1.66) |
106,699 |
1,368 |
12.8 (12.2–13.5) |
1.00 (reference) |
1.00 (reference) |
|
| GLP1RA vs. TZD | GLP1RA |
10,355 |
0.58 (0.41–1.17) |
9,895 |
122 |
12.3 (10.3–14.7) |
1.16 (0.94–1.42) |
0.75 (0.53–1.06) |
| TZD | 27,345 | 0.78 (0.42–1.57) | 34,232 | 334 | 9.8 (8.8–10.9) | 1.00 (reference) | 1.00 (reference) |
IQR, interquartile range.
*Analysis based on as-treated exposure definition, latency period is 30 days.
†PS-weighted HRs were standardized to the distribution of baseline covariates in IBT initiators.
Figure 1.
SMR-weighted Kaplan-Meier plots of ADRRT. A: DPP4i vs. SU cohort. HR 0.91 (95% CI 0.79–1.04). B: DPP4i vs. TZD cohort. HR 0.91 (95% CI 0.75–1.11). C: GLP1RA vs. LAI cohort. HR 0.50 (95% CI 0.39–0.65). D: GLP1RA vs. TZD cohort. HR 0.75 (95% CI 0.53–1.06). SMR weights create a pseudo-population of the untreated (comparators: SU, TZD, or LAI), which has the same covariate distribution as the treated (IBT). Every patient receiving IBT has a weight of 1, whereas every patient in the comparator group is weighted by PS/(1 − PS). The risks on the y-axis were obtained by a SMR-weighted Cox model (weighting comparator drug initiators by the PS odds [PS/(1 − PS)]). HR treats comparators as reference, and adjusted HR <1 indicates a lower risk for IBT.
The results for analysis based on multiple imputation for missing clinical measures (Supplementary Table 5) and subsequent analysis (Supplementary Table 6) stratified by HbA1c are consistent with primary results. When stratified by duration and preexisting retinopathy (Table 3) and baseline hypertension and ACE inhibitor/ARB treatment (Supplementary Table 7), the HRs of ADRRT did not differ meaningfully and the CIs widely overlap. We, therefore, refrained from statistical testing for interactions. When stratified by individual IBT, both exenatide and liraglutide showed a lower risk compared with LAI, and only exenatide showed a lower risk compared with TZD (Supplementary Table 8).
Table 3.
Crude and adjusted HRs for ADRRT associated with use of IBTs compared with therapeutic alternatives according to duration of use and preexisting retinopathy at cohort entry*
| Stratum | Comparison | Cohort | Patients, n | Median duration (years) of treatment (IQR)† | Person-years | ADRRT events, n | ADRRT incidence per 1,000 person-years (95% CI) | Crude HR (95% CI) | PS weighting,‡ HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Duration of use | |||||||||
| <6 months§ |
DPP4i vs. SU |
DPP4i |
39,292 |
0.50 (0.41–0.50) |
17,325 |
186 |
10.7 (9.3–12.4) |
1.09 (0.91–1.30) |
0.91 (0.75–1.10) |
| SU |
87,073 |
0.50 (0.42–0.50) |
39,344 |
385 |
9.8 (8.9–10.8) |
1.00 (reference) |
1.00 (reference) |
||
| DPP4i vs. TZD |
DPP4i |
51,410 |
0.50 (0.41–0.50) |
23,213 |
269 |
11.6 (10.3–13.1) |
0.77 (0.63–0.94) |
0.79 (0.62–1.02) |
|
| TZD |
22,231 |
0.50 (0.41–0.50) |
10,136 |
152 |
15.0 (12.8–17.6) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. LAI |
GLP1RA |
9,561 |
0.50 (0.41–0.50) |
4,105 |
46 |
11.2 (8.4–15.0) |
0.55 (0.41–0.74) |
0.54 (0.40–0.73) |
|
| LAI |
82,849 |
0.50 (0.41–0.50) |
36,108 |
732 |
20.3 (18.8–21.8) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. TZD |
GLP1RA |
10,355 |
0.50 (0.41–0.50) |
4,506 |
87 |
19.3 (15.6–23.8) |
1.33 (1.03–1.71) |
0.80 (0.52–1.23) |
|
| TZD |
27,345 |
0.50 (0.42–0.50) |
12,616 |
182 |
14.4 (12.5–16.7) |
1.00 (reference) |
1.00 (reference) |
||
| 6–12 months‖ |
DPP4i vs. SU |
DPP4i |
26,101 |
1.00 (0.75–1.00) |
10,099 |
67 |
6.6 (5.2–8.4) |
1.14 (0.85–1.52) |
1.01 (0.74–1.37) |
| SU |
61,308 |
1.00 (0.81–1.00) |
24,395 |
142 |
5.8 (4.9–6.9) |
1.00 (reference) |
1.00 (reference) |
||
| DPP4i vs. TZD |
DPP4i |
35,531 |
1.00 (0.75–1.00) |
13,673 |
86 |
6.3 (5.1–7.8) |
0.91 (0.62–1.33) |
0.95 (0.56–1.61) |
|
| TZD |
15,095 |
1.00 (0.72–1.00) |
5,663 |
39 |
6.9 (5.0–9.4) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. LAI |
GLP1RA |
5,658 |
1.00 (0.68–1.00) |
2,036 |
NTSR |
3.4 (1.6–7.2) |
0.34 (0.16–0.72) |
0.37 (0.17–0.79) |
|
| LAI |
52,365 |
1.00 (0.73–1.00) |
19,945 |
201 |
10.1 (8.8–11.6) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. TZD |
GLP1RA |
6,188 |
0.99 (0.66–1.00) |
2,163 |
18 |
8.3 (5.2–13.2) |
0.99 (0.59–1.67) |
0.69 (0.28–1.69) |
|
| TZD |
19,131 |
1.00 (0.73–1.00) |
7,212 |
61 |
8.5 (6.6–10.9) |
1.00 (reference) |
1.00 (reference) |
||
| >12 months |
DPP4i vs. SU |
DPP4i |
15,821 |
2.03 (1.40–3.14) |
22,914 |
96 |
4.2 (3.4–5.1) |
1.09 (0.86–1.38) |
0.85 (0.66–1.09) |
| SU |
39,678 |
2.17 (1.46–3.48) |
65,636 |
245 |
3.7 (3.3–4.2) |
1.00 (reference) |
1.00 (reference) |
||
| DPP4i vs. TZD |
DPP4i |
21,357 |
2.00 (1.39–3.09) |
30,597 |
165 |
5.4 (4.6–6.3) |
0.98 (0.73–1.31) |
1.32 (0.87–2.00) |
|
| TZD |
8,573 |
1.87 (1.34–2.86) |
11,250 |
62 |
5.5 (4.3–7.1) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. LAI |
GLP1RA |
2,934 |
1.75 (1.29–2.63) |
3,344 |
13 |
3.9 (2.3–6.7) |
0.45 (0.26–0.78) |
0.49 (0.28–0.86) |
|
| LAI |
31,550 |
2.16 (1.44–3.40) |
50,876 |
435 |
8.6 (7.8–9.4) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. TZD |
GLP1RA |
3,051 |
1.72 (1.30–2.49) |
3,251 |
17 |
5.2 (3.2–8.4) |
0.81 (0.48–1.36) |
0.61 (0.31–1.20) |
|
| TZD |
10,906 |
1.87 (1.34–2.88) |
14,487 |
91 |
6.3 (5.1–7.7) |
1.00 (reference) |
1.00 (reference) |
||
| Baseline retinopathy | |||||||||
| No preexisting retinopathy |
DPP4i vs. SU |
DPP4i |
34,737 |
0.72 (0.41–1.64) |
43,762 |
154 |
3.5 (3.0–4.1) |
0.98 (0.82–1.19) |
0.82 (0.67–1.00) |
| SU |
78,917 |
0.83 (0.41–1.98) |
115,848 |
389 |
3.4 (3.0–3.7) |
1.00 (reference) |
1.00 (reference) |
||
| DPP4i vs. TZD |
DPP4i |
45,316 |
0.75 (0.41–1.66) |
58,278 |
225 |
3.9 (3.4–4.4) |
0.73 (0.58–0.90) |
0.79 (0.59–1.04) |
|
| TZD |
19,520 |
0.69 (0.41–1.48) |
23,198 |
129 |
5.6 (4.7–6.6) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. LAI |
GLP1RA |
8,536 |
0.58 (0.41–1.17) |
8,269 |
38 |
4.6 (3.3–6.3) |
0.53 (0.38–0.73) |
0.47 (0.33–0.65) |
|
| LAI |
71,115 |
0.59 (0.41–1.56) |
87,616 |
673 |
7.7 (7.1–8.3) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. TZD |
GLP1RA |
8,973 |
0.58 (0.41–1.12) |
8,397 |
71 |
8.5 (6.7–10.7) |
1.34 (1.02–1.77) |
0.70 (0.44–1.11) |
|
| TZD |
23,898 |
0.73 (0.41–1.53) |
29,293 |
164 |
5.6 (4.8–6.5) |
1.00 (reference) |
1.00 (reference) |
||
| With preexisting retinopathy | DPP4i vs. SU |
DPP4i |
4,555 |
0.94 (0.53–1.88) |
6,460 |
195 |
30.2 (26.2–34.8) |
1.00 (0.84–1.19) |
1.02 (0.85–1.23) |
| SU |
8,156 |
1.11 (0.61–2.19) |
13,251 |
383 |
28.9 (26.1–32.0) |
1.00 (reference) |
1.00 (reference) |
||
| DPP4i vs. TZD |
DPP4i |
6,094 |
1.01 (0.59–1.97) |
9,048 |
295 |
32.6 (29.0–36.6) |
1.01 (0.82–1.25) |
1.04 (0.77–1.39) |
|
| TZD |
2,711 |
0.99 (0.61–1.81) |
3,786 |
124 |
32.8 (27.4–39.1) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. LAI |
GLP1RA |
1,025 |
0.82 (0.51–1.51) |
1,193 |
28 |
23.5 (16.2–34.1) |
0.60 (0.41–0.87) |
0.58 (0.39–0.85) |
|
| LAI |
11,734 |
1.07 (0.62–2.19) |
19,082 |
695 |
36.4 (33.8–39.3) |
1.00 (reference) |
1.00 (reference) |
||
| GLP1RA vs. TZD | GLP1RA |
1,382 |
0.78 (0.50–1.39) |
1,498 |
51 |
34.1 (25.8–45.0) |
0.93 (0.68–1.27) |
0.89 (0.56–1.41) |
|
| TZD | 3,447 | 1.00 (0.65–1.84) | 4,940 | 170 | 34.4 (29.6–40.1) | 1.00 (reference) | 1.00 (reference) | ||
IQR, interquartile range; NTSR, numbers too small (<11) to report based on Centers for Medicare & Medicaid Services rules and data use agreement.
*Analysis based on as-treated exposure definition, latency period is 30 days.
†Median duration of therapy for patients with a duration of treatment of more than 6 months was calculated by the actual duration of treatment received.
‡PS-weighted HRs were standardized to the distribution of baseline covariates in IBT initiators.
§Patients with a duration of treatment of more than 6 months were censored at 6 months postinitiation.
∥Patients with a duration of treatment of more than 12 months were censored at 12 months postinitiation.
Sensitivity Analyses
Overall, results of sensitivity analyses for ADRRT were consistent with our primary analysis (Supplementary Tables 9–15). However, we observed a decreased risk in the GLP1RA versus TZD comparison using a 180-day latency period (HR 0.68 [95% CI 0.50–0.93]) (Supplementary Table 9) or initial treatment analysis (0.75 [0.58–0.98]) (Supplementary Table 10). Our analyses for secondary outcome, IDR, also suggested similar results, except for a slightly lower risk for GLP1RA compared with TZD (e.g., HR 0.85 [0.75–0.97] using a 30-day latency period) (Supplementary Table 16).
Conclusions
Our study is the first large, population-based study to examine the effect of IBTs on the risk of DR among older adults with type 2 diabetes. Our results suggested that, compared with therapeutic alternatives, initiating IBTs is not associated with an increased risk of DR. Similar results were observed for DPP4i and GLP1RA, and, overall, the results were consistent across secondary and sensitivity analyses.
Unmeasured Confounding
Because some risk factors for DR may not be well captured in claims data (26), we mainly rely on the ACNU study design to reduce the potential for unmeasured confounding by indication. This study approach is a proven, effective method for minimizing the risk of confounding by indication. For example, in our previous study on cancer incidence among patients initiating insulin with glargine versus human NPH insulin (27), the strongest predictor of the need for insulin in patients with type 2 diabetes, BMI, had no influence on the choice of insulin because both cohorts in the ACNU design were candidates for either drug, thereby effectively removing unmeasured confounding that could be associated with differences in BMI between cohorts.
In this study, Table 1 presents evidence that treatment choice between IBTs and comparator was not meaningfully affected by measured markers of diabetes severity, including codes for neuropathy and nephropathy and broad categories of HbA1c levels, except for the GLP1RA versus LAI comparison. This finding is further evidence that if none of the measured risk factors for DR affect the choice between initiating these drug classes, it is unlikely that other unmeasured risk factors that would impact our study outcomes also influence treatment choice.
Additionally, we performed multiple imputation of key diabetes measures to further control for unmeasured confounding. Using this approach is statistically valid even though we have approximately 90% of missing data for the categories of HbA1c, blood pressure, and LDL cholesterol because the proportion of variables with missing data are small (4 out of 50 variables) and the size of the validation study (i.e., those with HbA1c, blood pressure, and LDL cholesterol values) is large (several hundred patients in each category) (28,29). The HRs changed slightly after multiple imputation for these clinical measures, indicating HbA1c had little effect on treatment choice except for GLP1RA versus insulin (both true and imputed clinical measures were not balanced for this comparison). However, as we only have broad HbA1c categories and have no separate category for very high HbA1c (e.g., >12% [108 mmol/mol]), there might be residual confounding by HbA1c, especially in our highest category (>9% [75 mmol/mol]).
IBT and Retinopathy Risk
As both albiglutide and dulaglutide were approved in 2014 in the U.S. (30,31), the majority of GLP1RA cohorts are exenatide and liraglutide initiators. GLP1RA therapies were not associated with an increased DR risk compared with therapeutic alternatives in our study, which is in line with the results of the EXSCEL (9) and LEADER trials (7). A few possible reasons may explain the inconsistency between our results and the significant findings in the SUSTAIN-6 (6) and TECOS (8) trials. First, the cardiovascular trials either did not assess DR (7–9) or grade DR severity (6) at baseline. Although randomization tends to minimize bias related to preexisting retinopathy, due to the relatively small numbers in these trials, it is possible that, by chance, the degree of retinopathy was not matched between the two arms (32). Second, it is possible that the effect of semaglutide on retinopathy truly exists, and the risk of DR differs across the class of GLP1RA, whereas our cohorts do not include semaglutide. Third, our study was limited to the elderly Medicare population and may not reflect outcomes in younger patients in trials who may have a lower risk of retinopathy. Last, the real-world adherence and persistence to treatment is typically lower (14) than trials, and the median duration for treatment in our cohorts ranged from 0.58 to 0.87 years.
GLP1RA was associated with a lower risk for DR (both ADRRT and IDR) compared with LAI and with a lower risk of IDR compared with TZD. Although results adjusting for HbA1c based on multiple imputation and subgroup analysis did not change much from primary analysis, residual confounding could not completely be ruled out. However, it is also possible that GLP1RA truly reduces the retinopathy risk. Topical administration of either GLP1RA (33) or DPP4i (34) prevents retinal neurodegeneration in mice. Further, the rapid glycemic control when initiating LAI is associated with early worsening of DR (23,35–37), which could contribute to this finding. TZD’s effects on macular edema (24,38) may contribute to the observed GLP1RA’s lower risk of IDR as well, although evidence has been conflicting (38,39). DPP4i shows a trend (statistically insignificant) of decreased risk of DR (both ADRRT and IDR), which could be explained by the observed preclinical beneficial effect on the retina (34).
Strengths
Our study has several strengths. With data that are representative of U.S. older adults, our large observational cohort study is the first real-world evidence designed to assess the effect of IBTs on degree of DR severity. Our ACNU design comparing IBT initiators with patients initiating a guideline-recommended clinical alternative both reduces the potential for unmeasured confounding by indication and provides a clinically appropriate comparison. We systemically assessed the potential for residual confounding and used rigorous statistical adjustment to minimize remaining imbalances between treatment cohorts compared.
We used several methods to minimize detection bias, recognizing that patients who do not have eye exams cannot have severe DR diagnosed or treatments applied. First, we required baseline eye exams within the year before cohort entry, and our data showed that the duration between last eye exam and index date was well balanced between IBTs and comparators. Second, as other eye conditions may evoke greater attention from eye care professionals, leading to earlier detection and treatment of DR, we systematically evaluated and balanced baseline eye diseases. Third, we used Medicare’s low-income subsidy as a surrogate marker for socioeconomic risk factors for DR diagnosis (16). Last, in a sensitivity analysis, we also restricted the analysis to patients with an eye exam after cohort entry, with results consistent with the primary analysis.
Limitations
Our study has limitations. First, we had no information on diabetes duration, and data on measures of glycemic control were available for only a small proportion of the population. However, we used surrogate measures of diabetes duration and glycemic control including age, diabetes complications, number of hyperglycemia diagnoses, and number of hospitalizations due to diabetes. The well-balanced covariates and the available clinical measures suggest our ACNU design removed unmeasured confounding except for in the GLP1RA versus LAI comparison. We also performed multiple imputation for missing clinical data and conducted several stratified analyses to minimize the risk that unmeasured confounding is responsible for our results.
Second, our algorithm for detection of the outcome has not been validated previously. However, we identified our primary outcome by treatments, which are specialist procedures that would be provided only by an ophthalmologist. Furthermore, for cases identified with an intravitreal injection procedure code (67028), we required that it be used jointly with a HCPCS drug code to minimize miscoding of treatments and excluded patients with specific forms of AMD that are treated by intravitreal injections. Therefore, it is unlikely that patients that met our disease definition (with diagnosis of diabetes or DR and received retinopathy treatment) did not receive treatment for retinopathy. A highly specific outcome definition minimizes bias in the HR, even in the presence of nonperfect sensitivity (40). Reassuringly, our sensitivity analysis that modified the outcome definition to be more specific (with DR diagnosis and evidence of receiving retinopathy treatments) produced results that were consistent with those of our primary analysis.
Last, we only assessed short-term IBT use, as the real-world adherence is low (14). The short follow-up in this study may impair the potential for detecting differences with long-term use of these drugs. Long-term specific studies with systematic grading of DR and consideration of significant clinical variables such as diabetes duration, HbA1c, and hypertension are urgently needed.
Summary
Our population-based, ACNU cohort study of older U.S. adults with diabetes suggests that the real-world use of IBTs over an average of less than 1 year does not increase the risk of DR compared with alternatives.
Supplementary Material
Article Information
Funding and Duality of Interest. This work was supported by a grant from Novo Nordisk. The sponsor had no role in analysis, drafting, or the decision to submit this manuscript. J.-L.H. is currently an employee of Takeda. V.P. receives salary support from R01 AG056479 and R01 HL118255 and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (UL1TR002489). S.G. is supported by the Diabetic Retinopathy Clinical Research Network, NIH (U10EY018817-03, U10EY014229-07, U10EY014231-09, EY14231, EY14269, and EY14229) and serves on the clinical advisory board for Welch Allyn. J.B.B. is supported by NIH (UL1TR001111). He has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Lexicon, Metavention, NovaTarg, Novo Nordisk, Sanofi, Senseonics, and vTv Therapeutics and grant support from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Lexicon, Novo Nordisk, Sanofi, Theracos, and vTv Therapeutics. He holds stock options in Mellitus Health and PhaseBio and has served on the board of the AstraZeneca HealthCare Foundation. T.S. receives investigator-initiated research funding and support from the National Institute on Aging as a principal investigator (R01/R56 AG023178 and R01 AG056479) and from NIH as co-investigator (R01 CA174453, R01 HL118255, and R21-HD080214). He also receives salary support as director of the Comparative Effectiveness Research Strategic Initiative, North Carolina Translational and Clinical Sciences Institute; from the UNC Clinical and Translational Science Award (UL1TR002489); and as director of the Center for Pharmacoepidemiology (current members are GlaxoSmithKline, UCB BioSciences, Merck, and Shire). He also receives research support from pharmaceutical companies (Amgen and AstraZeneca) to the Department of Epidemiology, UNC. He does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the population-based evaluation of drug benefits and harms in older U.S. adults (GIL200811.0010); the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the Comparative Effectiveness Research Strategic Initiative of UNC’s Clinical and Translational Science Award (UL1TR002489); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.W. did the statistical analysis and wrote the first draft of the manuscript. T.W. and J.-L.H. developed the protocol. T.W., J.-L.H., J.B.B., and T.S. were involved in data review and interpretation. T.W., J.-L.H., E.W.G., and S.G. participated in the design of the study. T.W., E.W.G., S.G., J.B.B., and T.S. contributed to critical revision of the manuscript for important intellectual content. J.-L.H. carried out initial analysis. V.P. oversaw and supported programing. J.B.B. and T.S. conceived and designed the study. All authors approved the final version of the manuscript. T.W. and T.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017, and at the 33rd International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Montreal, Canada, 26–30 August 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2285/-/DC1.
References
- 1.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 2010;33:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan AG, Blind E, Dunder K, et al. . Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–797 [DOI] [PubMed] [Google Scholar]
- 3.Gokhale M, Buse JB, Gray CL, Pate V, Marquis MA, Stürmer T. Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 2014;16:1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015;385:2107–2117 [DOI] [PubMed] [Google Scholar]
- 5.Gokhale M, Buse JB, Jonsson Funk M, et al. . No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase-4 inhibitors vs therapeutic alternatives. Diabetes Obes Metab 2017;19:970–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 9.Holman RR, Bethel MA, Mentz RJ, et al. l.; EXSCEL Study Group Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40:S64–S74 [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, Chew E, Duh EJ, et al. . Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh R, Ross JS, Sangaralingham LR, Adelman RA, Shah ND, Barkmeier AJ. Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and Medicare Advantage patients. Ophthalmology 2017;124:352–358 [DOI] [PubMed] [Google Scholar]
- 14.Carls GS, Tuttle E, Tan RD, et al. . Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care 2017;40:1469–1478 [DOI] [PubMed] [Google Scholar]
- 15.Varadhan L, Humphreys T, Hariman C, Walker AB, Varughese GI. GLP-1 agonist treatment: implications for diabetic retinopathy screening. Diabetes Res Clin Pract 2011;94:e68–e71 [DOI] [PubMed] [Google Scholar]
- 16.Martinell M, Dorkhan M, Stålhammar J, Storm P, Groop L, Gustavsson C. Prevalence and risk factors for diabetic retinopathy at diagnosis (DRAD) in patients recently diagnosed with type 2 diabetes (T2D) or latent autoimmune diabetes in the adult (LADA). J Diabetes Complications 2016;30:1456–1461 [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–686 [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49 [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Quality Assurance. The Healthcare Effectiveness Data and Information Set (HEDIS) measures. Available from http://www.ncqa.org/hedis-quality-measurement/hedis-measures. Accessed 10 April 2018
- 20.Bagley B. Measuring for Medicare: the Physician Quality Reporting Initiative. Available from https://www.aafp.org/fpm/2007/0600/p37.pdf. Accessed 10 April 2018 [PubMed]
- 21.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ, John Wiley & Sons, 2004 [Google Scholar]
- 22.Silva PS, Aiello LP. Telemedicine and eye examinations for diabetic retinopathy: a time to maximize real-world outcomes. JAMA Ophthalmol 2015;133:525–526 [DOI] [PubMed] [Google Scholar]
- 23.The Diabetes Control and Complications Trial Research Group Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol 1998;116:874–886 [DOI] [PubMed] [Google Scholar]
- 24.Makri OE, Georgalas I, Georgakopoulos CD. Drug-induced macular edema. Drugs 2013;73:789–802 [DOI] [PubMed] [Google Scholar]
- 25.Nencini C, Barberi L, Runci FM, Micheli L. Retinopathy induced by drugs and herbal medicines. Eur Rev Med Pharmacol Sci 2008;12:293–298 [PubMed] [Google Scholar]
- 26.Stürmer T, Glynn RJ, Rothman KJ, Avorn J, Schneeweiss S. Adjustments for unmeasured confounders in pharmacoepidemiologic database studies using external information. Med Care 2007;45(Suppl. 2):S158–S165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stürmer T, Marquis MA, Zhou H, et al. . Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes Care 2013;36:3517–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl S, Boulesteix AL, Zierer A, Thorand B, van de Wiel MA. Erratum to: Assessment of predictive performance in incomplete data by combining internal validation and multiple imputation. BMC Med Res Methodol 2016;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. Tanzeum Albiglutide. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125431. Accessed 17 October 2017
- 31.U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. Trulicity Dulaglutide. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125469. Accessed 17 October 2017
- 32.Simó R, Hernández C. GLP-1R as a target for the treatment of diabetic retinopathy: friend or foe? Diabetes 2017;66:1453–1460 [DOI] [PubMed] [Google Scholar]
- 33.Hernández C, Bogdanov P, Corraliza L, et al. . Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016;65:172–187 [DOI] [PubMed] [Google Scholar]
- 34.Hernández C, Bogdanov P, Solà-Adell C, et al. . Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017;60:2285–2298 [DOI] [PubMed] [Google Scholar]
- 35.Arun CS, Pandit R, Taylor R. Long-term progression of retinopathy after initiation of insulin therapy in type 2 diabetes: an observational study. Diabetologia 2004;47:1380–1384 [DOI] [PubMed] [Google Scholar]
- 36.The Kroc Collaborative Study Group Diabetic retinopathy after two years of intensified insulin treatment. Follow-up of the Kroc Collaborative Study. JAMA 1988;260:37–41 [DOI] [PubMed] [Google Scholar]
- 37.Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes 1985;34(Suppl. 3):74–79 [DOI] [PubMed] [Google Scholar]
- 38.Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med 2012;172:1005–1011 [DOI] [PubMed] [Google Scholar]
- 39.Gower EW, Lovato JF, Ambrosius WT, et al.; ACCORD Study Group . Lack of longitudinal association between thiazolidinediones and incidence and progression of diabetic eye disease: the ACCORD Eye study. Am J Ophthalmol 2018;187:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol 2012;65:343–349.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.